Summary:
Hyperbaric oxygen therapy (HBOT) serves as “primary” or “adjunctive” therapy in a wide range of pathologies. It is considered the mainstay of management for potentially life-threatening conditions such as carbon monoxide poisoning, decompression illness, and gas embolisms. Moreover, HBOT has been utilized for decades as an adjunctive therapy in a variety of medical disciplines, including chronic wounds, which affect approximately 6.5 million Americans annually. In general, chronic wounds are characterized by hypoxia, impaired angiogenesis, and prolonged inflammation, all of which may theoretically be ameliorated by HBOT. Nonetheless, the cellular, biochemical, and physiological mechanisms by which HBOT achieves beneficial results in chronic wounds are not fully understood, and there remains significant skepticism regarding its efficacy. This review article provides a comprehensive overview of HBOT, and discusses its history, mechanisms of action, and its implications in management of chronic wounds. In particular, we discuss the current evidence regarding the use of HBOT in diabetic foot ulcers, while digging deeply into the roots of controversy surrounding its efficacy. We discuss how the paucity of high-quality research is a tremendous challenge, and offer future direction to address existing obstacles.
Hyperbaric oxygen therapy (HBOT) serves as a “primary” or “adjunctive” therapy in a wide range of pathologies. It is considered the mainstay of management for potentially life-threatening conditions such as carbon monoxide poisoning, decompression illness, and gas embolisms.1–3 Additionally, HBOT has been utilized for decades as an adjunctive therapy in a variety of medical disciplines, including chronic wounds.4–9 A 2017 report by Kaiser Health News estimated that nearly 1,300 hospitals in the United States have installed hyperbaric facilities.10
Chronic cutaneous wounds are defined as “wounds that have failed to proceed through an orderly and timely series of events to produce a durable structural and functional closure.”11 Major etiologies that exhibit such wounds include diabetes, pressure, venous insufficiency, and peripheral arterial disease. Chronic wounds pose a significant burden of disease, affecting approximately 6.5 million Americans, with the care costs in the United States alone exceeding $50 billion annually.12 Those afflicted experience decreased quality-of-life, pain, restricted mobility, loss of limb, and even loss of life. The incidence of chronic wounds is on the rise due to an increasing elderly population and growing prevalence of obesity and diabetes.
In general, chronic wounds are characterized by hypoxia, impaired angiogenesis, and prolonged inflammation, all of which may theoretically be ameliorated by HBOT (Fig. 1). Nonetheless, the cellular, biochemical, and physiological mechanisms by which HBOT achieves beneficial results in chronic wounds are not fully understood, and there remains skepticism regarding its efficacy. This review provides a comprehensive overview of HBOT and discusses the developmental history of HBOT, its mechanisms of action, and recent findings regarding its efficacy as a treatment option for chronic wounds. This article digs deep into the roots of controversy surrounding the effectiveness of this treatment modality and offers future directions to address existing challenges.
Fig. 1.
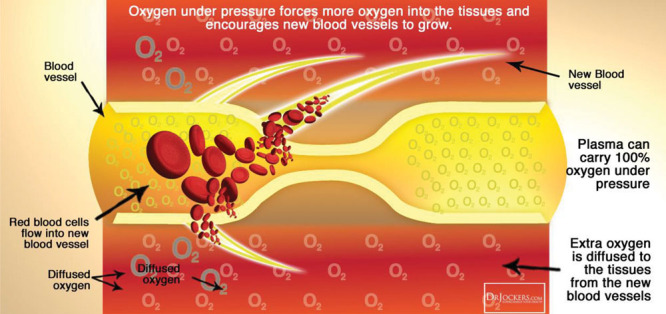
Pathology of chronic wounds. Chronic wounds are characterized by hypoxia, impaired angiogenesis, and prolonged inflammation.
METHODS
The data outlined in this article have been extracted from Systematic Reviews published in English from January 1, 2000 to July 1, 2019, extracted from The National Library of Medicine’s MEDLINE database, using the search terms “Hyperbaric,” “Hyperbaric Oxygen,” “Hyperbaric Oxygen Therapy,” and “Chronic Wound.”
