Abstract
Oral health conditions and cerebral small vessel disease, such as white matter lesions or cerebral microbleeds (CMBs), are associated with the incidence of stroke. The purpose of this study was to examine the associations between oral health conditions (serum IgG titers of periodontal pathogens) with the presence or severity of CMBs in acute stroke patients. From January 2013 to April 2016, acute stroke patients were registered in two hospitals. Serum samples were evaluated for antibody titers against 9 periodontal pathogens using the ELISA method. The cut-off points for reactivity (the positive decision point) to each antigen were defined as more than a mean ELISA unit + 1 standard deviation (after logarithmic transformation) in all subjects. CMBs were evaluated on T2*-weighted MRI. In all, 639 patients were evaluated (ischemic, n = 533 and hemorrhagic, n = 106; 73.1 ± 12.9 years old). Among these patients, 627 were available for CMB evaluation. Among the 9 evaluated periodontal pathogens, only Campylobacter rectus (C. rectus) was associated with the presence of CMBs. the prevalence of positive serum antibody titers against C. rectus was higher among patients with CMBs than among those without CMBs (14.6% vs. 8.7%, P = 0.025). In addition, positive serum antibody titers against C. rectus remained one of the factors associated with the presence of CMBs in multivariate logistic analysis (odds ratio 2.03, 95% confidence interval 1.19–3.47, P = 0.010). A positive serum antibody titer against C. rectus was associated with the presence of CMBs in acute stroke patients.
Introduction
Impaired oral health status, including periodontal disease, has an adverse impact on systemic health [1]. Periodontal disease associated with chronic systemic inflammation is thought to lead cardiovascular disease, stroke, and atherosclerosis progression [2]. A recent meta-analysis of cohort studies showed that the risk of ischemic or hemorrhagic stroke was significantly increased by 1.6-fold among patients with periodontitis [3]. Furthermore, an ARIC (Atherosclerosis Risk in Communities) cohort study performed in 10362 stroke-free participants over a 15-year follow-up period showed that periodontal disease was significantly associated with the incidence of cardioembolic and thrombotic stroke [4]. They also reported that access to regular dental examinations may reduce the risk of ischemic stroke. Therefore, the management or evaluation of periodontal disease is essential when considering stroke prevention.
Although accumulating evidence indicates an association between periodontal disease and stroke, few studies have investigated whether any specific pathogen related to periodontitis is associated with the incidence of stroke. Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) and Porphyromonas gingivalis (P. gingivalis) are classical and extensively explored “periodontal pathogens” [5]. Pussinen et al. found that systemic exposure to P. gingivalis but not A. actinomycetemcomitans was an independent risk factor for stroke in 8911 subjects [6]. Additionally, we previously reported that increased serum antibody titers against Prevotella intermedia (P. intermedia) were associated with atherothrombotic stroke patients [7]. Nishi et al. subsequently reported that increased serum antibody titers against Fusobacterium nucleatum (F. nucleatum) were associated with poor outcomes after stroke [8].
Cerebral small vessel disease (CSVD), such as cerebral microbleeds (CMBs) or white matter lesions (WMLs), are associated with the incidence of stroke [9]. The most well-established risk factor for CSVD is hypertension. Inflammation and endothelial dysfunction play key roles in the pathological cascade of CSVD [10–12]. Oral Microbacterium have recently been implicated in the pathogenesis of CSVD via an alternative pathway [13]. However, it is unclear whether periodontal disease or, in particular, specific pathogens that cause periodontitis, are associated with the presence or severity of CSVD. The aim of this study was to investigate whether serum IgG antibody titers against several periodontal pathogens are associated with CSVD among patients with acute stroke.
Materials and methods
Subjects
From January 2013 to April 2016, consecutive acute stroke patients who were categorized as ischemic or hemorrhagic were registered at Hiroshima University Hospital and Suiseikai Kajikawa Hospital. Each provided written informed consent according to a protocol approved by the Ethical Committee of Hiroshima University (Epd-614-2) and Suiseikai Kajikawa Hospital (2015–3) prior to undergoing examinations. Baseline data, including sex, age, body mass index (BMI), smoking habit, daily alcohol habit, comorbidities (hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, history of stroke and history of coronary artery disease), and serum C-reactive protein (CRP) levels (after logarithmic transformation) were collected for all patients. Imaging analysis was performed with computed tomography or magnetic resonance imaging (MRI) in all patients for the diagnosis of ischemic stroke or intracerebral hemorrhage. In the present study, patients who did not undergo MRI were excluded because the detail radiological findings for cerebral small vessel disease were evaluated by MRI. Ischemic stroke subtypes were classified using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria [14] by stroke specialists. Hemorrhagic infarction and trauma-induced hemorrhage were excluded from intracerebral hemorrhage. Hypertension was defined as the use of anti-hypertensive medication before admission or a confirmed blood pressure of ≥140/90 mmHg at rest measured 2 weeks after onset. Diabetes mellitus was defined as a glycated hemoglobin level of ≥6.5%, fasting blood glucose level of ≥126 mg/dl, or use of anti-diabetes medication. Dyslipidemia was defined as total cholesterol level of ≥220 mg/dl, a low-density lipoprotein cholesterol level of ≥140 mg/dl, a high-density lipoprotein cholesterol level of <40 mg/dl, triglyceride levels of ≥150 mg/dl, or the use of anti-dyslipidemia medication. Atrial fibrillation was defined as follows: (1) a history of sustained or paroxysmal atrial fibrillation or (2) atrial fibrillation detection upon arrival or during admission. Renal function was assessed by the estimated glomerular filtration rate (eGFR) using the following revised equation for the Japanese population as follows: eGFR (ml min-1 1.73 m-2) = 194 × (serum creatinine)-1.094 × (age)-0.287 × 0.739 (for women) [15]. Chronic kidney disease was defined as an eGFR<60 ml min−1 1.73 m−2. The patient’s history of stroke and coronary artery disease was collected from medical records or from the patient or the patient’s family.
