Abstract
Background
Given the significant morbidity and mortality of maternal sepsis, early identification is key to improve outcomes. This study aims to evaluate the performance characteristics of the systemic inflammatory response syndrome (SIRS), quick sequential organ failure assessment (qSOFA), and maternal early warning (MEW) criteria for identifying cases of impending sepsis in parturients. The secondary objective of this study is to identify etiologies and risk factors for maternal sepsis, and assess timing of antibiotics in patients diagnosed with sepsis.
Methods
Validated maternal sepsis cases during the delivery hospitalization from 1995 to 2012 were retrospectively identified at seven academic medical centers in the US and Israel. Control patients were matched by date of delivery in a 1:4 ratio. The sensitivity and specificity of SIRS criteria, qSOFA, and MEW criteria for identifying sepsis were calculated. Data including potential risk factors, vital signs, laboratory values, and clinical management were collected for cases and controls.
Results
Eighty-two sepsis cases during the delivery hospitalization were identified and matched to 328 controls. The most common causes of sepsis were the following: chorioamnionitis 20 (24.4%), endometritis 19 (23.2%), and pneumonia 9 (11.0%). Escherichia coli 12 (14.6%), other gram negative rods 8 (9.8%), and Group A streptococcus 6 (7.3%) were the most commonly found pathogens. The sensitivities and specificities for meeting criteria for screening tools were: 1) SIRS (0.93, 0.63); 2) qSOFA (0.50, 0.95); and 3) MEW criteria for identifying sepsis (0.82, 0.87). Of 82 women with sepsis, 10 (12.2%) died. The mortality rate for those who received antibiotics within one hour of diagnosis was 8.3%. The mortality rate was 20% for the patients who received antibiotics after more than one hour.
Conclusions
Chorioamnionitis and endometritis were the most common causes of sepsis, together accounting for about half of cases. Notable differences were observed in the sensitivity and specificity of sepsis screening tools with the highest to lowest sensitivity being SIRS, MEW, and qSOFA criteria, and the highest to lowest specificity being qSOFA, MEW, and SIRS. Mortality was doubled in the cohort of patients who received antibiotics after more than one hour. Clinicians need to be vigilant to identify cases of peripartum sepsis early in its course and prioritize timely antibiotic therapy.
Introduction
Sepsis is an important cause of maternal morbidity and mortality. According to the most recent estimate from the Centers for Disease Control and Prevention, sepsis is the third leading cause and accounts for approximately 12.7% of pregnancy-related deaths.1 It has been estimated that for each maternal death, there are 50 women with life-threatening morbidity from sepsis.2 In North Carolina, United Kingdom, and Michigan maternal mortality reviews, deaths due to sepsis were reportedly 43%, 47% and 73% preventable, respectively.3–5 Early identification and prompt treatment of patients with sepsis have been shown to improve outcomes.6 However, diagnosing sepsis during pregnancy and the postpartum period can be challenging as physiologic changes of pregnancy can mimic the signs of sepsis.7
Given the potential preventability of and significant morbidity and mortality associated with sepsis, early identification of sepsis is paramount, and many screening tools exist. The quick Sequential [Sepsis-related] Organ Failure Assessment (qSOFA) criteria are currently recommended as the screening tool for sepsis outside of the intensive care unit (ICU);8 previously, the Systemic Inflammatory Response Syndrome (SIRS) criteria were used.9 The Maternal Early Warning (MEW) criteria provides a screening tool to identify women with heightened risk for multiple causes of maternal morbidity and mortality (Table 1).10 While not developed specifically for patients with an infection, the parameters comprising MEW criteria have been adjusted particularly for pregnancy.
Table 1.
Definitions of Systemic Inflammatory Response Syndrome (SIRS) criteria, quick Sequential [Sepsis-related] Organ Failure Assessment (qSOFA) criteria, and Maternal Early Warning (MEW) criteria
| Term | Definition |
|---|---|
| SIRSa | Two or more of the following: |
| Temperature > 38°C or < 36°C | |
| Heart rate > 90 beats per minute | |
| Respiratory rate > 20 breaths per minute or PaCO2 < 32 mm Hg | |
| White blood cell count < 4 × 109/L or > 12 × 109/L | |
| qSOFAb | Two or more of the following: |
| Respiratory rate ≥ 22 breaths per minute | |
| Altered mentation | |
| Systolic blood pressure ≤ 100 mm Hg | |
| MEWc | One or more of the following: |
| Systolic blood pressure < 90 or > 160 mm Hg | |
| Diastolic blood pressure > 100 mm Hg | |
| Heart rate < 50 or > 120 beats per minute | |
| Respiratory rate < 10 or > 30 per minute | |
| Oxygen saturation on room air, at sea level < 95% | |
| Oliguria, <35 mL/hour for ≥ 2 hours | |
| Maternal agitation, confusion, or unresponsiveness; Patient with preeclampsia reporting a non-remitting headache or shortness of breath | |
Bone RC, Balk RA, Cerra FB, et al: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992 Jun; 101(6):1644–1655.
Singer M, Deutschman CS, Seymour CW, et al: The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016 Feb 23; 315(8):801–810.
Mhyre JM, D’Oria R, Hameed AB, et al: The maternal early warning criteria: a proposal from the national partnership for maternal safety. Obstet Gynecol 2014 Oct; 124(4):782–786.