Historical Notes
HBOT is not a novel concept, as the first reports of its use date back to 1662 when the British physician Henshaw first utilized compressed air for hyperbaric therapy in a chamber called a “Domicilium” (Fig. 2).13 In 1789, toxic effects of oxygen were first reported, thereby increasing a reluctance to use HBOT.13
Fig. 2.
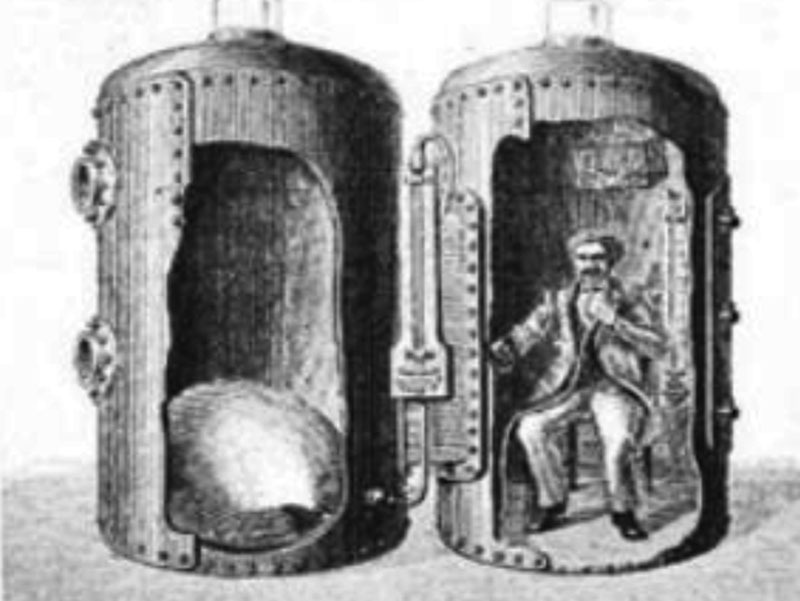
1662: Henshaw’s Domicilium.
A wide-spread use of HBOT was not adopted until the 20th century. In 1928, a Kansas City physician, Cunningham, built a large hyperbaric chamber spanning 5 stories, which was capable of accommodating up to 40 patients at a time (Fig. 3).13 Ite Boerema, recognized as the father of modern hyperbaric medicine, published the first clinical paper on HBOT in 1956 at the University of Amsterdam, describing the intraoperative use of hyperbaric oxygen to prolong safe operating times during cardiac surgery (Fig. 4). Boerema later reported on HBOT’s beneficial effects as a treatment for necrotizing infections and ischemic leg ulcers.14
Fig. 3.

1928: Cunningham’s steel ball hospital.
Fig. 4.
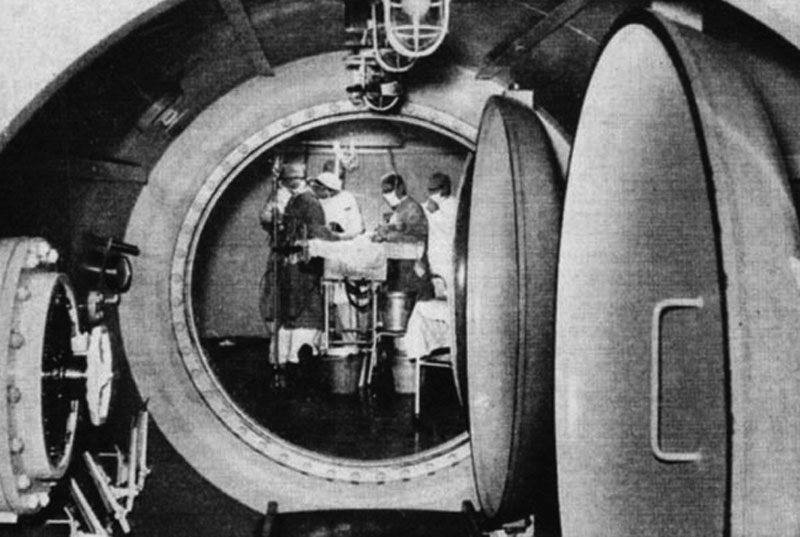
Ite Boerema operating in pure oxygen.
