Abstract
DNA methylation of the ELOVL2 (Elongation Of Very Long Chain Fatty Acids-Like 2) promoter is one of the most robust molecular biomarkers for chronological age, but whether ELOVL2 plays a functional role in aging has not been explored. ELOVL2 encodes a transmembrane protein involved in the synthesis of very long polyunsaturated fatty acids (VLC-PUFAs). These fatty acids play important roles in retinal biology and photoreceptor renewal, key processes implicated in age-related eye diseases such as age-related macular degeneration (AMD). Here, we summarize our work deciphering the role of ELOVL2 in the eye emphasizing the potential functional role of age-related DNA methylation in the pathophysiology of AMD.
Keywords: Polyunsaturated fatty acids, Aging, Membrane structure, Macular degeneration
Age is one of the most relevant clinical traits in predicting disease risk, mental and physical performance, and mortality [1]. In the eye, age is a strong risk factor for several blinding conditions (e.g., glaucoma and Age-Related Macular Degeneration – AMD) [2]. On a molecular level, aging is associated with a gradual decline in the efficiency and fidelity of molecular processes, associated with changes in gene expression and epigenetic modifications, leading to a deterioration of cell functions [3]. Identifying the cellular and molecular mechanisms that drive age-related physiological phenotypes still represents a major challenge in the field.
Epigenetic aging of tissues and organs has been tightly correlated with global genome DNA methylation changes in specific regions, called CpG islands. A number of recent studies have shown that CpG methylation (CpGme) patterns progressively change during aging in a variety of tissues and cells such as blood, muscle, brain, lung, and colon [4–7]. The rates of CpGme changes at subsets of affected sites were calculated and used to determine the cellular ‘epigenetic’ age which generally well correlates with chronological age and therefore can be used as a measure to assess biological aging in a quantitative manner [4,6]. One major question is whether these methylation changes merely correlate with aging, or if there any functional role of these epigenetic changes in regulating aging.
Interestingly, within the top ten markers predictive of human epigenetic age, four are localized in the CpG islands in the regulatory element of the ELOVL2 gene, accounting for over 70% of the one “methylation clock” model [8]. Consequently, methylation of the ELOVL2 regulatory region has been shown in many studies to correlate strongly with the biological age of individuals [9–12], as well as in rodents [7].
ELOVL2 is an enzyme that elongates long-chain omega-3 and omega-6 polyunsaturated fatty acids (LC-PUFAs), precursors of 22:6n-3, docosahexaenoic acid (DHA) and very-long-chain PUFAs (VLC-PUFAs), all playing important role in retina biology [13]. The fatty acids composition in the retina is unique - the retina is particularly enriched in PUFAs, with DHA constitutes 40–50% of the total fatty acids in the photoreceptor outer disc membranes [14]. VLC-PUFAs account for a unique ~5% of the total fatty acids in the disk membranes of photoreceptors, the second highest level in the body after testis. These features result in a highly fluid photoreceptors disc membranes that permit efficient conformational changes and signaling dynamics for visual chromophore necessary for the continuous detection of light [15–17].
PUFAs are well known to play important roles in the retina and deficiency of LC-PUFAs has been shown to be associated with increased risk of the dry form of AMD, a highly prevalent retinal disease [18]. Although no effective treatment is known for dry AMD, several clinical studies indicate that nutritional supplementation can help slow the disease progression [19]. In particular, recent studies suggest that individuals who self-reported intake of foods rich in omega 3 PUFAs were 30% less likely to develop central geographic atrophy (GA) and 50% less likely to develop AMD than subjects with the lowest self-reported intake [19–21].
While methylation of the ELOVL2 promoter is highly correlated with chronological age, whether ELOVL2 protein has a functional role in aging has not been investigated. In our recent paper published in Aging Cell, we demonstrated through both genetic and pharmacologic manipulation that Elovl2 has a functional role in a molecular aging program in the mammalian retina [22].
We first investigated whether there is increased methylation of the Elovl2 promoter in the mouse and, in particular, the retina, which has not been shown before. We observed an age-dependent increase in Elovl2 regulatory region methylation associated with concomitant downregulation of Elovl2 expression on mRNA and protein levels. Next, using the fluorescent in situ hybridization method, we observed Elovl2 expression in cone and rod photoreceptors, as well as the retinal pigment epithelium. We also observed a significant age-related decline of the expression of the Elovl2 in the eye. The same age-dependent changes of Elovl2 methylation and gene expression were observed in the mouse liver, indicating that age-associated methylation of Elovl2 occurs in multiple tissues in the mouse, similarly to what was observed previously in humans [23].
