Abstract
Background
The disease caused by the severe acute respiratory syndrome coronavirus 2 was named coronavirus disease 2019 and classified as a global public health emergency. The evidence related to the impact of coronavirus disease 2019 on pregnancy is limited to the second and third trimester of pregnancy, whereas data on the first trimester are scant. Many viral infections can be harmful to the fetus during the first trimester of pregnancy, and whether severe acute respiratory syndrome coronavirus 2 is one of them is still unknown.
Objective
With this study, we evaluated severe acute respiratory syndrome coronavirus 2 infection as a risk factor for early pregnancy loss in the first trimester of pregnancy. Furthermore, coronavirus disease 2019 course in the first trimester was assessed.
Study Design
Between February 22 and May 21, 2020, we conducted a case-control study at S. Anna Hospital, Turin, among pregnant women in their first trimester, paired for last menstruation. The cumulative incidence of coronavirus disease 2019 was compared between women with spontaneous abortion (case group, n=100) and those with ongoing pregnancy (control group, n=125). Current or past infection was determined by the detection of severe acute respiratory syndrome coronavirus 2 from nasopharyngeal swab and severe acute respiratory syndrome coronavirus 2 immunoglobulin G and immunoglobulin M antibodies in a blood sample. Patient demographics, coronavirus disease 2019–related symptoms, and the main risk factors for abortion were collected.
Results
Of 225 women, 23 (10.2%) had a positive test result for coronavirus disease 2019. There was no difference in the cumulative incidence of coronavirus disease 2019 between the cases (11/100, 11%) and the controls (12/125, 9.6%) (P=.73). Logistic regression analysis confirmed that coronavirus disease 2019 was not an independent predictor of early pregnancy loss (odds ratio, 1.28; confidence interval, 0.53–3.08). Coronavirus disease 2019–related symptoms in the first trimester were fever, anosmia, ageusia, cough, arthralgia, and diarrhea; no cases of pneumonia or hospital admission owing to coronavirus disease 2019–related symptoms were recorded. No difference in the incidence of symptoms was noted between the 2 groups.
Conclusion
Severe acute respiratory syndrome coronavirus 2 infection during the first trimester of pregnancy does not seem to predispose to early pregnancy loss; its cumulative incidence did not differ between women with spontaneous abortion and women with ongoing pregnancy. Coronavirus disease 2019 appears to have a favorable maternal course at the beginning of pregnancy, consistent with what has been observed during the second and third trimesters.
Key words: abortion, coronavirus, COVID-19, fetus, first trimester, miscarriage, pregnancy, pregnancy loss, preterm birth, SARS-CoV-2, seroprevalence, severe acute respiratory syndrome, vertical transmission
Introduction
The World Health Organization named the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease coronavirus disease 2019 (COVID-19) and declared it a pandemic. Coronaviruses are enveloped, nonsegmented, positive-sense RNA usually responsible for mild illness such as the common cold in adults and children.1 However, in the last decade, coronaviruses have caused 2 important epidemics: severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). COVID-19 was first reported in Wuhan (China) in December 2019 followed by outbreaks across the world.2 The first cases of COVID-19 in Italy were confirmed in January 2020, with a rapid rise in the number of cases in northern Italy starting in late February.
AJOG at a Glance.
Why was this study conducted?
Limited research exists on the outcomes of pregnant women with coronavirus disease 2019 (COVID-19), with published research mostly related to women in the second or third trimester. Evidence about maternal and obstetrical outcomes of women with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during the first trimester or about the risk of early pregnancy loss is lacking.
Key findings
We report the largest series of patients with COVID-19 in the first trimester of pregnancy to date. The study findings showed no significant difference in the cumulative incidence of COVID-19 in women who experienced spontaneous abortion (n=100) than those with ongoing pregnancies (n=125).
What does this add to what is known?
These findings may reassure women residing in COVID-19 epidemic areas who are planning pregnancy and may provide obstetricians with a guide for preconception counseling.