Image analysis
An MRI was performed with a 1.5-T scanner (SIGNA, GE Medical Systems, Fairfield, CT, USA or Magneton Symphony Advanced or Avanto, Siemens Medical Systems, Erlangen, Germany) or a 3.0-T scanner (SIGNA, GE Medical Systems, Fairfield, CT, USA or Spectra, Siemens Healthineers, Erlangen, Germany or Philips Ingenia, Philips Medical Systems, Best, the Netherlands) within 2 weeks of the day of admission in each hospital. WMLs were evaluated on fluid-attenuated inversion recovery imaging (FLAIR), and CMBs were evaluated on T2*-weighted gradient echo imaging (T2*WI GRE). The severity of WMLs on FLAIR was assessed based on the Fazekas classification (ranging from 0 to 3) [16], with grade 0 and 2 defined as mild and grade 3 defined as severe WMLs. In this study, we defined the WMLs as periventricular hyperintensity. CMBs were defined as small-foci signal loss with a diameter of 2 to 10 mm on T2*WI. CMBs were separated from vascular flow voids, calcifications or nonhemorrhagic iron deposits. Computed tomography can facilitate the identification of suspected calcification. The locations of CMBs were classified as follows: lobar (cortex, subcortex, and white matter), deep (basal ganglia, thalamus, brain stem, and cerebellum) and mixed (both lobar and deep). WMLs and CMBs were evaluated in each hospital by two neurologists who were blind to the patients’ clinical data. We examined coincidence among evaluators using 124 cases. The inter-rater agreement in WML scores (mild vs severe) and the presence of CMBs was good (Kappa coefficient = 0.85 or 0.65, respectively). In cases of disagreement, we sought consensus between the observers.
Measurement of serum antibody titers against periodontal pathogens
Serum IgG antibody titers against periodontal pathogens were determined using enzyme-linked immunosorbent assays (ELISA) as previously described [17]. Serum samples were collected from the patients within 3 days after stroke onset and stored at −80°C. Sonicated preparations of the following periodontal pathogens were used as bacterial antigens: P. gingivalis ATCC33277 (FimA type I), A. actinomycetemcomitans AUNY67 (Serotype c), P. intermedia ATCC26511, Prevotella nigrescens (P. nigrescens) ATCC33563, F. nucleatum ATCC10953, Treponema denticola (T. denticola) ATCC35405, Tannerella forsythensis (T. forsythensis) ATCC43037, Campylobacter rectus (C. rectus) ATCC33238, and Eikenella corrodens (E. corrodens) ATCC23834. We previously reported associations of these representative periodontal pathogens with serum antibody titers and stroke outcome [17]. The serum from 5 healthy subjects was pooled and used for calibration. Using serial dilutions of the pooled control serum, the standard reaction was defined based on the ELISA unit (EU), with 100 EU corresponding to a 1:3200 dilution of the calibrator sample. The cut-off points for reactivity (the positive decision point) to each antigen were defined as more than the mean EU + 1 SD (after logarithmic transformation) obtained from all subjects in this study.
Statistical analysis
Categorical variables are presented as numbers and percentages, and continuous variables are presented as means with standard deviations. The statistical significance of intergroup differences was assessed using χ2 tests for categorical variables and Student’s t tests or Mann-Whitney U tests for continuous variables. Multivariable logistic analysis was performed with factors that were found to show a considerable difference in a univariate analysis (P < 0.2). In all analyses, P < 0.05 was considered statistically significant. All analyses were performed using JMP 14.0 (SAS Institute, Inc., Cary, NC, USA).
Results
Among the 690 consecutive acute stroke patients, 51 who did not undergo MRI because of contraindications or unsteadiness were excluded. Thus, a total of 639 patients (73.1 ± 12.9 years old, 285 males) were enrolled in this study. The baseline clinical characteristics of these patients are presented in Table 1. There were 533 ischemic stroke patients and 106 intracerebral hemorrhage patients. The 533 ischemic stroke patients included 120 with cardioembolism, 123 with large-artery atherosclerosis, 111 with small-vessel occlusion and 179 others. The 106 intracerebral hemorrhage patients consisted of 78 hypertensives, 14 patients with cerebral amyloid angiopathy and 14 others.
