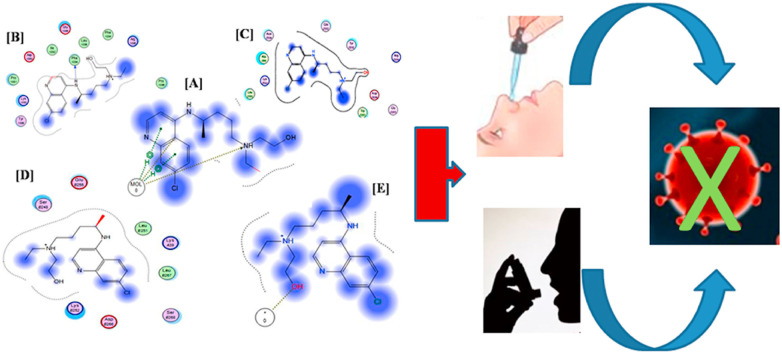
Abstract
Chloroquine (CQ) and hydroxychloroquine (HCQ) are undergoing several clinical trials for evaluating their efficacy and safety as antiviral drugs. Yet, there is still a great debate about their efficacy in combating COVID-19. This study aimed to evaluate the feasibility of intranasal and/or pulmonary administration of CQ/HCQ for COVID-19 using Bio/chemoinformatics tools. We, hereby, hypothesize the success of the intranasal and the pulmonary routes through a gelatin matrix to overcome several challenges related to CQ and HCQ pharmacodynamics and pharmacokinetics properties and to increase their local concentrations at the sites of initial viral entry while minimizing the potential side effects. Molecular docking on the gelatin-simulated matrix demonstrated high loading values and a sustained release profile. Moreover, the docking on mucin as well as various receptors including Angiotensin-converting enzyme 2 (ACE-2), heparin sulphate proteoglycan and Phosphatidylinositol binding clathrin assembly protein (PICALM), which are expressed in the lung and intranasal tissues and represent initial sites of attachment of the viral particles to the surface of respiratory cells, has shown good binding of CQ and HCQ to these receptors. The presented data provide an insight into the use of a novel drug formulation that needs to be tested in adequately powered randomized controlled clinical trials; aiming for a sustained prophylaxis effect and/or a treatment strategy against this pandemic viral infection.
Keywords: Chloroquine, COVID-19, Gelatin, Bioinformatics, Intranasal, Receptors, Pulmonary, Virus
Graphical abstract
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or COVID-19 has now been declared by the WHO as a new pandemic disease and hence contingency measures are urgently needed to face this immediate global challenge. Given the novelty of the infection and the lack of preventive vaccines or proven treatments, drug repurposing is being currently employed to combat this worldwide outbreak. This is an emerging strategy where existing medications that had already been tested safe in humans, are redeployed to combat difficult-to-treat diseases.
Chloroquine (CQ) and its derivative, Hydroxychloroquine (HCQ), have been used as prophylactic measures in malaria-endemic regions and also for the treatment of autoimmune diseases world-wide. CQ has shown therapeutic activity against viruses [40] including the human corona virus OC43 in animal models [29] and SARS-CoV in cell cultures [45]. Both CQ and HCQ were recently shown to have similar anti-SARS-CoV-2 effects in cell culture [32,59] and so may have prophylactic and/or therapeutic effects against COVID-19 [8]. Recently, Wang et al. demonstrated that at a low micromolar concentration, CQ was able to potently block viral infection at both entry and at post-entry stages of the SARS-CoV-2 in vitro. The effective concentration was that achievable in patients receiving 500 mg daily [49].
Recent evidence suggested that SARS-CoV entry (being a nanoparticle) [63] into host cells involves receptor-mediated endocytosis [64] despite that it was initially thought to directly fuse with the plasma membrane [48]. Heparan sulphate proteoglycans (HSPGs) were shown to provide the preliminary docking sites for the spike protein on the viral cell surface [46] and play an important role in SARS-CoV invasion at the early attachment phase [30]. The human Coronavirus NL63 (HCoV-NL63) has also been shown to utilize HSPGs for attachment to target cells [36].