Despite the rapidly growing number of cases worldwide, data on COVID-19 during pregnancy remain limited, being derived mainly from small sample studies.3, 4, 5, 6, 7, 8 A systematic review of published reports on coronaviruses (COVID-19, SARS, MERS) reported higher rates of preterm birth, preeclampsia, cesarean delivery, and perinatal death.9 The lack of data on spontaneous abortion because of COVID-19 during the first trimester precludes extrapolation of conclusive evidence for the effects of infection during early pregnancy. The paucity of reliable data has aroused concern in patients, and the disinformation reported by the media may lead pregnant women to embrace dramatic choices such as voluntary abortion.10
The wide range of clinical expression, the high rate of asymptomatic forms, and the poor accuracy of nasopharyngeal swab testing and its limited availability have been the main barriers to gaining a real understanding of the prevalence of the infection and its impact on pregnancy. In this complex scenario, the development of serologic tests for the detection of SARS-CoV-2 immunoglobulin G (IgG) and immunoglobulin M (IgM) could be useful to identify pregnant patients who were infected during early pregnancy. Although the quantity and quality of data on test performance are still limited, the level of accuracy has been reportedly moderate to good, so that patients infected by SARS-CoV-2 can be traced.11
This study aimed to evaluate the impact of COVID-19 on first-trimester pregnancy loss by comparing the cumulative incidence of SARS-CoV-2 infection in a cohort of women who experienced early spontaneous abortion and that of women with ongoing pregnancy at 12 weeks of gestational age. Furthermore, COVID-19 course in the first trimester was evaluated.
Materials and Methods
Women who had been referred to our hospital for pregnancy loss care during the first 13 weeks of pregnancy between February 22 and May 21, 2020 were contacted and enrolled (case group). All women who had access to our emergency room or to the pregnancy loss management service were contacted after being traced through our hospital’s database. Women 12 weeks pregnant who were admitted to our hospital for fetal nuchal translucency (NT) between April 16, 2020, and May 21, 2020, were the control group.
All pregnant women in Turin, Piedmont, are offered free of charge a comprehensive first-trimester risk assessment, performed at gestational age of 11 to 13 weeks as part of the public antenatal and obstetrical healthcare service. The attendance rate is high. The risk assessment includes a double test (blood sample for pregnancy-associated plasma protein A [PAPP-A] and free beta human chorionic gonadotropin [β-hCG]) and an ultrasound NT measurement (combined screening test) or NT measurement together with PAPP-A dosage, and a further blood sample for α-fetoprotein, free estriol, and β-hCG at gestational age of 15 to 18 weeks (integrated screening test).
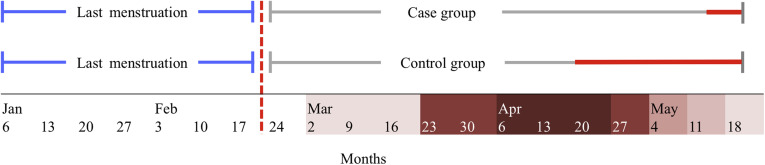
The first reported case of COVID-19 infection in Piedmont was dated February 22, 2020. To exclude the possibility of COVID-19 seroconversion before pregnancy, only women with last menstruation before that date were considered eligible for inclusion (Figure 1 ). This criterion allowed us to define seropositivity in the case group as a seroconversion that had occurred during pregnancy.
Figure 1.
Inclusion criteria and time of serologic and molecular sampling in the case and the control group
Time range for last menstruation inclusion (blue line); first reported case of COVID-19 in Piedmont, Italy (dotted red line); time of sera and nasopharyngeal swab sample collection (red line).
COVID-19 outbreak cases in Piedmont Region: weekly case increase.
Cosma et al. Coronavirus disease 2019 and early pregnancy loss. Am J Obstet Gynecol 2021.
Blood tests were performed for the detection of IgG and IgM nonneutralizing antibodies against SARS-CoV-2 and reverse transcriptase-polymerase chain reaction (RT-PCR) assays on nasopharyngeal swabs. Patients with a positive result by at least 1 test were also tested for the determination of specific neutralizing antibodies. Blood samples were centrifuged at 3000 rpm for 5 minutes to separate serum and analyzed the same day of collection.
