Supplemental Digital Content is available in the text
Keywords: Hepatocellular carcinoma, Liver resection, Liver transplantation, Local tumor destruction, Overall survival
Abstract
It is controversial regarding the treatment allocation for patients with stage I hepatocellular carcinoma (SI-HCC). The aim of the present study was to compare the long-term survival in SI-HCC patients undergoing liver transplantation (LT), liver resection (LR), local tumor destruction (LTD), or none. SI-HCC patients diagnosed between 2004 and 2015 were extracted from the SEER 18 registry database. Multivariable Cox models and propensity score matching (PSM) method were used to explore the association between surgical methods and long-term prognosis. A total of 5165 patients with stage I (AJCC, 6th or 7th) HCC were included in the study. Only 36.9% of patients diagnosed with HCC in stage I received surgical therapy. The incidence of LT was decreased over time (P < .001). In the multivariable-adjusted cohort (n = 5165), after adjusting potential confounding factors, a clear prognostic advantage of LT was observed in OS (P < .0001) compared with patients after LR. Patients undergoing LTD had a worse OS in comparison with patients who underwent LR (P < .0001). Patients who received no surgical treatment had the worst OS (P < .0001) among 4 treatment groups. In stratified analyses, the salutary effects of LT vs LR on OS were consistent across all subgroups except for a similar result in the noncirrhotic subgroup (P = .4414). The inferior survival effects of LTD vs LR on OS were consistent across all subgroups, and even in the subgroup with tumor size < 3 cm (P = .0342). In the PSM cohort, patients in LT group showed a better OS (P < .001) than patients in LR group (P < .0001) and patients undergoing LTD had a worse OS compared with patients who underwent LR (P = .00059). In conclusion, LT offered a survival advantage compared with LR among patients with Stage I HCC. LT is the best surgical treatment for stage I HCC in patients with advanced fibrosis, whereas LR provides comparable long-term outcomes to LT in patients without advanced fibrosis and should be considered as the first-line surgical option. LTD can be used as an alternative method when LR and LT are unavailable.
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second most lethal cancer worldwide.[1] Radical surgery, leading to the best outcomes of any treatment available, is the mainstay of HCC management.[1] Several staging systems have be established to determine long-term prognosis and enable the selection of the optimal treatment for the best candidates.[2–7] For patients with stage-I (American Joint Committee on Cancer pathological TNM stage, 6th or 7th) HCC (SI-HCC), surgical treatments, including liver resection (LR), liver transplantation (LT), and local tumor destruction (LTD) can be performed to achieve potentially curative treatments.[8–10]
According to the newest guideline of the European Association for the Study of the Liver (EASL) for HCC management,[1] LR is recommended for single HCC of any size when liver function is preserved. Consequently, for patients with SI-HCC (single tumor without vascular, lymphatic, and distant invasion), LR may be the primary treatment method. On the basis of Milan criterion and some expanded criteria,[11–14] LT can also be utilized in selected patients with SI-HCC, especially for patients with severe liver cirrhosis and impaired liver function. In addition, LTD such as radiofrequency ablation (RFA) represents treatment for nearly 30% of small HCC lesions (<5 cm).[15] A 3-cm cutoff value for RFA has also been proposed by several studies and currently is recommended by the American Hepato-Pancreato-Biliary Association.[16]
Owing to the controversy regarding the treatment allocation for patients with SI-HCC, the aim of the present study was to compare the treatment outcomes among patients with SI-HCC who received LR, LT, LTD, or none in the Surveillance, Epidemiology, and End Results (SEER) database, which is one of the most comprehensive and authoritative databases related to cancer. LR was referred as the primary treatment option for patients with SI-HCC and patients receiving other treatments, including LT, LTD, and none were compared with patients undergoing LR.
2. Patients and methods
2.1. Patient identification
SEER 18 registry for 2004 through 2015 was retrieved for the current research. We totally identified 68,505 cases diagnosed as HCC pathologically, which was based on the International Classification of Diseases for Oncology, 3rd Edition (site code: C22.0; histologic type ICD-O-3 codes: 8170-8175). All cases from the SEER database were treated between 2004 and 2015. The detailed process of case selection for this study is shown in Fig. 1. A total of 5165 cases with stage I (AJCC, 6th or 7th) HCC meeting the eligibility criteria were enrolled in the analyses. The following SEER codes for HCC treatment were selected: LTD: 10-17; LR: 20-25, 30, 36, 37, 50, 51, and 52; LT: 61. LTD included electrocautery, photodynamic therapy, fulguration, laser, cryosurgery, heat-radiofrequency ablation, percutaneous ethanol injection, or other. This study was reviewed and approved by the ethics committee of our hospital.
