Abstract
New insights have emerged from maturing long-term academic and commercial clinical trials regarding optimum management of chronic myeloid leukemia (CML). Velocity of response has unexpectedly proved less important than hitherto thought, does not predict survival, and is of unclear relevance for treatment-free remission (TFR). Serious and cumulative toxicity has been observed with tyrosine kinase inhibitors that had been expected to replace imatinib. Generic imatinib has become cost-effective first-line treatment in chronic phase despite chronic low-grade side-effects in many patients. Earlier recognition of end-phase by genetic assessment might improve prospects for blast crisis (BC). TFR has become an important new treatment goal of CML. To reflect this new situation ELN has recently revised and updated its recommendations for treating CML. After a brief review of 175 years of CML history this review will focus on recent developments and on current evidence for treating CML in 2020.
Introduction
Twenty-two years after the first patients with chronic myeloid leukemia (CML) were treated with the tyrosine kinase inhibitor (TKI) imatinib, outcome exceeds all expectations: most CML patients achieve a normal life expectancy, some in sustained treatment-free remissions (TFR) may operationally be cured.
Some expectations remain unmet, however. Most patients require life-long maintenance therapy. Also, progression to blast crisis still occurs in 5% to 7% of patients and remains a challenge. CML has not become the model disease for treating other leukemias or cancers. But the principle of elucidation of pathogenesis as a successful approach to treatment of cancer has been impressively shown in CML.
Success came a long way. CML was first described in 1844/5 when Virchow coined the term leukemia (Leukämie).1–5 Bone marrow was proposed early as possible tissue of origin of CML,6 but a definite diagnosis became possible only 82 years later when the Philadelphia (Ph)-chromosome was discovered and then the translocation t (9;22) was identified as hallmarks of the disease.7,8 The subsequent molecular dissection of the chromosomal breakpoints with identification of the BCR-ABL fusion products laid the groundwork for molecular CML-diagnostics and for targeted therapy with BCR-ABL Tyrosine kinase inhibitors (TKI) as the current treatment principle of choice. Molecular BCR-ABL1 monitoring in CML with derivation of the International Scale (IS) has become the posterchild for molecular monitoring of other leukemias and diseases.
Early palliative treatments were arsenic (Fowler's solution, 5 to 10 drops 3× daily for several weeks)9,10 and splenic irradiation,11 the mainstays of treatment until 1953 when busulfan was introduced.12 Hydroxyurea, available since 1963,13 was easier to handle, had fewer side effects than busulfan and prolonged survival modestly.14 Bone marrow transplantation was introduced in the late seventies15 and provided the first cures.16 At the same time interferon alpha (IFN) was shown to induce complete cytogenetic remissions (CCR) in a substantial minority of patients,17 usually younger patients. Randomized studies18–20 documented prolongation of survival with IFN which became the treatment of choice, although its exact mechanism of action is still not fully understood.21
The benefit by IFN had just been recognized (ASH management recommendations 1999)22 when BCR-ABL tyrosine kinase inhibition was introduced.
The detection of the ABL-oncogene was a byproduct of the search for a human leukemia virus in the 1960s and early 1970s. The first oncogenes (SRC, MYC) were isolated from chicken leukemia viruses.23,24 ABL was isolated from the acutely transforming murine Abelson leukemia virus in 1980.25 Numerous other oncogenes, isolated from retroviruses and from genomes of normal cells, followed.
Many oncogenes, amongst them SRC and ABL, encoded kinase activities that most notably phosphorylate tyrosine, a rarely phosphorylated amino acid.25,26 This finding gained significance for CML when it was recognized that the human ABL oncogene homologue was located on chromosome 9 at the breakpoint of t (9;22).27 The discovery of fusion transcripts of ABL with BCR sequences from chromosome 2228 led to transfection experiments and the observation that BCR-ABL sequences induced leukemia in mice.29,30 Since BCR-ABL1's oncogenic properties were mainly connected to its tyrosine kinase activity, it was the logical next step to define an inhibitor specific for bcr-abl tyrosine kinase suitable for therapeutic use in humans.31
The first trial with imatinib, a phase I study with poor risk CML patients, started in 1998.32 The stunning results convinced even skeptics that further studies were indicated. In 1999, a group of international investigators on CML met in Biarritz, France, to discuss the results and to convince Novartis to produce imatinib (at that time still STI571) in sufficient quantities for larger phase II and III trials. A letter sent by the group to Dr Daniel Vasella, then CEO of Novartis, recommending scale-up of the production of imatinib made the difference (The Magic Bullet33).
The development of tyrosine kinase inhibitor (TKI) therapy and of molecular monitoring has been extensively reviewed by ELN34–36 and will not be repeated here. But recent developments of current importance as discussed by ELN in its most recent recommendations,37 will be highlighted in this review.
Epidemiology
Median age at diagnosis of CML is approximately 56 to 57 years in Western countries as estimated from the EUTOS and SIMPLICITY registries.38,39 Patients older than 70 years make up more than 20%. In developing countries with younger populations median age is less than 50 years.40 The incidence per year per 100,000 population varies by age and ranges between 1 and 2 depending on the age of the respective populations.
Initial diagnostic workup
The workup at baseline includes the following examinations (Table 1).
Table 1.
Initial Diagnostic Workup.
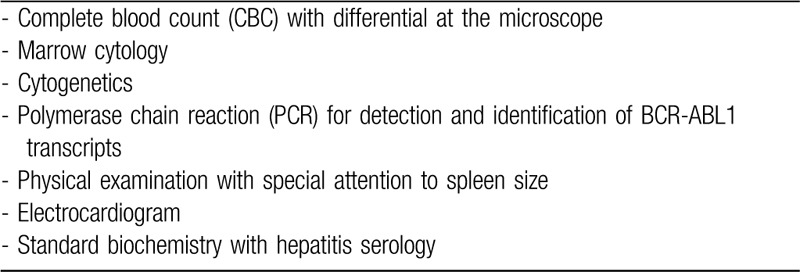
The preferred risk score for CML in the TKI era is the EUTOS long-term survival (ELTS) score whose accuracy to predict death from CML is higher than the Sokal score (Table 2).41
Table 2.
Assessment of Risk Score (ELTS score).
Identification of transcript type is important for molecular monitoring, since atypical transcripts may give false negative test results – and is also of prognostic importance. The shorter e13a2 transcript is reportedly associated with shorter survival and a longer time to DMR compared with the longer e14a2 transcript. Based on evidence from a registry of transcript types in 45,503 newly diagnosed patients from 45 countries transcript type may be helpful to predict response to treatment, outcome of treatment, and TFR.42
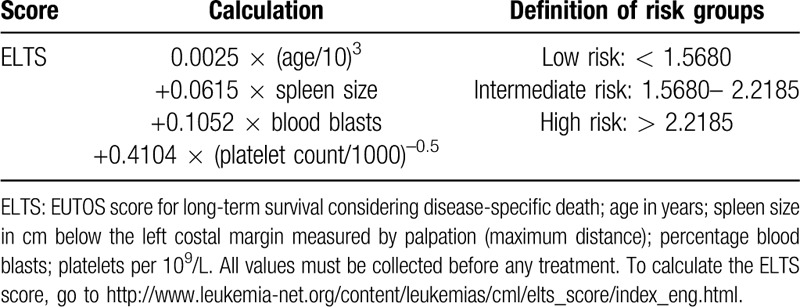
Several additional risk factors have been implicated, but so far none has been validated or found useful except reticulin content in a bone marrow biopsy43–45 and high-risk additional chromosomal abnormalities (ACA; Table 3). High-risk ACAs predict poorer response to TKIs and a higher risk of progression.46–48 Whereas the 2013 ELN-recommendations considered ACA a warning sign,36 the 2020 ELN recommendations upgraded ACA to a high-risk sign for treating patients.37
Table 3.
Genetically Based Risk Assessment.
First-line treatment
With few exceptions, the current first-line treatment is a TKI. A short course of hydroxyurea may be given in symptomatic patients while a diagnosis of CML is pending. Currently, 4 TKIs are approved for first-line treatment by the FDA and EMA: imatinib (Glivec®, Novartis), dasatinib (Sprycel®, Bristol-Myers Squibb), nilotinib (Tasigna®, Novartis), and bosutinib (Bosulif®, Pfizer). Radotinib (Supect®, Dae Wong Pharma) has been approved in South-Korea only49 and is not further considered here.