A rapid automated fluorescent lateral flow CE-approved immunoassay (AFIAS COVID-19, Boditech, Gang-won-do, Republic of Korea) was used for qualitative and semiquantitative detection of IgG and IgM nonneutralizing antibodies against the spike (S) and nucleocapsid (N) viral proteins; semiquantitative results are expressed as the cutoff index (COI), in which a COI of >1.1 indicates a positive result. Chemiluminescence CE-approved immunoassay technology was used for the semiquantitative determination of anti-S1 and anti-S2 specific IgG neutralizing antibodies to SARS-CoV-2 (Liaison SARS-CoV-2 S1/S2 IgG, DiaSorin, Saluggia, Italy); the antibody concentration is expressed as arbitrary units (AU/mL) and grades the results as positive when ≥15 AU/mL. Viral RNA extraction from the swab was performed on a MagNA Pure compact instrument (Roche, Basel, Switzerland) and analyzed using an RT-PCR assay (CFX-96, Bio-Rad, Hercules, CA) with the Liferiver Novel Coronavirus 2019-nCov real-time RT-PCR kit protocol, targeting genes N, E, and ORF1ab (Liferiver Bio-Tech, San Diego, CA).
Sample size calculation was not possible because the expected prevalence of disease was unknown at the time of population enrollment, and further recruitment beyond May 21 would have precluded the eligibility criterion for last menstruation.
Demographics, COVID-19–related symptoms, and data on exposure to possible risk factors for spontaneous abortion were collected by interview. The study was approved by the institutional review board of the City of Health and Science of Turin (reference number: 00171/2020). Written, informed consent was obtained from all participants. The results for quantitative variables are expressed as the mean ± standard deviation (SD), and qualitative categorical variables are expressed as frequency and percentages. Comparison of quantitative variables was performed using the t test or Wilcoxon-Mann-Whitney test based on normal or not distribution, respectively. Qualitative variables were compared using the chi-square test or Fisher exact test, as appropriate. When basic patient characteristics were present as confounding factors, regression analysis was performed to assess the relationship between COVID-19 infection and spontaneous abortion. Results are expressed as odds ratio (95% confidence interval [CI]). Statistical analyses were performed using SAS software version 9.4 for Windows (SAS Institute, Cary, NC).
Results
A total of 225 women in the first trimester of pregnancy attending our institute were included in the study. Overall, 100 women in the case group and 125 women in the control group were enrolled. The patient attendance rate was 87% (100/115) and 88% (125/142) in the case group and control group, respectively. Table 1 reports the patients’ characteristics at baseline; except for age, there were no statistically significant differences in demographics or risk factors for abortion between the 2 groups.
Table 1.
Baseline characteristics, clinical findings, and COVID-19 cumulative incidence in case and control groups
| Clinical findings | Case (n=100) | Control (n=125) | P value | |
|---|---|---|---|---|
| Age, y | 35.5 (±4.7) | 33.7 (±4.7) | .001 | |
| BMI before pregnancy, kg/m2 | 25.5 (±4.3) | 22.6 (±4.1) | .11 | |
| Pregnancy | 0 | 51 (51) | 77 (61.6) | .34 |
| 1 | 40 (40) | 37 (29.6) | ||
| 2 | 7 (7) | 9 (7.2) | ||
| 3 | 1 (1) | 2 (1.6) | ||
| 5 | 1 (1) | 0 (0) | ||
| Previous abortion | 0 | 66 (66) | 94 (75.2) | .11 |
| 1 | 27 (27) | 21 (16.8) | ||
| 2 | 6 (6) | 7 (5.6) | ||
| 3 | 0 (0) | 3 (2.4) | ||
| 6 | 1 (1) | 0 (0) | ||
| ART therapy | 7 (7) | 12 (9.6) | .48 | |
| Smoking history | 22 (22) | 16 (12.8) | .06 | |
| Thyroid disease | 10 (10) | 11 (8.8) | .75 | |
| Autoimmune diseases | 8 (8) | 4 (3.2) | .11 | |
| Thrombophilia | 5 (5) | 5 (4) | .75 | |
| Uncontrolled DM | 0 | 0 | >.99 | |
| Uterine abnormalities | 8 (8) | 9 (7.2) | .82 | |
| COVID-19 disease | 11 (11) | 12 (9.6) | .73 | |
Values are presented as number (percentage) or mean (±SD).