It has been established that Angiotensin-converting enzyme 2 (ACE2) serves as a functional receptor for SARS-CoV and HCoV-NL63, triggering endocytosis-driven cell entry [31,52,54]. Similarly, SARS-CoV-2 might use the ACE2-mediated mechanisms of host cell entry [53]. SARS-CoV has also been shown to utilize both clathrin-mediated and clathrin/caveolae-independent endocytosis mechanisms for its entry into human target cells [8,26]. Additionally, Phosphatidyl inositol binding clathrin assembly protein (PICALM) is a cargo-selecting clathrin adaptor that senses and drives membrane curvature, thereby regulating the rate of endocytosis [37].
CQ anti-SARS-CoV activity was demonstrated in the cell cultures even when administered after a viral infection [45]. This includes inhibition of pH-dependent viral fusion/replication by increasing the pH of endosomes and lysosomes [60]; prevention of glycosylation of the viral glycoprotein envelope and the host receptor protein [13]. Besides, CQ caused the inhibition of the virion assembly [9,40].
In addition to their direct antiviral actions, CQ and its derivatives were proven to exhibit host effects by attenuating the expression of pro-inflammatory factors and receptors [10] that can induce acute respiratory distress syndrome and are involved in the cytokine storm associated with the disease severity of COVID-19 [25]. Moreover, CQ and HCQ have demonstrated not only anti-viral and anti-inflammatory effects but also anti-thrombotic effects [39].
Nevertheless, CQ was proven to act as a zinc ionophore [57], where this element acts against viral replication by interfering with the viral RNA-dependent RNA-polymerase [28].
The efficacy and safety of CQ/HCQ in the clinical context of treatment and prophylaxis of COVID-19 were addressed in several clinical trials that showed inconsistent results. A clinical study with more than 100 patients demonstrated superiority of CQ phosphate to controls in inhibiting the exacerbation of pneumonia, and shortening the disease course without severe adverse events [13]. A non-randomized open-label trial in 20 patients showed that HCQ induced viral clearance in nasopharyngeal samples after 6 days of treatment, either alone or in combination with azithromycin [14]. On the other hand, an observational study involving 1446 hospitalized patients with COVID-19 showed that HCQ administration was not associated with either a greatly lowered or an increased risk of the composite end point of intubation or death [15]. A recently published systematic review concluded that COVID-19 patients should be treated with CQ/HCQ only if appropriately monitored and if the treatment lies within the context of high quality randomized controlled trials [11].
Understanding the mechanisms by which CQ and HCQ affect SARS-CoV-2 would be critical for optimizing and developing preventative and therapeutic strategies [24]. We sought to evaluate CQ and HCQ as potential anti-SARS-CoV-2 using Bio/chemoinformatics tools aiming to provide a help in the debate. The differences between the two investigated molecules (if any) could be attributed to the differences in the main structural, topological and electronic descriptors of the drugs [20,35]. Both drugs were evaluated at the therapeutic level pertaining to suppression of endocytosis in host cells through studying the interaction with HSPGs, ACE-2, and PICALM. They were also evaluated at the formulation level by comparing the loading in gelatin microsphere matrices as a basis for the formation of particulate systems (micro and nano); and at the biopharmaceutical level through studying the interaction with mucin.
CQ and its derivatives are reported to possess high volume of distribution after oral administration reaching a level of 100 l per one kg of the patient weight indicating a high distribution in the tissues compartment; and therefore have to be administered in relatively high oral dosages; a fact that leads to the associated side effects [19]. These effects may range from ocular toxicity [41] to myocardial infarction [5]. This may in turns limit its clinical use and hamper successful treatment of COVID-19 patients.
To this end, the aim of the current study is to evaluate the feasibility of a CQ/HCQ formulation that would be administered through an alternative route other than the oral delivery such as the intranasal and/or pulmonary routes. Uniting re-purposing of drugs with re-formulation aids in increasing the local concentrations of the drugs at the sites of initial SARS-CoV-2 entry and leads to a sustained prophylaxis effect and/or as a treatment strategy, while potentially reducing the adverse events.