Imatinib is effective in all phases of CML, and therapy has resulted in a normal life expectancy of most patients treated in chronic phase (CP) in clinical trials50,51 and population-based registries.52–54 No serious toxicity has surfaced after more than 20 years of use.37,55,56 DMR was achieved in more than 80% of patients which is stable in more than 70%57 allowing attempts at treatment discontinuation to achieve treatment-free remissions (TFR)58,59 alleviating chronic low-grade side-effects such as fatigue and muscle cramps.
Generic imatinib60–62 is now available worldwide and has become cost-effective initial therapy in CP.37,63 If a generic drug meets the national standards of a country involved in quality, bioavailability and efficacy, generic imatinib is an acceptable alternative to a branded product. The 2020 ELN recommendations37 state generic and brand product dosing should be the same. Monitoring the response to generics should also be the same as with branded drugs. If there is a change in therapy from a brand to a generic product, enhanced vigilance for the first six months is advised. Patients should continue the same generic brand if possible, to avoid potential side-effects due to changes in drug structure, bioavailability and drug preparation.
Imatinib resistance, second generation TKI, and second-line treatment
Second generation TKIs (2G-TKI, dasatinib, nilotinib, bosutinib) were developed following recognition of imatinib kinase domain (KD) resistance mutations64 which occur in 4.6% of 1551 CP CML patients over 10 years making it relatively rare.51 The higher potency of 2G-TKIs resulting in more rapid responses and relief of symptoms compared to imatinib when used in second-line65,66 led to their use also as first-line therapy. By recognizing imatinib resistant mutations, fewer patients progressed to blast crisis (BC).67,68 These positive effects, however, were counterbalanced by drug-induced adverse effects. 5- and 10-year data of randomized trials indicate survival with 2G-TKI first-line is similar to imatinib. The high rates of adverse effects to 2G-TKI (particularly pleural effusions in more than 25% of dasatinib-treated patients and serious vascular events with linear increase to 25% by 10 years in nilotinib-treated patients) argue against the use of 2G-TKI in first-line therapy.67–69
For second-line treatment, patients must be carefully selected considering the comorbidities and the side-effects of 2G-TKI. In the case of failure to imatinib, a change of therapy is mandatory and should be accompanied by investigating BCR-ABL1 KD mutations (Table 3). In the case of intolerance, the decision to change may be subjective depending in part on the patient, the physician and options of supportive care. Response criteria are the same as for first-line treatment.
Since dasatinib has pleuro-pulmonary toxicity, previous pleuro-pulmonary disease is a strong contraindication. A dose reduction from the approved dose of 100 mg/day in CP to 50 mg/day may reduce toxicity.70
Because of the cardiovascular toxicity of nilotinib a history of coronary heart disease, cerebrovascular accidents and/or peripheral arterial occlusive disease represent strong contraindications to nilotinib. Also, hypertension, diabetes mellitus, hypercholesterolemia and a history of pancreatitis may be contraindications to using nilotinib. A dose-increase from the approved dose of 300 mg twice daily is not recommended.
No relevant comorbidities and no strong contraindications to bosutinib have been identified. At the approved dose of 400 mg/day annoying, but typically transient diarrhea occurs. Owing to the shorter observation time compared to the other TKI, no firm statement can be made regarding long-term safety.
Selection criteria and dosing of TKI in first- and second-line have been extensively discussed in recent ELN recommendations.37,56
Indications of 2G-TKI and of the 3rd generation TKI (3G-TKI) ponatinib for second- and third-line treatments based on the most frequent KD resistance mutations are shown in Table 4.
Table 4.
TKI Indications Based on ABL1 Kinase Domain Mutation Status.

Ponatinib has been approved for patients resistant against 2 TKI and is the only approved TKI with activity against the T315I mutation.71,72 Dosing is critical; safety and efficacy must be considered.37
2G-TKI and ponatinib are effective against most KD resistance mutations, but cannot overcome resistance from other causes such as clonal evolution with emergence of ACA.
Table 5 summarizes the 5- and 10-year survival results of long term randomized and observational studies with imatinib or 2G-TKI. Similar survival rates are reported by population-based registries.52–54
Table 5.
Five- and Ten-year Survival Results in Clinical Trials.
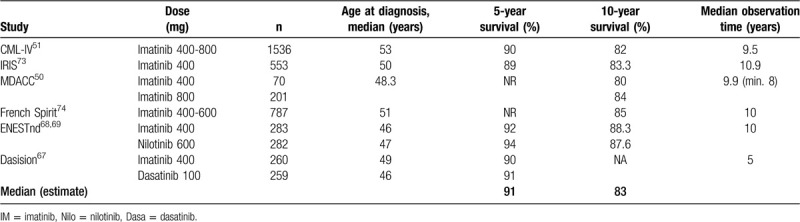
Current determinants of survival in CML are comorbidities,75 major route ACA,76 risk score, smoking77 and treatment center, but not initial treatment selection.51
Resistance to imatinib occurs in 10% to 15%, and to 2GTKI in <10% of patients in first-line treatment. In some patients, failure to respond may be related to poor compliance. Mutations account for resistance in about one third of resistant CP patients, and in about two thirds of resistant accelerated phase (AP) and BC patients. Alternative mechanisms of resistance include clonal evolution (emergence of high-risk ACA) and the activation of BCR-ABL1 independent pathways. A cytogenetic risk classification has been proposed to allow risk-based treatment adaptation.47,48,78
In about two-thirds of compliant TKI resistant CP patients and in about one third of resistant AP and BC patients, a mutation is neither detected, nor is it the only cause of resistance. Analyzing the genome and expression profiles of resistant CML cells may identify somatic mutations79–81 as early signs of progression, and lead to a genetically-based risk classification with the potential for non-BCR-ABL1 targeted therapy for resistant patients.82
BCR-ABL1 mutations can be detected with sensitivities of about 20% by Sanger sequencing and in about 3% by NGS. NGS is the recommended technology to detect clinically relevant BCR-ABL1 resistance mutations in patients not responding adequately to TKI.83,84
Allogeneic hematopoietic cell transplantation
Despite the superiority of drug treatment, allogeneic hematopoietic cell transplantation has retained a place in CP CML for patients with disease resistant to multiple TKIs or personal preferences.85,86 In resource poor countries the onetime expense of a transplant may be more economical than life-long treatment with a TKI.
Transplants should be strongly considered in persons resistant to 2G-TKIs. Someone resistant to the initial 2G-TKI therapy has a low chance of achieving a durable response to an alternative TKI and should be assessed early for a transplant. Early transplantation as a rule improves outcome.87 If the patient has also failed ponatinib, risk of progression is high. Someone progressing to AP under treatment is a candidate for an immediate transplant. For a patient presenting in BC a return to a second CP (CP2) should be attempted. Return to CP2 improves transplantation outcome.85,88 Also, in patients with high-risk ACA and low blast counts early transplantation may improve survival.48 Transplant mortality in CP is low,85 but GvHD remains a problem. Transplantation in BC is a high-risk procedure and not advised.37
Pregnancy and fertility
All TKIs are teratogenic and should be withheld during pregnancy.89,90 Low-level secretion of TKIs in breast milk contraindicates their use during breast-feeding.91 Sperm quality and morphology are unchanged after treatment with TKI.92 For more in-depth information see the ELN 2020 recommendations.37
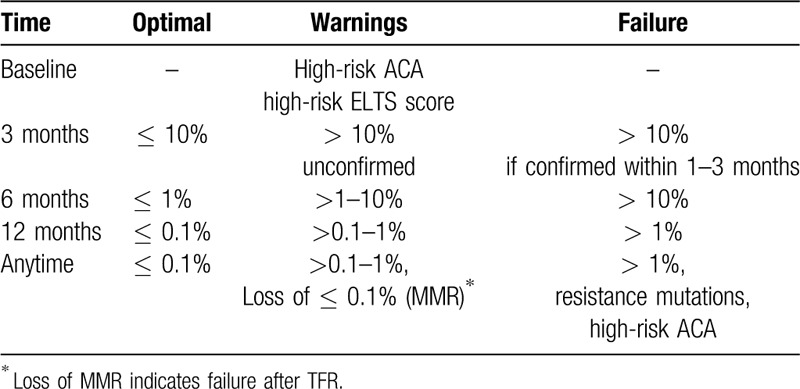
Response monitoring and milestones
Timely recognition of suboptimal response or resistance to TKI requires regular monitoring. Hematologic and cytogenetic monitoring have been replaced in most instances by the more sensitive molecular monitoring with quantitative PCR-techniques for BCR-ABL1 transcripts.93,94 Transcript levels are reported in a standardized fashion according to the International Scale (IS)95–97 which underlies the response milestones guiding treatment (Table 6). Complete cytogenetic remission (CCR) has been shown to be equivalent to 0.1% BCR-ABL1 on the IS.98
Table 6.