ART, assisted reproductive technique; BMI, body mass index; COVID-19, coronavirus disease 2019; DM, diabetes mellitus.
Cosma et al. Coronavirus disease 2019 and early pregnancy loss. Am J Obstet Gynecol 2021.
Of the 225 women tested for anti–SARS-CoV-2 IgG and IgM antibodies, 23 were found to be seropositive or their nasopharyngeal swab tested positive for COVID-19, yielding an overall cumulative incidence of 10.2% in the first trimester. There was no significant difference in the cumulative incidence of COVID-19 between the case patients (11/100, 11%) and the controls (12/125, 9.6%) (P=.73).
The age variable was entered into logistic regression analysis to evaluate COVID-19 infection in relation to confounders. There was no difference in the odds of being infected with SARS-CoV-2 between the 2 groups, indicating that COVID-19 infection was not an independent predictor of early pregnancy loss (1.282; 95% CI, 0.53–3.08).
Subgroup analysis of baseline characteristics of COVID-19–positive and COVID-19–negative patients with early pregnancy loss (case group) showed no statistically significant differences in demographics or risk factors for spontaneous abortion between the 2 groups, except for body mass index (BMI) (26.4±5.2 vs 23.2±4.2; P=.03).
In the case group, 5 of 11 (45.4%), 3 of 11 (27.2%), and 1 of 11 (9%) participants had a positive test result for SARS-CoV-2 IgG, SARS-CoV-2 IgM, or both SARS-CoV-2 IgG and IgM, respectively; RT-PCR of the nasopharyngeal swab was positive in 2 of 11 participants (18%) (Table 2 ). In the control group, 7 of 12 (58.3%), 3 of 12 (25%), and 2 of 12 (16.6%) had a positive test result for SARS-CoV-2 IgG, SARS-CoV-2 IgM, or both SARS-CoV-2 IgG and IgM, respectively; RT-PCR of the nasopharyngeal swab was positive in 5 of 12 participants (41.7%) (Table 3 ). No difference in positivity for IgG neutralizing antibodies was found between the case (6/11, 54.5%) and the control group (5/12, 41.7%) (P=.53) (Table 1). There was no statistically significant difference between the 2 groups for average antibody titer, both nonneutralizing (21.3 vs 18.3 COI; P=.42) and neutralizing antibodies (39.9 vs 46.9 AU/mL; P=.69).
Table 2.
Antibody levels and SARS-CoV-2 detection in sera and NS samples from patients with abortion
|
Diagnostic test |
Positive result | Patient |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Anti-NP IgM | COI>1.1 | <1.1 | 2.11 | <1.1 | 1.9 | <1.1 | <1.1 | <1.1 | <1.1 | 2.6 | <1.1 | 2.9 |
| Anti-NP IgG | COI>1.1 | <1.1 | 18.9 | <1.1 | <1.1 | 19.4 | <1.1 | 14.4 | 32.4 | <1.1 | 21.7 | <1.1 |
| Anti-RBD IgG | ≥15 AU/mL | <15 | 19.5 | <15 | <15 | 29.9 | 49.3 | 17.3 | 41 | <15 | 82.9 | <15 |
| NS | pos | neg | pos | neg | neg | neg | neg | neg | neg | neg | neg | |
COI, cutoff index; IgG, immunoglobulin G; IgM, immunoglobulin M; neg, negative; NP, nucleoprotein; NS, nasopharyngeal swab; pos, positive; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Cosma et al. Coronavirus disease 2019 and early pregnancy loss. Am J Obstet Gynecol 2021.
Table 3.