Kulonen first reported use of HBOT in chronic wounds in 1968. As research has begun to elucidate the oxygen-dependent cellular processes involved with tissue repair, such as collagen production by fibroblasts and the microbicidal activity of macrophages, the utilization of HBOT in the treatment of chronic wounds has become commonplace. This was followed by the decision by the Centers for Medicare & Medicaid Services to initiate reimbursement for HBOT for the treatment of diabetic foot ulcer (DFU) in 2002.
Overview and Description of the Technology
HBOT entails full body exposure and breathing of 100% oxygen while inside a hyperbaric chamber pressurized to greater than sea level (“sea level” is defined as 1 atmosphere absolute [ATA]).15,16 Typically, treatments involve pressurization to between 2.0 and 2.5 ATA, which would be equivalent to ~250 kPa/inch2, approximately the pressure at a depth of ~15 m of water. Treatment duration varies from 45 to 300 minutes depending upon the indication for which HBOT has been prescribed, with most treatment sessions lasting from 90 to 120 minutes.17 Therapy for acute indications may require only 1 or 2 treatment sessions, whereas chronic medical conditions may warrant up to 30 or more treatment sessions. Patients may receive up to 3 treatment sessions per day depending on the medical indication. Chambers are either single-occupant (mono-place) or multiple-occupant (multi-place).18
Mechanisms of Action
Most therapeutic benefits of HBOT can be attributed to the relationships between gas concentration, volume, and pressure. We know from Henry’s law that the amount of an ideal gas dissolved in a solution is directly proportional to its partial pressure (Fig. 5). Therefore, increasing partial pressure of oxygen in arterial blood during HBOT would improve the cellular delivery and supply of oxygen. This is the primary principle behind the effectiveness of HBOT in treating conditions in which oxygen delivery has been compromised, such as carbon monoxide poisoning and ischemia.
Fig. 5.
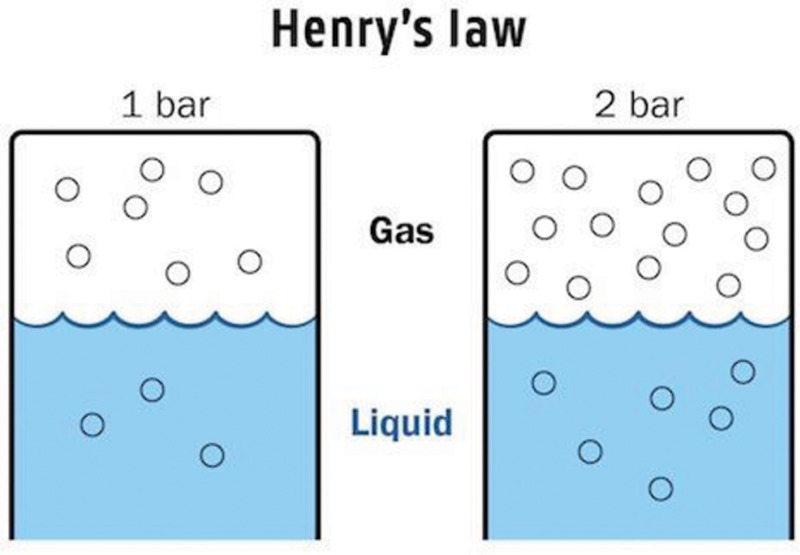
Henry’s Law: The concentration of a dissolved gas equals the pressure times the solubility coefficient of that gas.
Another major effect of HBOT can be explained by Boyle’s law, which indicates that the volume of a gas bubble is inversely related to the pressure exerted upon it (Fig. 6); this is the central concept underlying the beneficial properties of HBOT in management of conditions such as decompression illness and intravascular embolism.18
Fig. 6.