Figure 1.
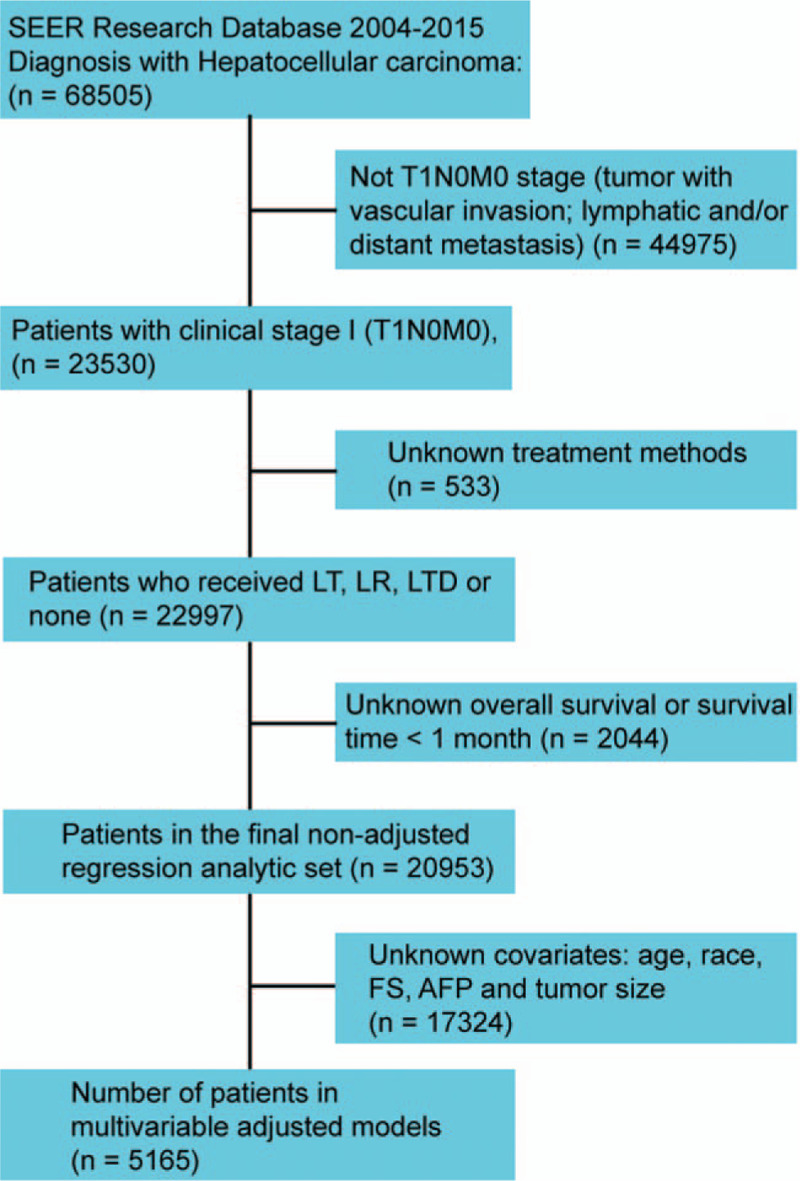
Flowchart representing selection process of patients included in this study.
2.2. Statistical analyses
The main endpoint was overall survival (OS). Continuous data was tested by t test or Kruskal–Wallis H test. Categorical data were examined by Chi-square test or Fisher exact test. Cochrane–Armitage trend test was utilized to assess for linear trends in the proportion of cases who underwent each type of treatment. Kaplan–Meier method was applied to plot the OS curves, and the log-rank test was used for testing. Multivariate Cox analysis was applied to estimate the hazard ratios (HRs) and 95% confidence intervals (95% CIs) for comparison among groups. The confounding factor was identified according to the following criterion: the variable was related to the main predictor (surgical method) or the dependent factor (long-term survival), but it was not in the causal pathway between the main outcome and the predictor. The interaction test was performed by the Wald test or likelihood ratio test.
Propensity score matching (PSM) was carried out based on the following covariates: sex, age, race, diagnostic year, fibrosis score (FS), alpha-fetoprotein (AFP) level, and tumor size. To achieve adequate matches, a 1:1 nearest-neighbor match with a preset caliber was performed (within a range of 0.02 standard deviations of the logit of the calculated propensity score). Statistical analysis was done with the R (http://www.R-project.org) and EmpowerStats software (www.empowerstats.com; X&Y solutions, Inc., Boston, MA).
