Abstract
Previous studies have indicated that the prevalence of gestational diabetes mellitus (GDM) was related to the season. However, there was no relevant information in Asia. The aim of this study was to determine whether there was seasonality of GDM and maternal blood glucose level in Taiwanese women.
A total of 6396 pregnancies were enrolled between 2012 and 2014 in this retrospective study. A 2-step approach according to the Carpenter-Coustan criteria was used for GDM diagnosis. A generalized linear mixed model was used to estimate the effect of season on GDM diagnosis by adjusting for age, prepregnancy body mass index, parity, history of GDM, fetal sex, and the rate of weight gain.
During the study period, 418 (6.5%) pregnancies were diagnosed as GDM. The model demonstrated an increased prevalence of GDM in spring and summer (odds ratio: 1.59, 95% confidence interval: 1.13–2.24; odds ratio: 1.59, 95% confidence interval: 1.14–2.23, respectively) compared to winter. For the glucose level variation, the model demonstrated an increase of 2.56 mg/dL glucose in the 50-g glucose challenge test in summer compared to winter. In glucose challenge test-positive pregnancies, the season also had an effect on the results of the 100-g 1-h, 2-h, and 3-h oral glucose tolerance tests, but no effect on the 100-g fasting oral glucose tolerance tests.
GDM prevalence in Taiwan presents seasonal variation, with the highest risk during spring and summer due to post-glucose load level variations. These findings could serve as reference data for countries in Southeast Asia or areas with a similar climate.
Keywords: Asia, gestational diabetes mellitus, glucose levels, season
1. Introduction
Gestational diabetes mellitus (GDM) is defined as diabetes diagnosed in the second or third trimester of pregnancy that is not clearly overt diabetes that was present prior to gestation.[1] GDM is an important medical and public health issue and is associated with adverse pregnancy outcomes. About 2.7% to 13.2% of pregnant women develop GDM during their pregnancies and the prevalence has increased considerably during the last decade.[2–4] Even the prevalence of GDM in high-risk populations reaches 25.7% in the second trimester and more than 30% in the third trimester.[5,6] If left untreated or undiscovered, perinatal morbidity, and mortality risk of mothers increase. Maternal diabetes during the pregnancy is strongly linked to adverse pregnancy outcomes, also at higher risk of subsequent development of type 2 diabetes.[7,8] There is evidence showing that exposure to maternal diabetes in utero has long-term adverse effects on the offspring, which likely occurs due to epigenetic modifications of the fetal genome, and could be averted by therapies applied early during pregnancy.[9] Therefore, it is important to realize the relevant factors associated with GDM, especially for the high-risk populations.
Differences in screening programs and diagnostic criteria may have resulted in different incidence and prevalence of GDM among various populations. Nevertheless, ethnicity has been proven to be an independent risk factor for GDM.[10] Women from Asia are a heterogeneous group by genetic background, culture, and lifestyle, such as diet. Previous studies showed that Southeast Asian women were more likely to have GDM, even with a lower (BMI).[2,11–14] From a pregnancy-outcome point of view, Southeast Asian women have lower rates of macrosomia compared with non-Hispanic White women, but preterm delivery with preeclampsia occurs more often compared with Japanese, and non-Hispanic White women.[15] Additionally, Southeast Asian women with GDM have a higher risk of developing type 2 diabetes compared with Caucasians.[16] A recent review reported a pooled GDM prevalence of 10% in Eastern and Southeast Asia. However, a difference in GDM prevalence can still be observed across countries.[17] Therefore, establishing appropriate diagnostic criteria of GDM and proper management strategies for Asian women with GDM is necessary.
Apart from the well-known risk factors, other parameters are also discussed in the literature, such as Vitamin D and temperature. A few interventional studies indicated that supplementation optimizes maternal vitamin D status or improves maternal glucose metabolism.[18,19] Vitamin D is the product of sun rays, which might explain the relationship between high temperature and GDM. However, observational studies about the association between maternal vitamin D status and risk of GDM are conflicting.[20,21] In the 1990s, Schmidt et al reported that ambient temperature may affect venous glucose concentrations after glucose tolerance tests.[22] However, subsequent research has shown that GDM is not seasonal.[23,24] Until recently, studies from different countries, including Sweden, Australia, Italy, and Greece, indicated that the prevalence of GDM was related to the season, with higher risk during summer.[25–28]
The prevalence of GDM varies among races and ethnicities, and climates vary around the world. The GDM risk factors have been explored in many research reports, and season is a factor that has been proposed in recent years to be associated with GDM. However, there has been no study on the relationship between GDM and season in Asian women. Therefore, the aim of this study is to determine whether there is a seasonal variation in GDM diagnosis and maternal blood glucose levels. The hypothesis was that the prevalence of GDM is related to the season in Taiwanese women.