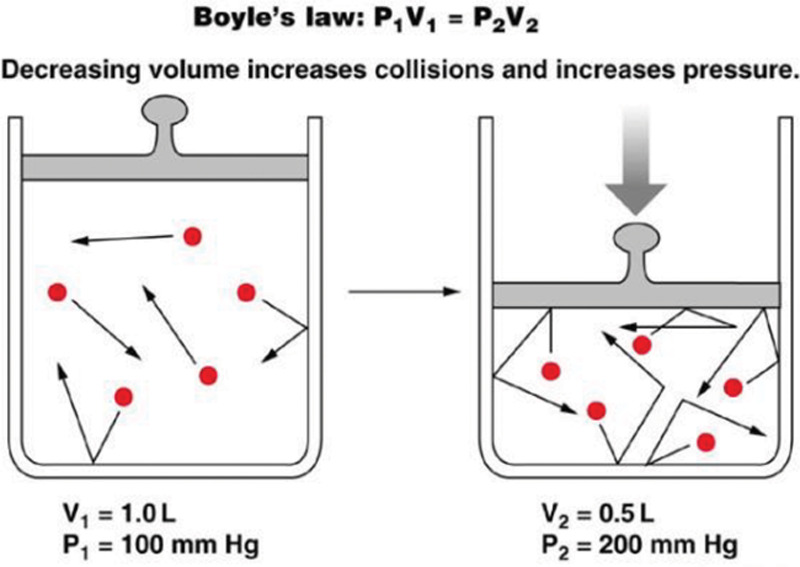
Boyle’s Law: Elevating hydrostatic pressure increases partial pressure of gases and causes a reduction in the volume of gas-filled spaces.
Several other therapeutic mechanisms of HBOT have been described in recent literature. It has been demonstrated that HBOT enhances neovascularization, and plays a role in improving the immune response, activating fibroblasts, downregulating inflammation, upregulating synthesis of growth factors, potentiating antibiotics and antibacterial processes, enhancing antioxidant response, and ameliorating ischemia-reperfusion injury.2,9,18–22
Contraindications and Adverse Effects
Although hyperbaric oxygen therapy remains relatively safe, several adverse side effects have been observed. Reversible myopia has occurred as a direct result of oxygen’s effects on the eye’s lens, whereas others have experienced barotrauma in the ears and sinuses and in rare cases, the teeth, and lungs.23 Middle ear barotrauma is among the most common side effects of HBOT, affecting up to 2% of the patients undergoing therapy. This can be prevented and managed by autoinflation techniques and inserting tympanostomy tubes, respectively. Other observed side effects include chest tightness, coughing, fatigue, headaches, vomiting, and a burning sensation in the chest.2,24 Although undesirable, these effects are reversible and nonfatal, leaving HBO therapy as a safe adjunctive treatment method for approved morbidities.
Oxygen toxicity is among the more serious complications associated with HBOT and can be associated with neurologic (eg, convulsions and psychological changes) and/or pulmonary (eg, pulmonary edema and respiratory failure) symptoms. Decompression sickness may occur in patients breathing compressed air that contains nitrogen. Fire hazard is considered the most common fatal complication of HBOT.9,18,21,25,26
HBOT may not be suitable for some individuals due to their current health or treatment regimen. “Absolute” contraindications for HBOT include untreated pneumothorax and concomitant use of certain chemotherapeutics such as doxorubicin or cisplatin. Additionally, there are several “relative” contraindications that warrant extreme caution; these include poorly controlled seizure disorder, hyperthyroidism, congestive heart failure with ejection fraction less than 30% (it is important to note that oxygen is a vasoconstrictor, and as a result HBOT may increase cardiac afterload), severe chronic obstructive pulmonary disease, asymptomatic pulmonary blebs, or bullae incidentally found on chest radiograph, active upper respiratory or sinus infections, recent ear or thoracic surgery, history of pneumothorax, uncontrolled fever, claustrophobia, and inability to equalize pressure in the middle ear.18,21
Indications and Clinical Use
HBOT serves as a “primary” therapy for a number of medical conditions. There exists an indisputable level of evidence that supports HBOT as the standard of care for the potentially fatal conditions of carbon monoxide poisoning, decompression illness, and arterial and venous gas embolisms.1–3 As such, HBOT has been approved by the Undersea and Hyperbaric Medical Society (UHMS) for 13 illnesses, including decompression sickness and arterial gas embolisms, though others propose it as a treatment for conditions outside of this list.27 (Please see Tables 1 and 2 for the full list of HBOT indications currently approved by “Undersea & Hyperbaric Medical Society” and “Centers for Medicare & Medicaid Services”, respectively.)