We first investigated the function of ELOVL2 in vitro utilizing Wi-38 cells, a human fibroblast line commonly used as a model for aging [24]. We observed that methylation of ELOVL2 increases with an associated decrease in ELOVL2 gene expression with an increased population doubling of Wi-38 cells. When we inhibited ELOVL2 expression using RNA interference, we observed increased senescence as well as decreased proliferation, both markers for aging, compared to controls. Next, by administering the global demethylating agent, 5-Aza, we demethylated the ELOVL2 regulatory region and, consequently, upregulated ELOVL2 expression. Remarkably, this was accompanied by the decreased senescence in Wi-38 cells. This suggests that the manipulation of ELOVL2 gene expression can have effects on aging in vitro.
Next, we investigated the function of Elovl2 in aging in vivo. As Elovl2 heterozygous mice are infertile [25], we created a knock-in point mutation using Crispr-Cas9 technology, Elovl2C234W, which has been previously shown to eliminate substrate specificity of Elovl2 elongation [26]. Using lipidomics, we confirmed that Elovl2C234W mutation results in loss of ELOVL2-specific function, i.e elongation of docosapentaenoic acid (DPA) (22:5n-3) to 24:5n-3, which is a precursor of DHA and other VLC-PUFAs. We further investigated the effect of Elovl2C234W mice on both anatomic and functional surrogates of aging in the mouse eye. These included autofluorescent (AF) deposits visualized with autofluorescence fundus imaging, which increases with age [27,28], as well as the electroretinogram (ERG), which shows a decrease in the maximum scotopic response with age [29]. In Elovl2C234W mice, we noticed an increase in AF deposits as well as a decrease in ERG compared to age-matched controls, suggesting that inhibiting Elovl2 accelerates aging in the mouse retina. In addition, on a microscopic level, we notice deposits below the retinal pigment epithelium, which contain many components of human drusen, included C3, C5b-9, Htra1, T-15 which have been implicated in the pathogenesis of macular degeneration [30].
Finally, we performed experiments to assess whether the upregulation of Elovl2 expression could slow the aging of the retina as assessed previously. We used the global demethylating agent 5-Aza to upregulate expression of Elovl2 and observed that administering 5-Aza by intravitreal injection decreased Elovl2 methylation, increased Elovl2 gene expression, and resulted in an increase in ERG scotopic response in wildtype aged mice compared to age-matched controls. These results suggest that pharmacological intervention, i.e. administration of 5-Aza, may slow the functional aging of the eye.
In summary (Fig. 1), our findings provide a molecular link between the metabolism of polyunsaturated fatty acids and aging of the eye by showing that changes in the membrane composition similar to those occurring in aging (“aging membranes” [31,32]) affect visual functions. Our work also presents the first example of age-dependent methylation of genes which serves as biomarkers of aging, may not just be epiphenomenon but could play a functional role in the aging process itself.
Fig. 1.
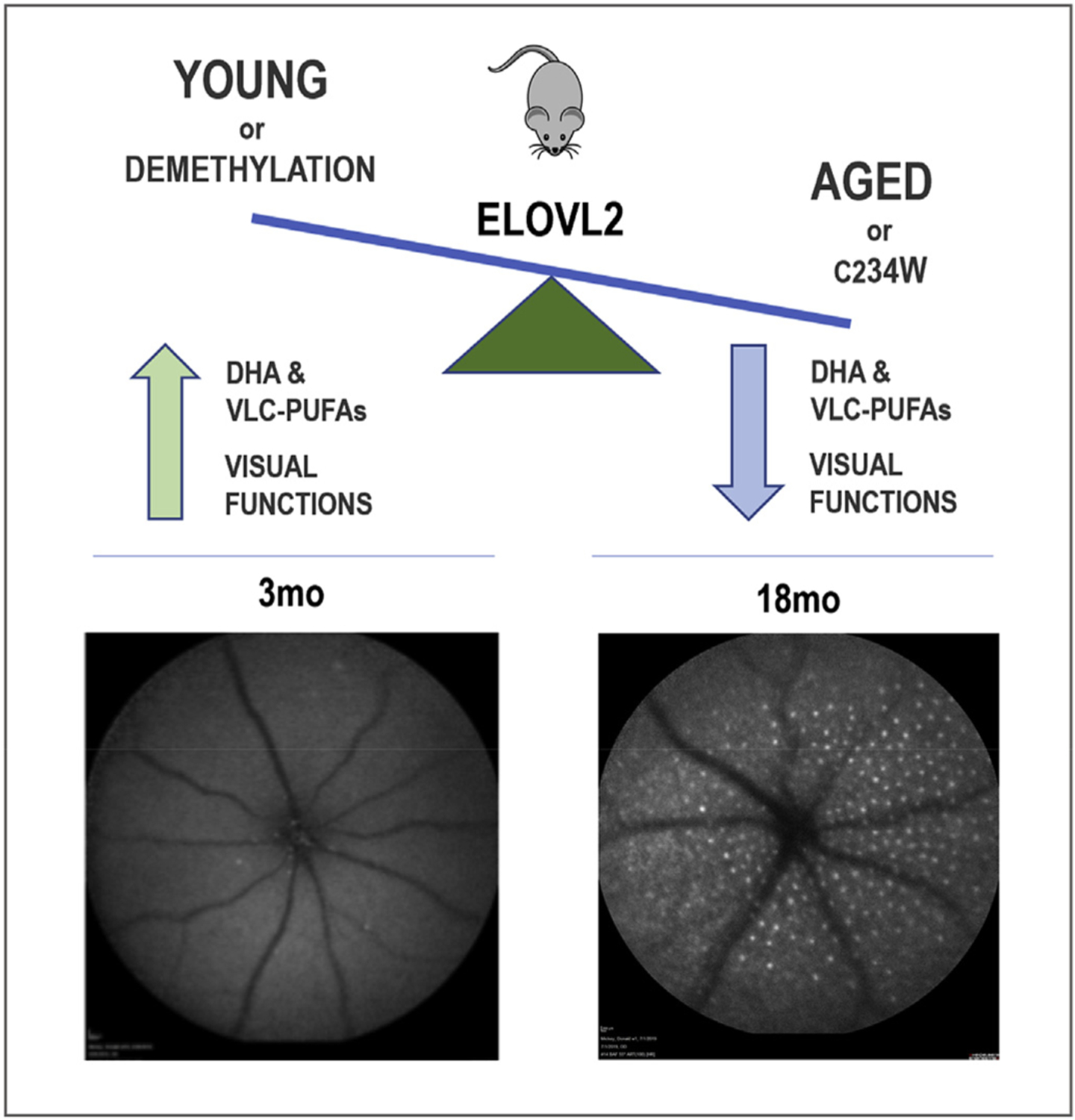
Age-related decline in ELOVL2 levels has a profound impact on eye structure and function by modulating the availability of DHA and VLC-PUFAs. These changes can be genetically and therapeutically modulated which provides solid foundations in developing novel strategies to treat age-related conditions in the eye including AMD.