3. Results
3.1. Patient characteristics
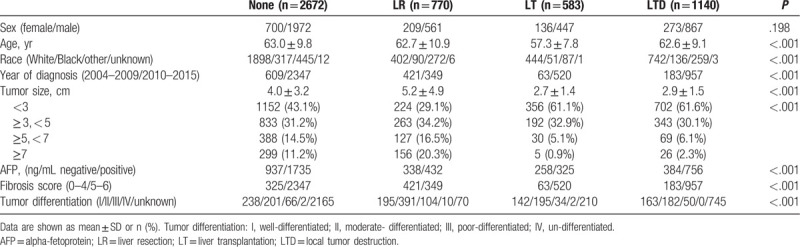
Figures 2 and 3 showed the incidence of 22,997 SI-HCC patients from 2004 to 2015 who received LR, LT, LTD, or none. The incidence of patients with SI-HCC who received no surgical treatment were increased over time (P < .001). The use of LR and LTD for HCC treatment remained unchanged over time (both P > .05), whereas incidence rate of LT was decreased over time (P < .001). Table 1 displayed the general demographics of the final group of 5165 HCC cases with available variables. Only 36.9% of cases diagnosed with HCC in stage I underwent surgical therapy. The mean age of patients undergoing LR, LT, LTD, or none was 62.7, 57.3, 62.6, and 63.0 years, respectively. Most of the cases were White (67.5%) or male (74.5%). When patients underwent LT and LTD, the tumors tended to measure < 3 cm (LT: 61.1%; LTD: 61.6%), and more patients had cirrhotic liver (LT: 89.2%; LTD: 83.9%). In the LR group, more patients were not cirrhotic (54.7% of patients had FS of 0–4), and 36.8% of patients had tumors measuring > 5 cm.
Figure 2.
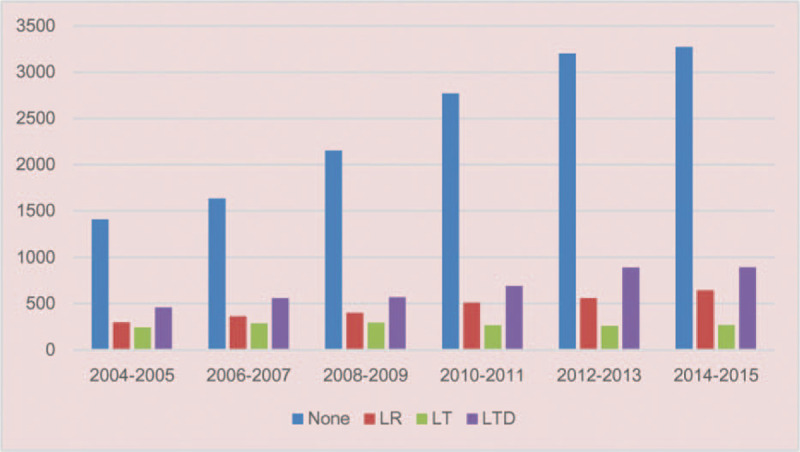
Number of patients with SI-HCC from 2004 to 2015 in the SEER cohort.
Figure 3.
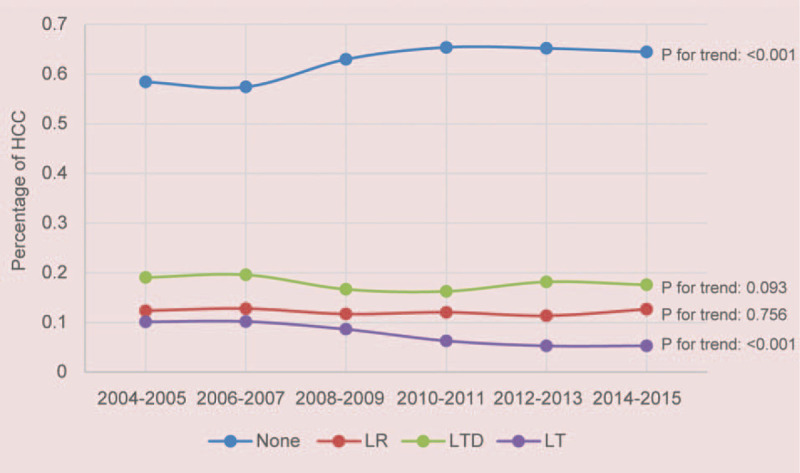
Incidence of patients with SI-HCC from 2004 to 2015 in the SEER cohort.
