Abstract
Monitoring for SARS-CoV-2 RNA in wastewater through the process of wastewater-based epidemiology (WBE) provides an additional surveillance tool, contributing to community-based screening and prevention efforts as these measurements have preceded disease cases in some instances. Numerous detections of SARS-CoV-2 RNA have been reported globally using various methods, demonstrating the technical feasibility of routine monitoring. However, in order to reliably interpret data produced from these efforts for informing public health interventions, additional quality control information and standardization in sampling design, sample processing, and data interpretation and reporting is needed. This review summarizes published studies of SARS-CoV-2 RNA detection in wastewater as well as available information regarding concentration, extraction, and detection methods. The review highlights areas for potential standardization including considerations related to sampling timing and frequency relative to peak fecal loading times; inclusion of appropriate information on sample volume collected; sample collection points; transport and storage conditions; sample concentration and processing; RNA extraction process and performance; effective volumes; PCR inhibition; process controls throughout sample collection and processing; PCR standard curve performance; and recovery efficiency testing. Researchers are recommended to follow the Minimum Information for Publication of Quantitative Real-Time PCR (MIQE) guidelines. Adhering to these recommendations will enable robust interpretation of wastewater monitoring results and improved inferences regarding the relationship between monitoring results and disease cases.
Keywords: SARS-CoV-2, COVID-19, RT-qPCR, WBE, Pandemic, Epidemic
1. Introduction
The current global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a public health emergency of international concern [1]. This pandemic has resulted in >30 million cases of COVID-19 and >950,000 deaths worldwide as of 18th September 2020 [1,2]. Non-pharmaceutical public health interventions including clinical testing, social distancing, contact tracing, isolation of infected individuals, and in extreme epidemics, complete restriction of human mobility in areas ranging in size from town-suburb to state or province have been adapted to slow down the spread of the community transmission [3, 4, 5]. While SARS-CoV-2 is predominantly a respiratory virus; SARS-CoV-2 RNA is also shed in faeces (102–107 gene copies (GC)/mL) and urine (102–105 GC/mL) in addition to saliva and sputum (105–1011 GC/mL), which are often collected via wastewater systems [6,7]. Scientists around the globe are now trying to develop reliable approaches to monitor the virus circulation within communities by measuring SARS-CoV-2 RNA in wastewater by an approach known as wastewater-based epidemiology (WBE) [8]. Several recent studies have reported the presence of SARS-CoV-2 RNA in wastewater in several countries [9*, 10, 11, 12**, 13*, 14].
Lodder and Husman [15] reported detection of SARS-CoV-2 RNA in wastewater in the Netherlands within four days of the first clinically-diagnosed cases in the country. Detections of SARS-CoV-2 RNA in wastewater have been reported in Milan, Italy, within a few days of the first national case [16] and in Brisbane, Australia, when the number of clinical cases were in the hundreds within a population of approximately 600,000 [9]. Interestingly, Medema and colleagues detected an RNA target associated with SARS-CoV-2 in wastewater from a city in the Netherlands six days before the first clinical cases were reported [12]. These observations indicate that WBE could be a feasible and sensitive means of monitoring SARS-CoV-2 infection presence and trends within communities. A modelling exercise has suggested that wastewater surveillance could theoretically be able to detect one SARS-CoV-2 infection among 2,000,000 individuals, but noted limitations including uncertainties around temperature-dependent RNA signal decay in wastewater and hydraulic residence times in wastewater collection systems prior to sample collection [17]. A preprint has reported a more modest detection limit of one fecal-shedding infection in 1,000 to two/10,000 as estimated from monitoring wastewater from a hospital with COVID19 treatment and isolation units [18]. However, it should be noted that this estimation was nor based on virus recovery corrections. Also, the authors extrapolated a single quantification cycle (Cq) value for a wastewater sample resulting from a known proportion of infections to the estimated threshold limit.
The sensitive detection of SARS-CoV-2 RNA in wastewater, and thereby the presence of infections within a community depends on both the wastewater sampling and the molecular-based methods employed, which remain diverse and unstandardized, and often lack important information needed by public health units to interpret and apply the information [19, 20, 21]. To date, little has been documented on the performance of concentration, extraction, and detection methods for SARS-CoV-2 in wastewater [22]. Few of the published SARS-CoV-2 WBE articles provide detailed experimental procedures which hinders our ability to replicate the experiments or to compare across studies, as is necessary to improve interpretation of the results of WBE to inform public health officials. In this opinion paper, we discuss the peer-reviewed journal articles that have reported the presence of SARS-CoV-2 RNA in wastewater and provide recommendations to encourage better quality control, allowing for more reliable and less ambiguous interpretation and application of WBE results.
2. Published studies detecting and enumerating SARS CoV-2 RNA in wastewater
Table 1 summarizes the peer-reviewed research on the prevalence and concentration of SARS-CoV-2 RNA in wastewater as observed in various countries. For untreated wastewater, sample volumes ranging from 40 to 500 mL are typically concentrated; however, for secondary and tertiary treated wastewater larger sample volumes, up to 70 L, have been concentrated. Of the 18 studies, 8 studies collected composite samples over durations ranging from 10 to 24 h, two studies collected both composite and grab samples, and eight studies collected grab samples as a single or a few points in time. Various virus concentration methods, including polyethylene glycol (PEG) precipitation, adsorption-extraction and adsorption-elution using electronegative membranes, ultrafiltration, ultracentrifugation, nanoCeram electropositive cartridge, and direct flocculation, have been used to detect SARS-CoV-2 RNA in wastewater. The majority (11 of 18) of the studies used a single concentration method, while the others used two different concentration methods without evaluating each method’s efficiency in concentrating enveloped SARS-CoV-2 from wastewater.
Table 1.