Response Milestones Expressed as BCR-ABL1 on the International Scale (IS).
DMR at the MR4 and MR4.5 levels is prognostic. Progression of CML is extremely rare at these levels.57 Patients may be operationally cured and require no further treatment. To test this possibility TKI discontinuation studies have been undertaken to determine optimum duration of treatment and of deep DMR, rate of TFR after discontinuation, and markers predictive of successful discontinuation,58,59,99 see paragraph on TFR below.
Quality of life
This is an important evolving field building on survival, but beyond the scope of this review.
In brief, since most patients receive TKIs for many years or even indefinitely, observation of quality of life in these patients and amelioration of chronic low-grade side-effects are important. Current research preferentially addresses tolerability of different TKIs.100,101 Replacement of one TKI by another may improve tolerability, but frequently at the expense of other, potentially more serious toxicity.102 Dose-reductions of TKIs are an option.70,103 Patient-reported outcome (PRO) questionnaires are encouraged to quantify chronic quality of life issues faced by CML patients.104
Treatment-free remission (TFR)
TFR is a new significant goal of CML management. A significant proportion of patients will achieve a DMR defined as BCR-ABL1 levels of MR4 and MR4.5 on the IS with current TKIs. Benchmark times for molecular response rates with imatinib are shown in Figure 1.
Figure 1.
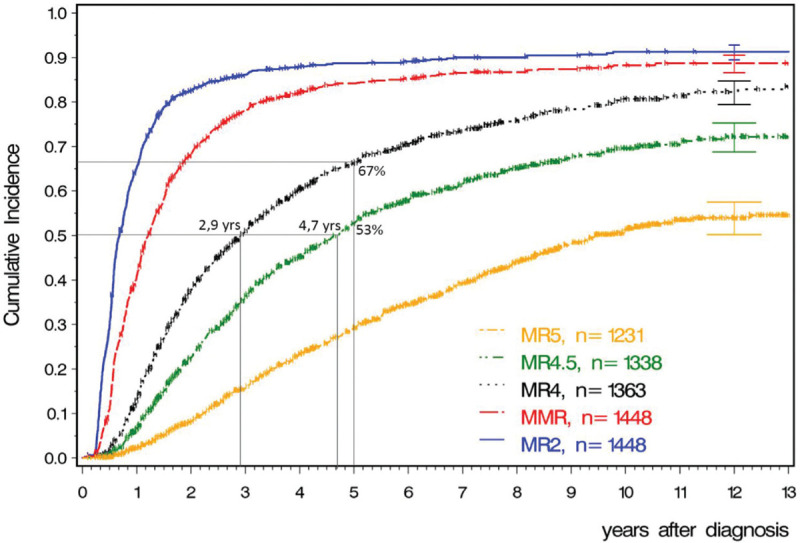
Benchmarks for molecular response rates with imatinib. 12-year incidences are 91% for MR2 (equivalent to CCR), 89% for MR3 (MMR), 82% for MR4, 72% for MR4.5 and 54% for MR5. Data updated from CML study IV. (M Pfirrmann, update of Ref. 55).
Median times to MR4 are 2.9 years, to MR4.5 4.7 years. 5-year rates are 67% for MR4 and 53% for MR4.5.
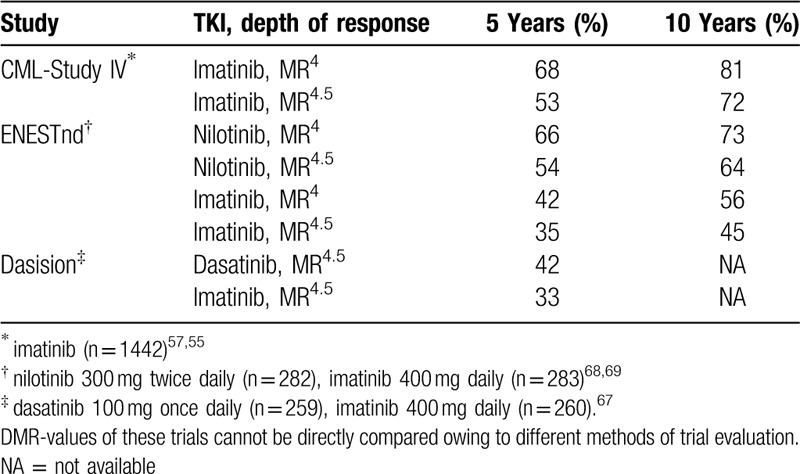
Table 7 lists benchmarks of DMR that can be expected by 5 and 10 years after treatment with imatinib, nilotinib and dasatinib.55,57,67–69 Five-year follow-up of first-line bosutinib is not yet available.105
Table 7.
Cumulative Incidences of DMR (MR4 and MR4.5) with Imatinib, Nilotinib and Dasatinib by 5 and 10 Years as Benchmarks.
An attempt at treatment discontinuation can be considered, if sustained DMR of sufficiently long duration has been achieved. An initial observation of 12 patients94 showed that about half of them in DMR (no detectable BCR-ABL transcripts by PCR) stayed in remission after cessation of imatinib. In a follow-up study of 100 patients (STop IMatinib or STIM study) 38% stayed in TFR after an observation period of 7 years.58,106 Most relapses occurred early within the first 6–12 months. Loss of MMR indicates failure of TFR.107 Virtually all relapsing patients regained their prior best response level after re-treatment.
A polymyalgia-like TKI withdrawal syndrome of musculo-skeletal pain may occur in a third of patients which is usually self-limited, but may require treatment with acetaminophen, non-steroidal anti-inflammatory drugs or rarely a short course of oral steroids.108,109 A patient study reported that the TKI withdrawal syndrome if unmanaged may cause more morbidity than hitherto thought.110
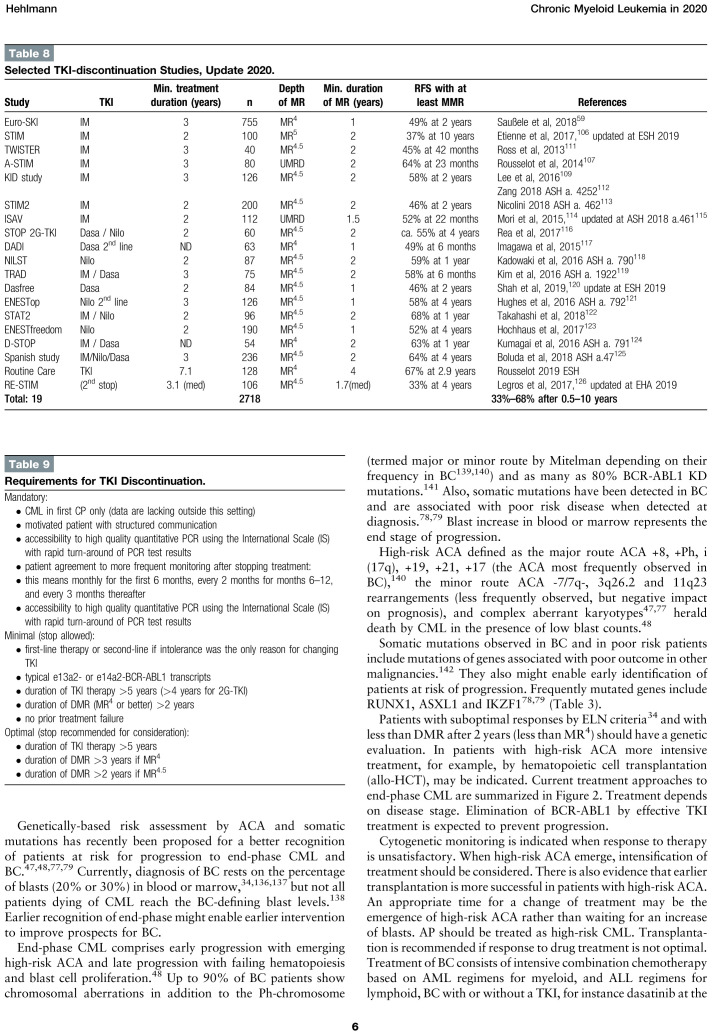
Table 8 shows a selection of discontinuation studies after treatment with imatinib or the 2G-TKI dasatinib and nilotinib.