Antibody levels and SARS-CoV-2 detection in sera and NS samples from pregnant patients
|
Diagnostic test |
Positive result | Patient |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| Anti-NP IgM | COI>1.1 | <1.1 | <1.1 | <1.1 | 2.1 | 1.6 | 1.2 | 1.2 | <1.1 | 1.2 | <1.1 | <1.1 | <1.1 |
| Anti-NP IgG | COI>1.1 | 19.3 | 19.3 | 15.6 | <1.1 | <1.1 | 21 | <1.1 | 21.5 | 23.2 | 21.9 | 2.45 | 20.7 |
| Anti-RBD IgG | ≥15 AU/mL | <15 | <15 | <15 | <15 | <15 | 52.7 | <15 | 21.1 | 103 | 30.5 | <15 | 27.5 |
| NS | neg | pos | neg | neg | neg | pos | neg | pos | neg | pos | neg | pos | |
COI, cutoff index; IgG, immunoglobulin G; IgM, immunoglobulin M; neg, negative; NP, nucleoprotein; NS, nasopharyngeal swab; pos, positive; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Cosma et al. Coronavirus disease 2019 and early pregnancy loss. Am J Obstet Gynecol 2021.
Of the patients diagnosed as having COVID-19, 12 reported previous symptoms (12/23, 52.2%), including fever (7/12, 58.3%), anosmia and ageusia (5/12, 41.7%), cough (5/12, 41.7%), arthralgia (4/12, 33.3%), and diarrhea (1/12, 8.3%); no pneumonia or hospital admission owing to COVID-19–related symptoms was recorded. No difference in the incidence of symptoms was noted between the case (4/11, 36.4%) and the control group (8/12, 66.6%) (P=.14).
Comment
Principal findings
With this case-control study, we evaluated the impact of COVID-19 on early pregnancy loss in a cohort of pregnant women with SARS-CoV-2 infection confirmed by antibody testing or RT-PCR assay of nasopharyngeal swabs. The results show that the risk of first-trimester spontaneous abortion is not affected by SARS-CoV-2 infection after being adjusted for age. No severe cases or hospital admission because of COVID-19–related symptoms were recorded, both in women who had ongoing pregnancies and in those with early pregnancy loss.
Results in context
Despite the large and rapidly growing number of cases worldwide, there are limited data on COVID-19 in pregnancy, mainly coming from case series and small sample studies related to the second and third trimesters of pregnancy. Concern is mounting about the impact of COVID-19 on pregnancy, possible vertical transmission,12, 13, 14, 15 and unfavorable obstetrical outcomes in particular. Reproductive medicine societies advised delaying the start of assisted reproductive treatments,16 and guidelines on the prevention and control of COVID-19 among pregnant women have been issued.17, 18, 19
Currently, data on the impact of coronaviruses on the first trimester of pregnancy are limited. Four of the 7 patients who presented with SARS coronavirus 1 (SARS-CoV-1) infection during their first trimester had a spontaneous abortion, likely the result of the hypoxia caused by SARS-CoV-1–related acute respiratory distress.20 Furthermore, 1 case of a woman with MERS during the first trimester has been reported. She was asymptomatic and went on to have a term delivery.21 As for SARS-CoV-2, a single pregnancy loss during the second trimester of pregnancy in a woman with COVID-19 was probably related to placental infection.22 Another study reported the first visualization by electron microscopy of the SARS-CoV-2 invading syncytiotrophoblasts in the placental villi.23 This evidence could suggest a potential impact of SARS-CoV-2 on spontaneous abortion.
Clinical implications
Our study findings may reduce concerns in patients during the first trimester of pregnancy. In this cohort of women who experienced a spontaneous abortion during the first trimester, the serologic prevalence of antibodies was similar to that in women with ongoing pregnancies. Furthermore, although viral infection at this stage could potentially affect embryogenesis and organ development, there is still no evidence for the intrauterine transmission of SARS-CoV-2. Our findings may reassure women who are planning a pregnancy in epidemic areas and may represent a guide for obstetricians during preconception counseling.
The course of COVID-19 varies widely: patients may remain asymptomatic or develop mild to severe symptoms leading to pneumonia, respiratory failure, and death.24 However, in this cohort, few patients were symptomatic and not more numerous in the case group. Severe disease was never observed. The lower incidence of severe manifestations during the first trimester could be explained by the minimal alteration in respiratory dynamics during this phase of pregnancy. Despite these reassuring data, pregnancies in women with COVID-19 can still have an unfavorable obstetrical outcome: inflammatory involvement of the placenta25 can be associated with preterm delivery.26 Obstetricians should discuss that although the first trimester seems not to expose the fetus to severe risks, pregnancy may still be complicated in the following weeks of gestation.