Table 1.
Clinical features of the 5165 patients with hepatocellular carcinoma in multivariable analytic set.
3.2. Long-term outcomes for SI-HCC patients
A total of 20,953 cases with available survival data were enrolled in survival analysis. Observed survival rates at 1, 3, 5, 7, 9, and 10 years in LR, LT, LTD, and no-surgery group are summarized in Table 2. Figure 4A showed the survival curves of patients who received the 4 treatments. The mean OS for patients after LR, LT, LTD, and none was 80.2, 107.4, 55.6, and 33.2 months, respectively. Significant differences were observed among 4 groups regarding OS (LT > LR > LTD > none).
Table 2.
Overall survival after different treatment methods in patients with stage I HCC.
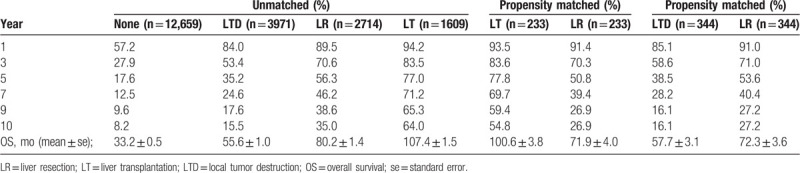
Figure 4.
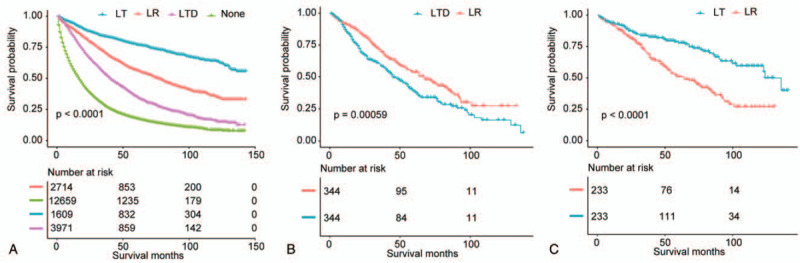
(A) Overall survival analysis for patients undergoing LR, LT, LTD, or none in nonadjusted population. (B) Overall survival analysis for patients after LR, LT, LTD, or none in propensity score matched population (LTD vs LR). (C) Overall survival analysis for patients after LR, LT, LTD, or none in propensity score matched population (LT vs LR).
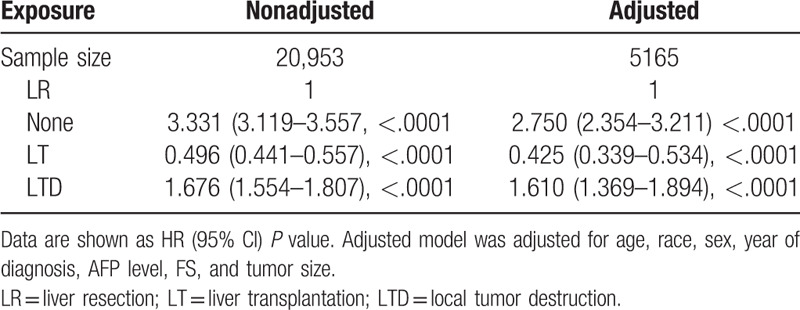
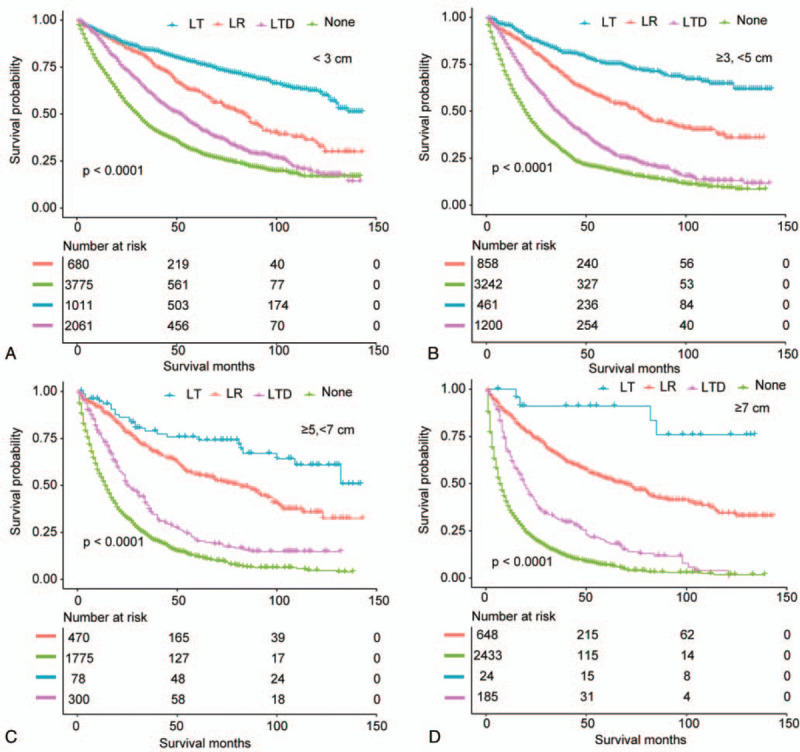
As displayed in Table 3, in the multivariate model (n = 5165), after adjusting potential confounding factors, a clear prognostic advantage of LT was observed in OS (HR, 0.425; 95% CI, 0.339–0.534; P < .0001) compared with patients after LR. Patients undergoing LTD had a worse OS in comparison with patients who underwent LR (HR, 1.610; 95% CI, 1.369–1.894; P < .0001). Patients received no surgical treatment had the worst OS (HR, 2.750; 95% CI, 2.354–3.211; P < .0001) among 4 treatment groups.