Table 1. Baseline characteristics.
| n = 639 | |
|---|---|
| Age (years) | 73.1 ± 12.9 |
| Female, n (%) | 285 (44.6) |
| Body mass index, kg/m2 | 22.8 ± 4.1 |
| Hypertension, n (%) | 487 (76.2) |
| Diabetes mellitus, n (%) | 166 (26.0) |
| Dyslipidemia, n (%) | 258 (40.4) |
| Atrial fibrillation, n (%) | 121 (18.9) |
| Chronic kidney disease, n (%) | 214 (33.5) |
| History of stroke, n (%) | 209 (32.7) |
| History of coronary artery disease, n (%) | 51 (8.0) |
| Current Smoker (n = 621), n (%) | 126 (19.9) |
| Daily alcohol habit (n = 619), n (%) | 137 (21.7) |
| Ischemic Stroke (n = 533) | |
| Cardioembolism, n (%) | 120 (22.5) |
| Large-artery atherosclerosis, n (%) | 123 (23.1) |
| Small-vessel occlusion, n (%) | 111(20.8) |
| Others, n (%) | 179 (33.6) |
| Intracerebral hemorrhage (n = 106) | |
| Hypertensive, n (%) | 78 (73.6) |
| Cerebral amyloid angiopathy, n (%) | 14 (13.2) |
| Others, n (%) | 14 (13.2) |
| MRI findings | |
| White matter lesions (WMLs) | |
| Fazekas Grade 0, n (%) | 39 (6.1) |
| Fazekas Grade 1, n (%) | 167 (26.1) |
| Fazekas Grade 2, n (%) | 236 (36.9) |
| Fazekas Grade 3, n (%) | 197 (30.8) |
| Presence of CMBs (n = 627), n (%) | 315 (50.2) |
| Multiple CMBs (n = 627), n (%) | 215 (34.3) |
Data are presented as the means ± SD for age and body mass index; and as the number of patients (%) for other parameters. MRI, magnetic resonance imaging; CMBs, cerebral microbleeds.
The association between serum antibody titers against periodontal pathogens and the severity of WMLs
Table 2 shows the baseline characteristics and serum antibody titers against each periodontal pathogen in patients with mild and severe WMLs. The patients with severe WMLs were significantly older, were more likely to be female, and had lower BMIs, lower rates of current smoking and daily alcohol habit, higher rates of hypertension, chronic kidney disease, and history of stroke, and higher serum CRP levels than patients with mild WMLs. In our evaluation of serum antibody titers against periodontal pathogens, there were no significant differences in the frequencies of subjects with positive titers among patient groups with different severities of WMLs.
Table 2. Baseline characteristics and serum IgG titers against periodontal pathogens of patients with mild and severer white matter lesions (WMLs).
| mild WMLs (0–2) n = 442 | severe WMLs (3) n = 197 | p | |
|---|---|---|---|
| Age (years) | 70.0 ± 13.0 | 80.0 ± 9.6 | < .001* |
| Female, n (%) | 180 (40.7) | 105 (53.3) | 0.003* |
| Body mass index, kg/m2 | 23.2 ± 4.1 | 21.8 ± 3.7 | < .001* |
| Hypertension, n (%) | 325 (73.5) | 162 (82.2) | 0.020* |
| Diabetes mellitus, n (%) | 112 (25.3) | 54 (27.4) | 0.625 |
| Dyslipidemia, n (%) | 185 (41.9) | 73 (37.1) | 0.258 |
| Atrial fibrillation, n (%) | 75 (17.0) | 46 (23.4) | 0.063 |
| Chronic kidney disease, n (%) | 126 (28.5) | 88 (44.7) | < .001* |
| History of stroke, n (%) | 108 (24.4) | 101 (51.3) | < .001* |
| History of coronary artery disease, n (%) | 31 (7.0) | 20 (10.2) | 0.206 |
| Current Smoking (n = 633), n (%) | 106 (24.3) (n = 436) | 20 (10.2) (n = 197) | < .001* |
| Daily alcohol habit, n (%) | 117 (26.9) (n = 435) | 20 (10.2) (n = 196) | < .001* |
| Serum CRP, log mg/dl | -0.82 ± 0.70 | -0.68 ± 0.72 | 0.007* |
| Frequencies of positivity for periodontal pathogens | |||
| P. gingivalis ATCC33277 (FimA type I), n (%) | 56 (12.7) | 24 (12.2) | 0.898 |
| A. actinomycetemcomitans AUNY67 (Serotype c), n (%) | 55 (12.4) | 18 (9.1) | 0.281 |
| P. intermedia ATCC26511, n (%) | 52 (11.8) | 27 (13.7) | 0.516 |
| P. nigrescens ATCC33563, n (%) | 49 (11.1) | 19 (9.6) | 0.677 |
| F. nucleatum ATCC10953, n (%) | 53 (12.0) | 34 (17.3) | 0.081 |
| T. denticola ATCC35405, n (%) | 71 (16.1) | 30 (15.2) | 0.816 |
| T. forsythensis ATCC43037, n (%) | 58 (13.1) | 30 (15.2) | 0.534 |
| C. rectus ATCC33238, n (%) | 47 (10.6) | 26 (13.2) | 0.349 |
| E. corrodens ATCC23834, n (%) | 58 (13.1) | 23 (11.7) | 0.700 |
Data are presented as means ± SD for age, body mass index, serum CRP and as the number of patients (%) for other parameters. CRP, C-reactive protein.