Prevalence and concentrations of SARS-CoV-2 RNA in wastewater.
| Country | Types of wastewater | Volume of wastewater concentrated (mL) | Sample storage prior to testing | Virus concentration methods used | Inclusion of process control | PCR inhibition checked | % recovery | RT-PCR assay/target gene used | Number of samples positive/number of samples tested (%) (concentration) | Sequencing | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Australia | Untreated wastewater | 100-200 (grab and 24-h composite) | Ice, 4°C | Adsorption-extraction and ultrafiltration | No | Yes | NR | N_Sarbeco NIID_2019-nCOV | 2/9 (22%) (1.9 to 12 GC/100 mL) | Sanger Illumina MiSeq |
[9] |
| Brazil | Untreated wastewater | Not provided (10-h composite) | NR | Ultracentrifugation | No | No | NR | CDC N2 | 5/12 (41.6%) | NU | [42] |
| China | Inlets of
pre-processing disinfection pool Outlet of pre-processing disinfection pool Final outlet of sewage disinfection pool |
NR (grab) | NR | NR | No | No | NR | SARS-Cov-2 nucleic acid detection kit | 3/3
(100%) 1/1 (100%) 0/1 (0%) |
NU | [43] |
| China | Hospital septic
tank Influent Hospital septic tank effluent |
2000 (grab) | NR | PEG precipitation | No | No | NR | CCDC-ORF1 CCDC-N |
0/4 (0%)
(500-1870 GC/L) 7/9 (78%) |
NU | [44] |
| Czech Republic | Untreated wastewater | 500 (mostly 24-h time or flow composite) | 5°C | Direct flocculation | Yes | Yes | 35.5 ± 13.0% using TGEV (whole process control) | EliGene® COVID19 BASIC A RT kit | 13/112 (11.6%) | NU | [45] |
| Germany | Untreated
wastewater Treated effluent |
45 (24-h flow composite samples) | Ice | Ultrafiltration and centrifugation | No | No | NR | M gene RdRP gene M gene RdRP gene |
9/9 (100)
(<10 GC/mL) 9/9 (100) (<100 GC/mL) 4/4 (100) (<10 GC/mL) 4/4 (100) (<100 GC/mL) |
Sanger | [46] |
| India | Untreated
Wastewater Final effluents |
50 (grab, 11:30am) | 4°C | Centrifugation, filtration, PEG precipitation | No | Yes | NR | TaqPath COVID-19 Combo Kit ORF1ab, N and S genes) | 2/2 (100%) (560
to 350 GC/L 0/2 (0%) |
NU | [36] |
| Italy | Untreated wastewater | 250 (24-h composite) | -20°C | Two-phase (PEG-dextran method) separation | No | No | NR | ORF1ab Spike protein RdRP |
6/12
(50%) 2/12 (16.7%) NR |
Direct sequence | [11] |
| Italy | Untreated wastewater | 250 (24-h composite) | -20°C | Two-phase (PEG-dextran method) separation | Yes | Yes | 2.04 ± 0.70% using Alphacoronavirus HcoV-229E) | ORF1ab E gene RdRP |
18/40 (45%) –
Nested RT-PCR 26/40 (65%) (290 to 56,000 GC/L) – RT-qPCR NR |
Direct sequence | [16] |
| Italy | Untreated
wastewater Treated wastewater |
NR (grab, 1pm) | NR | Membrane Filtration | Yes | Yes | NR | ORF1ab N gene E gene ORF1ab N gene E gene |
4/8
(50%) 0/4 (0%) |
Whole genome sequencing | [47] |
| Japan | Influent Secondary treated |
200
(grab) 5000 (grab) |
Ice | Electronegative
membrane-vortex (EMV) method Adsorption-extraction |
Yes | Yes | 71.6 ± 25.2%
using MS2 for EMV 8.5 ± 3.7% using MS2 for the adsorption- extraction (RNA extraction and RT-qPCR) |
N_Sarbeco NIID_2019-nCOV_N CDC N1 CDC N2 |
0/5 (0%) 1/5 (20%) (2,400 GC/L) |
Direct sequence | [10] |
| Spain | Influent Secondary Tertiary effluent |
200 (grab, 7-12 am) | 4°C | Al(OH)3 adsorption-precipitation | Yes | No | 10 ± 3.5% using
PEDV (influent) 3.3 ± 1.6% using PEDV (effluent) 10 ± 2.1% using MgV (influent) 6.2 ± 1.0% (MgV effluent) |
CDC N1, N2,
N3 CDC N1, N2, N3 CDC N1, N2, N3 |
35/42 (83%)
(5.10 to 5.50 log10 GC/L) 2/18 (11%) (5.40 log10 GC/L) 0/12 (0%) |
NU | [13] |
| Spain | Untreated wastewater | 200 (grab, 10 am – 12 pm) | 4°C | Al(OH)3 adsorption-precipitation | Yes | No | 2.56 - 18.8% using MgV | CDC N1 CDC N2 |
12/15 (80%)
(5.31 to 5.75 log10 GC/L) 13/15 (86.7%) (5.22 to 5.98 log10 GC/L) |
NU | [48] |
| The Netherlands | Untreated wastewater | 250 (24-h flow composite) | 4°C | Ultrafiltration | Yes | No | 73 ± 50% using
F-specific RNA phages for purification and concentration) 30.4 ± 22.3% using F-specific RNA phages for RNA extraction and RT-qPCR) |
CDC N1 CDC N2 CDC N3 E_Sarbeco |
18/29 (62.1%)
(12 to 790 GC/mL) 18/29 (62.1%) (12 to 2,200 GC/mL) 19/29(65.5%) (12 to 1,800 GC/mL) 18/29 (62.1%) |
NU | [12] |
| USA | Untreated wastewater | 40 (24-h composite) | 4°C | PEG precipitation | No | No | NR | CDC N1 CDC N2 CDC N3 |
10/10
(100%) 10/10 (100%) (57 to 303 GC/mL) 10/10 (100%) |
Sanger | [49] |
| USA | Untreated
wastewater Secondary treated effluent Final effluent |
100-750 (grabs at 7 - 11am and 24-h composite) | Ice, -80°C | Centrifugation
and ultrafiltration Adsorption and elution with electronegative membrane |
Yes | No | 54-56% using Phi 6 | CDC N1 CDC N2 |
2/15 (13%) (3,100 to -7,500 GC/L) | NU | [14] |
| USA | Sewage | 125 (24-h flow composite) | Ice | InnovaPrep Electronegative filtration |
Yes | Yes | 5.5 ± 2.1% using
BCoV) 7.6 ± 3.0 using BRSV) 4.8 ± 2.8% using BCoV) 6.6 ± 3.8 using BRSV) |
CDC N1 CDC N2 CDC N3 |
107/198
(54%) 125/198 (65%) 113/198 (57%) (10 to 10000 GC/100 mL) |
NU | [50] |
| USA | Untreated wastewater | 40-70 L (grab) | 4°C | NanoCeram electropositive cartridge | No | Yes | NR | CDC N1 | 54/54 (100%) (4 to 5 log10 GC/L) | NU | [51] |
NR: Not reported; NU: Not undertaken.