Table 8.
Selected TKI-discontinuation Studies, Update 2020.
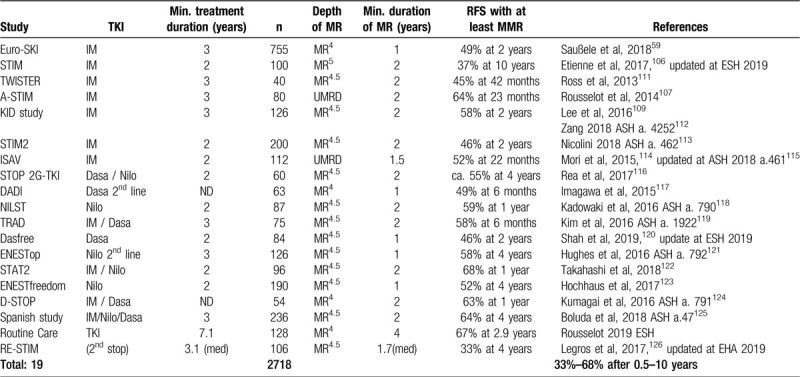
The largest of these studies = the Euro-SKI study of 755 mostly imatinib treated CML patients who had been in DMR at the MR4 level for at least 1 year = showed a TFR rate of 49% after 3 years.59 Duration of MR4 was determined as the most important predictor of TFR. Treatment discontinuation is feasible only in CP patients. Patients in advanced phases, particularly in BC, remain a challenge.
After failure of TFR, a second stop after additional treatment can result in a TFR-rate as high as 33% at 4 years,126 updated at EHA 2019.
Interestingly, dose reduction prior to complete discontinuation to reduce side-effects may improve successful TFR (Destiny study103). Another interesting observation is the finding in the ISAV study, by comparing TFR rates in younger and older patients, of significantly lower TFR rates in patients under 45 years of age114,115 which is in line with the observation of more aggressive disease in adolescents and young adults.127,128
Several studies addressed the issue of changing from imatinib to a 2G-TKI to shorten the interval to DMR and TFR. A more rapid response was generally observed, but toxicity of 2G-TKI limits this approach.
In the TIDEL-II study, the dose of patients receiving imatinib 600 mg/day failing to reach time benchmarks was increased to imatinib 800 mg/day or medication was changed to nilotinib 2 × 400 mg/day.129 This approach was considered feasible.
In the ENESTcmr study, imatinib-treated patients in CCR were randomized to remain on imatinib or to change to nilotinib. The rate of DMR by 4 years was, as expected, higher in the nilotinib group, but only 57% of nilotinib-treated patients completed 4 years of nilotinib therapy. The study provided no information whether patients in DMR subsequently achieved TFR successfully.130,131 It should be remembered that most patients in durable DMR still harbor residual BCR-ABL1 sequences in their genomic DNA.132
The ELN 2020 recommendations define the following requirements for TKI discontinuation for successfully achieving TFR (Table 9).37
Table 9.
Requirements for TKI Discontinuation.
It is recommended to consider TFR in appropriate patients after careful discussion employing the concept of shared decision making.133 First-line TKI, or a change to a 2G-TKI, for faster DMR are not recommended because of the more serious side-effects of 2G-TKI, their increased costs and absent information about the number of patients who might actually benefit. A change to 2G-TKI to improve the depth of response can be considered in selected patients in whom DMR has not been reached such as the motivated patient with a high priority for TFR, younger patients with low or intermediate risk disease or women who wish to become pregnant.
End phase CML and blast crisis
Outcome of patients in blast crisis (BC) treated with single agents, combination chemotherapy, and TKI alone and in combination with intensive chemotherapy134,135 remains unsatisfactory. Once BC has occurred, survival is generally less than one year with death due to infection or bleeding. New approaches are urgently needed.
Genetically-based risk assessment by ACA and somatic mutations has recently been proposed for a better recognition of patients at risk for progression to end-phase CML and BC.47,48,77,79 Currently, diagnosis of BC rests on the percentage of blasts (20% or 30%) in blood or marrow,34,136,137 but not all patients dying of CML reach the BC-defining blast levels.138 Earlier recognition of end-phase might enable earlier intervention to improve prospects for BC.
End-phase CML comprises early progression with emerging high-risk ACA and late progression with failing hematopoiesis and blast cell proliferation.48 Up to 90% of BC patients show chromosomal aberrations in addition to the Ph-chromosome (termed major or minor route by Mitelman depending on their frequency in BC139,140) and as many as 80% BCR-ABL1 KD mutations.141 Also, somatic mutations have been detected in BC and are associated with poor risk disease when detected at diagnosis.78,79 Blast increase in blood or marrow represents the end stage of progression.
High-risk ACA defined as the major route ACA +8, +Ph, i(17q), +19, +21, +17 (the ACA most frequently observed in BC),140 the minor route ACA -7/7q-, 3q26.2 and 11q23 rearrangements (less frequently observed, but negative impact on prognosis), and complex aberrant karyotypes47,77 herald death by CML in the presence of low blast counts.48
Somatic mutations observed in BC and in poor risk patients include mutations of genes associated with poor outcome in other malignancies.142 They also might enable early identification of patients at risk of progression. Frequently mutated genes include RUNX1, ASXL1 and IKZF178,79 (Table 3).
Patients with suboptimal responses by ELN criteria34 and with less than DMR after 2 years (less than MR4) should have a genetic evaluation. In patients with high-risk ACA more intensive treatment, for example, by hematopoietic cell transplantation (allo-HCT), may be indicated. Current treatment approaches to end-phase CML are summarized in Figure 2. Treatment depends on disease stage. Elimination of BCR-ABL1 by effective TKI treatment is expected to prevent progression.
Figure 2.
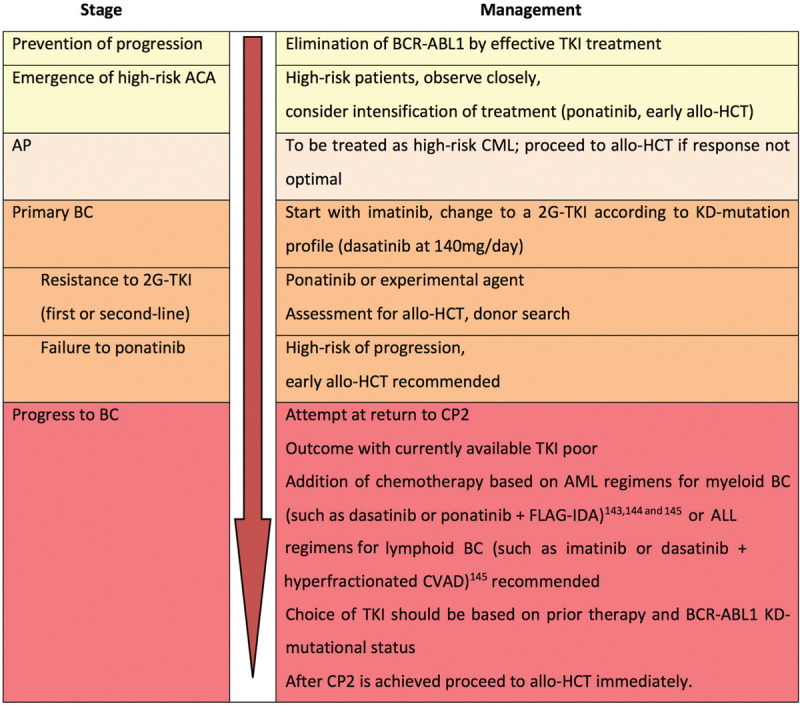
Management strategy for end-phase CML. The red arrow indicates progression to the worse. CP2 = second chronic phase.