2. Materials and methods
2.1. Materials
This retrospective study involved collecting the laboratory data and medical records of pregnant women without overt diabetes who underwent a 50-g 1-h glucose challenge test (GCT) at 24 to 30 weeks of gestation between January 2012 and December 2014 and delivered at the Ditmanson Medical Foundation Chia-Yi Christian Hospital (DMF-CYCH). Women with multifetal pregnancies, preexisting diabetes, and chronic/preexisting or unspecified hypertension were excluded. This study was approved by the Institutional Review Board of the DMF-CYCH (CYCH IRB No: 100006). We collected data from medical records; thus, the committee agreed that the informed consent of each participant was not necessary. Plasma glucose levels were measured using a Hitachi 7170 automatic analyzer (Hitachi Co., Tokyo, Japan) at the DMF-CYCH central laboratory according to a standard clinical protocol.
2.2. GDM diagnosis
The included women underwent the 2-step approach for GDM screening. The 50-g 1-h GCT was considered positive if the screening value was ≥ 140 mg/dL. If the GCT was positive, the women were referred for a 100-g 3-h oral glucose tolerance test (OGTT) to confirm whether they had GDM. Women were diagnosed with GDM when 2 or more plasma glucose level results exceeded or equaled 95, 180, 155, or 140 mg/dL in the fasting, 1-, 2- or 3-h plasma glucose tests, respectively (the Carpenter-Coustan criteria).[29]
2.3. Statistical analyses
Normality was assessed by using the Shapiro–Wilk test. The differences between the season groups and the characteristics of the pregnancies were analyzed using Kruskal–Wallis tests for the continuous variables because of nonnormal distributions and are presented as medians (interquartile ranges). The GDM rate between the season groups and the characteristics of the pregnancies were analyzed using Wilcoxon rank sum tests for the continuous variables because of nonnormal distributions and are presented as medians (interquartile ranges). Continuous variables with normal distributions were compared by Student t test and are presented as means ± standard deviations. The Chi-squared test was used for the categorical variables, which are presented as counts (percentages). Pregnant women with more than 1 pregnancy during the study period were not excluded. Therefore, we applied generalized linear mixed models to estimate the effects of different seasons on GDM prevalence and the glucose levels by adjusting for maternal age at GCT, prepregnancy BMI, parity, history of GDM,[10,30–32] fetal sex,[33] and the rate of weight gain from prepregnancy to GDM screening (50-g GCT).[34–36] In the mixed models, all covariates were included as fixed effects. We considered correlation of data from the same individual as a random effect that incorporated an unstructured variance-covariance matrix. The associations were described in terms of adjusted odds ratios (ORs) with 95% confidence intervals (CIs) for binary outcomes and estimates with 95% CIs for continuous glucose values. We managed to consider “Maternal age at GCT” and “Prepregnancy BMI” as continuous variables or categorical variables in different models in analyzing the relationship between season and GDM. The months of March to May were defined as spring; June to August were defined as summer; September to November were defined as autumn; and December to February were defined as winter. BMI was calculated as the ratio of body weight (in kilograms) divided by the square of height (in meters). According to the Bureau of Health Promotion, Department of Health, Taiwan, a BMI of <18.5 kg/m2 denotes underweight, 18.5 to <24 kg/m2 normal weight, 24 to <27 kg/m2 overweight, and ≥27 kg/m2 obesity (https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=542&pid=9734). The rate of weight gain was calculated by dividing weight gain from prepregnancy to GDM screening by the corresponding number of weeks. The average monthly temperature during the study period was obtained from the nearby fixed-site Chia-Yi monitoring station, operated by the Taiwan Environmental Protection Administration, which measured the weather data hourly throughout Taiwan (https://taqm.epa.gov.tw/taqm/tw/default.aspx). We calculated the daily average temperature and matched these data with the women's screening dates. A 2-sided P value < .05 was considered statistically significant. All data were merged and analyzed using SAS 9.4 (SAS Institute, Cary, NC).
3. Results
A total of 6809 pregnancies occurred in patients without overt diabetes who underwent a 50-g 1-h GCT at 24 to 30 weeks of gestation between January 2012 and December 2014 and delivered at the DMF-CYCH; 287 pregnancies in patients with multifetal pregnancies, preexisting diabetes, or chronic/preexisting or unspecified hypertension were excluded. A total of 126 subjects did not complete the OGTTs and were excluded. Finally, 5923 women with 6396 pregnancies were enrolled in the study. Among them, 468 (7.9%) women had multigravida during the study period, and the pregnancies of these women were analyzed separately. During this period, 418 (6.5%) pregnancies were associated with GDM (Fig. 1).
Figure 1.