Table 1.
Indications for Hyperbaric Oxygen Therapy per Undersea and Hyperbaric Medical Society
| Indications for HBOT per Undersea and Hyperbaric Medical Society |
|---|
| Air or gas embolism |
| Carbon monoxide poisoning |
| Cyanide poisoning |
| Clostridial myositis and myonecrosis (gas gangrene) |
| Crush injury, compartment syndrome, and other acute traumatic ischemias |
| Decompression sickness |
| Arterial inefficiencies: central retinal artery occlusion |
| Arterial inefficiencies: enhancement of healing in selected problem wounds |
| Severe anemia |
| Intracranial abscess |
| Necrotizing soft–tissue infections |
| Osteomyelitis (refractory) |
| Delayed radiation injury (soft tissue and bony necrosis) |
| Compromised grafts and flaps |
| Acute thermal burn injury |
| Idiopathic sudden sensorineural hearing loss |
Table 2.
Indications for Hyperbaric Oxygen Therapy per Centers for Medicare and Medicaid Services
| Indications for HBOT per Centers for Medicare and Medicaid Services |
|---|
| Acute carbon monoxide intoxication |
| Decompression illness |
| Gas embolism |
| Gas gangrene |
| Acute traumatic peripheral ischemia |
| Crush injuries and suturing of severed limbs |
| Acute peripheral arterial insufficiency |
| Progressive necrotizing infections |
| Preparation and preservation of compromised skin grafts |
| Chronic refractory osteomyelitis |
| Osteoradionecrosis |
| Soft-tissue radionecrosis |
| Cyanide poisoning |
| Actinomycosis |
| Diabetic wounds of the lower extremity with type 1 or 2 diabetes, a Wagner Grade 3 or higher ulcer, and failure of adequate course of standard wound therapy |
Albeit not as strong as the available evidence for its “primary” use, research has shown HBOT to be beneficial as an “adjunctive” therapy in the case of a diverse range of other pathologies including but not limited to those of neurology, oncology, orthopedic, rheumatology, cardiovascular, genitourinary, gastrointestinal, and hepatobiliary origin, as well as acute and chronic wounds. Moreover, some studies have displayed HBOTs favorable impact on radiation-induced injuries where fibrotic deposition, diminished vascularity, and tissue hypoxia play role in the disease pathogenesis.18,28–32 Although there appears to be a correlation between the use of HBOT and an improved outcome, causation has yet to be definitively established. Conditions such as diabetic foot ulcers, ischemic stroke, sports injuries, and multiple sclerosis are common diseases that are treated with HBOT but a lack of strong support from peer-reviewed research, with many studies being underpowered. As such, HBOT has been described as “a therapy in search of disease.”27,33 Further studies need to be performed that are properly randomized, controlled, and conducted so that its proper uses may be identified.
Over the past decade, Cochrane Reviews has assessed potential “adjunctive” indications for HBOT. The results of these Systematic Reviews are summarized in Table 3. It is important to point out that the authors have unanimously taken note of the fact that the majority of the trials included in these Systematic Reviews suffered from small sample sizes, methodological deficiencies, and/or poor reporting outcomes, concluding that the results should be interpreted “cautiously.” The one common consensus in these Systematic Reviews was that “appropriately Powered trials of high methodological rigor is required to define which patients, if any, can be expected to benefit most from HBOT.”4,34–47
Table 3.