Cytogenetic monitoring is indicated when response to therapy is unsatisfactory. When high-risk ACA emerge, intensification of treatment should be considered. There is also evidence that earlier transplantation is more successful in patients with high-risk ACA. An appropriate time for a change of treatment may be the emergence of high-risk ACA rather than waiting for an increase of blasts. AP should be treated as high-risk CML. Transplantation is recommended if response to drug treatment is not optimal. Treatment of BC consists of intensive combination chemotherapy based on AML regimens for myeloid, and ALL regimens for lymphoid, BC with or without a TKI, for instance dasatinib at the approved dose 140 mg/day for BC or ponatinib, in preparation for a prompt transplantation if possible. Flow cytometry distinguishes between lymphoid and myeloid BC allowing appropriate selection of treatment. Lymphoid BC has more treatment options and a better outcome than myeloid BC. In patients who cannot tolerate intensive chemotherapy regimens, a more palliative approach with less intensive therapy according to immunophenotype should be considered such as vincristine and prednisone in lymphoid BC.
There is evidence that emergence of high-risk ACA is an indication for a timelier change of treatment with better outcome.48 Comparing transplantation outcome in early and late end-phase, a clinically relevant, though not statistically significant difference of 30% in 2-year survival suggests that outcome of transplanted patients with high-risk ACA depends on disease stage similar to patients without ACA.87
Summary and prospects
Based on the results of maturing long-term clinical trials management of CP-CML is again changing profoundly. All randomized studies that compare imatinib 400 mg once daily with 2G-TKIs, imatinib 400 mg with dose increase, or imatinib combined with IFN alpha or low-dose cytarabine have failed to improve OS. Although deeper molecular responses occurred more rapidly with 2G-TKIs, with imatinib dose increase or with imatinib in combination with peg-IFN alpha, these events did not translate into better OS than with imatinib at a standard dose of 400 mg daily. Nevertheless, these studies provided greater insights in the safety and efficacy of the drugs, as well as benchmarks for molecular response as a basis for individualized treatment and eventually treatment discontinuation. The studies showed that survival has moved close to that of the general population. Now more patients die of CML-unrelated causes than from CML. The goal of treatment in these patients is better supportive care and management of side-effects of treatments aiming at best possible quality of life.
A new important development has been recognizing that treatment can be successfully stopped in a substantial minority of patients depending upon whether duration of both treatment and DMR are long enough to make TFR a feasible option. TFR is an important new goal of CML management which should be discussed with appropriate patients.
Regarding changing therapy from imatinib to a 2G-TKI in a patient with stable CCR or MMR, but in whom the level of DMR (< MR4) was insufficient to warrant consideration of discontinuation, no recommendation can be made in view of the high toxicity and costs of 2G-TKI. Also, there is no information about the rate of successful TFR from large randomized trials with different initial treatment regimens addressing this specific issue.
Regarding changing from 2G-TKI to imatinib, this can be considered when no DMR is achieved within 3 years to avoid the risk of serious cumulative toxicity of 2G-TKI.
Current challenges on the path to cure of CML are increasing the proportion of patients in whom treatment can be successfully discontinued, and the further decrease of patients who progress to BC. This can be achieved by optimizing treatment with available drugs, by developing new drugs with better efficacy and by better recognition of patients at risk for progression and of optimum conditions for treatment discontinuation (duration of DMR, duration of treatment, other factors such as risk score, age, gender), and by more intensive treatment of patients not responding well enough, respectively. Of urgency is still the management of refractory disease of those 6% who progress to BC in spite of seemingly adequate treatment. Earlier recognition of such patients seems possible.
Finally, factors causing CML remain of interest. The only established risk factor is still radiation as observed after the atomic bombs on Hiroshima and Nagasaki. Better epidemiologic studies and registries may provide an answer.143–145
Acknowledgements
The author thanks Drs. Richard T Silver and Robert P. Gale for critically reading the manuscript, and Johannes Hehlmann for support.
Footnotes
Citation: Hehlmann R. Chronic Myeloid Leukemia in 2020. HemaSphere, 2020;4:5(e468). http://dx.doi.org/10.1097/HS9.0000000000000468
The author declares no conflicts of interest.
References
- 1.Donné A. Cours de microscopie complementaire des etudes medicales. Balière, Paris. 1844;135:196.
- 2.Bennett JH. Case of hypertrophy of the spleen and liver in which death took place from suppuration of the blood. Edinburgh Med Surg J. 1845;64:413–423.
- 3.Virchow R. Weisses Blut. Frorieps Notizen. 1845;36:151–156.
- 4.Virchow R. Die Leukämie. In: Gesammelte Abhandlungen Zur Wissenschaftlichen Medizin. 1856, 19-211. Meidinger, Frankfurt.
- 5.Geary CG. The story of chronic myeloid leukemia. Brit J Haematol. 2000;110:2–11. [DOI] [PubMed] [Google Scholar]
- 6.Neumann E. Über myelogene Leukämie. Berliner Klin Wochenschr. 1878;15:69–135.
- 7.Nowell PC, Hungerford DA. A minute chromosome in human chronic granulocytic leukemia. Science. 1960;132:1497–1501. [Google Scholar]
- 8.Rowley J. A new consistent chromosomal abnormality in chronic myelogenous leukemia identified by quinacrine fluorescence and giemsa staining. Nature. 1973;243:290–293. [DOI] [PubMed] [Google Scholar]
- 9.Lissauer. Zwei Fälle von Leucaemie. Berliner Klin. Wochenschrift. 1865;2:403.