The performance characteristics of these screening tools (SIRS, qSOFA, and MEW criteria) for the identification of sepsis in pregnancy are currently unknown and it is unclear which screening criteria should be used for maternal sepsis. Therefore, the primary objective of this multicenter case-control study was to evaluate the performance characteristics of the SIRS, qSOFA, and MEW criteria for identifying cases of impending sepsis during the delivery hospitalization. The secondary objectives were to characterize the etiologies, risk factors, and to assess the timing of antibiotics in patients diagnosed with sepsis.
Methods
Institutional review board approval was obtained at each of the seven participating centers: University of Michigan Health System [UMHS] (Ann Arbor, Michigan), Brigham and Women’s Hospital [BWH] (Boston, Massachusetts), Massachusetts General Hospital [MGH] (Boston, Massachusetts), Northwestern Memorial Hospital [NM] (Chicago, Illinois), Beaumont Health [BH] (Royal Oak, Michigan), Intermountain Healthcare [IH] (Salt Lake City, Utah), Shaare Zedek Medical Centre [SZMC] (Jerusalem, Israel). As this was a retrospective chart review, informed written consent was waived. This manuscript adheres to the applicable STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines.
Delivery hospitalization (defined as a hospitalization in which both sepsis and delivery occurred) admissions were screened for sepsis. International Classification of Diseases Ninth Revision (ICD-9) codes indicating sepsis, severe sepsis, or septic shock were searched using one of the following methods: 1) delivery admissions were identified and then administrative claims were screened for ICD-9 codes consistent with sepsis, severe sepsis, septic shock, or other severe infection codes (UMHS 1995–2012, IH 1997–2012); or 2) delivery admissions were searched for ICD-9 codes indicating sepsis, severe sepsis, septic shock, and other severe infection codes (BWH and MGH 1997–2012, NM 2007–2012, BH 2000–2012, SZMC 1994–2012). Each chart identified by screening was manually reviewed to verify that the patient met the defined clinical criteria for severe sepsis: sepsis along with organ failure, hypotension, or hypoperfusion, which was the definition in use during the period when patients included in the study were hospitalized.9
To evaluate risk factors, sensitivity, and specificity of screening criteria, the delivery hospitalization patient group was matched to healthy control patients. Controls for delivery hospitalization cases were matched by date of delivery and hospital in a 1:4 ratio to maximize power.11 Four controls were selected for the delivery date from the available pool of patients using a random number generator. Vital signs were collected for sepsis cases for the 24-hour period prior to and including the time of diagnosis. Time of diagnosis was defined by the earliest time any one of the following criteria was met: 1) time of initiation of broad-spectrum antibiotics or broadening of existing antibiotic regimen, 2) time of documentation of sepsis in the chart, or 3) time of transfer to the intensive care unit for sepsis. For controls, vital signs for controls were collected for the 24-hour period corresponding with the sepsis patient’s diagnosis in relation to delivery (e.g., if the sepsis patient was diagnosed on postpartum day one, then the vital signs and white blood cell count for the matched control patients were collected for the 24-hour period on postpartum day one). The vital signs and daily laboratory values were collected at the same time point relative to delivery for cases and controls to account for the dynamic physiological changes (heart rate, white blood cell count, respiratory rate) occurring during delivery and the postpartum period.7
Data were collected that were expected to be routinely documented in both controls and cases; thus, not all diagnostic criteria for the MEW criteria scoring system were collected (urine output and oxygen saturation, as these are not routinely collected for parturients in the postpartum period). For modified MEW criteria, data regarding systolic blood pressure, respiratory rate, heart rate, and neurological changes were collected and analyzed. The MEW criteria parameters inconsistent with the physiology of sepsis (systolic blood pressure >160 mmHg, diastolic blood pressure >100 mmHg, heart rate <50, and respiratory rate <10) were not included in the analysis. For qSOFA and modified MEW criteria, neurological changes were collected for the sepsis cases and were assumed to have occurred at the time of diagnosis; it was assumed that none of the control patients exhibited neurological changes. Data were collected by chart review and placed into REDCap™ (Research Electronic Data Capture). Data collected at each site were screened by the coordinating center (University of Michigan) for any inconsistencies or outlying values. In those instances, the primary site was contacted to verify the data through chart review.
Medical records, including documentation pertaining to prenatal care, labor and delivery records, and the intensive care unit (ICU) admission charts were reviewed. Laboratory data and medication administration records were also reviewed. Demographic information, medical comorbidities, obstetric variables, vital sign data, microbiology results, white blood cell count values, hospital course, antibiotics types ordered, time from diagnosis until antibiotic administration, and details of infection type and treatment were collected.
Statistical Analysis
The discriminatory capability of SIRS, qSOFA, and modified MEW criteria were assessed by calculating sensitivity and specificity for each individual criterion and combinations of multiple criteria. Cases and matched controls with vital signs and laboratory values for the various criteria were included in the analysis. All analyses were performed using SAS software v9.4 (SAS Institute, Care, NC).
Descriptive statistics summarized the type of infection, organism type, and clinical course for sepsis cases and were reported in n (%). Patient demographic, obstetric, and medical comorbidity characteristics were compared for sepsis cases and matched controls. To account for the small sample size and matching of cases and controls, exact conditional logistic regression estimated the association between each potential risk factor and the outcome of sepsis; exact unadjusted odds ratios (OR) and 95% confidence intervals (CI) were calculated.