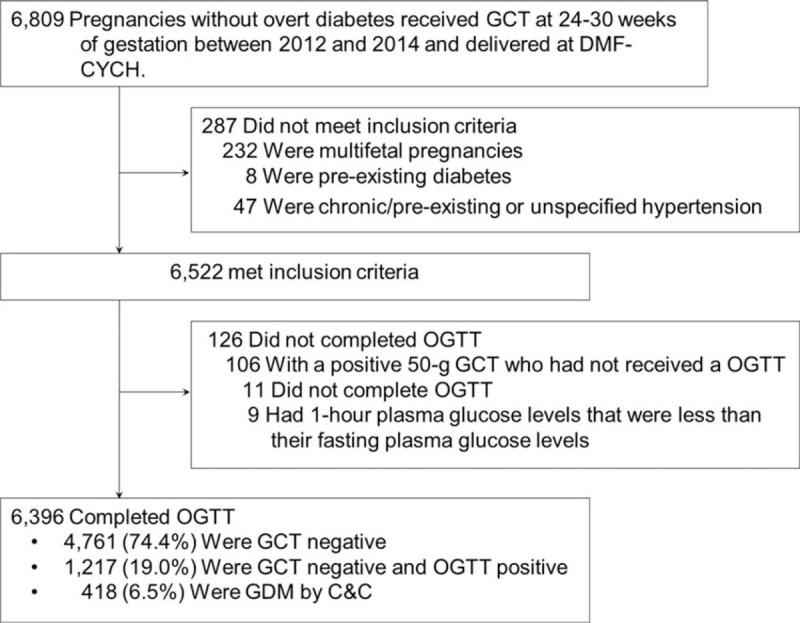
Screening and enrollment of the pregnant women included in the study. C-C = Carpenter-Coustan, GCT = glucose challenge test, GDM = glucose diabetes mellitus, OGTT = oral glucose tolerance test.
Table 1 shows the associations between the season and the characteristics of the pregnancies. The pregnancies screened during the winter and spring were associated with an increased rate of weight gain (P < .001). There were no significant correlations between the season and the other characteristics.
Table 1.
The association between season and the characteristics of the pregnancies.
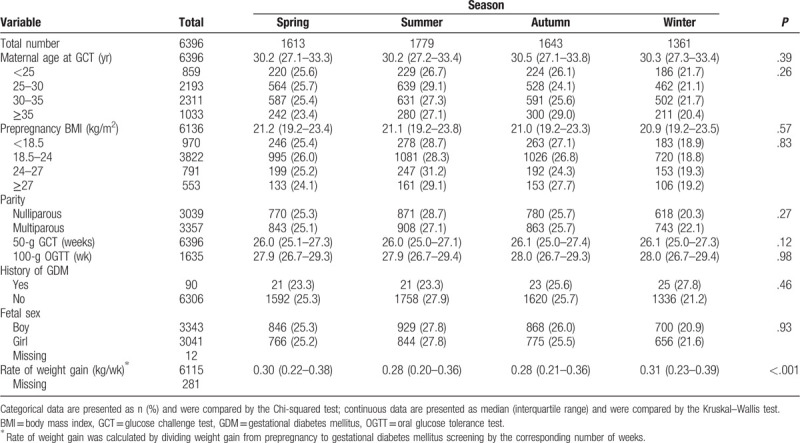
Table 2 shows the characteristics of the GDM and non-GDM groups. In univariate analysis, the overall GDM prevalence was 6.5%. The highest rate of GDM occurred in summer, and the lowest rate occurred in winter (7.4% vs 4.8%, P = .02). Women with increasing age, increasing prepregnancy BMI, or a history of GDM all had a significant higher rate of being diagnosed with GDM (all P < .001). Multiparous women also were more likely to develop GDM (7.3% vs 5.7%, P = .01). A male fetus tended to cause GDM more often than female (7.1% vs 6.0%, P = .07). The rate of weight gain had no difference between GDM and non-GDM pregnancies, both having a median of 0.29 kg/wk. In multivariate analysis, after adjusting for maternal age (continuous), BMI (continuous), parity, history of GDM, fetal sex, and rate of weight gain, the model demonstrated an increased prevalence of pregnancies associated with GDM in spring and summer (OR: 1.59, 95% CI: 1.13–2.24; OR: 1.59, 95% CI: 1.14–2.23, respectively) compared to winter in model 1. In model 2, when maternal age and prepregnancy BMI were classified as categorical variables, the relationship between GDM, and season was stronger. The rate of weight gain carried a significant risk for GDM; a weight gain increase of 1 kg/wk made a GDM diagnosis 2.97 and 2.57 more likely in model 1 and model 2, respectively.
Table 2.
The correlates of gestational diabetes mellitus in univariate and multivariate analysis.