- 10.Forkner CE, Scott TF. Arsenic as a therapeutic agent in chronic myelogenous leukemia. JAMA. 1931;97:3–6. [Google Scholar]
- 11.Minot GR, Buckman TE, Isaacs R. Chronic Myelogenous Leukemia: age, incidence, duration, and benefit derived from irradiation. JAMA. 1924;82:1489–1494. [Google Scholar]
- 12.Haut A, Abbott WS, Wintrobe MM, et al. Busulfan in the treatment of chronic myelocytic leukemia. The effect of long-term intermittent therapy. Blood. 1961;17:1–19. [PubMed] [Google Scholar]
- 13.Kennedy BJ. Hydroxyurea therapy in chronic myelogenous leukemia. Cancer. 1972;29:1052–1056. [DOI] [PubMed] [Google Scholar]
- 14.Chronic Myeloid Leukemia Trialists’ Collaborative Group Hydroxyurea versus busulphan for chronic myeloid leukemia: an individual patient data meta-analysis of three randomized trials. Br J Haematol. 2000;110:573–576. [DOI] [PubMed] [Google Scholar]
- 15.Fefer A, Buckner CD, Thomas ED, et al. Cure of hematologic neoplasia with transplantation of marrow from identical twins. N Engl J Med. 1977;297:146–148. [DOI] [PubMed] [Google Scholar]
- 16.Goldman JM, Apperley JF, Jones L, et al. Bone marrow transplantation for patients with chronic myeloid leukemia. N Engl J Med. 1986;314:202–207. [DOI] [PubMed] [Google Scholar]
- 17.Talpaz M, Kantarjian HM, McCredie K, et al. Hematologic remission and cytogenetic improvement induced by recombinant human interferon alpha A in chronic myelogenous leukemia. N Engl J Med. 1986;314:1065–1069. [DOI] [PubMed] [Google Scholar]
- 18.Italian Cooperative Study Group on Chronic Myeloid Leukemia Interferon alfa-2a as compared with conventional chemotherapy for the treatment of chronic myeloid leukemia. N Engl J Med. 1994;330:820–825. [DOI] [PubMed] [Google Scholar]
- 19.Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood. 1994;84:4064–4077. [PubMed] [Google Scholar]
- 20.Allan NC, Richards SM, Shepherd PCA. On behalf of the UK Medical Research Council's Working Parties for Therapeutic Trials in Adult Leukemia. UK Medical Research Council randomized, multicenter trial of interferon-alpha n1 for chronic myeloid leukemia: improved survival irrespective of cytogenetic response. Lancet. 1995;345:1392–1397. [DOI] [PubMed] [Google Scholar]
- 21.Talpaz M, Mercer J, Hehlmann R. The interferon-alpha revival in CML. Ann Hematol. 2015;94 (Suppl 2):S195–207. [DOI] [PubMed] [Google Scholar]
- 22.Silver RT, Woolf SH, Hehlmann R, et al. An evidence-based analysis of the effect of busulfan, hydroxyurea, interferon, and allogeneic bone marrow transplantation in treating the chronic phase of chronic myeloid leukemia: developed for the american society of hematology: presented in part at the ASH Education Session, December 5, 1998, Miami Beach, FL. Blood. 1999;94:1517–1536. [PubMed] [Google Scholar]
- 23.Duesberg PH, Vogt PK. Differences between the ribonucleic acids of transforming and non-transforming avian tumor viruses. Proc Natl Acad Sci U S A. 1970;67:1673–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bister K, Vogt PK. Genetic analysis of the defectiveness in strain MC29 avian leukosis virus. Virology. 1978;88:213–221. [DOI] [PubMed] [Google Scholar]
- 25.Witte ON, Dasgupta A, Baltimore D. Abelson murine leukemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980;283:826–831. [DOI] [PubMed] [Google Scholar]
- 26.Collett MS, Erikson RL. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978;75:2021–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heisterkamp N, Stephenson JR, Groffen J, et al. Localization of the c-abl oncogene adjacent to a translocation break point in chronic myelocytic leukemia. Nature. 1983;306:239–242. [DOI] [PubMed] [Google Scholar]
- 28.Shtivelman E, Lifshitz B, Gale RP, et al. Fused transcript of abl and bcr genes in chronic myelogenous leukemia. Nature. 1985;315:550–554. [DOI] [PubMed] [Google Scholar]
- 29.Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–830. [DOI] [PubMed] [Google Scholar]
- 30.Heisterkamp N, Jenster G, Ten Hoeve J, et al. Acute leukemia in bcr/abl transgenic mice. Nature. 1990;344:251–253. [DOI] [PubMed] [Google Scholar]
- 31.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr–Abl positive cells. Nat Med. 1996;2:561–566. [DOI] [PubMed] [Google Scholar]
- 32.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. [DOI] [PubMed] [Google Scholar]
- 33.Vasella D, Slater R. Magic Cancer Bullet. 2004;Harper Business, New York:[2003]. ISBN: 0060010304. [Google Scholar]
- 34.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. [DOI] [PubMed] [Google Scholar]
- 35.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann VS, Baccarani M, Hasford J, et al. The EUTOS population-based registry: incidence and clinical characteristics of 2904 CML patients in 20 European Countries. Leukemia. 2015;29:1336–1343. [DOI] [PubMed] [Google Scholar]
- 39.Hehlmann R, Cortes JE, Zyczynski T, et al. Tyrosine kinase inhibitor interruptions, discontinuations and switching in patients with chronic-phase chronic myeloid leukemia in routine clinical practice: SIMPLICITY. Am J Hematol. 2019;94:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malhotra H, Radich J, Garcia-Gonzalez P. Meeting the needs of CML patients in resource-poor countries. Hematology Am Soc Hematol. 2019;2019:433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfirrmann M, Baccarani M, Saußele S, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30:48–56. [DOI] [PubMed] [Google Scholar]
- 42.Baccarani M, Castagnetti F, Gugliotta G, et al. The proportion of different BCR-ABL1 transcript types in chronic myeloid leukemia. An international overview. Leukemia. 2019;33:1173–1183. [DOI] [PubMed] [Google Scholar]
- 43.Büsche G, Hehlmann R, Hecker H, et al. Marrow fibrosis, indicator of therapy failure in chronic myeloid leukemia–prospective long-term results from a randomized-controlled trial. Leukemia. 2003;17:2444–2453. [DOI] [PubMed] [Google Scholar]
- 44.Büsche G, Ganser A, Schlegelberger B, et al. Marrow fibrosis and its relevance during imatinib treatment of chronic myeloid leukemia. Leukemia. 2007;21:2420–2427. [DOI] [PubMed] [Google Scholar]
- 45.Hidalgo-López JE, Kanagal-Shamanna R, Quesada AE, et al. Bone marrow core biopsy in 508 consecutive patients with chronic myeloid leukemia: Assessment of potential value. Cancer. 2018;124:3849–3855. [DOI] [PubMed] [Google Scholar]
- 46.Fabarius A, Kalmanti L, Dietz CT, et al. Impact of unbalanced minor route versus major route karyotypes at diagnosis on prognosis of CML. Ann Hematol. 2015;94:2015–2024. [DOI] [PubMed] [Google Scholar]
- 47.Wang W, Cortes JE, Tang G, et al. Risk stratification of chromosomal abnormalities in chronic myelogenous leukemia in the era of tyrosine kinase inhibitor therapy. Blood. 2016;127:2742–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hehlmann R, Voskanyan A, Lauseker M, et al. High-risk additional chromosomal abnormalities at low blast counts herald death by CML. Leukemia. 2020 May 7. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 49.Do YR, Kwak JY, Kim JA, et al. Long-term data from a phase 3 study of radotinib versus imatinib in patients with newly diagnosed, chronic myeloid leukemia in the chronic phase (RERISE). Brit J Haematol. 2020;189:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasaki K, Strom SS, O’Brien S, et al. Relative survival in patients with chronic-phase chronic myeloid leukemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. Lancet Haematol. 2015;2:e186–e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hehlmann R, Lauseker M, Saußele S, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31:2398–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thielen N, Visser O, Ossenkoppele G, et al. Chronic myeloid leukemia in the Netherlands: a population-based study on incidence, treatment, and survival in 3585 patients from 1989 to 2012. European J Haematol. 2016;97:145–154. [DOI] [PubMed] [Google Scholar]
- 53.Bower H, Björkholm M, Dickman PW, et al. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34:2851–2857. [DOI] [PubMed] [Google Scholar]
- 54.Welch HG, Kramer BS, Black WC. Epidemiologic signatures in cancer. N Engl J Med. 2019;381:1378–1386. [DOI] [PubMed] [Google Scholar]
- 55.Kalmanti L, Saußele S, Lauseker M, et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. Leukemia. 2015;29:1123–1132. [DOI] [PubMed] [Google Scholar]
- 56.Steegmann JL, Baccarani M, Breccia M, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukemia. Leukemia. 2016;30:1648–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hehlmann R, Müller MC, Lauseker M, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014;32:415–423. [DOI] [PubMed] [Google Scholar]