*P < 0.05 (statistically significant).
The association between serum antibody titers against periodontal pathogens and the presence of CMBs
Of the 639 evaluated patients, 12 were excluded because of artifacts caused by body movement during the evaluation of CMBs on T2*WI. Hence, 627 patients were analyzed (ischemic, n = 522 and hemorrhagic, n = 105). The clinical characteristics of patients with and without CMBs are shown in Table 3. The patients with CMBs were significantly older and had higher frequencies of hypertension and history of stroke than were found in those without CMBs. Of the 9 periodontal pathogens for which we evaluated serum antibody titers, we found that only serum antibody titers against C. rectus were associated with the presence of CMBs. The frequency of positive serum antibody titers against C. rectus was higher among patients with CMBs than those without CMBs (14.6% vs. 8.7%, P = 0.025). Multivariate logistic analysis revealed that a positive serum antibody titer against C. rectus was associated with the presence of CMBs after adjustment for factors that showed considerable differences (odds ratio 2.04, 95% confidence interval 1.19–3.48, P = 0.010) (Table 4). CRP levels were not associated with serum antibody titers against C. rectus (ρ = 0.019, p = 0.64, n = 627; S1 Fig).
Table 3. Baseline characteristics and serum IgG titers against periodontal pathogens of patients with and without cerebral microbleeds (CMBs).
| Patients without CMBs n = 312 | Patients with CMBs n = 315 | p | |
|---|---|---|---|
| Age (years) | 71.7 ± 13.4 | 74.6 ± 12.2 | 0.008* |
| Female, n (%) | 148 (47.4) | 132 (41.9) | 0.173 |
| Body mass index, kg/m2 | 23.1 ± 4.1 | 22.5 ± 4.0 | 0.091* |
| Hypertension, n (%) | 214 (68.6) | 267 (84.8) | < .001* |
| Diabetes mellitus, n (%) | 74 (23.7) | 88 (27.9) | 0.237 |
| Dyslipidemia, n (%) | 134 (43.0) | 120 (38.1) | 0.223 |
| Atrial fibrillation, n (%) | 68 (21.8) | 50 (15.9) | 0.066 |
| Chronic kidney disease, n (%) | 93 (29.8) | 118 (37.5) | 0.052 |
| History of stroke, n (%) | 82 (26.3) | 123 (39.1) | < .001* |
| History of coronary artery disease, n (%) | 24 (7.7) | 26 (8.3) | 0.883 |
| Current smoking, n (%) | 68 (21.9) (n = 310) | 55 (17.7) (n = 311) | 0.192 |
| Daily alcohol habit, n (%) | 69 (22.3) (n = 310) | 64 (20.7) (n = 309) | 0.696 |
| Serum CRP, log mg/dl | -0.81 ± 0.73 | -0.76 ± 0.69 | 0.191 |
| Frequencies of positivity for periodontal pathogens | |||
| P. gingivalis ATCC33277 (FimA type I), n (%) | 36 (11.5) | 44 (14.0) | 0.403 |
| A. actinomycetemcomitans AUNY67 (Serotype c), n (%) | 40 (12.8) | 32 (10.2) | 0.318 |
| P. intermedia ATCC26511, n (%) | 35 (11.2) | 44 (14.0) | 0.336 |
| P. nigrescens ATCC33563, n (%) | 30 (9.6) | 37 (11.8) | 0.439 |
| F. nucleatum ATCC10953, n (%) | 42 (13.5) | 45 (14.3) | 0.818 |
| T. denticola ATCC35405, n (%) | 49 (15.7) | 52 (16.5) | 0.828 |
| T. forsythensis ATCC43037, n (%) | 43 (13.8) | 45 (14.3) | 0.909 |
| C. rectus ATCC33238, n (%) | 27 (8.7) | 46 (14.6) | 0.025* |
| E. corrodens ATCC23834, n (%) | 38 (12.2) | 42 (12.8) | 0.720 |
Data are presented as means ± SD for age, body mass index, serum CRP and as the number of patients (%) for other parameters. CRP, C-reactive protein.
*P < 0.05 (statistically significant).
Table 4. Indicators associated with the presence of CMBs.
| Odds ratio | 95%CI | p-value | |
|---|---|---|---|
| Age | 1.02 | 1.00–1.03 | 0.047* |
| Female | 0.63 | 0.43–0.90 | 0.013* |
| BMI | 0.96 | 0.91–1.00 | 0.054 |
| Hypertension | 2.66 | 1.75–4.03 | <0.001* |
| Atrial fibrillation | 0.52 | 0.33–0.82 | 0.005* |
| Chronic kidney disease | 1.16 | 0.80–1.67 | 0.436 |
| History of stroke | 1.56 | 1.09–2.24 | 0.016* |
| Current smoking | 0.82 | 0.53–1.29 | 0.401 |
| Serum CRP | 1.12 | 0.88–1.41 | 0.356 |
| C. rectus positive | 2.04 | 1.19–3.48 | 0.010* |
Multivariate logistic analysis was performed with the factors in Table 3 that showed considerable differences in univariate analysis (p < 0.2) for the presence of CMBs. C. rectus, indicates Campylobacter rectus; CMBs, cerebral microbleeds; CI, confidence interval; BMI, body mass index; CRP, C-reactive protein.