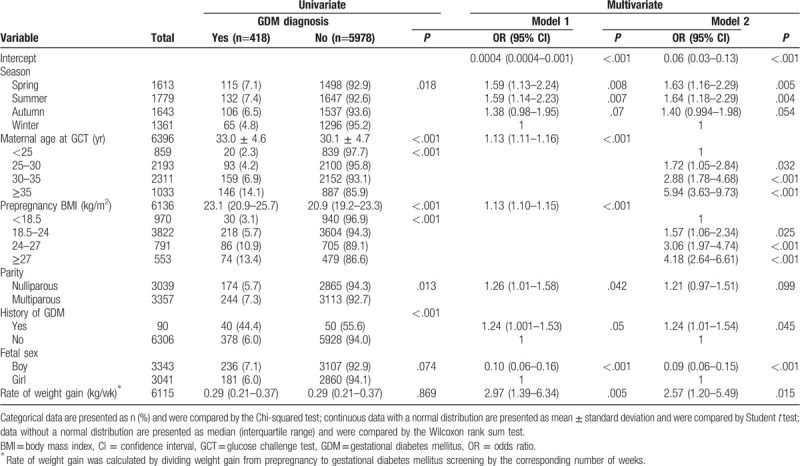
Figure 2 shows the trend of GDM rate and temperature by month. As the temperature rose and as the standard deviation of temperature grew smaller, the GDM rate was higher. In contrast, as the temperature got lower and the standard deviation rose, the GDM rate was lower. The highest prevalence of GDM was noted in August (8.1%), and the lowest one was in February (4.7%).
Figure 2.
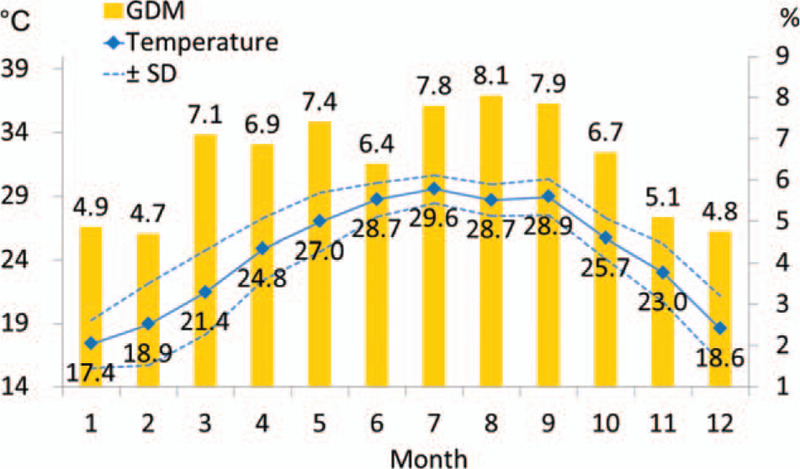
The trend of gestational diabetes mellitus rate and temperature by month. GDM = gestational diabetes mellitus, SD = standard deviation.
Table 3 shows the abnormal glucose rates according to season. The mean ± standard deviation of temperature in summer was 29.0 ± 1.3°C, and in winter it was 18.3 ± 2.7°C. The highest positive rate of 50-g GCT occurred in summer, and the lowest rate occurred in winter (27.3% vs 23.0%, P = .06). After adjusting for confounding factors, the model demonstrated an increased prevalence of pregnancies associated with abnormal 50-g GCT in spring and summer (OR: 1.22, 95% CI: 1.02–1.47; OR: 1.27, 95% CI: 1.06–1.53, respectively) compared to winter. In GCT-positive pregnancies, the highest abnormal rates and adjusted ORs of the 100-g 1-h, 2-h and 3-h OGTTs occurred in spring (27.4%, P = .08; OR: 1.55, 95% CI: 1.04–2.31), spring (32.8%, P = .07; OR: 1.70, 95% CI: 1.170–2.48), and autumn (19.2%, P = .01; OR: 2.00, 95% CI: 1.23–3.24 ), respectively, but the results of the 100-g fasting OGTT showed no difference according to season (P = .98).
Table 3.
Abnormal glucose rates and estimated odds ratios according to season.
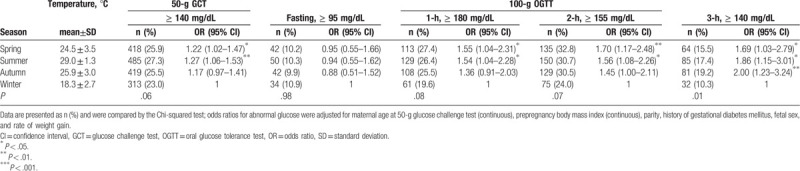
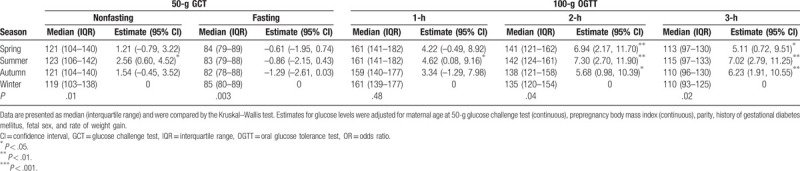
Table 4 shows the association between season and oral glucose level. Compared to other seasons, winter had a lower median glucose in the 50-g GCT (P = .01), 100-g OGTT at 2 h (P = .04), and 100-g OGTT at 3 h (P = .02) but a higher median in the 100-g OGTT at fasting (P = .003) than other seasons. After adjusting for confounding factors, the model demonstrated an increase of 2.56 mg/dL glucose in the 50-g GCT in summer compared to winter. In GCT-positive pregnancies, the season had no effect on the results of the 100-g fasting OGTT, but it did have an effect on the results of the 100-g 1-h, 2-h, and 3-h OGTTs.