Table 3.
Association of surgical methods with patient overall survival.
3.3. Stratified analyses
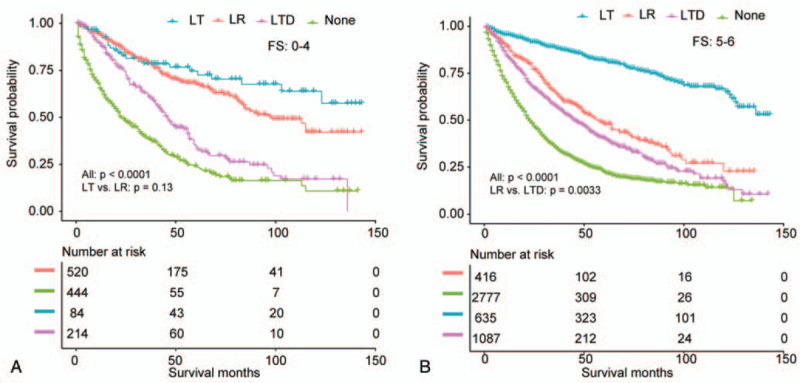
To analyze the consistency of the relationships between surgical methods and long-term prognosis, we conducted stratified analyses. Tables 4 and 5 demonstrated the association of surgical methods and the survival stratified by FS (P value for interaction: .0131) and tumor size (P value for interaction: .0141), respectively. The other subgroup analyses based on the other variables (all interaction P values > .05) are shown in supplementary Table 1 (http://links.lww.com/MD/E928). In exploratory stratified analysis, the salutary effects of LT vs LR on OS were consistent across all subgroups with the exception of a similar result in the noncirrhotic subgroup (HR, 0.811; 95% CI, 0.476–1.382; P = .4414). Specially, for cases with tumor size of 5 to 7 cm, LT was still associated with a significant OS benefit (HR, 0.338; 95% CI, 0.152–0.754; P = .0081). Survival curves stratified by FS and tumor size are shown in Figs. 5 and 6.
Table 4.
Association of surgical methods with patient overall survival based on FS subgroups (Interaction P: .0131).
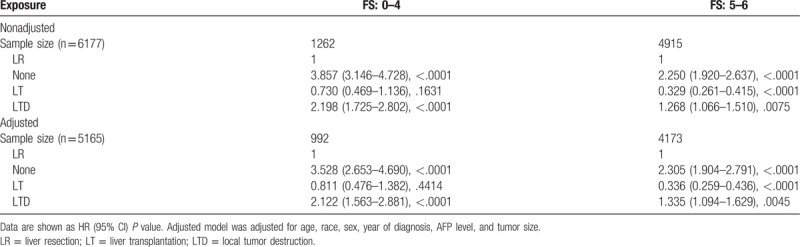
Table 5.
Association of surgical methods with patient overall survival based on tumor size subgroups (Interaction P: .0141).
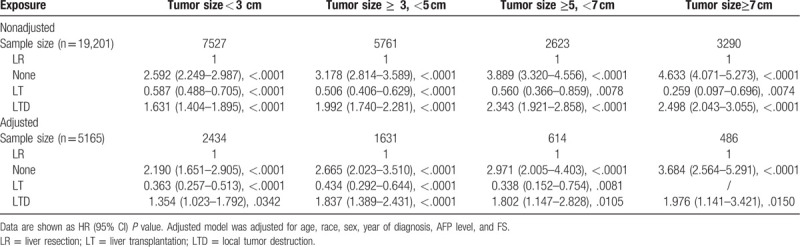
Figure 5.