Research implications
Serologic tests, in conjunction with SARS-CoV-2 RT-PCR assays, may offer a more feasible opportunity to identify both active and past infections and evaluate the real spread of SARS-CoV-2 to the point that some governments have suggested their use in large-scale population tracking.27 Determination of seroconversion in pregnant women could answer some concerns about unfavorable pregnancy outcomes, which are not otherwise resolvable. The nonnegligible prevalence of infection in asymptomatic pregnant women reported in our cohort and elsewhere8 , 28 makes universal screening of all pregnant patients seem desirable. Long-term follow-up of ongoing pregnancies will respond to other doubts about the impact of COVID-19 in pregnant patients.
Strengths and limitations
One of the strengths of this study is the enrollment of women with serologically confirmed COVID-19 by means of 2 different serologic assays; the combined results of RT-PCR and nasopharyngeal swab samples is another major strength of the study. The high attendance rate to the study protocol limited confounding factors such as population selection bias. Antibodies to COVID-19 were detected in about 1 of 10 pregnant patients in the cohort; however, this finding should be carefully interpreted, as it cannot be generalized given that the cohort was derived from a single center located in a region with a high incidence of COVID-19.
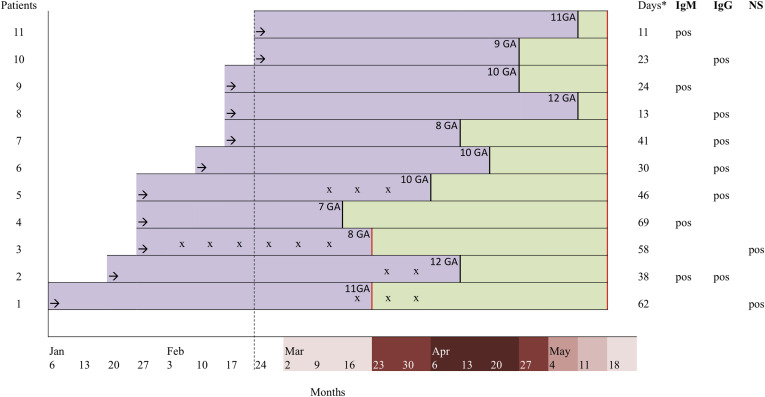
Although the number of COVID-19–positive patients in the case group is low, except for BMI, the group does not differ in baseline characteristics from the COVID-19–negative patients with early pregnancy loss. This suggests that the study conclusions may be extended to larger samples. Overweight among COVID-19 patients has been reported in other series of nonpregnant patients and is being increasingly described as an underappreciated risk factor for COVID-19.29A major limitation of the study is that we were unable to accurately backdate the time of infection in women with spontaneous abortion. In the absence of an IgG avidity test, we evaluated the time elapsed between the abortion and the blood test for antibody detection. The profile of antibodies against SARS-CoV-2 in this cohort was comparable with previous findings. Seroconversion of IgG or IgM within 20 days after symptom onset has recently been reported.30 The median day of seroconversion for both IgG and IgM was 13 days with a synchronous or a discordant pattern. In light of this evidence, seroconversion during pregnancy could be excluded (or be controversial) only in 1 patient (no. 4, Figure 2 ) in the case group. The detection of IgM antibodies at 66 days after abortion does not preclude that seroconversion might have occurred after the loss of pregnancy. In view of future research addressing the issue on the relationship between COVID-19 and spontaneous abortion, it will be difficult for researchers to precisely define the timing of infection and the effective seroconversion during pregnancy. Inclusion criteria, together with the beginning of the study at pandemic outbreak, allowed us to fairly overcome this issue.
Figure 2.
Time elapsed between spontaneous abortion care, diagnostic testing and seromolecular profiles
Last menstruation (black arrow); hospital care for early pregnancy loss (black vertical line); first reported case of COVID-19 in Piedmont (dotted black line); time elapsed between the spontaneous abortion and diagnostic testing (rectangular green box); pregnancy (rectangular violet box); serological and/or molecular sampling (red line); reported COVID-19–related symptoms (x); days elapsed between the spontaneous abortion and diagnostic testing (asterisk).