Table 4.
The glucose levels and estimates according to season.
4. Discussion
During the study period, 6.5% of the pregnancies were associated with a diagnosis of GDM. In this retrospective study, we observed clear seasonality in the prevalence of GDM by using a 2-step approach for GDM diagnosis: an increased GDM prevalence occurred among pregnancies in spring and summer compared to winter due to post-glucose load level variations after adjusting for maternal age, BMI, parity, history of GDM, fetal sex, and rate of weight gain. The GDM prevalence peak occurred in spring and summer, and the lowest prevalence was during winter. The degree of variation in the glucose level in the 50-g GCT in summer was significantly higher than in winter. In GCT-positive pregnancies (either abnormal glucose rates or glucose levels), the season had an effect on the results of the 100-g 1-h, 2-h, and 3-h OGTTs, but it did not have effect on the results of the 100-g fasting OGTT.
Advanced maternal age is an independent risk factor for GDM.[37] Our study has the concordant result. After controlling for other risk factors (such as BMI and parity), women older than 35 years old had 6-time odds ratio to be diagnosed with GDM compared with those who were less than 25 years old. Recently, a meta-analysis demonstrates that the risk of GDM increases linearly with successive age-groups.[38] Subgroup analyses also indicated that from the age of 25, Asian women had a significantly higher risk of developing GDM than Europid women. On the other hand, the average age of childbearing over the past 4 decades has been increasing.[39] This may be the reason for the incidence of GDM increased year by year.[2–4] Therefore, it is important to realize the relevant factors associated with GDM, especially for the high-risk populations.
Recent studies have indicated that the prevalence of GDM is related to the season[25–27] and that GDM prevalence increases during the summer compared to winter. The diagnosis of GDM in this study was in line with those results. A large multicenter study including 11,538 pregnant women in southern Sweden reported that summer was associated with an increased frequency of GDM compared to all other months (OR 1.51, 95% CI 1.24–1.83) and that there was seasonal variation in the 2-h glucose concentration in the OGTT (P < .001); the fasting glucose levels were unaffected by temperature.[26] In the same year, another study in a coastal city of Australia found that the prevalence of GDM according to the 1-h or 2-h diagnostic test was higher in summer and lower in winter than the overall prevalence.[25] Recently, Chiefari et al[27] found that the prevalence of GDM, according to the Italian Health Ministry guidelines, in Italian patients was also significantly higher in summer than in spring, autumn and winter. Regarding glucose levels, no differences were observed between the median fasting glycemia values among the 4 seasons; rather, serum glucose levels according to the 1-h and 2-h OGTTs in summer were higher than those in other seasons.[27] These results indicated that seasonal variation affected the GDM diagnosis rate due to post-glucose load level variation and not to the fasting OGTT measurement. This study had similar findings on the post-glucose load data in the 50-g 1-h GCT and 100-g 1-h, 2-h, and 3-h OGTT.
A 50-g 1-h GCT is the first step in the 2-step GDM diagnosis approach. Only 1 previous study conducted in Israel has investigated the seasonality of GCT results.[15] Wainstock et al found that the GCT results in winter were independently associated with the lowest risk of pathological GCT values compared to all other seasons. In our study, winter was also associated with the lowest median GCT value and lowest risk for abnormal rates of GCT. Taiwan is in a subtropical region, where the temperature varies less between the 4 seasons than in Israel, with a Mediterranean climate, but there is still seasonal variation (Fig. 2), and the diagnostic GCT-positive rates in spring and summer were significantly higher than that in winter (ORs: 1.22 and 1.27, respectively). Considering only the glucose level, a GCT during summer had significantly different effect compared to winter.