Overall survival analysis based on FS for patients undergoing LR, LT, LTD, or none (A, FS: 0-4; B, FS: 5-6).
Figure 6.
Overall survival analysis based on tumor size for patients undergoing LR, LT, LTD, or none (A, <3 cm; B, ≥3, <5 cm; C, ≥5 cm, <7 cm; D, ≥7 cm).
The inferior survival effects of LTD vs LR on OS were consistent across all subgroups, and even in the subgroup with tumor size < 3 cm (HR, 1.354; 95% CI, 1.023–1.792; P = .0342) (Tables 4 and 5; Supplementary Table 1, http://links.lww.com/MD/E928).
3.4. Outcomes in propensity score matched population
As displayed in supplementary Tables 2 (http://links.lww.com/MD/E929) (LT vs LR) and 3 (http://links.lww.com/MD/E930) (LTD vs LR), in the matched group, prognostic variables were well-balanced for most baseline features. In the PSM cohort, cases in LT group showed a better OS (P < .001) than cases in LR group (Fig. 4C). The mean OS in LT and LR groups was 100.6 and 71.9 months, respectively (Table 2). Patients undergoing LTD had a worse OS (P = .00059) compared with cases who underwent LR (Fig. 4B). In the matched groups, the mean OS in LTD and LR groups was 57.7 and 72.3 months, respectively (Table 2).
4. Discussion
The selection of surgical methods including LT, LR, and LTD is dependent on a series of interacting factors such as organ supply, tumor burden, liver function, remnant liver volume, medical comorbidities, and patient performance status.[1] In stage I (AJCC 6th and 7th) HCC, all 3 surgical methods were utilized for selected patients. However, the long-term prognosis for patients in this stage after different surgical procedures was controversial,[8,10,17–21] thus a comprehensive analyses is needed to illustrate the prognostic effect of these treatment methods. Using LR as a reference, we compared the long-term prognosis among SI-HCC patients after LR, LT, LTD, or none treatment. In the current study, we observed that patients with SI-HCC treated with LT had a better OS than their counterparts who received LR, whereas patients after LTD showed inferior OS compared with patients after LR. This prognostic effect was consistent after PSM was performed. Besides, our exploratory analysis demonstrated that the treatment effect was consistent across all subgroups except for a similar result in the noncirrhotic subgroup (LT vs LR).
The strength of this study lies in that the outcomes were confirmed by adequate statistical analyses in a representative population on a national scale. Using data form SEER, we were able to adjust a series of variables, including fibrosis score (Ishak), tumor size, AFP levels, and AJCC tumor stage to analyze the independent role of surgical methods in long-term survival of cases with SI-HCC. To date, the existed literatures are insufficient to illustrate the survival differences of HCC cases after different treatments. The majority of previous studies that compared LT or LTD with LR for HCC were single-center studies.[22–24] In these studies, because basic characteristics (i.e., liver cirrhosis and tumor stage) between groups showed significant differences, multivariable analysis was usually used to adjust confounders in majority of the studies. However, there was inherent defect of regression analyses, because it was comprehensively limited by sample size. In addition, in our study, we performed subgroup analysis and interaction test to get rid of the variables affecting the association between surgical methods and patient survival.
According to conventional Milan criterion, patients with tumor > 5 cm were surgically ineligible for LT. However, in the present study, patients with tumor of 5 to 7 cm undergoing LT still had a better OS compared with those after LR, which is consistent with some expanded criterion such as Up-to-7 criteria[13] and Hangzhou criterion.[14] Due to the limited number of patients who received LT with tumor > 7 cm, we failed to validate the potential breakpoints for LT in SI-HCC patients. However, in an era of organ shortage, a decrease of LT rate was observed in the present study. In addition, patients with noncirrhotic liver after LR had a comparable OS compared with those after LT. Hence, LT may be better reserved for HCC patients with severe cirrhosis and/or unresectable tumor, while LR should be considered as the first surgical selection.[25,26]
Over the past decades, several techniques for thermal or chemical tumor destruction have been introduced and clinically used. In previous studies, controversial therapeutic results were observed for patients undergoing RFA and other locoregional therapy methods, including percutaneous ethanol injection, microwave ablation, and cryoablation.[19,27] In the present study, all these methods were defined as LTD and compared with patients who received LR. However, in our study, LTD resulted in inferior OS when compared with LR in SI-HCC patients, even in patients with small tumor size. In sensitivity analyses, we only included patients undergoing RFA, and an inferior OS was still observed when compared with LR (data not shown). However, owing to the national shortage of organs, LTD can be used for patients with liver cirrhosis illegible for LR. In addition, LTD might be related to fewer postoperative complications and a shorter hospital stay than LR. More randomized clinical trials with low risk of bias evaluating the effect of LTD are needed.