Acknowledgments
This work was supported by NIH/NEI R01 EY02701 and RPB Special Scholar Award to D.S.K, by NIH/NEI K08EY030510 to D.L.C as well as by RPB Unrestricted Grants to Gavin Herbert Eye Institute and Shiley Eye Institute.
Footnotes
Declaration of competing interest
D.L.C. and D.S.-K. are scientific advisors at Visgenx.
References
- [1].Glei DA, et al. , Predicting survival from telomere length versus conventional predictors: a multinational population-based cohort study, PloS One 11 (2016), e0152486, 10.1371/journal.pone.0152486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lin JB, Tsubota K, Apte RS, A glimpse at the aging eye, NPJ Aging. Mech. Dis 2 (2016) 16003, 10.1038/npjamd.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Newgard CB, Sharpless NE, Coming of age: molecular drivers of aging and therapeutic opportunities, J. Clin. Invest 123 (2013) 946–950, 10.1172/JCI68833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Horvath S, DNA methylation age of human tissues and cell types, Genome Biol 14 (2013) R115, 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Levine ME, et al. , An epigenetic biomarker of aging for lifespan and healthspan, bioRxiv (2018) 276162, 10.1101/276162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hannum G, et al. , Genome-wide methylation profiles reveal quantitative views of human aging rates, Mol. Cell 49 (2013) 359–367, 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Spiers H, et al. , Age-associated changes in DNA methylation across multiple tissues in an inbred mouse model, Mech. Ageing Dev 154 (2016) 20–23, 10.1016/j.mad.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hannum G, et al. , Genome-wide methylation profiles reveal quantitative views of human aging rates, Mol. Cell 49 (2013) 359–367, 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bacalini MG, et al. , Systemic age-associated DNA hypermethylation of ELOVL2 gene: in vivo and in vitro evidences of a cell replication process, J. Gerontol. A Biol. Sci. Med. Sci (2016), 10.1093/gerona/glw185 glw185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Garagnani P, et al. , Methylation of ELOVL2 gene as a new epigenetic marker of age, Aging Cell 11 (2012) 1132–1134, 10.1111/acel.12005. [DOI] [PubMed] [Google Scholar]
- [11].Mansego ML, Milagro FI, Zulet MÁ, Moreno-Aliaga MJ, Martínez JA, Differential DNA methylation in relation to age and health risks of obesity, Int. J. Mol. Sci 16 (2015) 16816–16832, 10.3390/ijms160816816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rönn T, et al. , Impact of age, BMI and HbA1c levels on the genome-wide DNA methylation and mRNA expression patterns in human adipose tissue and identification of epigenetic biomarkers in blood, Hum. Mol. Genet (2015), 10.1093/hmg/ddv124 ddv124. [DOI] [PubMed] [Google Scholar]
- [13].Leonard AE, et al. , Identification and expression of mammalian long-chain PUFA elongation enzymes, Lipids 37 (2002) 733–740. [DOI] [PubMed] [Google Scholar]
- [14].Fliesler SJ, Anderson RE, Chemistry and metabolism of lipids in the vertebrate retina, Prog. Lipid Res 22 (1983) 79–131. [DOI] [PubMed] [Google Scholar]
- [15].Oates J, Watts A, Uncovering the intimate relationship between lipids, cholesterol and GPCR activation, Curr. Opin. Struct. Biol 21 (2011) 802–807, 10.1016/j.sbi.2011.09.007. [DOI] [PubMed] [Google Scholar]