2. Methodology
2.1. Construction of the virtual carrier using molecular dynamics simulations
GROMACS (17) v4.6.5 software package was used to carry-out all-atom molecular dynamics simulations. The atom typing and assignment of parameters and charges of the gelatin matrix [35] were carried-out online (https://cgenff.paramchem.org/) according to CHARMM general force field (CgenFF). To prepare the gelatin system, 48 peptide molecules were constructed, comprising 18 amino acids in each molecule. The primary sequence of the peptides was AGPRGQ (Hyp)GPAGPDGQ (Hyp)GP. The matrix was then subjected to a molecular dynamics run, with full periodic boundary conditions, a time step of 2 fs, and a cut-off distance for van der Waals's and electrostatic interactions of 1 nm. PME was used to calculate electrostatic interactions and LINCS algorithm was used to constrain all bonds. The systems were equilibrated for 7 ns at 298K using a v-rescale thermostat at a pressure of 1 bar using a Berendsen barostat.
2.2. Construction of the Heparan Sulphate proteoglycan (HSPG)
The chemical structure of HSPG was obtained from PubChem®, drawn using the ChemDraw® Ultra package version 10 where two molecules (simulating the HSPG present in cell membranes) were utilized for docking after energy minimization using MMFF94x forcefield found in MOE® version 2014.0901 (Chemical Computing Group Inc., Montreal, Canada).
2.3. Obtaining the target peptides and proteins virtual matrices
The crystal structure of the relevant delivery and the related receptor targets were obtained from the protein data bank (http://www.rcsb.org). The codes 2ACM corresponded to Mucin. The codes 6m17, 3zyk corresponded to the ACE-2 and PICALM, respectively. The polar hydrogens were added to the obtained pdb files using MOE® version 2014.0901 (Chemical Computing Group Inc., Montreal, Canada).
2.4. Preparing the drugs chemical structures for docking
The isomeric SMILES corresponding to the chemical structures of the studied antimalarial drugs; CQ and HCQ were obtained using PubChem®. The corresponding 3D chemical structures were generated using the builder function of MOE® version 2014.0901 (Chemical Computing Group Inc., Montreal, Canada). Further, energy minimization was carried out for all the investigated molecules using MMFF94x forcefield of the same software [21].
2.5. Docking of the investigated drugs on the investigated carrier
The docking analysis was employed using MOE version 2014.0901 (Chemical Computing Group Inc., Montreal, Canada). The pdb file of the protein nanoparticles matrix was imported to MOE where the identification of the binding site was performed using MOE's “Site finder” tool [23]. The docking experiment was conducted using the “triangle matcher” as a placement method.
The used software creates dummy atoms around the docking target atoms. These dummy atoms are considered the docking positions. The London ΔG score was utilized for calculating the binding energies scoring values. The London ΔG scoring function estimates the free energy of binding of the ligand from a given optimum pose.
2.6. Calculating the main descriptors of the investigated drugs
In order to explain the differences in docking scores observed for the studied drugs, some crucial constitutional, electronic and topological descriptors were calculated. The selected descriptors were the molecular weight, xLogP, topological polar surface area, number of H-atoms donors and acceptors and finally the fragment complexity. The descriptors were calculated using Bioclipse® version 2.6 (Bioclipse project, Uppsala University, Sweden) using the molecules mol files generated using ChemDraw® Ultra version 10.
2.7. Statistical analysis
Allstatistical analyses in this study was performed using GraphPad Prism® v.5.0 (GraphPad software, San Diego, CA) and at a level of significance P < 0.05.
3. Results and discussion
The global impact of COVID-19 infection is profound as it represents a public health threat that is entering a phase beyond containment. De novo drug development is a lengthy and a costly process and hence impractical to face the immediate global demand. The antimalarial drugs, CQ and HCQ, have shown to block COVID-19 infection in cell culture studies [50,59]. CQ, specifically, was recently found to be highly effective in reducing the viral replication that can be easily achievable with standard dosing due to their favourable penetration in tissues such as the lung [33], [7][7,33].
Deepening in the several suggested mechanisms by which these drugs could affect SARS-CoV-2 would provide a more profound insight into the rational formulation that is needed for the treatment of this infection. In this study, Bio/Chemoinformatics tools are deployed to study the docking of CQ and HCQ into a gelatin matrix as the basis for the formation of particulate systems (micro/nano). Table 1 shows the obtained binding energies after docking of the two investigated drugs HCQ and CQ on the related macromolecules.
Table 1.