Patients who developed sepsis prior to the time of delivery were excluded from the risk factor analysis for only the specific variables examining cesarean delivery, preterm delivery, stillbirth, induction of labor, and corticosteroid administration, in order to respect temporality in the analysis of risk factors (e.g., cesarean delivery cannot cause sepsis prior to delivery). However, they were included in all other variables for the risk factor analyses.
The primary objective of evaluation of screening tools and secondary objectives of descriptive clinical characteristics, defining risk factors, and antibiotic timing were determined a priori. During data collection the MEW and qSOFA criteria were published. While it was planned a priori to collect and evaluate vital signs for the identification of sepsis, it was then determined ad hoc to group by screening tools for presentation purposes only. The sample size was a convenience sample with all centers reporting all available data from cases and matched controls during the study period indicated.
Missing Data
If there were missing values for a covariate, it was excluded from the exact logistic regression model to calculate the unadjusted odds ratio for that variable. Missing data regarding the screening tool analysis were handled as follows: 1) for sepsis case patients missing all vital sign and laboratory information (e.g., not available since the diagnosis was made at an outside hospital and subsequently transferred), the case and four matched controls were excluded from the screening tool analysis (n=8 sepsis cases and 32 controls); 2) if all control patients were not admitted to the hospital at the same time point relative to delivery that the case developed sepsis (e.g., several days prior to or after delivery), the case and four matched controls were excluded from the screening tool analysis (n= 92 controls and 23 sepsis cases); and 3) if vital signs were missing for one or more of the control patients, those particular controls were excluded from the analysis (n=15 controls).
Results
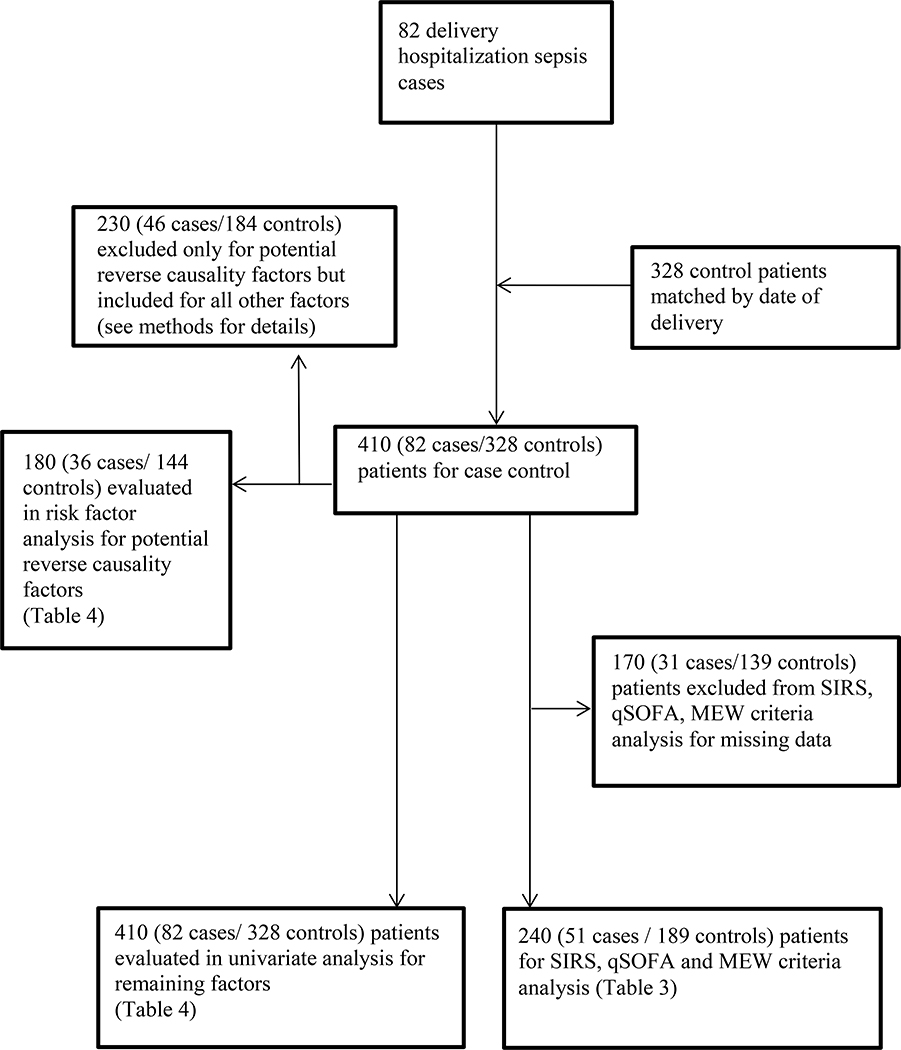
Eighty-two cases of sepsis during hospitalization for delivery and 328 controls were identified. A flowchart of patient selection and controls for the tables is indicated in Figure 1. The cases reported from each center are the following: UMHS (13 cases), BWH (17 cases), IH (18 cases), MGH (5 cases), BH (10 cases), NM (12 cases), and SZMC (7 cases). A description of organisms, type of infection, and severity of infection is presented in Table 2. The most common type of infection associated with the development of maternal sepsis was chorioamnionitis 20 (24.4%). The types of infection designated as “other” contained mostly patients who presented with disseminated infections with an unclear primary source. The most common organism type identified was Escherichia coli 12 (14.6%). Out of 82 women with sepsis, 10 (12.2%) died, and 6 (7.7%) were discharged to an extended care facility. Of note, among patients for whom the timing of the administration of antibiotics was available, n=56, [24 (29.3%) exact timing not available], 4 (57.1%) of non-survivors, 16 (32.7%) of survivors, and 20 (35.7%) of all sepsis cases (both survivors and non-survivors) received antibiotics more than one hour after diagnosis. The mortality rate for those who received antibiotics within one hour was 8.3% (95% CI 1.2% to 22.5%). The mortality rate was 20% (5.7% to 43.7%) for the patients who received antibiotics after more than one hour.