COVID-19 outbreak cases in Piedmont Region: weekly case increase.
GA, gestational age; IgG, immunoglobulin G; IgM, immunoglobulin M; neg, negative; NS, nasopharyngeal swab.
Cosma et al. Coronavirus disease 2019 and early pregnancy loss. Am J Obstet Gynecol 2021.
Another limitation is that patients with very early pregnancy loss may not have been enrolled in our study because they did not require obstetrical care, which is performed before the patient has her first obstetrical visit. We believe, however, that there is no reason to think that within this small patient group the cumulative incidence of COVID-19 would have been so high as to question our results. It is difficult to hypothesize that preclinical abortions could be caused by SARS-CoV-2 in a stage when pregnancy loss is much more likely to occur because of chromosomal defects in the embryo rather than because of virus-induced detrimental effects at the maternal-fetal interface.31, 32, 33 Recent evidence shows that SARS-COV-2 binds to angiotensin-converting enzyme 2 (ACE2) receptors and the cellular transmembrane serine protease 2 (TMPRSS2) to facilitate the fusion of viral and cellular target membranes. Because coexpression of ACE2 and TMPRSS2 at such an early stage of pregnancy is negligible,34 we believe our groups are reasonably representative and our analysis realistic.
Conclusions
Our study provides reassuring findings for women who intend to become pregnant during the SARS-CoV-2 pandemic or who became infected during their first trimester of pregnancy. COVID-19 appears to have a favorable maternal course at the beginning of pregnancy, consistent with what has been observed during the third trimester when the clinical characteristics of COVID-19–positive pregnant women were similar to those found in women from the general population.35 More importantly, no significant difference in the early pregnancy loss rate was observed.
Acknowledgments
We thank the staff of the Laboratory of S. Anna Hospital for sample collection and storage.
Footnotes
S.C. and A.R.C. are joint first authors.
The authors report no conflict of interest.
This work was primarily supported by funding from the University of Turin. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Cite this article as: Cosma S, Carosso AR, Cusato J, et al. Coronavirus disease 2019 and first trimester spontaneous abortion: a case-control study of 225 pregnant patients. Am J Obstet Gynecol 2021;224:391.e1-7.
References
- 1.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan J., Guo J., Fan C., et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223:111.e1–111.e14. doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao D., Yin H., Chen J., et al. Clinical analysis of ten pregnant women with COVID-19 in Wuhan, China: a retrospective study. Int J Infect Dis. 2020;95:294–300. doi: 10.1016/j.ijid.2020.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrazzi E., Frigerio L., Savasi V., et al. Vaginal delivery in SARS-CoV-2-infected pregnant women in northern Italy: a retrospective analysis. BJOG. 2020 doi: 10.1111/1471-0528.16278. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao J., He X., Gong Q., Yang L., Zhou C., Li J. Analysis of vaginal delivery outcomes among pregnant women in Wuhan, China during the COVID-19 pandemic. Int J Gynaecol Obstet. 2020;150:53–57. doi: 10.1002/ijgo.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breslin N., Baptiste C., Gyamfi-Bannerman C., et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020;2:100118. doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutton D., Fuchs K., D’Alton M., Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Mascio D., Khalil A., Saccone G., et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2:100107. doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y.T., Li C., Zhang C.J., Huang H.F., FRCOG Is termination of early pregnancy indicated in women with COVID-19? Eur J Obstet Gynecol Reprod Biol. 2020;251:271–272. doi: 10.1016/j.ejogrb.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tré-Hardy M., Wilmet A., Beukinga I., Dogné J.M., Douxfils J., Blairon L. Validation of a chemiluminescent assay for specific SARS-CoV-2 antibody. Clin Chem Lab Med. 2020;58:1357–1364. doi: 10.1515/cclm-2020-0594. [DOI] [PubMed] [Google Scholar]