Docking binding energy (ΔG) values after docking of the investigated drugs on the related macromolecules.
| Macromolecule (Carrier/Protein/Proteoglycan) – PDB code | Binding Energy (kcal/mole) |
|
|---|---|---|
| Chloroquine | Hydroxychloroquine | |
| Gelatin matrix | −8.72 ± 0.1 | −10.09 ± 0.01 |
| (Gelatin nanospheres) | ||
| Mucin – 2ACM | −9.22 ± 0.1 | −10.10 ± 0.1 |
| ACE-2 – 6m17 | −8.71 ± 0.2 | −8.75 ± 0.2 |
| PICALM- 3zyk | −8.29 ± 0.1 | −10.49 ± 0.2 |
| Heparan Sulphate Proteoglycan | −8.83 ± 0.1 | −11.65 ± 0.1 |
* ACE-2: Angiotensin Converting Enzyme −2.
* PICALM: phosphatidylinositol binding clathrin assembly protein.
Both molecules showed good interactions with the investigated carrier and receptors. After performing an un-paired t-test using GraphPad Prism® v.5.0, the slightly significant (P < 0.05) lower score of the binding energy (more negative values of ΔG) indicated better interaction of HCQ on the gelatin matrix. This could be attributed to its more hydrophilic nature demonstrated by its higher total polar surface area and more H-bond donors and acceptors that can interact with the gelatin carboxylic and amino groups [22,38] compared to the CQ counterparts. This higher interaction may result in a higher loading potential and more sustained release effect of HCQ, specifically, from the gelatin matrix. Gelatin has been rationally selected as the nanoparticulate matrix material for loading the investigated drugs due to its high biocomptability, suitability for nasal and pulmonary delivery, mucoadhesive properties and its efficiency on loading several hydrophilic and hydrophobic drugs [1,22,42]. The successful construction of the gelatin matrix using the adopted molecular dynamics method was obtained after following the same protocols of the authors previous studies [23,35].
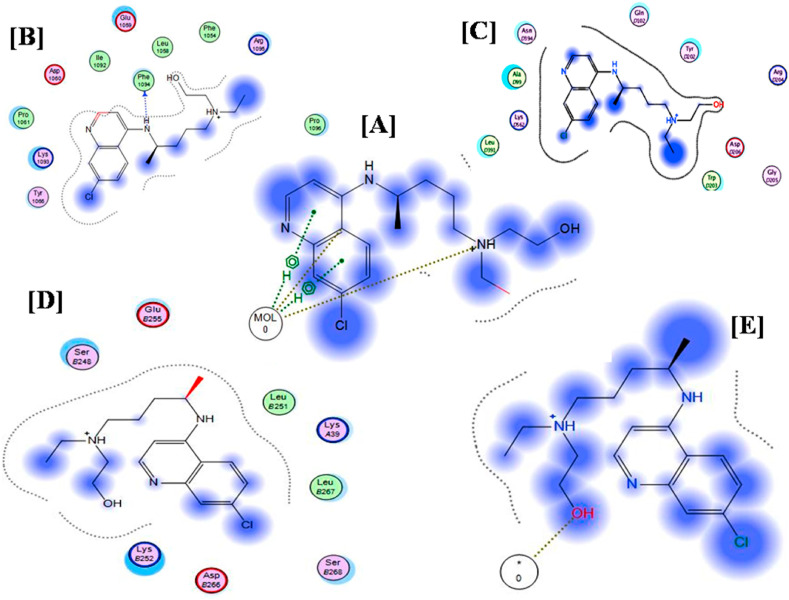
On the delivery level, the docking results of the two drugs on mucin as an indicator of the ability of the drug for better mucoadhesion was also in favor of HCQ. Mucin is an abundant micromolecule that is present in the airways [47]. Furthermore, higher affinity of HCQ to HSPGs, ACE -2 and PICALM was observed as revealed by the lower binding energy scores. This may be attributed to the higher polarity and hydrophilicity nature of HCQ as demonstrated by its physico-chemical descriptors; higher total polar surface area, lower LogP value and higher numbers of H-bond donors and acceptors (Table 2 ). These are crucial properties that are warranted for the compatibility with hydrophilic amino acids that are present in mucin, ACE-2 and PICALM such as: Aspartic acid, Glutamic acid, arginine and phenylalanine. Moreover, other HCQ structural and topological descriptors such as: less globularity, higher molecular flexibility and lower molecular weight contributes to the overall better affinity and interaction whether with the aforementioned enzymes or with HSPG (Table 2). Nevertheless, the obtained binding energies (Table 1) for HCQ specifically indicate significant interactions with the investigated macromolecules and high affinity (Fig. 1 ).