Figure 1.
Flowchart for patient selection
Table 2.
Clinical characteristics of women with sepsis during the delivery hospitalization
| Clinical Characteristic | Delivery Hospitalization Cases N=82 |
|---|---|
| n(%) | |
| Type of Infectiona | |
| Chorioamnionitis | 20 (24.4) |
| Endometritis | 19 (23.2) |
| Pneumonia | 9 (11.0) |
| Wound infection | 7 (8.5) |
| Genitourinary infection | 5 (6.1) |
| Endocarditis | 3 (3.7) |
| Pyelonephritis | 3 (3.7) |
| Meningitis | 2 (2.4) |
| Central line associated blood stream infection | 2 (2.4) |
| Mastitis | 0 |
| Other | 17 (20.7) |
| Unknown | 6 (7.3) |
| Organism Identified | |
| Escherichia coli | 12 (14.6) |
| Group A streptococcus | 6 (7.3) |
| Other streptococcus | 2 (2.4) |
| Staphylococcus | 5 (6.1) |
| Other gram negative rods | 8 (9.8) |
| Multiple organisms | 6 (7.3) |
| Other | 6 (7.3) |
| Unknown | 37 (45.1) |
| Clinical Course | |
| Died | 10 (12.2) |
| Discharged to an extended care facilityb | 6 (7.7) |
| Antibiotic timing not available | 24 (29.3) |
| Antibiotic administration more than one hour after order | 20 (35.7) |
| Survivorsc | 16 (32.7) |
| Non-survivorsd | 4 (57.1) |
| Intensive care unit admission | 71 (86.6) |
| Mechanical ventilation | 54 (65.9) |
| Hemodialysis | 9 (11.0) |
| Neurological changesb | 32 (44.4) |
| Vasopressor/inotropic support | 38 (46.3) |
| Enteral feeding | 20 (24.4) |
| Timing of sepsis in relation to delivery | |
| Antepartum | 14 (17.1) |
| Intrapartum | 32 (39.0) |
| Postpartum | 36 (43.9) |
More than one type of infection may have been present
Missing values are the following: neurological changes=10, discharged to an extended care facility=4
Survivors (n=49) for which antibiotic timing was available, (n=2) excluded due to already on broad spectrum antibiotics at the time of diagnosis
Non-survivors (n=7) for which antibiotic timing was available
Fifty-one cases and 189 controls were included in the screening tool analysis. All screening criteria (SIRS, qSOFA, and modified MEW criteria) and corresponding sensitivities and specificities are listed in Table 3 for each criterion separately and also grouped according to meeting criteria and combinations of criteria met. Two SIRS criteria and two qSOFA criteria are required to meet criteria for sepsis, while one MEW criterion is required to indicate a positive trigger. SIRS criteria had a sensitivity of 0.93 (95% CI 0.81 to 0.99) and a specificity of 0.63 (95% CI 0.55 to 0.71). The qSOFA criteria had a sensitivity of 0.50 (95% CI 0.33 to 0.67) and a specificity of 0.95 (95% CI 0.91 to 0.98). The modified MEW criteria had a sensitivity of 0.82 (95% CI 0.66 to 0.92) and specificity of 0.87 (95% CI 0.81 to 0.91).
Table 3.