Of the 18 studies, nine studies used either a whole (concentration to RT-qPCR) process control or a molecular (RNA extraction to RT-qPCR) process control. Only eight of the studies tested wastewater samples for the presence of PCR inhibitors (that impede amplification) using process control but very limited information has been provided with respect to magnitude and frequency of PCR inhibitors in wastewater samples. Ten studies did not provide any information on the method’s recovery efficiency, and the remaining studies determined the recovery efficiency using a variety of enveloped (Phi 6 phage, porcine epidemic diarrhea virus (PEDV), bovine coronavirus (BCoV), bovine respiratory syncytial virus (BRSV), and transmissible gastroenteritis virus (TGEV)) and nonenveloped viruses (F-specific RNA phage, mengovirus (MgV)). Also, a wide array of RT-qPCR assays was used to detect and enumerate molecular targets associated with the SARS-CoV-2 genome, often with little or no information provided on the RT-qPCR assay performance characteristics. All of these factors influence the ability to compare results among studies. Several studies provided quantitative data on the numbers of gene copies (GC)/L of wastewater, whereas some studies provided positive/negative results. Only six studies confirmed the identity of SARS-CoV-2 RNA using sequencing approaches, and of these studies, none reported false positive results.
3. Recommendations for SARS-CoV-2 WBE
While the published studies have helped to establish the technical feasibility of routine monitoring for SARS-CoV-2 RNA in wastewater, it is apparent that there is generally a lack of quality control information in the published literature for WBE of SARS-CoV-2. This lack of reporting on quality control has the potential to limit the interpretation and usefulness of the produced data for advancing the WBE field, and ultimately implementing public health interventions. A list of variables that are likely to impact the detection and quantification of SARS-CoV-2 RNA in wastewater and therefore the sensitivity and accuracy of WBE for public health surveillance are shown in Table 2 . Increasing interest in WBE among public health officials and the introduction of state-wide or national monitoring programs in several countries demands improved reporting of methodological details and quality control metrics [23]. A recent review even suggested the need for an optimised and univocal methodological framework concerning the detection and quantification of SARS-CoV-2 RNA in wastewater [20]. Scaling SARS-CoV-2 wastewater surveillance to deliver national programs will also likely require that testing be performed not only by research laboratories but also by commercial laboratories. Such a rapid expansion in testing capacity makes robust and reproducible methods and quality control vital to produce actionable public health information. In view of this need, we recommend methodological and quality assurance approaches for SARS-CoV-2 RNA detection in wastewater using molecular methods.
Table 2.
List of variables that may impact the detection of SARS-CoV-2 RNA in wastewater and accuracy of WBE.
| Analytical steps | Variables | Examples | Key considerations |
|---|---|---|---|
| Wastewater sampling | Sampling method | Grab
sample 24-h composite sample |
Sampling time of
day Autosampler sampling frequency Size of the sewer catchment Diurnal variation of faecal load Hydraulic retention time (HRT) |
| Sampling frequency | Hourly Multiple days per week Daily Weekly Bi-weekly |
Available
resources Auto-sampler Accessibility to the WWTP Co-operation from utilities and/or councils |
|
| Sampling types/locations | Influent Primary effluent Secondary effluent Treated effluent |
HRT Chlorine dose Suspended solids |
|
| Sample processing | Sample storage conditions | Refrigerated Frozen |
Storage
space Storage temperature |
| Sample pre-treatment | Pasteurization Pre-filtration Centrifugation |
Virus
loss Time required for sample pre-treatment |
|
| Virus concentration methods | Adsorption-extraction Adsorption-elution PEG precipitation, Ultrafiltration, Ultracentrifugation |
Virus recovery
efficiency Effective volume analysed Cost Speed |
|
| Process control | Whole process
control Molecular process control |
Surrogate
virus Same group of viruses Non-infectious Easy to source Easy to cultivable |
|
| Molecular detection | Viral RNA extraction | Direct
extraction from membrane Extraction from the concentrated sample |
Extraction
efficiency Commercial extraction kit, In-house extraction method Manual extraction Robotic, extraction Cost Speed |
| RT step | One-step Two-steps |
cDNA synthesis
kit, Enzyme RT primer Cost Efficacy |
|
| PCR format | RT-PCR, Nested RT-PCR RT-qPCR dPCR |
Platform
availability Sensitivity Speed Downstream analysis (e.g., sequencing) |
|
| RT-PCR assay | CDC N1 CDC N2 E_Sarbeco N_Sarbeco NIID HKU CDC RdRP |
Assay limit of
detection Specificity Sensitivity Repeatability Intra and inter CV Duplexing or multiplexing |
|
| PCR performance characteristics | R2
value Efficiency Slope Y-intercept ALOD ALOQ |
Inter
CV Intra CV Repeatability Reproducibility Assay sensitivity |
|
| Cut off Cq value | Cq value >40 | Fluorescence
intensity threshold Inhibition |
|
| Sequencing confirmation | Sanger
sequencing Illumina MiSeq Direct sequencing Whole genome sequencing |
Sequencing
format PCR product amount Amplicon size Cost Cover |
|
| Data analysis | Reproducibility | Replication in
sample processing Detection procedures |
Reproducibility
in biological and technical replicates PCR replicate (positive/negative) |
| Adjustment of quantification data | Adjustment with process control recovery and/or virus recovery by concentration method | Reproducibility
in biological and technical replicates PCR replicate (positive/negative) |
|
| Analysis of left-censored data | Adjustment with process control recovery and/or virus recovery by concentration method | Adaptation of adjustment |
3.1. Wastewater sampling