Taiwan is an island on the border of 2 climatic zones. The northern part of the island has a subtropical climate with wet summers and dry, cool winters. The southern part has a monsoon tropical climate, which experiences an extraordinarily rainy wet season (summer) and pronounced dry season (winter). Our study was conducted in the monsoon climate in southern Taiwan, where the average monthly temperature is higher (17.4°C to 29.6°C, see Fig. 2) than that of other countries where similar research topics have been studied (e.g., UK,[24] Australia,[25] Sweden,[26] Greece).[28] In our study area, for every degree increase in temperature, the likelihood of a diagnosis of GDM increased by 3% (OR 1.03, 95% CI 1.01–1.06). To further explore the reasons for the seasonal variation in GDM, we further conducted a stratified analysis to investigate the correlation between temperature changes and GDM. After adjusting for other factors, temperatures within seasons had no influence on GDM. We speculate that the temperature variation within seasons is too small to affect GDM. Although the temperature is higher and does not vary much within a year in the study area, there is still the phenomenon of seasonality. And we still found that GDM was affected by the season after controlling for the confounding factors. Therefore, season may be considered one of the parameters of GDM diagnosis in Taiwanese women.
We further explored the mechanism of GDM seasonality. The effect of season on the diagnosis rate of GDM was due to post-glucose load level variation.[25–27] In 1997, the effects of changes in temperature on 1-h and 2-h OGTT results were investigated in healthy males in a climate-controlled chamber.[40] The 2-h glucose result increased nonlinearly with each 5°C increase, and the rise was most apparent between 25°C and 30°C. A recent report also found a significantly higher glucose level in the heat (43°C) compared with both cold (7.2°C) and neutral (22°C) environments from the 15- to the 120-minute time point (P < .002).[41] The pancreas anticipates the increase in insulin resistance and thus elevated glucose that occur late in pregnancy and therefore increases β-cell number and function early in pregnancy.[42] A recent study conducted by Retnakaran et al, in pregnant women, showed that a rising mean daily temperature in the 3 to 4 weeks prior to the OGTT may have an adverse effect on maternal β-cell function and thereby increase the risk of GDM.[43] Another possible mechanism might be the activation of brown adipose tissue upon cold exposure. Orava et al reported that cold exposure resulted in a 12-fold increase in glucose disposal in brown adipose tissue.[44] In addition, its activation increases whole-body glucose disposal and insulin sensitivity in humans.[45]
A strength of this study is that it is the first to investigate the effect of seasonality on the diagnosis of GDM in a large clinical sample of Asian women. These findings could serve as reference data for countries in Southeast Asia or areas with a similar climate. Some mothers had more than 1 pregnancy during the study period; therefore, we used generalized linear mixed models to address the repeated gravidity data and adjust for risk factors of GDM to retain complete gravidity information and increase the precision of the analysis.
A limitation of this study was that we used only the variables that had been recorded in the hospital information system, so we lacked information on some GDM risks, such as diet and physical activity, that might change according to the weather; thus, these risks were not evaluated. Notably, seasonal dietary changes were noted in a meta-analysis of the literature.[46] The winter or the postharvest season is associated with increased energy intake. In the winter in Taiwan, because of the Chinese New Year, individuals consume more food and engage in less physical activity than in other seasons. Our research bears out these trends. The pregnant patients who underwent GDM screening during winter presented a faster rate of weight gain than those who underwent screening in other seasons; however, they had a lower prevalence of GDM, and the model was adjusted for this factor. Second, to further explore the reasons for the seasonal variation of GDM, we conducted a stratified analysis to investigate the correlation between temperature variation and GDM. The results did not show a significant correlation between temperature and GDM within a season. Temperature seems a part of the explanation, but not all. To determine whether this was because stratified analysis reduced the number of study samples or there was interference from seasonal-related factors, further research is needed.
5. Conclusions
Asian women were more likely to have GDM. GDM prevalence in Taiwan presents seasonal variation, with increased risk during spring and summer due to post-glucose load level variations. These findings could serve as reference data for countries that still use the 2-step approach for GDM screening and can also serve as a reference for countries in Southeast Asia or areas with a similar climate. Season may be considered as one of the factors that poses influence of GDM diagnosis in Southeast Asian women, especially for the women who were at high risk of GDM. More research is warranted to determine the seasonality of GDM in the general population from the other parts of Asia.
Author contributions
Conceptualization: Panchalli Wang, Chung-Shing Wu, Chun-Pai Yang, Mei-Chun Lu.
Data curation: Panchalli Wang, Mei-Chun Lu.
Formal analysis: Chung-Yi Li, Mei-Chun Lu.
Methodology: Mei-Chun Lu.
Resources: Panchalli Wang, Chung-Shing Wu.
Software: Chung-Yi Li, Mei-Chun Lu.
Supervision: Mei-Chun Lu.
Writing – original draft: Panchalli Wang, Chung-Shing Wu, Mei-Chun Lu.
Writing – review & editing: Panchalli Wang, Chung-Shing Wu, Chung-Yi Li, Chun-Pai Yang, Mei-Chun Lu.