Sensitivity and specificity of criteria for sepsis
| Criteria | N (%) Sepsis Cases | N (%) Controls | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|
| SIRSa | ||||
| WBC˂ 4 or >12 | 38 (74.5) | 62 (41.1) | 0.75 (0.60, 0.86) | 0.59 (0.51, 0.67) |
| HR>90 | 49 (96.1) | 104 (55.3) | 0.96 (0.87, 1.00) | 0.45 (0.37, 0.52) |
| RR>20 | 28 (62.2) | 18 (9.9) | 0.62 (0.47, 0.76) | 0.90 (0.85, 0.94) |
| T<36°C or T>38°C | 33 (68.7) | 52 (28.7) | 0.69 (0.54, 0.81) | 0.71 (0.64, 0.78) |
| [T>38 or <36] and [HR>90] | 33 (68.8) | 33 (18.2) | 0.69 (0.54, 0.81) | 0.82 (0.75, 0.87) |
| [T>38 or <36] and [RR>20] | 23 (53.5) | 7 (4.0) | 0.53 (0.38, 0.69) | 0.96 (0.92, 0.98) |
| [T>38 or <36] and [WBC>12 or<4] | 27 (56.3) | 14 (9.8) | 0.56 (0.41, 0.71) | 0.90 (0.84, 0.95) |
| [HR>90] and [RR>20] | 28 (62.2) | 15 (8.3) | 0.62 (0.47, 0.76) | 0.92 (0.87, 0.95) |
| [HR>90] and [WBC>12 or <4] | 36 (70.6) | 32 (21.3) | 0.71 (0.56, 0.83) | 0.79 (0.71, 0.85) |
| [RR>20] and [WBC>12 or <4] | 20 (44.4) | 6 (4.2) | 0.44 (0.30, 0.60) | 0.96 (0.92, 0.98) |
| Any 2 SIRS | 40 (93.0) | 51 (36.7) | 0.93 (0.81, 0.99) | 0.63 (0.55, 0.71) |
| qSOFAa | ||||
| RR≥22 | 28 (62.2) | 17 (9.4) | 0.62 (0.47, 0.76) | 0.91 (0.85, 0.94) |
| SBP≤100 mmHg | 26 (55.3) | 76 (40.4) | 0.55 (0.40, 0.70) | 0.60 (0.52, 0.67) |
| Neurological changes | 17 (37.8) | 0 | 0.38 (0.24, 0.53) | 1.00 (0.98, 1.00) |
| [RR≥22] and [SBP≤100 mmHg] | 14 (33.3) | 9 (5.0) | 0.33 (0.20, 0.50) | 0.95 (0.91, 0.98) |
| Any 2 qSOFA | 19 (50.0) | 9 (5.0) | 0.50 (0.33, 0.67) | 0.95 (0.91, 0.98) |
| Modified MEWa | ||||
| SBP< 90 mmHg | 17 (36.2) | 13 (6.9) | 0.36 (0.23, 0.51) | 0.93 (0.88, 0.96) |
| HR>120 | 30 (58.8) | 12 (6.4) | 0.59 (0.44, 0.72) | 0.94 (0.89, 0.97) |
| RR>30 | 14 (31.1) | 0 | 0.31 (0.18, 0.47) | 1.00 (0.98, 1.00) |
| Neurological changes | 17 (37.8) | 0 | 0.38 (0.24, 0.53) | 1.00 (0.98, 1.00) |
| Any MEW trigger | 31 (81.6) | 24 (13.3) | 0.82 (0.66, 0.92) | 0.87 (0.81, 0.91) |
WBC=white blood cell (109/L), HR=heart rate (beats per minute), RR= respiratory rate (breaths per minute), T= temperature (°C)
Missing for each variable was the following: WBC˂ 4 × 109/L or >12 × 109/L =38, HR>90=1, RR>20=14, T<36°C or T>38°C =11, T<36°C or T>38°C and HR>90 =11, T<36°C or T>38°C and RR>20=20, T<36°C or T>38°C and WBC˂ 4 × 109/L or >12 × 109/L =49, HR>90 and RR>20=15, HR>90 and WBC˂ 4 × 109/L or >12 × 109/L =39, RR>20 and WBC˂ 4 × 109/L or >12 × 109/L =52, RR≥22=14, SBP<100 mmHg=5, Neurological changes=6, RR≥22 and SBP≤100 mmHg=18, SBP< 90 mmHg =5, HR>120=1, RR>30=14, Any modified MEW trigger=22, Any 2 SIRS=58, Any 2 qSOFA=22
Characteristics that were evaluated as potential risk factors for development of sepsis with corresponding unadjusted OR and 95% confidence intervals (CI) are presented in Table 4. Risk factors for sepsis with point estimates of 5 or greater and significant confidence intervals were the following: cesarean delivery-in labor (OR 20.9, 95% CI 5.1 to 128.3), cesarean delivery-not in labor (OR 15.6, 95% CI 4.2 to 87.2), PROM > 24 hours prior to labor (OR 8.9, 95% CI 2.5 to 39.4), stillbirth (OR 15.4, 95% CI 2.3 to infinity), preterm delivery (OR 6.1, 95% CI 2.4 to 16.8), retained products of conception (OR 12.9, 95% CI 2.4 to 128.1), multiple gestation (OR 5.7, 95% CI 1.8 to 19.5), congestive heart failure (OR 9.7, 95% CI 1.2 to infinity), chronic renal disease (OR 9.7, 95% CI 1.2 to infinity), and chronic liver disease (OR 15.4, 95% CI 2.3 to infinity).
Table 4.