*P < 0.05 (statistically significant).
The association between C. rectus and the number or locations of CMBs
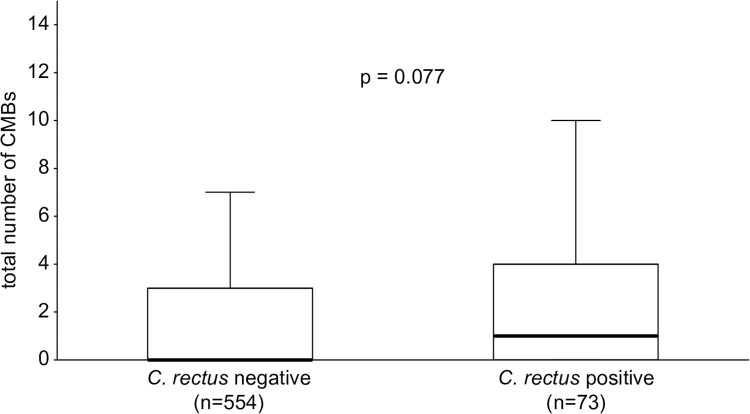
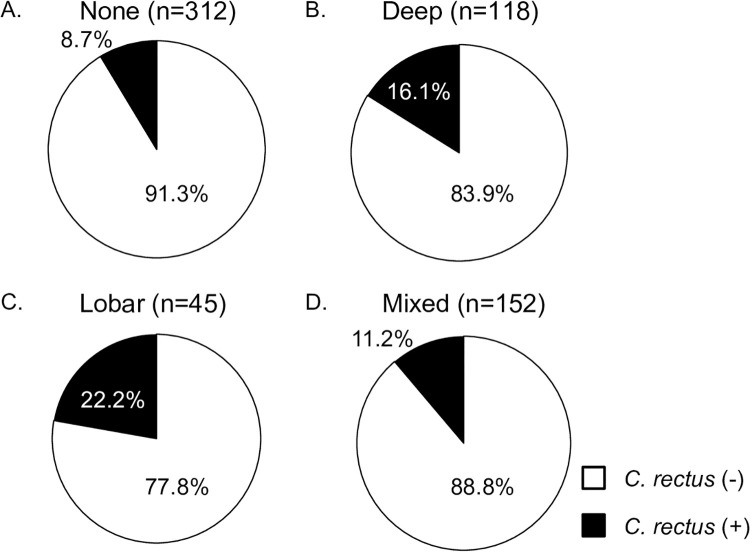
Although the difference was not significant, the total number of CMBs was slightly higher in patients with a positive antibody titer against C. rectus than in those without (median [interquartile range], 1 [0–4] vs. 0 [0–3], P = 0.077) (Fig 1). A positive serum antibody titer against C. rectus was not associated with multiple CMBs (more than 2) in either the univariate or multivariate logistic analysis. The patients were divided into four categories according to the location of CMBs (none, deep, lobar, and mixed). In all, 27 of the 312 patients (8.7%) with no CMBs, 19 of the 118 patients (16.1%) with deep CMBs, 10 of the 45 patients (22.2%) with lobar CMBs, and 17 of the 152 patients (11.2%) with mixed CMBs were positive for serum antibody titers against C. rectus (P = 0.019) (Fig 2). These distributions were also similar after the patients were divided into those with ischemic stroke and those with intracerebral hemorrhage (S2 and S3 Figs).
Fig 1. Association between the total number of CMBs and positive serum antibody titers against C. rectus.
The total number of CMBs was non-significantly higher in patients with positive serum antibody titers against C. rectus than in patients with negative tests (median (IQR), 1 (0–4) vs. 0 (0–3), P = 0.077). The error bars show the local minimum (10%) and local maximum (90%) after excluding outliers. C. rectus: Campylobacter rectus; CMBs: cerebral microbleeds.
Fig 2. Association between the locations of CMBs and positive serum antibody titers against C. rectus.
C. rectus was detected in 16.1% of patients with deep CMBs, in 22.2% of those with lobar CMBs, in 8.7% of those with no CMBs and in 11.2% of those with mixed CMBs. C. rectus: Campylobacter rectus; CMBs: cerebral microbleeds.
Discussion
This study demonstrates that a positive serum IgG antibody titer against C. rectus is associated with the presence of CMBs in acute stroke patients after adjusting for baseline covariate factors.