- [16].Agbaga MP, et al. , Differential composition of DHA and very-long-chain PUFAs in rod and cone photoreceptors, J. Lipid Res 59 (2018) 1586–1596, 10.1194/jlr.M082495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Agbaga MP, Mandal MN, Anderson RE, Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein, J. Lipid Res 51 (2010) 1624–1642, 10.1194/jlr.R005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bazan NG, Molina MF, Gordon WC, Docosahexaenoic acid signal-olipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases, Annu. Rev. Nutr 31 (2011) 321–351, 10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Skowronska-Krawczyk D, Chao DL, Long-chain polyunsaturated fatty acids and age-related macular degeneration, Adv. Exp. Med. Biol 1185 (2019) 39–43, 10.1007/978-3-030-27378-1_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sangiovanni JP, et al. , {omega}−3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study, Am. J. Clin. Nutr 90 (2009) 1601–1607, 10.3945/ajcn.2009.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Keenan TD, et al. , Adherence to the Mediterranean diet and progression to late age-related macular degeneration in the Age-Related Eye Disease Studies 1 and 2, Ophthalmology (2020), 10.1016/j.ophtha.2020.04.030. [DOI] [PubMed] [Google Scholar]
- [22].Chen D, et al. , The lipid elongation enzyme ELOVL2 is a molecular regulator of aging in the retina, Aging Cell (2020), e13100, 10.1111/acel.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Slieker RC, Relton CL, Gaunt TR, Slagboom PE, Heijmans BT, Age-related DNA methylation changes are tissue-specific with ELOVL2 promoter methylation as exception, Epigenet. Chromatin 11 (2018) 25, 10.1186/s13072-018-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hayflick L, The limited in vitro lifetime of human diploid cell strains, Exp. Cell Res 37 (1965) 614–636. [DOI] [PubMed] [Google Scholar]
- [25].Zadravec D, et al. , ELOVL2 controls the level of n-6 28:5 and 30:5 fatty acids in testis, a prerequisite for male fertility and sperm maturation in mice, J. Lipid Res 52 (2011) 245–255, 10.1194/jlr.M011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gregory MK, Cleland LG, James MJ, Molecular basis for differential elongation of omega-3 docosapentaenoic acid by the rat Elovl5 and Elovl2, J. Lipid Res 54 (2013) 2851–2857, 10.1194/jlr.M041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chavali VR, et al. , A CTRP5 gene S163R mutation knock-in mouse model for late-onset retinal degeneration, Hum. Mol. Genet 20 (2011) 2000–2014, 10.1093/hmg/ddr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xu HC, M, A. Manivannan, N. Louis, J.V. Forrester, Early Retinal Autofluorescence is from subretinal microglia in aged experimental rodents, Invest. Ophthalmol. Vis. Sci 48 (2007). [Google Scholar]
- [29].Kolesnikov AV, Fan J, Crouch RK, Kefalov VJ, Age-related deterioration of rod vision in mice, J. Neurosci 30 (2010) 11222–11231, 10.1523/JNEUROSCI.4239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Crabb JW, The proteomics of drusen, Cold Spring Harb. Perspect. Med 4 (2014) a017194, 10.1101/cshperspect.a017194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Skowronska-Krawczyk D, Budin I, Aging membranes: unexplored functions for lipids in the lifespan of the central nervous system, Exp. Gerontol 131 (2019) 110817, 10.1016/j.exger.2019.110817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zs-Nagy I, Aging of cell membranes: facts and theories, Interdiscipl. Top Gerontol 39 (2014) 62–85, 10.1159/000358900. [DOI] [PubMed] [Google Scholar]