Demographic, obstetric, and maternal risk factors for sepsis
| Characteristic | Delivery Hospitalization Cases n=82 (%) | Controls n=328 (%) | Unadjusted Odds Ratio 95% CI | |
|---|---|---|---|---|
| Patient Demographics | Age | |||
| ≤24 years old | 17 (20.7) | 65 (19.8) | Reference | |
| 25 to 34 years old | 48 (58.5) | 200 (61.0) | 0.9 (0.5, 1.8) | |
| ≥35 years old | 17 (20.7) | 63 (19.2) | 1.0 (0.4, 2.3) | |
| Race/Ethnicitya | ||||
| White (non-Hispanic) | 41 (52.6) | 230 (74.2) | Reference | |
| African American (non-Hispanic) | 16 (20.5) | 28 (9.0) | 4.0 (1.7, 11.3) | |
| Native American /Alaska Native | 1 (1.3) | 0 (0) | 4.0 (0.2, Infinity) | |
| Asian | 9 (11.5) | 15 (4.8) | 4.0 (1.4, 11.5) | |
| Native Hawaiian /Pacific Islander | 2 (2.6) | 1 (0.3) | 9.3 (0.5, 563.4) | |
| Other | 3 (3.8) | 2 (0.6) | 6.8 (0.8, 82.3) | |
| Hispanic | 6 (7.7) | 34 (11.0) | 0.8 (0.2, 2.5) | |
| Obstetric Variables | Nulliparousa | 45 (55.6) | 131 (40.3) | 1.8 (1.1, 3.1) |
| Type of deliveryb | ||||
| Spontaneous Vaginal | 8 (22.2) | 102 (70.8) | Reference | |
| Operative Vaginal | 0 (0) | 9 (6.3) | 1.4 (0, 8.7) | |
| Cesarean delivery (in labor) | 14 (38.9) | 16 (11.1) | 20.9 (5.1, 128.3) | |
| Cesarean delivery (not in labor) | 14 (38.9) | 17 (11.8) | 15.6 (4.2, 87.2) | |
| Cerclagea | ||||
| None | 77 (95.1) | 323 (98.8) | Reference | |
| Rescue | 1 (1.2) | 1 (0.3) | 4.0 (0.1, 314) | |
| Prophylactic | 3 (3.7) | 3 (0.9) | 4.0 (0.5, 29.9) | |
| PROM > 24 hours prior to labora | 10 (12.7) | 6 (1.8) | 8.9 (2.5, 39.4) | |
| Stillbirthb | 3 (8.3) | 0 (0) | 15.4 (2.3, Infinity) | |
| Preterm delivery (<37 weeks)b | 16 (44.4) | 16 (11.1) | 6.1 (2.4, 16.8) | |
| Retained products of conceptiona | 7 (8.5) | 3 (0.9) | 12.9 (2.4, 128.1) | |
| Steroids during pregnancya,b | ||||
| None | 26 (74.3) | 134 (93.1) | Reference | |
| Maternal indication | 1 (2.9) | 2 (1.4) | 2.4 (0, 215.6) | |
| Fetal indication | 8 (22.9) | 8 (5.6) | 4.7 (1.4, 16.7) | |
| Induction of labora,b | 7 (24.1) | 45 (33.6) | 0.6 (0.2, 1.9) | |
| Multiple gestation | 9 (11.0) | 7 (2.1) | 5.7 (1.8, 19.5) | |
| GBS positivea | 13 (33.3) | 47 (17.7) | 2.3 (1.0, 5.2) | |
| Maternal comorbidities | BMI≥40a | 14 (19.7) | 20 (7.4) | 3.7 (1.4, 9.6) |
| Congestive heart failurea | 2 (2.5) | 0 (0) | 9.7 (1.2, Infinity) | |
| Chronic renal diseasea | 2 (2.5) | 0 (0) | 9.7 (1.2, Infinity) | |
| Chronic liver diseasea | 3 (3.7) | 0 (0) | 15.4 (2.3, Infinity) | |
| Diabetes Mellitus | ||||
| Type 1 | 0 (0) | 1 (0.3) | 4.0 (0, 76.0) | |
| Type 2 | 2 (2.4) | 3 (0.9) | 2.7 (0.2, 23.3) | |
| GDM | 7 (8.5) | 11 (3.4) | 3.1 (0.9, 10.8) | |
| None | 73 (89.0) | 313 (95.4) | Reference | |
| Malignancy | 1 (1.2) | 0 (0) | 4.0 (0.2, Infinity) | |
| Smoking | 15 (18.3) | 26 (7.9) | 2.7 (1.2, 5.8) | |
PROM= Premature rupture of membranes, GBS=Group B streptococcus, BMI=Body mass index, GDM=gestational diabetes mellitus
Missing for each variable was the following: Race/ethnicity=22, Nulliparous=4, cerclage=2, PROM>24 hours prior to labor=4, Retained products of conception=1, Steroids during pregnancy=1, Induction of labor=17, GBS positive=105, BMI≥40kg/m2=68, Congestive heart failure=7, chronic renal disease =6, chronic liver disease=6
Data for patients with sepsis prior to delivery were excluded for specific variables of the analysis due to potential for reverse causality (46 sepsis cases, 184 controls)
Discussion
In our retrospective cohort of pregnant or recently postpartum women hospitalized for delivery, 59% of sepsis cases were caused by chorioamnionitis, endometritis, and pneumonia. Escherichia coli, other gram negative rods, and Group A streptococcus were the most common pathogens. We observed notable differences in the sensitivity and specificity of the three most commonly used screening tools for impending sepsis (i.e., SIRS, qSOFA, and MEW criteria) in this population.