Table 2.
Main physico-chemical descriptors of the investigated drugs.
| Molecule | Canonical SMILES | Total polar surface area | Number of H-bond acceptors | Number of H-bond donors | Molecular globularity | Molecular Flexibility | logP (o/w) | Molecular weight |
|---|---|---|---|---|---|---|---|---|
| Chloroquine | Clc1cc2nccc (NC(CCC [NH+](CC)CC)C)c2cc1 | 28.20 | 3 | 1 | 0.173 | 5.996 | 4.287 | 320.888 |
| Hydroxychlor-oquine | Clc1cc2nccc (NC(CCC [NH+](CCO)CC)C)c2cc1 | 48.40 | 4 | 2 | 0.186 | 6.618 | 3.252 | 336.89 |
Fig. 1.
Docking results of Hydroxy-Chloroquine on [A] Gelatin matrix, [B] Mucin, [C] PICALM, [D] ACE-2 and [E] Heparan Sulphate.
HSPGs acts as primary binding sites, promoting viral docking and facilitating subsequent interaction with the specific receptors and also function as storage sites on non-permissive cells mediating ‘‘in trans’’ infection by presenting the viruses to their target cells [46]. Furthermore, Heparan sulphate is present in the lung tissues and is necessary for pulmonary haemostasis [17]. ACE-2 has been shown to be a co-receptor for SARS-CoV-2 viral entry with increasing evidence that it has a protracted role in the pathogenesis of COVID-19 [61]. ACE-2 is expressed in many body tissues, including the lungs [27,56,62]. CQ can interfere with ACE-2 receptor glycosylation thus prevents SARS-CoV-2 attachment to the target cells [33,49]. PICALM shows medium distribution in nasopharynx, bronchial and lung tissues (https://www.proteinatlas.org/ENSG00000073921-PICALM/tissue). PICALM is one of the three most abundant proteins in clathrin-coated pits. The depletion of this receptor has previously been shown to inhibit clathrin-mediated endocytosis; an important mechanism for the SARS-viruses internalization [24,37]. This is considered the main mechanism of nanoparticles internalization as well [2,12]. Thoughtfully, exploiting nanoparticulate formulations can impose competitive inhibition with the virus entry. It was previously shown that binding of CQ and HCQ to PICALM may induce the down-regulation of this protein [51].
The safety of CQ/HCQ is well established in malaria or autoimmune disease. However, some serious side effects (cardiotoxicity, retinopathy and neuromypathy) may occur with long-term usage of high doses or in overdose [3,18,19]. A clinical study of CQ phosphate in the treatment of COVID-19 associated pneumonia did not show severe adverse reactions in the patients [13]. On the other hand, in a cohort study of 90 hospitalized COVID-19 patients, the use of HCQ with or without azithromycin for treatment of pneumonia associated with COVID-19 increased the risk of corrected QT (QTc) prolongation, and concurrent treatment with azithromycin was associated with greater changes in QTc [34]. COVID-19 patients could be more vulnerable to these side effects because of their co-morbidities (such as cardiovascular disease, diabetes and obesity), advanced age, and subsequent co-medication [16].
4. Hypothesized prophylaxis protocol
Both CQ and HCQ have shown to have good binding scores to the aforementioned receptors as shown in Table 1. Furthermore, in an interesting study conducted by Bernard et al. [4], the intranasal CQ was proven effective in reducing the SARS-virus titres in BalB/c mice treated with 30 mg/kg dose by about a one-half logarithmic magnitude. In a more recent study, SARS-CoV-2 entry factors were found to be highly expressed in the nasal epithelial cells [43]. This finding encourages and warrants the usage of the intranasal route of administration of the suggested virus combating drugs. Hence, we propose testing the intranasal and the pulmonary routes in ongoing clinical trials as alternative routes to the oral delivery. The advantages of administering the drugs directly to the sites of viral entry include higher drug concentration in the affected tissues (the lungs and intranasal tissues) while avoiding large systemic concentrations [6], and minimizing the potential side effects as well. This is of great benefit for any prophylactic treatment that requires prolonged administration of a drug, particularly as in the case of CQ or HCQ.