- 58.Mahon FX, Réa D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukemia who have maintained complete molecular remission for at least 2 years: the prospective, multicenter Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–1035. [DOI] [PubMed] [Google Scholar]
- 59.Saussele S, Richter J, Guilhot J, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukemia (EURO-SKI): a prespecified interim analysis of a prospective, multicenter, non-randomized, trial. Lancet Oncol. 2018;19:747–757. [DOI] [PubMed] [Google Scholar]
- 60.Malkan UY, Salih AKSU, Aktimur SH, et al. Generic imatinib mesylate is as effective as original glivec in the clinical management of CML. Int J Hematol Oncol. 2015;28:215–221. [Google Scholar]
- 61.Islamagic E, Hasic A, Kurtovic S, et al. The efficacy of generic imatinib as first-and second-line therapy: 3-year follow-up of patients with chronic myeloid leukemia. Clin Lymph Myel Leuk. 2017;17:238–240. [DOI] [PubMed] [Google Scholar]
- 62.Eskazan AE, Sadri S, Keskin D, et al. Outcomes of chronic myeloid leukemia patients with early molecular response at 3 and 6 months: a comparative analysis of generic imatinib and Glivec. Clin Lymph Myeloma Leuk. 2017;17:804–811. [DOI] [PubMed] [Google Scholar]
- 63.Padula WV, Larson RA, Dusetzina SB, et al. Cost-effectiveness of tyrosine kinase inhibitor treatment strategies for chronic myeloid leukemia in chronic phase after generic entry of imatinib in the United States. JNCI. 2016;108:djv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. [DOI] [PubMed] [Google Scholar]
- 65.Shah NP, Rousselot P, Schiffer C, et al. Dasatinib in imatinib-resistant or-intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034. Am J Hematol. 2016;91:869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giles FJ, Le Coutre PD, Pinilla-Ibarz J, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27:107–112. [DOI] [PubMed] [Google Scholar]
- 67.Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patient trial. J Clin Oncol. 2016;34:2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hughes TP, Saglio G, Larson RA, et al. Long-term outcomes in patients with chronic myeloid leukemia in chronic phase receiving frontline nilotinib versus imatinib: Enestnd 10-year analysis. Blood. 2019;134:2924–2924. [Google Scholar]
- 70.Naqvi K, Jabbour E, Skinner J, et al. Long-term follow-up of lower dose dasatinib (50 mg daily) as frontline therapy in newly diagnosed chronic-phase chronic myeloid leukemia. Cancer. 2020;126:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cortes JE, Kim DW, Pinilla-Ibarz JL, et al. A phase 2 trial of ponatinib in Philadelphia chromosome–positive leukemias. N Engl J Med. 2013;369:1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lipton JH, Chuah C, Guerci-Bresler A, et al. Ponatinib versus imatinib for newly diagnosed chronic myeloid leukemia: an international, randomized, open-label, phase 3 trial. Lancet Oncol. 2016;17:612–621. [DOI] [PubMed] [Google Scholar]
- 73.Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guilhot F. ESH 14.9.2019 Bordeaux, update to: Preudhomme, C., Guilhot, J., Nicolini, F et al (2010). Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N Engl J Med. 2010;363:2511–2521. [DOI] [PubMed] [Google Scholar]
- 75.Saußele S, Krauss MP, Hehlmann R, et al. Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: results of the randomized CML study IV. Blood. 2015;126:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760–6768. [DOI] [PubMed] [Google Scholar]
- 77.Lauseker M, Hasford J, Saussele S, et al. Smokers with chronic myeloid leukemia are at a higher risk of disease progression and premature death. Cancer. 2017;123:2467–2471. [DOI] [PubMed] [Google Scholar]
- 78.Gong Z, Medeiros LJ, Cortes JE, et al. Cytogenetics-based risk prediction of blastic transformation of chronic myeloid leukemia in the era of TKI therapy. Blood Adv. 2017;1:2541–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grossmann V, Kohlmann A, Zenger M, et al. A deep-sequencing study of chronic myeloid leukemia patients in blast crisis (BC-CML) detects mutations in 76.9% of cases. Leukemia. 2011;25:557–560. [DOI] [PubMed] [Google Scholar]
- 80.Branford S, Wang P, Yeung DT, et al. Integrative genomic analysis reveals cancer-associated mutations at diagnosis of CML in patients with high-risk disease. Blood. 2018;132:948–961. [DOI] [PubMed] [Google Scholar]
- 81.Kok CH, Yeung DT, Lu L, et al. Gene expression signature that predicts early molecular response failure in chronic-phase CML patients on frontline imatinib. Blood Adv. 2019;3:1610–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Branford S, Kim DDH, Apperley JF, et al. Laying the foundation for genomically-based risk assessment in chronic myeloid leukemia. Leukemia. 2019;33:1835–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kizilors A, Crisà E, Lea N, et al. Effect of low-level BCR-ABL1 kinase domain mutations identified by next-generation sequencing in patients with chronic myeloid leukemia: a population-based study. Lancet Haematol. 2019;6:e276–e284. [DOI] [PubMed] [Google Scholar]
- 84.Soverini S, Bavaro L, De Benedittis C, et al. Prospective assessment of NGS-detectable mutations in CML patients with nonoptimal response: the NEXT-in-CML study. Blood. 2020;135:534–541. [DOI] [PubMed] [Google Scholar]
- 85.Hehlmann R, Berger U, Pfirrmann M, et al. Drug treatment is superior to allografting as first-line therapy in chronic myeloid leukemia. Blood. 2007;109:4686–4692. [DOI] [PubMed] [Google Scholar]
- 86.Saussele S, Lauseker M, Gratwohl A, et al. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood. 2010;115:1880–1885. [DOI] [PubMed] [Google Scholar]
- 87.Gratwohl A, Pfirrmann M, Zander A, et al. Long-term outcome of patients with newly diagnosed chronic myeloid leukemia: a randomized comparison of stem cell transplantation with drug treatment. Leukemia. 2016;30:562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jain P, Kantarjian HM, Ghorab A, et al. Prognostic factors and survival outcomes in patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era: cohort study of 477 patients. Cancer. 2017;123:4391–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abruzzese E, Trawinska MM, Perrotti AP, et al. Tyrosine kinase inhibitors and pregnancy. Mediterr J Hematol Infect Dis. 2014;6:e2014038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cortes JE, Abruzzese E, Chelysheva E, et al. The impact of dasatinib on pregnancy outcomes. Am J Hematol. 2015;90:1111–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chelysheva E, Aleshin S, Polushkina E, et al. Breastfeeding in patients with chronic myeloid leukemia: case series with measurements of drug concentrations in maternal milk and literature review. Mediterranean J Hematol Infect Dis. 2018;10:e2018027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nicolini FE, Alcazer V, Huguet F, et al. CML patients show sperm alterations at diagnosis that are not improved with imatinib treatment. Leuk Res. 2016;48:80–83. [DOI] [PubMed] [Google Scholar]
- 93.Cross NC. Standardization of molecular monitoring for chronic myeloid leukemia. Best Pract Res Clin Hematol. 2009;22:355–365. [DOI] [PubMed] [Google Scholar]
- 94.Cross NCP, White HE, Müller MC, et al. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012;26:2172–2175. [DOI] [PubMed] [Google Scholar]
- 95.Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Branford S, Fletcher L, Cross NC, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008;112:3330–3338. [DOI] [PubMed] [Google Scholar]
- 97.Cross NCP, White HE, Colomer D, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015;29:999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lauseker M, Hanfstein B, Haferlach C, et al. Equivalence of BCR-ABL transcript levels with complete cytogenetic remission in patients with chronic myeloid leukemia in chronic phase. J Cancer Res Clin Oncol. 2014;140:1965–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rousselot P, Huguet F, Rea D, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109:58–60. [DOI] [PubMed] [Google Scholar]
- 100.Efficace F, Castagnetti F, Martino B, et al. Health-related quality of life in patients with chronic myeloid leukemia receiving first-line therapy with nilotinib. Cancer. 2018;124:2228–2237. [DOI] [PubMed] [Google Scholar]
- 101.Efficace F, Stagno F, Iurlo A, et al. Health-related quality of life of newly diagnosed chronic myeloid leukemia patients treated with first-line dasatinib versus imatinib therapy. Leukemia. 2020;34:488–498. [DOI] [PubMed] [Google Scholar]