The results of this study showed that in the SEER cohort, SI-HCC patients who received surgical treatment experienced a substantial prognostic advantage over their peers not receiving radical therapies. Given the clear relationship with improved prognosis, it is surprising that surgery rates (36.9%) are low for patients with SI-HCC. In the study of Shah et al,[28] they reported that approximately 39.8% of patients without therapy were diagnosed with stage I or II HCC. On the basis of these data, it is reasonable to conclude that surgery is underutilized for HCC patients with treatable early-stage HCC. Reasons for not receiving therapy is unclear. It may stem from curative surgery are more often available for SI-HCC patients with compensated liver function.[29,30] Second, patients of low socioeconomic status cannot receive timely and proper treatment, while they usually have higher incidence of chronic viral hepatitis, the main risk factor for HCC.[28] Barriers to treatment and its underutilization must be explored and eliminated to improve long-term survival in HCC patients in the US.
The current study had many limitations. First, SEER database did not provide enough data for evaluation of liver function including Child–Pugh score and prothrombin time/international normalized ratio, bilirubin, creatinine, model for end-stage liver disease (MELD) score, while liver function was important for clinical decision-making process to decide among LR, LTD, or LT. Second, details on postoperative morbidities were extremely limited, and thus, we could not assess the influence of surgical methods to the short-term prognosis. Third, PSM methods may bring selection bias. Lastly, we cannot get access to the data related to pre- or postoperative management (such as TACE and chemotherapy); thus, the impact of adjuvant treatment was not analyzed in the multivariable analyses.
In conclusion, due to the decreased LT rate, LR should be recommended as the basic treatment for patients with SI-HCC, and LT may be better reserved for HCC patients with severe cirrhosis and/or insufficient liver volume. LTD can be used as an alternative method when LR and LT are unavailable. Patients after surgical treatments were associated with statistically better survival for patients with SI-HCC compared with patients who received no surgical treatment. On the basis of these results, if surgery rates were to increase in select patients, then average survival would also be expected to increase. Measurements of liver function in the database would further allow us to define the preferential surgical methods for patients diagnosed with SI-HCC.
Author contributions
Conceptualization: Zhaoping Wu.
Formal analysis: Zhaoping Wu.
Supervision: Lingling Cao.
Glossary
Abbreviations: AFP = alpha-fetoprotein, CI = confidence intervals, EASL = European Association for the Study of the Liver, FS = fibrosis score, HCC = hepatocellular carcinoma, HRs = hazard ratios, OS = overall survival, PSM = propensity score matching, LR = liver resection, LT = liver transplantation, LTD = local tumor destruction, SEER = Surveillance, Epidemiology, and End Results.
References
- [1].Kolarich AR, Shah JL, George TJ, Jr, et al. Non-surgical management of patients with intrahepatic cholangiocarcinoma in the United States, 2004-2015: an NCDB analysis. J Gastrointest Oncol 2018;9:536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Addissie BD, Roberts LR. Classification and staging of hepatocellular carcinoma: an aid to clinical decision-making. Clin Liver Dis 2015;19:277–94. [DOI] [PubMed] [Google Scholar]
- [3].Minagawa M, Ikai I, Matsuyama Y, et al. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg 2007;245:909–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chan AC, Fan ST, Poon RT, et al. Evaluation of the seventh edition of the American Joint Committee on Cancer tumour-node-metastasis (TNM) staging system for patients undergoing curative resection of hepatocellular carcinoma: implications for the development of a refined staging system. HPB (Oxford) 2013;15:439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sirivatanauksorn Y, Tovikkai C. Comparison of staging systems of hepatocellular carcinoma. HPB Surg 2011;2011:818217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vauthey JN, Ribero D, Abdalla EK, et al. Outcomes of liver transplantation in 490 patients with hepatocellular carcinoma: validation of a uniform staging after surgical treatment. J Am Coll Surg 2007;204:1016–27. discussion 1027-1028. [DOI] [PubMed] [Google Scholar]