Cochrane Review Results on Potential Indications for Hyperbaric Oxygen Therapy
| Cochrane Study Title | Publication Year | Authors’ Conclusions |
|---|---|---|
| HBOT for chronic wounds4 | 2015 | In diabetic foot ulcers, HBOT significantly improved healing in the short term, but not in the long term. |
| HBOT for chronic wounds34 | 2004 | In diabetic foot ulcers, HBOT significantly reduced the risk of major amputation and may improve the chance of healing at 1 year. The routine management of chronic wounds associated with other pathologies with HBOT is not justified |
| HBOT for late radiation tissue injury35 | 2016 | For LRTI affecting tissues of the head, neck, anus, and rectum, HBOT is associated with improved outcome. HBOT appears to reduce the chance of osteoradionecrosis following tooth extraction in an irradiated field. No evidence of important clinical effect on neurological tissues. |
| HBOT for autism spectrum disorder36 | 2016 | No evidence that HBOT improves symptoms of ASD |
| HBOT for necrotizing fasciitis37 | 2015 | Failed to support or refute the effectiveness of HBOT |
| HBOT for acute coronary syndrome38 | 2015 | There is some evidence from small trials to suggest that HBOT is associated with a reduction in the risk of death, the volume of damaged muscle, the risk of major adverse cardiac events, and time to relief from ischemic pain. The routine application of HBOT cannot be justified. |
| HBOT for migraine and cluster headache39 | 2015 | There was some evidence that HBOT was effective for the termination of acute migraine in an unselected population |
| HBOT for acute ischemic stroke40 | 2014 | No good evidence to show that HBOT improves clinical outcomes, but the possibility of clinical benefit has not been excluded |
| HBOT for malignant otitis externa41 | 2013 | No clear evidence to demonstrate the efficacy of HBOT when compared with antibiotics and/or surgery |
| HBOT for acute surgical and traumatic wounds42 | 2013 | No high-quality evidence. Although 2 small trials suggested that HBOT may improve the outcomes of skin grafting and trauma, these trials were at risk of bias. |
| HBOT for bony fractures43 | 2012 | No evidence to support or refute the effectiveness of HBOT for the management of delayed or nonunion bony fractures |
| HBOT for idiopathic sudden sensorineural hearing loss and tinnitus44 | 2012 | For people with acute ISSHL, the application of HBOT significantly improved hearing, but the clinical significance remains unclear. No evidence of a beneficial effect of HBOT on chronic ISSHL or tinnitus |
| HBOT for traumatic brain injury45 | 2012 | Although the addition of HBOT may reduce the risk of death and improve the final GCS, there is little evidence that the survivors have a good outcome. The routine application of HBOT to these patients cannot be justified. |
| HBOT for vascular dementia46 | 2012 | Insufficient evidence to support HBOT as an effective treatment |
CCS, Glasgow Coma Scale; ISSHL, Idiopathic Sudden Sensorineural Hearing Loss; LRTI, late radiation tissue injury.
Financial Cost
Cost-effectiveness is a central issue in modern healthcare. The cost of a full course of HBO treatment for diabetic foot ulcers varies by location and depends upon several factors, such as setup costs and ongoing costs, reimbursement systems, and the number of patients treated per center. Costs also differ geographically. In the United States, charges are typically between $200 and $1,250 per treatment session, with a full course of treatment averaging 50–60 hours in the HBO chamber and costing from $50,000 (Medicare) to $200,000 (private pay).21,48 In 2011, a full HBO treatment in the Netherlands was about €6,920 (equaled $7,762), displaying the cost differential outside the United States.49
According to market research, the global HBOT devices market size was estimated at USD 2.21 billion in 2016.50 A rising number of university and private companies funded clinical trials indicates an ongoing adoption of the technique and contributes to propel growth of the HBOT market. Additionally, technological development in the field of hyperbaric oxygen therapy devices is expected to push/increase the demand over the next years and further impel their growth. As HBO can be used to treat several conditions noninvasively, market research found that nearly 90% (1800 out of 2000) of hospitals and 71% (500 out of 700) of clinics are already offering hyperbaric oxygen therapies for many of the diseases previously detailed, including chronic wounds.