Glossary
Abbreviations: BMI = body mass index, CI = confidence interval, DMF-CYCH = Ditmanson Medical Foundation Chia-Yi Christian Hospital, GCT = glucose challenge test, GDM = gestational diabetes mellitus, OGTT = oral glucose tolerance test, OR = odds ratio.
References
- [1].American Diabetes, Association, 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care 2018;41:S13–27. [DOI] [PubMed] [Google Scholar]
- [2].Yeung RO, Savu A, Kinniburgh B, et al. Prevalence of gestational diabetes among Chinese and South Asians: a Canadian population-based analysis. J Diabetes Complications 2017;31:529–36. [DOI] [PubMed] [Google Scholar]
- [3].Melchior H, Kurch-Bek D, Mund M. The prevalence of gestational diabetes. Dtsch Arztebl Int 2017;114:412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Feig DS, Hwee J, Shah BR, et al. Trends in incidence of diabetes in pregnancy and serious perinatal outcomes: a large, population-based study in Ontario, Canada, 1996-2010. Diabetes Care 2014;37:1590–6. [DOI] [PubMed] [Google Scholar]
- [5].Perovic M, Gojnic M, Arsic B, et al. Relationship between mid-trimester ultrasound fetal liver length measurements and gestational diabetes mellitus. J Diabetes 2015;7:497–505. [DOI] [PubMed] [Google Scholar]
- [6].Perović M, Garalejić E, Gojnić M, et al. Sensitivity and specificity of ultrasonography as a screening tool for gestational diabetes mellitus. J Matern Fetal Neonatal Med 2012;25:1348–53. [DOI] [PubMed] [Google Scholar]
- [7].Bellamy L, Casas JP, Hingorani AD, et al. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773–9. [DOI] [PubMed] [Google Scholar]
- [8].Noussitou P, Monbaron D, Vial Y, et al. Gestational diabetes mellitus and the risk of metabolic syndrome: a population-based study in Lausanne, Switzerland. Diabetes Metab 2005;31:361–9. [DOI] [PubMed] [Google Scholar]
- [9].Monteiro LJ, Norman JE, Rice GE, et al. Fetal programming and gestational diabetes mellitus. Placenta 2016;48: (Suppl 1): S54–60. [DOI] [PubMed] [Google Scholar]
- [10].Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med 2004;21:103–13. [DOI] [PubMed] [Google Scholar]
- [11].Gunton JE, Hitchman R, McElduff A. Effects of ethnicity on glucose tolerance, insulin resistance and beta cell function in 223 women with an abnormal glucose challenge test during pregnancy. Aust N Z J Obstet Gynaecol 2001;41:182–6. [DOI] [PubMed] [Google Scholar]
- [12].Hunsberger M, Rosenberg KD, Donatelle RJ. Racial/ethnic disparities in gestational diabetes mellitus: findings from a population-based survey. Womens Health Issues 2010;20:323–8. [DOI] [PubMed] [Google Scholar]
- [13].Chu SY, Abe K, Hall LR, et al. Gestational diabetes mellitus: all Asians are not alike. Prev Med 2009;49:265–8. [DOI] [PubMed] [Google Scholar]
- [14].Yuen L, Wong VW. Gestational diabetes mellitus: challenges for different ethnic groups. World J Diabetes 2015;6:1024–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wainstock T, Yoles I. Pregnant women may be sweeter in the summer: seasonal changes in glucose challenge tests results. A population-based study. Diabetes Res Clin Pract 2019;147:134–7. [DOI] [PubMed] [Google Scholar]
- [16].Lee AJ, Hiscock RJ, Wein P, et al. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes: a retrospective cohort study using survival analysis. Diabetes Care 2007;30:878–83. [DOI] [PubMed] [Google Scholar]
- [17].Nguyen CL, Pham NM, Binns CW, et al. Prevalence of gestational diabetes mellitus in eastern and Southeastern Asia: a systematic review and meta-analysis. J Diabetes Res 2018;2018:6536974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yin WJ, Tao RX, Hu HL, et al. The association of vitamin D status and supplementation during pregnancy with gestational diabetes mellitus: a Chinese prospective birth cohort study. Am J Clin Nutr 2020;111:122–30. [DOI] [PubMed] [Google Scholar]
- [19].Benaim C, Cocate PG, de Barros EG, et al. Longitudinal association of 25-hydroxyvitamin D with adipokines and markers of glucose metabolism among Brazilian pregnant women. Br J Nutr 2019;121:42–54. [DOI] [PubMed] [Google Scholar]
- [20].Joergensen JS, Lamont RF, Torloni MR. Vitamin D and gestational diabetes: an update. Curr Opin Clin Nutr Metab Care 2014;17:360–7. [DOI] [PubMed] [Google Scholar]
- [21].Zhao Y, Teng Y, Wang J, et al. Effects of vitamin D supplementation in early pregnancy on high-risk groups of gestational diabetes mellitus. Wei Sheng Yan Jiu 2019;48:226–31. [PubMed] [Google Scholar]