- [7].Camma C, Di Marco V, Cabibbo G, et al. Survival of patients with hepatocellular carcinoma in cirrhosis: a comparison of BCLC CLIP and GRETCH staging systems. Aliment Pharmacol Therap 2008;28:62–75. [DOI] [PubMed] [Google Scholar]
- [8].Pugalenthi A, Cutter CS, Fong Y. Current treatment for small (< 5 cm) hepatocellular carcinoma: evolving roles for ablation and resection. Adv Surg 2014;48:97–114. [DOI] [PubMed] [Google Scholar]
- [9].Cauchy F, Soubrane O, Belghiti J. Liver resection for HCC: patient's selection and controversial scenarios. Best Pract Res Clin Gastroenterol 2014;28:881–96. [DOI] [PubMed] [Google Scholar]
- [10].Akoad ME, Pomfret EA. Surgical resection and liver transplantation for hepatocellular carcinoma. Clin Liver Dis 2015;19:381–99. [DOI] [PubMed] [Google Scholar]
- [11].Mazzaferro V, Bhoori S, Sposito C, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Trans 2011;17: (suppl 2): S44–57. [DOI] [PubMed] [Google Scholar]
- [12].Yao FY, Xiao L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant 2007;7:2587–96. [DOI] [PubMed] [Google Scholar]
- [13].Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35–43. [DOI] [PubMed] [Google Scholar]
- [14].Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726–32. [DOI] [PubMed] [Google Scholar]
- [15].Berger NG, Tanious MN, Hammad AY, et al. External radiation or ablation for solitary hepatocellular carcinoma: a survival analysis of the SEER database. J Surg Oncol 2017;116:307–12. [DOI] [PubMed] [Google Scholar]
- [16].Munene G, Vauthey JN, Dixon E. Summary of the 2010 AHPBA/SSO/SSAT Consensus Conference on HCC. Int J Hepatol 2011;2011:565060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hasegawa K, Aoki T, Ishizawa T, et al. Comparison of the therapeutic outcomes between surgical resection and percutaneous ablation for small hepatocellular carcinoma. Ann Surg Oncol 2014;21: (suppl 3): S348–55. [DOI] [PubMed] [Google Scholar]
- [18].Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–55. [DOI] [PubMed] [Google Scholar]
- [19].Weis S, Franke A, Mossner J, et al. Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev 2013;CD003046. [DOI] [PubMed] [Google Scholar]
- [20].Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010;252:903–12. [DOI] [PubMed] [Google Scholar]
- [21].Kutlu OC, Chan JA, Aloia TA, et al. Comparative effectiveness of first-line radiofrequency ablation versus surgical resection and transplantation for patients with early hepatocellular carcinoma. Cancer 2017;123:1817–27. [DOI] [PubMed] [Google Scholar]
- [22].Baccarani U, Isola M, Adani GL, et al. Superiority of transplantation versus resection for the treatment of small hepatocellular carcinoma. Transplant Int 2008;21:247–54. [DOI] [PubMed] [Google Scholar]
- [23].Fonseca AL, Cha CH. Hepatocellular carcinoma: a comprehensive overview of surgical therapy. J Surg Oncol 2014;110:712–9. [DOI] [PubMed] [Google Scholar]
- [24].Wu L, Swan P, McCall J, et al. Intention-to-treat analysis of liver transplantation, resection and thermal ablation for hepatocellular carcinoma in a single centre. HPB (Oxford) 2018;20:966–76. [DOI] [PubMed] [Google Scholar]
- [25].Seshadri RM, Besur S, Niemeyer DJ, et al. Survival analysis of patients with stage I and II hepatocellular carcinoma after a liver transplantation or liver resection. HPB (Oxford) 2014;16:1102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Groeschl RT, Clark Gamblin T, Turaga KK. Surgical resection in hepatocellular carcinoma patients with minimal background fibrosis: a strategy in the era of organ shortage. Ann Surg Oncol 2013;20:2043–8. [DOI] [PubMed] [Google Scholar]
- [27].Germani G, Pleguezuelo M, Gurusamy K, et al. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol 2010;52:380–8. [DOI] [PubMed] [Google Scholar]
- [28].Shah SA, Smith JK, Li Y, et al. Underutilization of therapy for hepatocellular carcinoma in the medicare population. Cancer 2011;117:1019–26. [DOI] [PubMed] [Google Scholar]
- [29].Burman B, Helton WS. Disparities in care for patients with curable hepatocellular carcinoma. HPB (Oxford) 2015;17:745–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hoehn RS, Hanseman DJ, Jernigan PL, et al. Disparities in care for patients with curable hepatocellular carcinoma. HPB (Oxford) 2015;17:747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]