CSVDs, including lacunar infarction, WMLs, and CMBs, are common phenomena associated with ageing that worsen with hypertension and diabetes mellitus [18]. It has been suggested that chronic inflammation and blood-brain barrier disruption are associated with CSVDs [19]. Several studies have demonstrated an association between lacunar infarction and chronic periodontal disease, which is thought to induce chronic inflammation [20, 21]. Chronic periodontal disease has also been independently associated with the presence of lacunar infarction after adjustment for several vascular risk factors [20]. In addition, the severity of periodontitis tended to be associated with the number of lacunar infarctions. The progression of periodontitis may lead to an increase in the number of lacunar infarctions [21]. Whether periodontal disease is associated with the severity of WMLs has not been determined. Tooth loss caused by the progression of periodontal disease is associated with the severity of WMLs [22, 23]. However, in our study, we did not find that any specific periodontal disease antibody titer was associated with the severity of WMLs.
Of the 9 antibodies against periodontal pathogens for which we checked serum titer levels, a positive serum antibody titer for C. rectus was associated with the presence of CMBs. When we considered the locations of CMBs, we found that the frequency of patients with a positive serum antibody titer against C. rectus was high in both patients with deep and patients with lobar CMBs. In addition, patients with a positive serum antibody titer against C. rectus did not have higher numbers of CMBs than were found in those without. These results may indicate that a positive serum antibody titer against C. rectus is associated with the initial occurrence of CMBs but not with an increase in the number of CMBs. In general, deep CMBs were associated with hypertension, and lobar CMBs were associated with amyloid angiopathy [24]. Hypertension was the most important factor that was related to the number of CMBs [25]. Indeed, hypertension was closely associated with the presence of CMBs in the present study. In recent years, it has been suggested that cnm gene-positive Streptococcus mutans (cnm-positive S. mutans) is related to the occurrence of CMBs in deep regions. Bacteria residing in the oral cavity may be a source of CMBs, which may be induced via different pathways in these patients than has been observed in hypertension and cerebral amyloid angiopathy [26]. Therefore, the association between oral bacteria and CMBs has received considerable attention. Our results indicate that a positive serum antibody titer against C. rectus is associated with the presence of CMBs and provide further evidence supporting the notion that oral health conditions play a role in the occurrence of CMBs.
C. rectus is a gram-negative, microaerophilic, and motile bacterium associated with periodontal disease [27, 28]. C. rectus is also thought to play a pathogenic role in human disease. It has been reported that the prevalence of periodontal pathogens, including C. rectus, is higher in the subgingival biofilms of patients with coronary artery disease than in non-cardiac subjects. Furthermore, C. rectus and other periodontal pathogens were significantly associated between the subgingival and atherosclerotic plaques in cardiac patients [29]. Although some case reports have shown that there is an association between C. rectus and cavernous sinus thrombosis [30, 31], no cohort study has investigated the association between C. rectus and neurological disease. However, Misaki et al. have reported that the coexistence of C. rectus with cnm-positive S. mutans in saliva was associated with high urinary protein levels in patients with immunoglobulin A nephropathy (IgAN) [32]. Microalbumin reflects endothelial dysfunction, and endothelial dysfunction is thought to play a role in the mechanisms leading to CSVD-related brain changes [33]. Therefore, C. rectus and cnm-positive S. mutans may be associated with systemic small vessel disease outbreaks. Future studies must therefore examine whether the coexistence of C. rectus with cnm-positive S. mutans is related to CMB occurrence.
This study has several limitations. First, we could not evaluate the clinical periodontal examination of all patients. It is not fully clear how serum antibody titers against periodontal disease are influenced by the presence or severity of periodontal disease. Second, this study targeted acute ischemic stroke patients, and various factors, such as systemic inflammation involving CRP or several cytokines, might therefore affect the association between serum antibody titers and both periodontal disease and CSVD [34, 35]. Although we could not evaluate cytokine levels, we found no significant associations between serum antibody titers and CRP levels (S1 Table). Hence, the association between serum periodontal antibody titers and CSVD is likely not due to systemic inflammation alone. Third, there may be a lot of variability in the healthy controls due to the small number included (5 healthy subjects). This might lead to potential concerns about the statistical analysis of the serum antibody data. Fourth, we did not focus on increased levels of titers of each periodontal pathogen, although we focused on the positive rate. In fact, there is no significant difference in the association with serum IgG titer against C. rectus and the presence of CMBs (S2 Table). The limitation of this study was that we revealed only the association with positive rate of serum IgG titers and presence of CMBs. Finally, because this is a cross-sectional study, the causal relationship between the progression of periodontal diseases and the occurrence of CSVD was not explored. Further studies should investigate the association between serum antibody titers and both periodontal disease and CSVD in the general population.
In conclusion, serum IgG antibody titers against C. rectus are high in acute stroke patients with CMBs. Further clinical and basic studies should be performed to determine the influence of C. rectus on the pathophysiological status of CSVD.
Supporting information
The analyses were performed using Spearman’s rank correlation coefficient.
(TIF)
(TIF)
(TIF)
The analyses were performed using Spearman’s rank correlation coefficient.
(TIF)
(TIF)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by research grants from the Japan Society for the Promotion 350 of Science KAKENHI (Grant Numbers 17K17350, 17K17907, 18K10746, and 351 20K16579).