- [22].Schmidt MI, Matos MC, Branchtein L, et al. Variation in glucose tolerance with ambient temperature. Lancet 1994;344:1054–5. [DOI] [PubMed] [Google Scholar]
- [23].Moses R, Griffiths R. Is there a seasonal variation in the incidence of gestational diabetes? Diabet Med 1995;12:563–5. [DOI] [PubMed] [Google Scholar]
- [24].Janghorbani M, Stenhouse E, Jones RB, et al. Gestational diabetes mellitus in Plymouth, U.K.: prevalence, seasonal variation and associated factors. J Reprod Med 2006;51:128–34. [PubMed] [Google Scholar]
- [25].Moses RG, Wong VC, Lambert K, et al. Seasonal changes in the prevalence of gestational diabetes mellitus. Diabetes Care 2016;39:1218–21. [DOI] [PubMed] [Google Scholar]
- [26].Katsarou A, Claesson R, Ignell C, et al. Seasonal pattern in the diagnosis of gestational diabetes mellitus in southern Sweden. J Diabetes Res 2016;2016:8905474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chiefari E, Pastore I, Puccio L, et al. Impact of seasonality on gestational diabetes mellitus. Endocr Metab Immune Disord Drug Targets 2017;17:246–52. [DOI] [PubMed] [Google Scholar]
- [28].Vasileiou V, Kyratzoglou E, Paschou SA, et al. The impact of environmental temperature on the diagnosis of gestational diabetes mellitus. Eur J Endocrinol 2018;178:209–14. [DOI] [PubMed] [Google Scholar]
- [29].Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 1982;144:768–73. [DOI] [PubMed] [Google Scholar]
- [30].Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr 2011;94:1975s–9s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cypryk K, Pertynska-Marczewska M, Szymczak W, et al. Overweight and obesity as common risk factors for gestational diabetes mellitus (GDM), perinatal macrosomy in offspring and type-2 diabetes in mothers. Przegl Lek 2005;62:38–41. [PubMed] [Google Scholar]
- [32].Solomon CG, Willett WC, Carey VJ, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 1997;278:1078–83. [PubMed] [Google Scholar]
- [33].Retnakaran R, Kramer CK, Ye C, et al. Fetal sex and maternal risk of gestational diabetes mellitus: the impact of having a boy. Diabetes Care 2015;38:844–51. [DOI] [PubMed] [Google Scholar]
- [34].Zhong C, Li X, Chen R, et al. Greater early and mid-pregnancy gestational weight gain are associated with increased risk of gestational diabetes mellitus: a prospective cohort study. Clin Nutr ESPEN 2017;22:48–53. [DOI] [PubMed] [Google Scholar]
- [35].Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol 2010;115:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cho EH, Hur J, Lee KJ. Early gestational weight gain rate and adverse pregnancy outcomes in Korean Women. PLoS One 2015;10:e0140376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Marozio L, Picardo E, Filippini C, et al. Maternal age over 40 years and pregnancy outcome: a hospital-based survey. J Matern Fetal Neonatal Med 2019;32:1602–8. [DOI] [PubMed] [Google Scholar]
- [38].Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract 2020;162:108044. [DOI] [PubMed] [Google Scholar]
- [39].Matthews TJ, Hamilton BE. First births to older women continue to rise. NCHS Data Brief 2014;152:1–8. [PubMed] [Google Scholar]
- [40].Moses RG, Patterson MJ, Regan JM, et al. A non-linear effect of ambient temperature on apparent glucose tolerance. Diabetes Res Clin Pract 1997;36:35–40. [DOI] [PubMed] [Google Scholar]
- [41].Dumke CL, Slivka DR, Cuddy JS, et al. The effect of environmental temperature on glucose and insulin after an oral glucose tolerance test in healthy young men. Wilderness Environ Med 2015;26:335–42. [DOI] [PubMed] [Google Scholar]
- [42].Baeyens L, Hindi S, Sorenson RL, et al. β-Cell adaptation in pregnancy. Diabetes Obes Metab 2016;18: (Suppl 1): 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Retnakaran R, Ye C, Kramer CK, et al. Impact of daily incremental change in environmental temperature on beta cell function and the risk of gestational diabetes in pregnant women. Diabetologia 2018;61:2633–42. [DOI] [PubMed] [Google Scholar]
- [44].Orava J, Nuutila P, Lidell ME, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 2011;14:272–9. [DOI] [PubMed] [Google Scholar]
- [45].Chondronikola M, Volpi E, Børsheim E, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014;63:4089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Stelmach-Mardas M, Kleiser C, Uzhova I, et al. Seasonality of food groups and total energy intake: a systematic review and meta-analysis. Eur J Clin Nutr 2016;70:700–8. [DOI] [PubMed] [Google Scholar]