Efficacy in Chronic Wounds
HBOT has been used as an “adjunctive” therapy for chronic wounds since the mid 1960s. The mechanisms by which HBOT may augment healing in chronic wounds are not fully understood, though several rationales have been proposed throughout years. It has been demonstrated that HBOT can modulate the local and systemic effects witnessed in both acute and chronic injuries. In general, the common denominators in chronic wounds are hypoxia, prolonged inflammation, and impaired angiogenesis, all of which may potentially be ameliorated by HBOT.18,51
The data on efficacy of HBOT in chronic wounds are often inconsistent and inconclusive.51–56 Among various etiologies involved in the development of chronic wounds, the highest number of studies and the bulk of HBOT literature have been devoted to the subject of DFUs. A 2004 Cochrane Review evaluated the role of HBOT in chronic wounds, concluding that HBOT may reduce the risk of major amputation in DFU patients and may improve healing at 1 year. Unfortunately, many of the studies reviewed suffered from limited sample sizes and methodological flaws. The same study evaluated the role of HBOT in chronic wounds of venous, arterial, and pressure etiology, and concluded that the routine utilization of HBOT for these indications was not justified based on the evidence (Table 3).34
In 2015, an updated Cochrane Review was conducted. The evidence from this study revealed that HBOT may improve the healing rate of DFU in the short term (ie, 6 wks), but not the long term (ie, 1 y). The authors further found no significant difference in major amputation rate in DFU population, while once again emphasizing the various flaws in the study design and reporting outcomes of the trials included (Table 3).4 Löndahl et al57 conducted a randomized, double-blinded, placebo-controlled clinical trial in 2010 evaluating 94 patients with Wagner Grade 2, 3, or 4 DFUs. They concluded that adjunctive HBOT facilitates healing in selected patients.57 A 2017 report by Lam et al demonstrated that HBOT may improve healing and decrease amputation in “ischemic” DFUs; however, there was limited evidence on its effect on nonischemic DFUs and nondiabetic arterial ulcers.51
Zhao et al58 conducted a meta-analysis on DFUs in 2017 studying 9 randomized clinical trials. They found that although HBOT was associated with a greater reduction in the wound size compared with the standard therapy, no differences existed with respect to the rate of complete healing, amputation risk, and adverse events.58 The following year, in 2018, Ennis et al53 conducted a retrospective study of over 600,000 Wagner Grades 3 and 4 DFUs concluding that HBOT may be of benefit in the case of “advanced” ulcers. Most recently, in 2019, Golledge and Singh59 carried out a systematic review and meta-analysis of 9 clinical trials in the field of DFUs. Authors concluded that HBOT improves the healing of DFUs and reduces the amputation rate.59
In contrast, 2 recent studies by Fedorko et al52 and Santema et al55 found that HBOT did not offer any significant advantages toward complete wound healing in DFUs associated with lower-limb ischemia. However, these studies too have been subject to criticism due to several methodological errors.60–62
While definitive proof for HBOT as a therapeutic has yet to be established, it appears that by and large, among the potential indications for HBOT in the field of chronic wounds, the strongest favorable evidence exists for ischemic, infected (ie, Wagner Grade 3 or worse) DFUs.51–56
Why the Controversy?
As we have highlighted in this article, much controversy exists with regard to the adjunctive therapeutic effects of HBOT on chronic wounds. There are several culprits for the existing discord. First and foremost, a comprehensive mechanistic understanding of the technology is lacking. HBOT acts through diverse and not-fully-understood mechanisms to promote angiogenesis and decrease inflammation. Moreover, many of the initial HBOT studies that demonstrate favorable outcomes were performed in the inpatient/hospital setting, which ensured proper patient, physician, and staff compliance; it is not completely unexpected to see that these results have not fully translated to the reality of the outpatient/clinic setting. Also, there are inherent impediments to an ideal study design investigating HBOT; as an example, the unique environment of hyperbaric chambers generates significant challenges to ideal blinding of both patients as well as investigators.7 Finally, trials investigating HBOT are faced with the same challenges such as “procedural variations” and others that are almost impractical to account for, which have plagued clinical studies in this particular field for decades.63–66
To make the matter even worse, similar to the efficacy trials, there have been contradictory reports on economics and cost-effectiveness of HBOT in the field of chronic wounds. The cost of diabetic foot disease in the United States in 2007 was $30 billion, of which $19 billion was due to foot ulceration and $11 billion to amputations. It was estimated in 2007 that effective diabetic foot ulcer and amputation prevention could realistically save the US healthcare system up to $21.8 billion annually.67 Unfortunately, studies have failed to prove unanimously that HBOT has the potential to lower the costs of care for DFUs. The 2008 Study by Canadian Agency for Drugs and Technologies in Health reported that adjunctive HBOT for DFUs is cost-effective compared with standard care alone.68 The more recent study conducted in 2017 by Health Quality Ontario indicated that adjunctive HBOT for DFUs may lower costs due to reduced amputation rate, but overall authors concluded that “there is uncertainty” regarding cost-effectiveness.69
This overall environment of uncertainty has inevitably led to discrepancies between “accepted,” “covered,” and “off-label” indications for HBOT. This has brought several stakeholders with differing motivations into play, paving the way for the utilization of HBOT for unregulated and unwarranted indications, whereby little to no supportive evidence exist.70 Not surprisingly, the skepticism ensued has made it even more challenging to vindicate this potential therapy or to see its merits.