Sampling design is a pivotal factor for detecting SARS-CoV-2 RNA in wastewater. The concentration of SARS-CoV-2 RNA in influent wastewater is expected to vary diurnally, based on defecation frequency and timing, as well as the sampling technique and frequency. Defecation in the general population is most frequent in the early morning compared to other times [24]. Therefore, wastewater collected during periods of peak fecal loading may be more enriched in SARS-CoV-2 RNA than wastewater generated at other times in the day. In situations where an autosampler is not available, these periods of increased fecal loading should be targeted for grab sampling, as one or more grab samples taken during these periods would provide a higher probability of SARS-CoV-2 RNA detection and quantification owing to higher RNA concentrations. We recommend that peak fecal loading times be identified prior to a sampling campaign dependent on grab samples. While the exact peak fecal loading periods will vary between WWTPs due to differences in sewer infrastructure, total influent flow may be a useful proxy for anthropogenic activity and fecal shedding in the morning.
Although a toilet flush cycle lasts only for several seconds, the resultant pulse of wastewater disperses across time depending on wastewater collection system characteristics, such as pumping stations [25]. For example, flushes of anthropogenic gadolinium (used as a MRI contrast agent) in a WWTP catchment (approx. 100,000 inhabitants) were found to arrive at the WWTP in discrete pulses ranging from 4 to 20 min wide [26]. Compared to grab samples, composite wastewater samples collected with an autosampler are much better suited to adequately sampling these pulses. Where an autosampler is available, autosamplers should composite as frequently (e.g., 10-15 mins) as possible. This is particularly important for sampling campaigns aiming to detect shedding by very few individuals. A flow-weighted composite sample is strongly recommended as it accounts for the often-numerous fluctuations in flow experienced at the inlet of a WWTP [26]. If this is not possible, a time-based composite sample is recommended. If an autosampler is unavailable, sampling during peak fecal loading is recommended as above. Details regarding autosampler setup and grab sampling time has been poorly reported to date (Table 1) and should be regularly reported to aid the interpretation of results.
Sampling frequency (i.e. number of discrete samples collected) is another important factor that needs to be considered for WBE of COVID-19 in the community. While most adults defecate once every 24-h or less [24], studies published to date indicate that some infected people do not shed the virus consistently, if at all. Consequently, two or more 24-h composite samples per week, or one 48 or 72-h composite sample per week is ideal for sampling programs aiming to sample the majority of shedding events in a community. Depending on the resources available, weekly sampling is recommended as a minimum with bi-weekly sampling preferably on weekends providing increased resolution. Where sample analysis costs or resources are restrictive, pooling samples from adjacent catchments for analysis may be a useful, particularly in areas served by multiple small WWTPs and/or in scenarios where a positive detection is unlikely. For WWTP catchments with a significant transient population (e.g., day workers or weekend visitors), comparison of morning or afternoon samples, or weekend and weekday samples may provide some insight as to the movement pattern of the shedder(s). Information on sample volume collected, collection points (e.g., influent, before/after grit removal, primary clarifier), transport conditions, and inclusion of a field blank must be clearly detailed for comparison between studies.
3.2. Wastewater sample storage and pre-treatment
Following sample collection, storage conditions can also affect the detection of SARS-CoV-2 RNA signals in wastewater samples owing to stability. It is recommended that samples should be transported on ice from the collection point to the laboratory. Upon arrival at the laboratory, samples should be stored at 4°C and should be concentrated within 48-72 h. Several studies have reported prolonged persistence of enveloped viruses, including SARS-CoV-2 and the RNA in wastewater samples at ∼4°C [27, 28, 29, 30]. Therefore, short-term storage, for 1-5 days at 4°C may be appropriate followed by concentration and extraction for RT-qPCR analysis. The impacts of storing untreated bulk wastewater samples at -20 and -80°C are not known. Furthermore, the impacts of pasteurization and repeated freeze-thaw cycles on the degradation of SARS-CoV-2 RNA in wastewater are not well understood and should be avoided until more data are available. We acknowledge that pasteurization is often undertaken to minimize the risk associated with handling wastewater. However, when the concentration of SARS-CoV-2 RNA is expected to be low in wastewater, this approach is not recommended as it may produce false negative results. We also encourage researchers to provide critical information on sample metadata such as biochemical oxygen demand (BOD), chemical oxygen demand (COD), total suspended solid (TSS), pH, etc, storage temperature, storage time prior to sample processing, whether the samples were frozen prior to virus concentration, as well as any pre-treatment before the concentration step.
3.3. SARS-CoV-2 concentration
Several virus concentration methods have been developed for the detection of enteric viruses in water and wastewater matrices [31]. However, some of the approaches may not be appropriate for enveloped viruses including SARS-CoV-2 [7,19]. Virus concentration is particularly important because the concentration of SARS-CoV-2 in wastewater is expected to be low in the beginning or at the tail end of an epidemic. To provide an effective early warning system or to inform decisions on an easing of restrictions safely, the methods must be sensitive enough to detect a very small number (low concentration) of viruses in a wastewater sample.