Few screening tools for sepsis have been applied to the pregnant and immediately postpartum periods, and comparisons between available tools are lacking. A novel aspect of this report is that notable differences in the sensitivity and specificity of sepsis screening tools in a multicenter parturient population were observed, with the highest to lowest sensitivity being SIRS, MEW, and qSOFA criteria, and the highest to lowest specificity being qSOFA, MEW, and SIRS. This is similar to the findings of a recent meta-analysis evaluating the sensitivity and specificity of SIRS and qSOFA in a general population of sepsis patients. That study reported SIRS as superior for sensitivity and qSOFA as superior for specificity.12 The Sepsis in Obstetrics Score is not a screening tool for sepsis in all pregnant women, rather it is a scoring system to predict the need for ICU admission in patients with a known or suspected infection. Therefore, it was not assessed in this study as we limited our analyses specifically to screening tools for sepsis.13
Our study provides further insight into the causes (i.e. chorioamnionitis, endometritis and pneumonia) and specific organisms commonly associated with sepsis in pregnant women,2,14 and highlighted the importance of initiating early therapy. The Surviving Sepsis Campaign recommends administration of an appropriate broad-spectrum antibiotic therapy within the first hour of diagnosis15, something that was not achieved in 35.7% of our sepsis cases; in these cases, mortality was 20% compared with 8.3% for those receiving antibiotics within one hour of diagnosis. With each hour of antibiotic therapy delay in a general population with sepsis, mortality increases by 7.6%.16 Our data suggest a similar pattern occurs in pregnancy with increased mortality when antibiotics are delayed more than one hour. Strategies to encourage early antibiotic treatment include placing broad spectrum antibiotics in automated medication dispensing systems on labor and delivery (avoiding pharmacy delays), requiring providers to close the loop of communication that prioritizes antibiotic administration (by triaging multiple orders with the nursing staff), ensuring early and adequate intravenous access, and administering antibiotics immediately while awaiting transfer to another part of the hospital (many patients did not receive antibiotics until they arrived in the ICU despite orders written several hours prior).
The strengths of this study are the detailed information obtained from chart review including vital signs, timing of antibiotic administration, and temporal relation between sepsis and risk factors (allowing the evaluation of risk factors without potential bias due to reverse causality). This study also includes data from seven large academic centers with differing practice patterns and patient populations leading to the generalizability of the results.
Because sepsis during pregnancy and the peripartum period is rare, our data are limited in statistical power to define the significance of some potential risk factors for sepsis. We were unable to compute a multivariable logistic regression model due to the limited sample size. Sepsis was defined using the operant definition at the time the patients were hospitalized. Verifying sepsis diagnosis using sepsis-related SOFA scoring at the time of diagnosis would have been difficult because many criteria are not routinely documented in pregnant women (bilirubin level, PaO2, specific Glasgow coma scores) and creatinine levels do not correlate to the scoring system since they are much lower during pregnancy at baseline. In addition, for the purposes of this study, urine output and SpO2 values were not collected; however these variables may be abnormal in septic patients and could enhance the sensitivity of the MEW criteria. There were also a substantial number of patients excluded from the screening tool analysis because it was essential to have both controls and patients at the same time period in relation to delivery to account for the physiological changes occurring peripartum. If the sepsis cases occurred many days prior or after delivery, it was not always possible to match controls because women with uneventful deliveries are generally discharged quickly. An additional limitation is the frequency with which complete vital sign data were missing in the patients’ charts, necessitating exclusion of some patients from the analysis of the performance characteristics of the scoring systems. This finding is consistent with previous reports indicating the often incomplete and infrequent evaluation of vital signs in obstetric patients.17 Without proper measurement and recording of vital signs, physiological abnormalities are unlikely to be identified in a timely manner; this is an important area for future quality improvement projects. As an example, although respiratory rate has been correlated with outcomes in septic patients, it is very poorly recorded in medical charts.5,18 Ancillary staff should be educated about the importance of respiratory rate in detecting potentially significant metabolic derangement.
In conclusion, although MEW criteria demonstrated a sensitivity of 82% and a specificity of 87%, if adopted, potentially 18% of patients would not be identified. SIRS criteria demonstrated a sensitivity of 93%, but with a specificity of 63%. SIRS criteria would identify more patients, but at the potential expense of alarm fatigue. The qSOFA criteria, with a sensitivity of 50% could potentially miss 50% of patients with sepsis. Clinical, physiologic, and laboratory features should be more robustly investigated to determine if additional or altered criteria should be employed in this population of patients. Obstetric care units should be encouraged to obtain timely and complete vital sign information for all patients to readily identify patients with sepsis and to facilitate providing prompt antibiotic treatment.
“Key Points” Summary.
Question: Which of the screening tools (SIRS, qSOFA, MEW criteria) best identifies impending sepsis in pregnant or recently postpartum women?
Findings: Notable differences were observed in the sensitivity and specificity of sepsis screening tools with the highest to lowest sensitivity being SIRS, MEW, and qSOFA criteria, and the highest to lowest specificity being qSOFA, MEW, and SIRS.
Meaning: An ideal screening tool for maternal sepsis has yet to be identified.
Acknowledgments
The authors would like to acknowledge Keerthana Sankar B.S., Robert Schoenfeld B.S., Katherine Boyer B.S., and Danielle Isham B.S. (research assistants, Department of Anesthesiology, University of Michigan, Ann Arbor, MI) for the maintenance of REDCap™ database and assistance with organizing data. We would also like to thank Claire Kalpakjian PhD (Associate Professor, Department of Physical Medicine and Rehabilitation, University of Michigan, Ann Arbor, MI), Richard Smiley MD PhD (Professor, Department of Anesthesiology, Columbia University Medical Center, New York, NY), David Aronoff MD (Associate Professor, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN) and Digna Velez-Edwards MD (Assistant Professor, Department of Obstetrics and Gynecology, Vanderbilt University Medical Center, Nashville, TN) for participation in the early planning stages of this study.
Disclosure of Funding
This work was supported by the University of Michigan Health System Department of Anesthesiology. Support for REDCap (Research Electronic Data Capture) reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000433. B.T.B. is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the NIH (Bethesda, Maryland, United States) under Award Number K08HD075831. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. E.A.S.C. is supported by a grant from the Burroughs Wellcome Foundation. P.T. was supported by a grant from the Robert Wood Johnson Foundation (Princeton, NJ) Harold Amos Medical Faculty Development Program (award 69779). No source of funding had a role in any stage of the study, analysis, or writing of this manuscript.