- 102.Kim DW, Saussele S, Williams LA, et al. Outcomes of switching to dasatinib after imatinib-related low-grade adverse events in patients with chronic myeloid leukemia in chronic phase: the DASPERSE study. Ann Hematol. 2018;97:1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clark RE, Polydoros F, Apperley JF, et al. Initial reduction of therapy prior to complete treatment discontinuation in chronic myeloid leukemia: final results of the British DESTINY Study. Lancet Haematol. 2019;6:e375–e383. [DOI] [PubMed] [Google Scholar]
- 104.Khoury HJ, Williams LA, Atallah E, et al. Chronic myeloid leukemia: what every practitioner needs to know in 2017. Am Soc Clin Oncol Educ Book. 2017;37:468–479. [DOI] [PubMed] [Google Scholar]
- 105.Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Etienne G, Guilhot J, Rea D, et al. Long-term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35:298–305. [DOI] [PubMed] [Google Scholar]
- 107.Rousselot P, Charbonnier A, Cony-Makhoul P, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32:424–430. [DOI] [PubMed] [Google Scholar]
- 108.Richter J, Söderlund S, Lübking A, et al. Musculoskeletal pain in patients with chronic myeloid leukemia after discontinuation of imatinib: a tyrosine kinase inhibitor withdrawal syndrome. J Clin Oncol. 2014;32:2821–2823. [DOI] [PubMed] [Google Scholar]
- 109.Lee SE, Choi SY, Song HY, et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: the KID study. Haematologica. 2016;101:717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sharf G, Marin C, Bradley JA, et al. Treatment-free remission in chronic myeloid leukemia: the patient perspective and areas of unmet needs. Leukemia. 2020 May 26. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 111.Ross DM, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122:515–522. [DOI] [PubMed] [Google Scholar]
- 112.Zang DY, Lee WS, Mun YC, et al. Long-term follow-up after treatment discontinuation in patients with chronic myeloid leukemia: the Korean Imatinib Discontinuation (KID) study. Blood. 2018;132 (Supplement 1):4252–14252. [Google Scholar]
- 113.Nicolini FE, Dulucq S, Guilhot J, et al. The evaluation of residual disease by digital PCR and TKI duration are critical predictive factors for molecular recurrence after stopping imatinib first-line in chronic phase CML patients: results of the STIM2 Study. Blood. 2018;132:462.30072412 [Google Scholar]
- 114.Mori S, Vagge E, Le Coutre P, et al. Age and d PCR can predict relapse in CML patients who discontinued imatinib: The ISAV study. Am J Hematol. 2015;90:910–914. [DOI] [PubMed] [Google Scholar]
- 115.Mori S, le Coutre P, Abruzzese E, et al. Imatinib Suspension and Validation (ISAV) study: final results at 79 months. Blood. 2018;132 (Supplement 1):461–1461.30072411 [Google Scholar]
- 116.Rea D, Nicolini FE, Tulliez M, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood. 2017;129:846–854. [DOI] [PubMed] [Google Scholar]
- 117.Imagawa J, Tanaka H, Okada M, et al. Discontinuation of dasatinib in patients with chronic myeloid leukemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicenter phase 2 trial. Lancet Haematol. 2015;2:e528–e535. [DOI] [PubMed] [Google Scholar]
- 118.Kadowaki N, Kawaguchi T, Kuroda J, et al. Discontinuation of nilotinib in patients with chronic myeloid leukemia who have maintained deep molecular responses for at least 2 years: a multicenter phase 2 stop nilotinib (Nilst) trial. Blood. 2016;128:790–1790. [DOI] [PubMed] [Google Scholar]
- 119.Kim DDH, Bence-Bruckler I, Forrest DL, et al. Treatment-free remission accomplished by dasatinib (TRAD): Preliminary results of the Pan-Canadian tyrosine kinase inhibitor discontinuation trial. Blood. 2016;128:1922–11922. [Google Scholar]
- 120.Shah N, García-Gutiérrez JV, Jiménez-Velasco A, et al. Dasfree 2-year update: Dasatinib discontinuation in patients (PTS) with chronic myeloid leukemia in chronic phase (CML-CP) and deep molecular response (DMR): PF408. HemaSphere. 2019;3 (S1):156. [DOI] [PubMed] [Google Scholar]
- 121.Hughes TP, Boquimpani CM, Takahashi N, et al. Treatment-free remission in patients with chronic myeloid leukemia in chronic phase according to reasons for switching from imatinib to nilotinib: subgroup analysis from ENESTop. Blood. 2016;128:792–1792. [Google Scholar]
- 122.Takahashi N, Nishiwaki K, Nakaseko C, et al. Treatment-free remission after two-year consolidation therapy with nilotinib in patients with chronic myeloid leukemia: STAT2 trial in Japan. Haematologica. 2018;103:1835–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hochhaus A, Masszi T, Giles FJ, et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31:1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kumagai T, Nakaseko C, Nishiwaki K, et al. Discontinuation of dasatinib after deep molecular response for over 2 years in patients with chronic myelogenous leukemia and the unique profiles of lymphocyte subsets for successful discontinuation: a prospective, multicenter Japanese trial (D-STOP Trial). Blood. 2016;128:791–1791. [Google Scholar]
- 125.Hernández-Boluda JC, Pereira A, Pastor-Galán I, et al. Feasibility of treatment discontinuation in chronic myeloid leukemia in clinical practice: results from a nationwide series of 236 patients. Blood Cancer J. 2018;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Legros L, Nicolini FE, Etienne G, et al. Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer. 2017;123:4403–4410. [DOI] [PubMed] [Google Scholar]
- 127.Pemmaraju N, Kantarjian H, Shan J, et al. Analysis of outcomes in adolescents and young adults with chronic myelogenous leukemia treated with upfront tyrosine kinase inhibitor therapy. Haematologica. 2012;97:1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kalmanti L, Saussele S, Lauseker M, et al. Younger patients with chronic myeloid leukemia do well in spite of poor prognostic indicators: results from the randomized CML study IV. Ann Hematol. 2014;93:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yeung DT, Tang C, Vidovic L, et al. KIR2DL5B genotype predicts outcomes in CML patients treated with response-directed sequential imatinib/nilotinib strategy. Blood. 2015;126:2720–2723. [DOI] [PubMed] [Google Scholar]
- 130.Hughes TP, Lipton JH, Spector N, et al. Deep molecular responses achieved in patients with CML-CP who are switched to nilotinib after long-term imatinib. Blood. 2014;124:729–736. [DOI] [PubMed] [Google Scholar]
- 131.Hughes TP, Leber B, Cervantes F, et al. Sustained deep molecular responses in patients switched to nilotinib due to persistent BCR-ABL1 on imatinib: final ENESTcmr randomized trial results. Leukemia. 2017;31:2529–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ross DM, Branford S, Seymour JF, et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia. 2010;24:1719–1724. [DOI] [PubMed] [Google Scholar]
- 133.Lichtin AE, Woolf SH, Silver RT, et al. Chronic myeloid leukemia- ASH practice guideline and beyond. Hematology. 1998;435–453. [Google Scholar]
- 134.Hehlmann R. How I treat CML blast crisis. Blood. 2012;120:737–747. [DOI] [PubMed] [Google Scholar]
- 135.Hehlmann R, Saußele S, Voskanyan A, et al. Management of CML-blast crisis. Best Pract Res Clin Haematol. 2016;29:295–307. [DOI] [PubMed] [Google Scholar]
- 136.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. [DOI] [PubMed] [Google Scholar]
- 137.Radich JP, Deininger M, Abboud CN. NCCN Clinical practice guidelines in oncology. Chronic myeloid leukemia version. J Natl Compr Canc Netw. 2018;16:1108–1135. [DOI] [PubMed] [Google Scholar]
- 138.Spiers AS. Metamorphosis of chronic granulocytic leukaemia: diagnosis, classification, and management. Br J Haematol. 1979;41:1–7. [DOI] [PubMed] [Google Scholar]
- 139.Mitelman F, Levan G, Nilsson PG, et al. Non-random karyotypic evolution in chronic myeloid leukemia. Int J Cancer. 1976;18:24–30. [DOI] [PubMed] [Google Scholar]
- 140.Johansson B, Fioretos T, Mitelman F. Cytogenetic and molecular genetic evolution of chronic myeloid leukemia. Acta Haematol. 2002;107:76–94. [DOI] [PubMed] [Google Scholar]
- 141.Soverini S, Hochhaus A, Nicolini FE, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118:1208–1215. [DOI] [PubMed] [Google Scholar]
- 142.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Milojkovic D, Ibrahim A, Reid A, et al. Efficacy of combining dasatinib and FLAG-IDA for patients with chronic myeloid leukemia in blastic transformation. Haematologica. 2012;97:473–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Copland M, Slade D, Byrne J, et al. FLAG-IDA and ponatinib in patients with blast phase chronic myeloid leukemia: results from the phase I/II UK trials acceleration programme matchpoint trial. Blood. 2019;497. [Google Scholar]
- 145.Strati P, Kantarjian H, Thomas D, et al. HCVAD plus imatinib or dasatinib in lymphoid blastic phase chronic myeloid leukemia. Cancer. 2014;120:373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]