According to the FDA, the therapeutics that are administered through alternative routes to the intravenous and the oral routes, should be normalized to the area of application [44]. The human intranasal and pulmonary areas are taken as 5 times those of the Balb/c mice. Moreover, it is now well acquainted that the intranasal and pulmonary doses are more effective than their oral counterparts and hence down-regulation of the doses is usually attained [55]. Therefore we suggest a daily dose of 50–100 mg CQ or HCQ [58] administered intranasally or through inhalation in a combination of gelatin micro/nanospheres matrices together with a zinc oral supplementation therapy [28]. The microspheres would impart better contact and localization of the drug associated with a sustained release effect. Complimentary to the microspheres action, the nanospheres would suggest another mechanism for combating the virus through competitive inhibition with the virus via the PICALM mechanism and would also impart better penetration into the lung cells (in case of treatment purposes).
5. Conclusion and future perspective
We hereby suggest evaluating the efficacy and safety of the use of CQ or HCQ through the intranasal and the pulmonary routes in adequately designed and powerful clinical trials aiming for the prophylaxis and/or the treatment of COVID-19. We proposed a novel idea of formulating these related re-purposed drugs in a combined gelatin micro/nano particulate matrices systems that would be administered through the intranasal and the pulmonary routes. This will lead to the prevention and the competitive inhibition of the cellular viral invasion in one hand and to the increase in the drugs penetration, the enhancement and the sustainment of their antiviral activity while reducing their dosing and hence the unfavourable side effects on the other hand. It is worth noting, that this virtual approach could be beneficial in assessing any other re-purposed drugs or new molecules, not only on the biological but also on the formulation level.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None Declared.
References
- 1.Abdelrady H., Hathout R.M., Osman R., Saleem I., Mortada N.D. Exploiting gelatin nanocarriers in the pulmonary delivery of methotrexate for lung cancer therapy. Eur J Pharmaceut Sci. 2019;133:115–126. doi: 10.1016/j.ejps.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Abozeid S.M., Hathout R.M., Abou-Aisha K. Silencing of the metastasis-linked gene, AEG-1, using siRNA-loaded cholamine surface-modified gelatin nanoparticles in the breast carcinoma cell line MCF-7. Colloids Surf B Biointerfaces. 2016;145:607–616. doi: 10.1016/j.colsurfb.2016.05.066. [DOI] [PubMed] [Google Scholar]
- 3.Ashley E.A., Recht J., White N.J. Primaquine: the risks and the benefits. Malar J. 2014;13:418. doi: 10.1186/1475-2875-13-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnard D.L., Day C.W., Bailey K., Heiner M., Montgomery R., Lauridsen L., Chan P.K., Sidwell R.W. Evaluation of immunomodulators, interferons and known in vitro SARS-coV inhibitors for inhibition of SARS-coV replication in BALB/c mice. Antivir Chem Chemother. 2006;17:275–284. doi: 10.1177/095632020601700505. [DOI] [PubMed] [Google Scholar]
- 5.Blignaut M., Espach Y., van Vuuren M., Dhanabalan K., Huisamen B. Revisiting the cardiotoxic effect of chloroquine. Cardiovasc Drugs Ther. 2019;33:1–11. doi: 10.1007/s10557-018-06847-9. [DOI] [PubMed] [Google Scholar]
- 6.Borghardt J.M., Kloft C., Sharma A. Inhaled therapy in respiratory disease: the complex interplay of pulmonary kinetic processes. Can. Respir. J. 2018:2732017. doi: 10.1155/2018/2732017. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colson P., Rolain J.M., Lagier J.C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020:105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colson P., Rolain J.M., Lagier J.C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020:105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colson P., Rolain J.M., Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020;55:105923. doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortegiani A., Ippolito M., Ingoglia G., Iozzo P., Giarratano A., Einav S. Update I. A systematic review on the efficacy and safety of chloroquine/hydroxychloroquine for COVID-19. J Crit Care. 2020;59:176–190. doi: 10.1016/j.jcrc.