Conflicts of Interest
BTB is an investigator for grants supported by Lilly, Pfizer, GSK, Pacira, and Baxalta for unrelated projects. BTB also serves as a consultant to Optum, Inc. SE has several patents with Oridion/Medtronic and has received funding for travel from Medtronic, Zoll, Diasorin and Laerdal all for unrelated projects. All other authors declare no disclosures.
Footnotes
Contribution to Authorship
Melissa E. Bauer: This author helped with study planning, data collection, data analysis, interpretation of analyzed data, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Michelle Housey: This author helped with study planning, data analysis, interpretation of analyzed data, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Samuel T. Bauer: This author helped with study planning, data collection, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Sydney Behrmann: This author helped with data collection, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Anthony Chau: This author helped with data collection, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Caitlin Clancy: This author helped with data collection, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Erin A.S. Clark: This author helped with study planning, data collection, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Sharon Einay: This author helped with interpretation of analyzed data, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Elizabeth Langen: This author helped with interpretation of analyzed data, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Lisa Leffert: This author helped with interpretation of analyzed data, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Stephanie Lin: This author helped with data collection, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Manokanth Madapu: This author helped with data collection, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Michael Maile: This author helped with interpretation of analyzed data, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Emily McQuaid-Hanson: This author helped with data collection, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Kristina Priessnitz: This author helped with data collection, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Hen Y Sela: This author helped with data collection, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Anuj Shah: This author helped with data collection, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Paul Sobolewski: This author helped with data collection, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Paloma Toledo: This author helped with study planning, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Lawrence Tsen: This author helped with study planning, writing or editing of the manuscript for critical content, and approval of the final manuscript.
Brian T Bateman: This author helped with study planning, interpretation of analyzed data, writing or editing of the manuscript for critical content, and approval of the final manuscript.
References
- 1.Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130(2):366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acosta CD, Kurinczuk JJ, Lucas DN, Tuffnell DJ, Sellers S, Knight M. Severe maternal sepsis in the UK, 2011–2012: A national case-control study. PLoS Med. 2014;11(7):e1001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg CJ, Harper MA, Atkinson SM, et al. Preventability of pregnancy-related deaths: Results of a state-wide review. Obstet Gynecol. 2005;106(6):1228–1234. [DOI] [PubMed] [Google Scholar]
- 4.Knight M, Nair M, Tuffnell D, Shakespeare J, Kenyon S, Kurinczuk JJ. Saving Lives, Improving Mothers’ Care - Lessons Learned to Inform Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2013–15. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2017. [Google Scholar]
- 5.Bauer ME, Lorenz RP, Bauer ST, Rao K, Anderson FW. Maternal deaths due to sepsis in the state of Michigan, 1999–2006. Obstet Gynecol. 2015;126(4):747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. [DOI] [PubMed] [Google Scholar]
- 7.Bauer ME, Bauer ST, Rajala B, et al. Maternal physiologic parameters in relationship to systemic inflammatory response syndrome criteria: A systematic review and meta-analysis. Obstet Gynecol. 2014;124(3):535–541. [DOI] [PubMed] [Google Scholar]
- 8.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. [DOI] [PubMed] [Google Scholar]
- 10.Mhyre JM, D’Oria R, Hameed AB, et al. The maternal early warning criteria: A proposal from the national partnership for maternal safety. Obstet Gynecol. 2014;124(4):782–786. [DOI] [PubMed] [Google Scholar]
- 11.Grimes DA, Schulz KF. Compared to what? Finding controls for case-control studies. Lancet. 2005;365(9468):1429–1433. [DOI] [PubMed] [Google Scholar]
- 12.Serafim R, Gomes JA, Salluh J, Povoa P. A comparison of the quick-SOFA and systemic inflammatory response syndrome criteria for the diagnosis of sepsis and prediction of mortality: A systematic review and meta-analysis. Chest. 2017. [DOI] [PubMed] [Google Scholar]
- 13.Albright CM, Ali TN, Lopes V, Rouse DJ, Anderson BL. The sepsis in obstetrics score: A model to identify risk of morbidity from sepsis in pregnancy. Am J Obstet Gynecol. 2014;211(1):39.e31–38. [DOI] [PubMed] [Google Scholar]
- 14.Bauer ME, Bateman BT, Bauer ST, Shanks AM, Mhyre JM. Maternal sepsis mortality and morbidity during hospitalization for delivery: Temporal trends and independent associations for severe sepsis. Anesth Analg. 2013;117(4):944–950. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2016. Intensive Care Med. 2017;43(3):304–377. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. [DOI] [PubMed] [Google Scholar]
- 17.Knight M, Kenyon S, Brocklehurst P, Neilson J, Shakespeare J, Kurinczuk JJ. Saving Lives, Improving Mothers’ Care - Lessons Learned to Inform Future Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009–2012.: Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2014. [Google Scholar]
- 18.Kenzaka T, Okayama M, Kuroki S, et al. Importance of vital signs to the early diagnosis and severity of sepsis: Association between vital signs and sequential organ failure assessment score in patients with sepsis. Intern Med. 2012;51(8):871–876. [DOI] [PubMed] [Google Scholar]