References
- 1.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–20. 10.1016/S0140-6736(05)67728-8 . [DOI] [PubMed] [Google Scholar]
- 2.Orlandi M, Suvan J, Petrie A, Donos N, Masi S, Hingorani A, et al. Association between periodontal disease and its treatment, flow-mediated dilatation and carotid intima-media thickness: a systematic review and meta-analysis. Atherosclerosis. 2014;236(1):39–46. Epub 2014/06/17. 10.1016/j.atherosclerosis.2014.06.002 . [DOI] [PubMed] [Google Scholar]
- 3.Lafon A, Pereira B, Dufour T, Rigouby V, Giroud M, Béjot Y, et al. Periodontal disease and stroke: a meta-analysis of cohort studies. Eur J Neurol. 2014;21(9):1155–61, e66-7. Epub 2014/04/08. 10.1111/ene.12415 . [DOI] [PubMed] [Google Scholar]
- 4.Sen S, Giamberardino LD, Moss K, Morelli T, Rosamond WD, Gottesman RF, et al. Periodontal Disease, Regular Dental Care Use, and Incident Ischemic Stroke. Stroke. 2018;49(2):355–62. Epub 2018/01/15. 10.1161/STROKEAHA.117.018990 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Consensus report. Periodontal diseases: epidemiology and diagnosis. Ann Periodontol. 1996;1(1):216–22. 10.1902/annals.1996.1.1.216 . [DOI] [PubMed] [Google Scholar]
- 6.Pussinen PJ, Alfthan G, Jousilahti P, Paju S, Tuomilehto J. Systemic exposure to Porphyromonas gingivalis predicts incident stroke. Atherosclerosis. 2007;193(1):222–8. Epub 2006/07/26. 10.1016/j.atherosclerosis.2006.06.027 . [DOI] [PubMed] [Google Scholar]
- 7.Hosomi N, Aoki S, Matsuo K, Deguchi K, Masugata H, Murao K, et al. Association of serum anti-periodontal pathogen antibody with ischemic stroke. Cerebrovasc Dis. 2012;34(5–6):385–92. Epub 2012/11/29. 10.1159/000343659 . [DOI] [PubMed] [Google Scholar]
- 8.Nishi H, Hosomi N, Ohta K, Aoki S, Nakamori M, Nezu T, et al. Serum IgG antibody titer to Fusobacterium nucleatum is associated with unfavorable outcome after stroke. Clin Exp Immunol. 2020. Epub 2020/03/10. 10.1111/cei.13430 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. 10.1016/S1474-4422(10)70104-6 . [DOI] [PubMed] [Google Scholar]
- 10.Fornage M, Chiang YA, O’Meara ES, Psaty BM, Reiner AP, Siscovick DS, et al. Biomarkers of Inflammation and MRI-Defined Small Vessel Disease of the Brain: The Cardiovascular Health Study. Stroke. 2008;39(7):1952–9. 10.1161/STROKEAHA.107.508135 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevenson SF, Doubal FN, Shuler K, Wardlaw JM. A systematic review of dynamic cerebral and peripheral endothelial function in lacunar stroke versus controls. Stroke. 2010;41(6):e434–42. Epub 2010/04/15. 10.1161/STROKEAHA.109.569855 . [DOI] [PubMed] [Google Scholar]
- 12.Nezu T, Hosomi N, Aoki S, Kubo S, Araki M, Mukai T, et al. Endothelial dysfunction is associated with the severity of cerebral small vessel disease. Hypertens Res. 2015;38(4):291–7. Epub 2015/02/12. 10.1038/hr.2015.4 . [DOI] [PubMed] [Google Scholar]
- 13.Ihara M, Yamamoto Y. Emerging Evidence for Pathogenesis of Sporadic Cerebral Small Vessel Disease. Stroke. 2016;47(2):554–60. Epub 2016/01/07. 10.1161/STROKEAHA.115.009627 . [DOI] [PubMed] [Google Scholar]
- 14.Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. 10.1161/01.str.24.1.35 . [DOI] [PubMed] [Google Scholar]
- 15.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–92. Epub 2009/04/01. 10.1053/j.ajkd.2008.12.034 . [DOI] [PubMed] [Google Scholar]
- 16.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–6. 10.2214/ajr.149.2.351 . [DOI] [PubMed] [Google Scholar]
- 17.Nakahara T, Hyogo H, Ono A, Nagaoki Y, Kawaoka T, Miki D, et al. Involvement of Porphyromonas gingivalis in the progression of non-alcoholic fatty liver disease. J Gastroenterol. 2018;53(2):269–80. Epub 2017/07/24. 10.1007/s00535-017-1368-4 . [DOI] [PubMed] [Google Scholar]
- 18.Schmidt H, Zeginigg M, Wiltgen M, Freudenberger P, Petrovic K, Cavalieri M, et al. Genetic variants of the NOTCH3 gene in the elderly and magnetic resonance imaging correlates of age-related cerebral small vessel disease. Brain. 2011;134(Pt 11):3384–97. Epub 2011/10/17. 10.1093/brain/awr252 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34(3):806–12. Epub 2003/02/27. 10.1161/01.STR.0000058480.77236.B3 . [DOI] [PubMed] [Google Scholar]