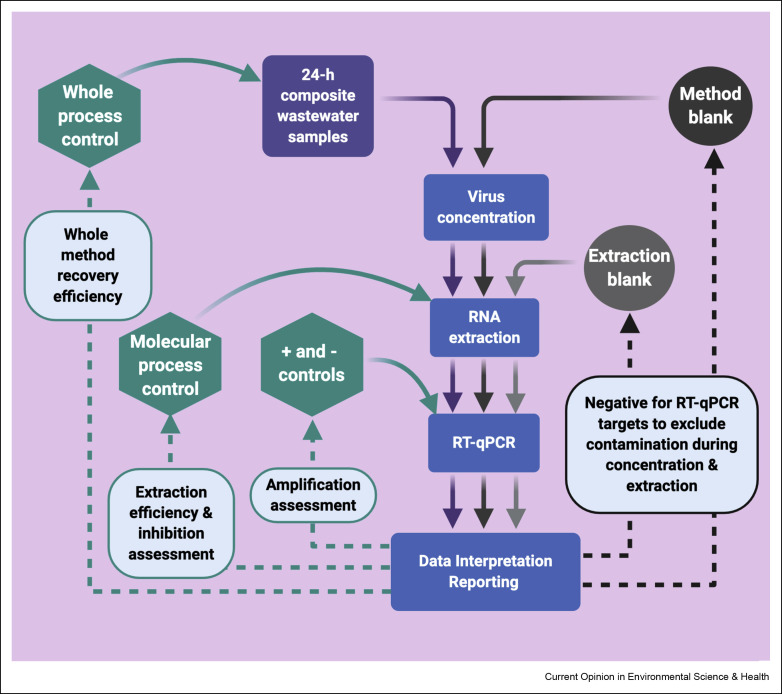
A recent study evaluated seven concentration methods for the recovery of murine hepatitis virus (MHV), a surrogate of SARS-CoV-2, in a small volume (e.g., 50 mL) of untreated wastewater. Among the methods tested, the electronegative membrane, with the addition of MgCl2 resulted in the greatest mean recovery rate [32]. Such a method is suitable for several reasons: it does not involve any prefiltration and pre-centrifugation step, and viruses are adsorbed on the membrane from both the liquid and solid fractions of the wastewater. If a larger diameter membrane is used, up to 200 mL wastewater can be processed with this method. However, this method may not be suitable for highly turbid and medium-sized wastewater samples (500 mL to 1 L). For wastewater volumes greater than 200 mL, the PEG precipitation method can be a suitable option [19]. Both adsorption-extraction and PEG precipitation methods can be performed in most laboratories with basic equipment and do not require extensive technical expertise. Further studies need to be conducted and validated to better understand the difference in recovery between enveloped and non-enveloped viruses using various concentration methods. We also recommend including a method blank (analyte free matrix) for each batch of wastewater samples processed to assess cross contamination from sample analysis. A flow chart showing steps and quality control measures should be taken for reliable and comparable data from wastewater epidemiology for SARS-CoV-2 is presented in Fig. 1 .
Fig 1.
A flow chart showing steps and quality controls for producing reliable and comparable information of SARS-CoV-2 RNA for wastewater-based epidemiology.
3.4. PCR inhibition and process control
PCR-based assays are prone to the inhibitors that can be found in wastewater which affect the sensitivity of the assay and may result in false‐negative results [33]. Wastewater influent compositions are highly variable within a single WWTP and the variability in composition is even greater between WWTPs. Wastewater samples contain polysaccharides, metal ions, and RNases, which can inhibit RT-PCR amplification [33,34]. These PCR inhibitors may be co-concentrated during virus concentration, depending on the method used. Therefore, the effect of inhibitors in wastewater samples needs to be investigated, and if present, inhibition should be both reported, and efforts should be taken to minimize overall inhibition. To achieve this, we recommend that each wastewater sample should be seeded with a surrogate virus as a whole-process control to obtain information on the surrogate virus recovery and RNA recovery and RT-PCR inhibition for the entire process starting from sample concentration to RT-PCR detection [31]. If an appropriate process control is not available, molecular process controls can also be included to obtain information on the RNA extraction efficiency, and RT-PCR inhibition should, at a minimum, be evaluated (e.g., through target dilution) and reported. Based on the process control data, samples may need to be reanalyzed (e.g., after dilution to reduce PCR inhibitors to suitable levels) or switch to an alternative method for increased sensitivity [31].
For a reliable process control, it is appropriate to select a virus which is morphologically and genetically similar to the target virus and is expected not to be present in the tested water. For example, for SARS-CoV-2, low-pathogenic animal CoVs such as MHV, BCoV, FIPV or enveloped bacteriophages, such as Phi6 phage, represent ideal controls. We acknowledge that obtaining a suitable process control may be difficult for many laboratories, especially during a pandemic. Nonetheless, the selection of already established process controls for enteric viruses, such as single-stranded murine norovirus (MnV) and MgV, may be preferable to not including a process control [35]. Viruses that are already present in wastewater in high numbers, such as pepper mild mottle virus (PMMoV) or MS2 phage may be used as a process control as long as their concentrations in the original and in the concentrated samples are compared to assess recovery levels. Nonetheless, the structure and size of these viruses are substantially different from the structure of SARS-CoV-2, therefore, they may provide an inaccurate estimation of recovery. However, these viruses can be used as a qualitative control to check the successful extraction of RNA from wastewater samples.