- 12.Alzamora M.C., Paredes T., Caceres D., Webb C.M., Valdez L.M., La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020;37:861–865. doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carosso A., Cosma S., Borella F., et al. Pre-labor anorectal swab for SARS-CoV-2 in COVID-19 pregnant patients: is it time to think about it? Eur J Obstet Gynecol Reprod Biol. 2020;249:98–99. doi: 10.1016/j.ejogrb.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong L., Tian J., He S., et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323:1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carosso A.R., Cosma S., Benedetto C. Vaginal delivery in COVID-19 pregnant women: anorectum as a potential alternative route of SARS-CoV-2 transmission. Am J Obstet Gynecol. 2020;223:612. doi: 10.1016/j.ajog.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaiarelli A., Bulletti C., Cimadomo D., et al. COVID-19 and ART: the view of the Italian Society of Fertility and Sterility and Reproductive Medicine. Reprod Biomed Online. 2020;40:755–759. doi: 10.1016/j.rbmo.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poon L.C., Yang H., Kapur A., et al. Global interim guidance on coronavirus disease 2019 (COVID-19) during pregnancy and puerperium from FIGO and allied partners: information for healthcare professionals. Int J Gynaecol Obstet. 2020;149:273–286. doi: 10.1002/ijgo.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carosso A., Cosma S., Serafini P., Benedetto C., Mahmood T. How to reduce the potential risk of vertical transmission of SARS-CoV-2 during vaginal delivery? Eur J Obstet Gynecol Reprod Biol. 2020;250:246–249. doi: 10.1016/j.ejogrb.2020.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020;222:415–426. doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong S.F., Chow K.M., Leung T.N., et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alfaraj S.H., Al-Tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV) infection during pregnancy: report of two cases & review of the literature. J Microbiol Immunol Infect. 2019;52:501–503. doi: 10.1016/j.jmii.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baud D., Greub G., Favre G., et al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 2020;323:2198–2200. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Algarroba G.N., Rekawek P., Vahanian S.A., et al. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am J Obstet Gynecol. 2020;223:275–278. doi: 10.1016/j.ajog.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muhidin S., Behboodi Moghadam Z., Vizheh M. Analysis of maternal coronavirus infections and neonates born to mothers with 2019-nCoV; a systematic review. Arch Acad Emerg Med. 2020;8:e49. [PMC free article] [PubMed] [Google Scholar]
- 27.Zullo F., Di Mascio D., Saccone G. Coronavirus disease 2019 antibody testing in pregnancy. Am J Obstet Gynecol MFM. 2020;2:100142. doi: 10.1016/j.ajogmf.2020.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vintzileos W.S., Muscat J., Hoffmann E., et al. Screening all pregnant women admitted to labor and delivery for the virus responsible for coronavirus disease 2019. Am J Obstet Gynecol. 2020;223:284–286. doi: 10.1016/j.ajog.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kass D.A., Duggal P., Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet. 2020;395:1544–1545. doi: 10.1016/S0140-6736(20)31024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long Q.X., Liu B.Z., Deng H.J., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 31.Massalska D., Zimowski J.G., Bijok J., et al. First trimester pregnancy loss: clinical implications of genetic testing. J Obstet Gynaecol Res. 2017;43:23–29. doi: 10.1111/jog.13179. [DOI] [PubMed] [Google Scholar]
- 32.Choi T.Y., Lee H.M., Park W.K., Jeong S.Y., Moon H.S. Spontaneous abortion and recurrent miscarriage: a comparison of cytogenetic diagnosis in 250 cases. Obstet Gynecol Sci. 2014;57:518–525. doi: 10.5468/ogs.2014.57.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angiolucci M., Murru R., Melis G., Carcassi C., Mais V. Association between different morphological types and abnormal karyotypes in early pregnancy loss. Ultrasound Obstet Gynecol. 2011;37:219–225. doi: 10.1002/uog.7681. [DOI] [PubMed] [Google Scholar]
- 34.Pique-Regi R., Romero R., Tarca A.L., et al. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? Elife. 2020;9:e58716. doi: 10.7554/eLife.58716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Z., Wang M., Zhu Z., Liu Y. Coronavirus disease 2019 (COVID-19) and pregnancy: a systematic review. J Matern Fetal Neonatal Med. 2020 doi: 10.1080/14767058.2020.1759541. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]