- 20.Leira Y, López-Dequidt I, Arias S, Rodríguez-Yáñez M, Leira R, Sobrino T, et al. Chronic periodontitis is associated with lacunar infarct: a case-control study. Eur J Neurol. 2016;23(10):1572–9. Epub 2016/07/15. 10.1111/ene.13080 . [DOI] [PubMed] [Google Scholar]
- 21.Taguchi A, Miki M, Muto A, Kubokawa K, Migita K, Higashi Y, et al. Association between oral health and the risk of lacunar infarction in Japanese adults. Gerontology. 2013;59(6):499–506. Epub 2013/08/07. 10.1159/000353707 . [DOI] [PubMed] [Google Scholar]
- 22.Minn YK, Suk SH, Park H, Cheong JS, Yang H, Lee S, et al. Tooth loss is associated with brain white matter change and silent infarction among adults without dementia and stroke. J Korean Med Sci. 2013;28(6):929–33. Epub 2013/06/03. 10.3346/jkms.2013.28.6.929 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Brutto OH, Mera RM, Zambrano M. The Influence of Age in the Relationship between Cerebral Small Vessel Disease and Edentulism. The Atahualpa Project. Eur Neurol. 2016;76(3–4):112–6. Epub 2016/08/17. 10.1159/000448839 . [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Soo YO, Mok VC. Cerebral microbleeds: is antithrombotic therapy safe to administer? Stroke. 2014;45(9):2811–7. Epub 2014/07/15. 10.1161/STROKEAHA.114.004286 . [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Liu R, Sun W, Peng Q, Zhang W, Xu E, et al. Different impacts of blood pressure variability on the progression of cerebral microbleeds and white matter lesions. Stroke. 2012;43(11):2916–22. Epub 2012/09/04. 10.1161/STROKEAHA.112.658369 . [DOI] [PubMed] [Google Scholar]
- 26.Tonomura S, Ihara M, Kawano T, Tanaka T, Okuno Y, Saito S, et al. Intracerebral hemorrhage and deep microbleeds associated with cnm-positive Streptococcus mutans; a hospital cohort study. Sci Rep. 2016;6:20074 Epub 2016/02/05. 10.1038/srep20074 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rams TE, Feik D, Slots J. Campylobacter rectus in human periodontitis. Oral Microbiol Immunol. 1993;8(4):230–5. 10.1111/j.1399-302x.1993.tb00565.x . [DOI] [PubMed] [Google Scholar]
- 28.Mahlen SD, Clarridge JE. Oral abscess caused by Campylobacter rectus: case report and literature review. J Clin Microbiol. 2009;47(3):848–51. Epub 2008/12/24. 10.1128/JCM.01590-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahendra J, Mahendra L, Nagarajan A, Mathew K. Prevalence of eight putative periodontal pathogens in atherosclerotic plaque of coronary artery disease patients and comparing them with noncardiac subjects: A case-control study. Indian J Dent Res. 2015;26(2):189–95. 10.4103/0970-9290.159164 . [DOI] [PubMed] [Google Scholar]
- 30.Oka K, Nakano Y, Sazumi Y, Michitani T, Horiguchi S, Ocho K, et al. Clival Osteomyelitis with Cavernous Sinus Thrombosis Due to Fusobacterium nucleatum and Campylobacter rectus Induced by Tooth Extraction. Intern Med. 2018;57(22):3325–8. Epub 2018/07/06. 10.2169/internalmedicine.1025-18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leo QJ, Bolger DT. Septic cavernous sinus thrombosis due to Campylobacter rectus infection. BMJ Case Rep. 2014;2014 Epub 2014/05/19. 10.1136/bcr-2013-203351 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Misaki T, Naka S, Wato K, Hatakeyama R, Nagasawa Y, Ito S, et al. Campylobacter rectus in the Oral Cavity Correlates with Proteinuria in Immunoglobulin A Nephropathy Patients. Nephron. 2018;139(2):143–9. Epub 2018/02/09. 10.1159/000487103 . [DOI] [PubMed] [Google Scholar]
- 33.Poggesi A, Pasi M, Pescini F, Pantoni L, Inzitari D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: A review. J Cereb Blood Flow Metab. 2016;36(1):72–94. 10.1038/jcbfm.2015.116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sobowale OA, Parry-Jones AR, Smith CJ, Tyrrell PJ, Rothwell NJ, Allan SM. Interleukin-1 in Stroke: From Bench to Bedside. Stroke. 2016;47(8):2160–7. Epub 2016/03/01. 10.1161/STROKEAHA.115.010001 . [DOI] [PubMed] [Google Scholar]
- 35.Nambi V, Hoogeveen RC, Chambless L, Hu Y, Bang H, Coresh J, et al. Lipoprotein-associated phospholipase A2 and high-sensitivity C-reactive protein improve the stratification of ischemic stroke risk in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40(2):376–81. Epub 2008/12/18. 10.1161/STROKEAHA.107.513259 . [DOI] [PMC free article] [PubMed] [Google Scholar]