3.4. RNA extraction
In order to obtain high quality RNA of SARS-CoV-2 for RT-qPCR analysis, several factors need to be considered, including the RNA extraction procedures, the concentration, purity, and integrity of the extracted RNA, risks of cross-contamination by DNA/RNA that may be present in the lab (equipment, surfaces, air), or the presence of other contaminants. Several studies have employed various commercially available RNA extraction kits for the extraction of SARS-CoV-2 RNA from wastewater. The recovery of RNA may vary greatly depending on the kits used, and the quality of the what ostensibly are identical kits may vary considerably between manufacturers. The majority of studies used 140-450 μL of concentrated samples for RNA extraction and obtained 30 to 100 μL of viral RNA extracts [9,10,12,13,36]. The volume of concentrated samples used for the viral RNA extraction step and the resulting volume of RNA extracts may also need to be optimized to reduce the inhibition during the downstream RT-qPCR process that also influence the probability of detecting SARS-CoV-2 RNA. Little has been documented on the performance of various commercial RNA extraction kits for the recovery of SARS-CoV-2 from concentrated wastewater samples. In our opinion, RNA extraction kits that have fewer steps and are equipped with PCR inhibitor removal techniques are likely to be more useful in order to reduce the chance of contamination and downstream inhibition. Both virus concentration and RNA extraction methods need to be equally effective for the isolation of low levels of SARS-CoV-2 from wastewater. We also recommend including a reagent blank (negative extraction control) for each batch of RNA extraction to document the absence of cross-contamination from reagents during the extractions.
3.5. RT-qPCR/dPCR QA/QC
For detection and quantification of SARS-CoV-2 RNA in wastewater, RT-PCR, RT-qPCR and droplet digital PCR have been used. These PCR technologies use different platforms, reagents, protocols, analysis methods, and reporting formats which results in a lack of methodological consistency in PCR experiments and the resulting data. Bustin and colleagues [37] and Huggett and colleagues [38], recommended a minimum information for publication of both qPCR and digital PCR experiments to ensure the experiment’s accuracy, correct interpretation, and repeatability. To generate data of the highest integrity, WBE researchers should adhere to the published Minimum Information for Publication of Quantitative Real-time PCR (MIQE) and the Digital MIQE guidelines as strictly as possible. All PCR experiments should include the appropriate no-template controls and positive controls (for RT-PCR) and standards (for RT-qPCR). Standard curves can be prepared from a diverse array of materials including synthetic DNA or RNA of the amplicon, plasmid constructs, cDNA cloned into plasmids, and RNA extracted from the biological samples [37]. Each of these standards materials confers certain advantages and disadvantages [39]. Therefore, it is vital that the type of standard material used is fully described including the manufacturer and method of determining the copy number for use in calibration. Additionally, any required treatment, such as digestion or linearization of circular control plasmids, should be reported as circular plasmids have been observed to cause quantification bias [40]. In the case of RT-qPCR experiments, standard curve characteristics (slope, y-intercept, r2 value), Cq values, and estimated copy numbers should be reported. A fresh diluted standard curve should be used when a Cq shift of 0.5-1.0 is observed [37]. The standard curve should be used in every RT-qPCR run if possible. Alternatively, a master stadard curve compiled from multiple independent experiments can also be used [41]. For digital PCR experiments the metrics required to calculate the most probable copy number (total number of partitions and number of positive partitions, partition volume, etc.) should be reported. We also recommend that PCR experiments include technical replicates for each sample and SARS-CoV-2 specific targets. For each SARS-CoV-2 specific assay, assay limits of detection should be reported, including the method of determining such limits. Ideally, the specificity of the assays used should be confirmed by sequencing or analysis of the resulting PCR amplicons.
3.6. Reporting turnaround time
While wastewater surveillance for SARS-CoV-2 has the potential to act as an early warning system, the merit for WBE will be influenced by site selection and can only be realised with rapid turnround. The value of results from wastewater surveillance decreases with increasing turnaround time, particularly in cases where other methods do not provide rapid, objective information. To maximise this value, sampling programs should aim to minimise the time take between the stages of approvals, wastewater sampling, analysis, reporting and consequent actioning based on the results. We recommend that each program develop its own unique operating protocols for each of these four aspects in order to maximise the value of surveillance efforts. Sites may be selected for many reasons based on the status of the pandemic in a particular region. The fast commencing of sampling will require an efficient approval process and close collaboration with those responsible for sewer networks, such as city councils, water utility companies and service provider companies. In the case where WBE is being applied to small populations, as may be the case with age care facilities, prisons, and university campus accommodation, ethics approvals may be needed. Rapid response coupled with rapid turnround clearly provides the best chance of capturing early detects.
4. Concluding remark
WBE has been shown to be a powerful and effective tool to assess viral infections at a community level. Extraordinary efforts have been made globally to investigate SARS-CoV-2 in wastewater as well; however, to date, there is no identified gold standard method for the concentration, extraction and detection of the virus in complex environmental matrices, such as sewage. In order to obtain accurate results, sampling, sample process and viral quantification methods should be evaluated and validated. Samples should be taken on a regular basis and transported chilled to laboratories where they should be stored at 4°C and processed withing 2-3 days. Several sample concentration protocols are available and may be useful for SARS-CoV-2 recovery; however, their performance may vary among samples and hence appropriate process controls should be used. Quantitative and digital PCR quantification methods have been shown to detect SARS-CoV-2; however, these methods may be affected by inhibitors. Efforts should be taken to reduce the amount of inhibitors during RNA extraction and appropriate controls should be used to assess false negative and positive readings. Following these guidelines, actionable and reliable SARS-CoV-2 RNA concentrations in wastewater can be obtained. Results then can be compared and further evaluated on an international level to assist the mitigation of the pandemic.
CRediT authorship contribution statement
Warish Ahmed: Conceptualization, Writing - original draft. Aaron Bivins: Conceptualization, Writing - original draft. Paul M. Bertsch: Writing - original draft. Kyle Bibby: Writing - original draft. Phil M. Choi: Writing - original draft. Kata Farkas: Writing - original draft. Pradip Gyawali: Writing - original draft. Kerry A. Hamilton: Writing - original draft. Eiji Haramoto: Writing - original draft. Masaaki Kitajima: Writing - original draft. Stuart L. Simpson: Writing - original draft. Sarmila Tandukar: Writing - original draft. Kevin Thomas: Writing - original draft. Jochen F. Mueller: Writing - original draft.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
KF was funded by the UK Natural Environment Research Council (NERC) under the COVID-19 URGENCY programme (Ref. NE/V004883/1).