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farid M.M., Hathout R.M., Fawzy M., bou-Aisha K. Silencing of the scavenger receptor (Class B - type 1) gene using siRNA-loaded chitosan nanaoparticles in a HepG2 cell model. Colloids Surf B Biointerfaces. 2014;123:930–937. doi: 10.1016/j.colsurfb.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 13.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 14.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honore S., Colson P., Chabriere E., La S.B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., Labella A., Manson D.K., Kubin C., Barr R.G., Sobieszczyk M.E., Schluger N.W. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A., Satlin M.J., Campion T.R., Nahid M., Ringel J.B., Hoffman K.L., Alshak M.N., Li H.A., Wehmeyer G.T., Rajan M., Reshetnyak E., Hupert N., Horn E.M., Martinez F.J., Gulick R.M., Safford M.M. Clinical characteristics of covid-19 in New York city. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haeger S.M., Yang Y., Schmidt E.P. Heparan sulfate in the developing, healthy, and injured lung. Am J Respir Cell Mol Biol. 2016;55:5–11. doi: 10.1165/rcmb.2016-0043TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haeusler I.L., Chan X.H.S., Guerin P.J., White N.J. The arrhythmogenic cardiotoxicity of the quinoline and structurally related antimalarial drugs: a systematic review. BMC Med. 2018;16:200. doi: 10.1186/s12916-018-1188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haladyj E., Sikora M., Felis-Giemza A., Olesinska M. Antimalarials - are they effective and safe in rheumatic diseases? Reumatologia. 2018;56:164–173. doi: 10.5114/reum.2018.76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hathout R.M., El-Ahmady S.H., Metwally A.A. Curcumin or bisdemethoxycurcumin for nose-to-brain treatment of Alzheimer disease? A bio/chemo-informatics case study. Nat Prod Res. 2018;32:2873–2881. doi: 10.1080/14786419.2017.1385017. [DOI] [PubMed] [Google Scholar]
- 21.Hathout R.M., Metwally A.A. Towards better modelling of drug-loading in solid lipid nanoparticles: molecular dynamics, docking experiments and Gaussian Processes machine learning. Eur J Pharm Biopharm. 2016;108:262–268. doi: 10.1016/j.ejpb.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Hathout R.M., Metwally A.A. Gelatin nanoparticles. Methods Mol Biol. 2019:71–78. doi: 10.1007/978-1-4939-9516-5_6. 2000. [DOI] [PubMed] [Google Scholar]
- 23.Hathout R.M., Metwally A.A., Woodman T.J., Hardy J.G. Prediction of drug loading in the gelatin matrix using computational methods. ACS Omega. 2020;5:1549–1556. doi: 10.1021/acsomega.9b03487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu T.Y., Frieman M., Wolfram J. Insights from nanomedicine into chloroquine efficacy against COVID-19. Nat Nanotechnol. 2020;15:247–249. doi: 10.1038/s41565-020-0674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue Y., Tanaka N., Tanaka Y., Inoue S., Morita K., Zhuang M., Hattori T., Sugamura K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J Virol. 2007;81:8722–8729. doi: 10.1128/JVI.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J., Farzan M., Wohlford-Lenane C., Perlman S., McCray P.B., Jr. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaushik N., Subramani C., Anang S., Muthumohan R., Shalimar Nayak B., Ranjith-Kumar C.T., Surjit M. Zinc salts block hepatitis E virus replication by inhibiting the activity of viral RNA-dependent RNA polymerase. J Virol. 2017;91 doi: 10.1128/JVI.00754-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keyaerts E., Li S., Vijgen L., Rysman E., Verbeeck J., Van R.M., Maes P. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob Agents Chemother. 2009;53:3416–3421. doi: 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang J., Yang N., Deng J., Liu K., Yang P., Zhang G., Jiang C. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PloS One. 2011;6 doi: 10.1371/journal.pone.0023710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 34.Mercuro N.J., Yen C.F., Shim D.J., Maher T.R., McCoy C.M., Zimetbaum P.J., Gold H.S. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiology. 2020;5:1036–1041. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metwally A.A., El-Ahmady S.H., Hathout R.M. Selecting optimum protein nano-carriers for natural polyphenols using chemoinformatics tools. Phytomedicine. 2016;23:1764–1770. doi: 10.1016/j.phymed.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Milewska A., Zarebski M., Nowak P., Stozek K., Potempa J., Pyrc K. Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. J Virol. 2014;88:13221–13230. doi: 10.1128/JVI.02078-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller S.E., Mathiasen S., Bright N.A., Pierre F., Kelly B.T., Kladt N., Schauss A., Merrifield C.J., Stamou D., Honing S., Owen D.J. CALM regulates clathrin-coated vesicle size and maturation by directly sensing and driving membrane curvature. Dev Cell. 2015;33:163–175. doi: 10.1016/j.devcel.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ossama M., Hathout R.M., Attia D.A., Mortada N.D. Enhanced allicin cytotoxicity on HEPG-2 cells using glycyrrhetinic acid surface-decorated gelatin nanoparticles. ACS Omega. 2019;4:11293–11300. doi: 10.1021/acsomega.9b01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quiros Roldan E., Biasiotto G., Magro P., Zanella I. The possible mechanisms of action of 4-aminoquinolines (chloroquine/hydroxychloroquine) against Sars-Cov-2 infection (COVID-19): a role for iron homeostasis? Pharmacol Res. 2020;158:104904. doi: 10.1016/j.phrs.2020.104904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartzman S., Samson C.M. Are the current recommendations for chloroquine and hydroxychloroquine screening appropriate? Rheum Dis Clin N Am. 2019;45:359–367. doi: 10.1016/j.rdc.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Shokry M., Hathout R.M., Mansour S. Exploring gelatin nanoparticles as novel nanocarriers for Timolol Maleate: augmented in-vivo efficacy and safe histological profile. Int J Pharm. 2018;545:229–239. doi: 10.1016/j.ijpharm.2018.04.059. [DOI] [PubMed] [Google Scholar]
- 43.Sungnak W., Huang N., Becavin C., Berg M., Queen R., Litvinukova M. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.U.S.Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research CDER . 2005. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Ref Type: Data File. [Google Scholar]
- 45.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vives R.R., Lortat-Jacob H., Fender P. Heparan sulphate proteoglycans and viral vectors : ally or foe? Curr Gene Ther. 2006;6:35–44. doi: 10.2174/156652306775515565. [DOI] [PubMed] [Google Scholar]
- 47.Voynow J.A. What does mucin have to do with lung disease? Paediatr Respir Rev. 2002;3:98–103. doi: 10.1016/s1526-0550(02)00007-0. [DOI] [PubMed] [Google Scholar]
- 48.Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G., Jiang C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolfram J., Nizzero S., Liu H., Li F., Zhang G., Li Z., Shen H., Blanco E., Ferrari M. A chloroquine-induced macrophage-preconditioning strategy for improved nanodelivery. Sci Rep. 2017;7:13738. doi: 10.1038/s41598-017-14221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu K., Li W., Peng G., Li F. Crystal structure of NL63 respiratory coronavirus receptor-binding domain complexed with its human receptor. Proc Natl Acad Sci U S A. 2009;106:19970–19974. doi: 10.1073/pnas.0908837106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu L., Boyd J.L., Daniels V., Wang Z., Chow D.S., Putcha L. Dose escalation pharmacokinetics of intranasal scopolamine gel formulation. J Clin Pharmacol. 2015;55:195–203. doi: 10.1002/jcph.391. [DOI] [PubMed] [Google Scholar]
- 56.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xue J., Moyer A., Peng B., Wu J., Hannafon B.N., Ding W.Q. Chloroquine is a zinc ionophore. PloS One. 2014;9 doi: 10.1371/journal.pone.0109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang A., Yang C., Yang B. Use of hydroxychloroquine and interferon alpha-2b for the prophylaxis of COVID-19. Med Hypotheses. 2020;144:109802. doi: 10.1016/j.mehy.2020.109802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou D., Dai S.M., Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020;75:1667–1670. doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hathout R.M., Kassem D.H. Positively charged electroceutical spun chitosan nanofibers can protect health care providers from COVID-19 infection: An opinion. Front Bioeng Biotechnol. 2020;8:885. doi: 10.3389/fbioe.2020.00885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abdel-Hafez S.M., Hathout R.M., Sammour O.A. Curcumin-loaded ultradeformable nanovesicles as a potential delivery system for breast cancer therapy. Colloids Surf B Biointerfaces. 2018;167:63–72. doi: 10.1016/j.colsurfb.2018.03.051. [DOI] [PubMed] [Google Scholar]