References
Papers of particular interest, published within the period of review, have been highlighted as:
*of special interest
** of outstanding interest
- 1.WHO: Coronavirus disease (COVID-19) situation report-209. WHO. Geneva. 2020.
- 2.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real-time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung K., Wu J.T., Liu D., Leung G.M. First-wave COVID-19 transmissbility and severity in China outside Hubei after control measures, and second-wave scenario planning: a modelling impact assessment. The Lancet. 2020;395:1382–1393. doi: 10.1016/S0140-6736(20)30746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan A., Liu L., Wang C., Guo H., Hao X., Wang Q., Huang J., He N., Yu H., Lin X. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020;323:1–9. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prem K., Liu Y., Russel T.W., Kucharski A.J., Eggo R.M., Davies N. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: A modelling study. The Lancet Public Health. 2020;5:e261–e270. doi: 10.1016/S2468-2667(20)30073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones D.L., Baluja Q.M., Graham D.W., Corbishley A., McDonald J.E., Malham S.K., Hillary L.K., Connor T.R., Gaze H.W., Moura I.B. Shedding of SARS-CoV-2 in feces and urine and its potential role in person-to-person transmission and the environment-based spread of COVID-19. Sci Total Environ. 2020;749:141364. doi: 10.1016/j.scitotenv.2020.141364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci Total Environ. 2020;739:139076. doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bivins A., North D., Ahmad A., Ahmed W., Alm E., Been F., Bhattacharya P., Bijlsma L., Boehm A.B., Brown J. Wastewater-based epidemiology: Global collaborative to maximize contributions in the fight against COVID-19. Environ Sci Technol Lett. 2020;13:7754–7757. doi: 10.1021/acs.est.0c02388. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed W., Angel N., Edson J., Bibby K., Bivins A., Brien J.W.O., Choi P.M., Kitajima M., Simpson S.L., Li J. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci Total Environ. 2020;731:140405. doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Rosa G., Iaconelli M., Mancini P., Bonanno G., Ferraro G.B., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medema G., Heijnen L., Elsinga G., Italiaander R. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ Sci Technol Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]; First application of wastewater based epidemiology, reports detection of SARS-CoV-2 in wastewater a week before pandemic hits Netherlands.
- 13.Randazzo W., Truchado P., Ferranfo E.C., Simon P., Allende A., Sanchez G. SARS-CoV-2 RNA titers in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports detection of SARS-CoV-2 in wastewater with appropriate quality control measures.
- 14.Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmiz B., Ahmed W., Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: A study in Louisiana, USA. Sci Total Environ. 2020;743:140621. doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol Hepatol. 2020;5:533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Rosa G., Mancini P., Bonanno G., Veneri C., Iaconelli M., Bonadonna L., Lucentini L., Suffredini E. SARS-CoV-2 has been circulating in northen Italy since December 2019: Evidence from environmental monitoring. Sci Total Environ. 2020;750:141711. doi: 10.1016/j.scitotenv.2020.141711. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that SARS-CoV-2 was circulating in different geographical regions in northern Italy long before the first detection of clinical case. This study also suggests that SARS-CoV-2 RNA monitoring in wastewater can provide early warning system for COVID-19 in the community.
- 17.Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: Feasibility, economy, opportunities and challenges. Sci Total Environ. 2020;730:138875. doi: 10.1016/j.scitotenv.2020.138875. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorgensen A.D., Gamst J., Hansen L.V., Knudsen I.I.H., Jensen S.K.S. Eurofins Covid-19 sentinel TM wastewater test provide early warning of a potential COVID-19 outbreak. MedRXiv. 2020 doi: 10.1101/2020.07.10.20150573. 2020. [DOI] [Google Scholar]
- 19.Lu D., Huang Z., Luo J., Zhang X., ShaSha Primary concentration – the critical step in implementing the wastewater based epidemiology for the COVID-19 pandemic: a mini-review. Sci Total Environ. 2020;747:141245. doi: 10.1016/j.scitotenv.2020.141245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michael-Kordatou I.M., Karaolia P., Fatta-Kassinos D. Sewage-analysis as a tool for the COVID-19 pandemic response and management: the urgent need fr optimised protocols for SARS-CoV-2 detection and quantification. J Environ Chem Eng. 2020;8:104306. doi: 10.1016/j.jece.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson J.R., Nancharaiah Y.V., Gu X., Lee W.L., Rajal B.B., Haines M.B., Girones R., Ng L.C., Alm E.J., Wuertz S. Making waves: Wastewater surveillance of SARS-CoV-2 for population-based health management. Water Res. 2020;184:116181. doi: 10.1016/j.watres.2020.116181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Reilly K.M., Allen D.J., Fine P., Asghar H. The challenges of informative wastewater sampling for SARS-CoV-2 must be met: lessons from polio eradication. The Lancet Microbe. 2020 doi: 10.1016/S2666-5247(20)30100-2. (online early) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baraniuk C. Sewage monitoring is the UK’s next defence against COVID-19. BMJ. 2020;370:m2599. doi: 10.1136/bmj.m2599. [DOI] [PubMed] [Google Scholar]
- 24.Heaton K.W., Radvan J., Cripps H., Mountford R.A., Braddon F.E., Hughes A.O. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut. 1992;33:818–824. doi: 10.1136/gut.33.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rieckermann J., Neumann M., Ort C., Huisman L.J., Guzer W. Dispersion coefficients of sewers from trace experiments. Water Sci Technol. 2005;52:123–133. [PubMed] [Google Scholar]
- 26.Ort C., Lawrence M.G., Rieckermann J., Joss A. Sampling for pharmaceuticals and personal care products (PPCPs) and illicit drugs in wastewater systems: Are your conclusions valid? A critical review. Environ Sci Technol. 2010;44:6024–6035. doi: 10.1021/es100779n. [DOI] [PubMed] [Google Scholar]
- 27.Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43:1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronavirus in water and wastewater. Food Environ Virol. 2009;1:10. [Google Scholar]
- 29.Ahmed W., Bertsch P.M., Bibby K., Haramoto E., Hewitt J., Huygens F., Gyawali P., Korajkic A., Riddel S., Sherchan S.P. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ Res 2020b. 2020 doi: 10.1016/j.envres.2020.110092. (accepted). This study evaluates the decay of SARS-CoV-2 and surrogate coronavirus in wastewater at various temperatures. SARS-CoV-2 RNA is likely to persist long enough in untreated wastewater to permit reliable detection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muirhead A., Zhu K., Brown J., Basu M., Brinton M.A., Costa F., Hayat M.J., Stauber C.E. Zika virus persistence in sewage. Environ Sci Technol Lett. 2020 doi: 10.1021/acs.estlett.0c00535. (online early) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haramoto E., Kitajima M., Hata A., Torrey J.R., Masago Y., Sano D., Katayama H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018;135:168–186. doi: 10.1016/j.watres.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed W., Bertsch M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci Total Environ. 2020;739:139960. doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study evaluates seven different concentration methods and reports recovery rates of murine hepatitis virus, a surrogate for SARS-CoV-2. This study suggests that absorption-extraction method is cost-effective and relatively straightforward for recovery of enveloped viruses in wastewater.
- 33.Schraeder C, Schielke A, Ellerbroek L, Johne R: PCR inhibitors – occurrence, properties and removal. J Appl Microbiol 113:1014-1026. [DOI] [PubMed]
- 34.Rock C., Alum A., Abbaszadegan M. PCR inhibitor levels in concentrates of biosolid samples predicted by a new method based on extraction-emission matrix specteroscopy. Appl Environ Microbiol. 2010;76:8102–8109. doi: 10.1128/AEM.02339-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hennechart-Collette C, Martin-Latil S, Guillier L, Perelle S: Determination of which virus to use as a process control when testing the presence of hepatitis A virus and norovirus in food and water. Int J Food Microbiol 202, 57-65. [DOI] [PubMed]
- 36.Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. The first proof of the capability of wastewater surveillance for COVID-19 in India through the detection of the genetic material of SARS-CoV-2. Sci Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 38.Huggett J.F., Foy C.A., Benes V., Emslie K., Garson J.A., Haynes R., Hellemans J., Kubista M., Mueller R.D., Nolan T. The digital MIQE guidelines: Minimum information for publication of quantitative digital PCR experiments. Clin Chem. 2013;59:892–902. doi: 10.1373/clinchem.2013.206375. [DOI] [PubMed] [Google Scholar]
- 39.Bustin SA: A to Z of Quantitative PCR. 2004. IUL Biotechnology Series, International University Line, 2004-2006.
- 40.Lin C.-H., Chen Y.-C., Pan T.-M. Quantification bias caused by plasmid DNA conformation in quantitative real-time PCR assay. PLoS One. 2011;6 doi: 10.1371/journal.pone.0029101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shanks O.C., Atikovic E., Blackwood A., Lu J., Noble R.T., Domingo J.S., Seifring S., Sivaganesan M., Haugland R.A. Quantitative PCR for detection and enumeration of genetic markers of bovine fecal pollution. Appl Environ Microbiol. 2008;74:745–752. doi: 10.1128/AEM.01843-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prado T., Fumian T.M., Mannarino C.F., Maranhao A.G., Siqueira M.M., Miagostovich M.P. Preliminary results of SARS-CoV-2 detection in sewerage system in Niteroi municipality, Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 2020;115 doi: 10.1590/0074-02760200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J., Feng H., Zhang S., Ni Z., Ni L., Chena Y., Zhoud L., Zhong Z., Qua T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int J Infect Dis. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang D., Ling H., Huang X., Li J., Li W., Yi C., Zhang T., Jiang Y., He Y., Deng S. Potential spreading risks and disinfection challenges of medical wastewater by the presence of Severe Acute respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Sci Total Environ. 2020;741:140445. doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mlejnkova H., Sovova K., Vasickova P., Ocenaskova V., Jasikova L., Juranova E. Preliminary study of SARS-CoV-2 occurrence in wastewater in the Czech Republic. Int J Environ Res Public Health. 2020;17:5508. doi: 10.3390/ijerph17155508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westhaus S, Weber F-A, Schiwy S, Linnemann V, Brinkmann M, Widera M, Greve C, Janke A, Hollert H, Wintgens T., et al.: Detection of SARS-CoV-2 in raw and treated wastewater in Germany – suitability for COVID-10 surveillance and potential transmission risks. Sci Total Environ 751:141750. [DOI] [PMC free article] [PubMed]
- 47.Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci Total Environ. 2020;744:140911. doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Randazzo W., Cuevas-Ferrando E., Sanjuan R., Domingo-Capal P. Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. Int J Hyg Environ Health. 2020:113621. doi: 10.1016/j.ijheh.2020.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu F., Xiao A., Zhang J., Gu X., Lee W., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystem. 2020 doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports concentration of SARS-CoV-2 in wastewater and compared with clinical confirmed cases.
- 50.Gonzalez R., Curtis K., Bivins A., Bibby K., Weir M.H., Yetka K., Thompson H., Keeling D., Mitchell J., Gonzalez D. COVID-19 surveillance in Southern Virginia using wastewater-based epidemiology. Water Res. 2020;186:116296. doi: 10.1016/j.watres.2020.116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyani B., Fonoll X., Norton J., Mohrotra A., Xagoraraki I. SARS-CoV-2 in Detroit Wastewater. J Environ Eng. 2020;11 [Google Scholar]