CONCLUSIONS
Compressed air and hyperbaric oxygen have been utilized in medicine for centuries. HBOT is now considered the mainstay of treatment for a number of life-threatening conditions such as carbon monoxide poisoning, decompression illness, and gas embolism.1–3,71 Moreover, HBOT has the distinctive ability to remedy tissue hypoxia, reduce inflammation, and alleviate ischemia-reperfusion injury.7 The current evidence in the field of chronic wounds suggests that HBOT may have favorable effects on ischemic, infected (ie, Wagner Grade 3 or worse) DFUs.51–56 Despite many studies highlighting the potential benefits of HBOT, much controversy remains with regard to its efficacy in wound healing.15 The paucity of high-quality randomized controlled trials makes it difficult to properly assess the efficacy of HBOT. To accurately validate the potential benefits of HBOT, more vigorous investigations with adequately powered sample sizes are warranted.
Footnotes
Published online 25 September 2020.
Drs. Hajhosseini and Kuehlmann contributed equally to this work.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Weaver LK, Hopkins RO, Chan KJ, et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med. 2002;347:1057–1067. [DOI] [PubMed] [Google Scholar]
- 2.Leach RM, Rees PJ, Wilmshurst P.Hyperbaric oxygen therapy. BMJ. 1998;317:1140–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver LK.Hyperbaric oxygen in the critically ill. Crit Care Med. 2011;39:1784–1791. [DOI] [PubMed] [Google Scholar]
- 4.Kranke P, Bennett MH, Martyn-St James M, et al. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2015;2015:CD004123. [DOI] [PubMed] [Google Scholar]
- 5.de Smet GHJ, Kroese LF, Menon AG, et al. Oxygen therapies and their effects on wound healing. Wound Repair Regen. 2017;25:591–608. [DOI] [PubMed] [Google Scholar]
- 6.Sepehripour S, Dhaliwal K, Dheansa B.Hyperbaric oxygen therapy and intermittent ischaemia in the treatment of chronic wounds. Int Wound J. 2018;15:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fife CE, Eckert KA, Carter MJ.An update on the appropriate role for hyperbaric oxygen: indications and evidence. Plast Reconstr Surg. 2016;1383 Suppl107S–116S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dauwe PB, Pulikkottil BJ, Lavery L, et al. Does hyperbaric oxygen therapy work in facilitating acute wound healing: a systematic review. Plast Reconstr Surg. 2014;133:208e–215e. [DOI] [PubMed] [Google Scholar]
- 9.Thom SR.Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127Suppl 1131S–141S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galewitz P.Hospitals ramp up hyberbaric therapy for diabetics, despite concerns. Kaiser Health News. 2017. June 28 Available at: https://khn.org/news/hospitals-put-more-stock-in-hyperbaric-therapy-for-diabetics-despite-concerns/. Accessed September 9, 2020.
- 11.Administration UF. a. D. FDA Executive Summary Classification of Wound Dressings Combined with Drugs. Available at: https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/GeneralandPlasticSurgeryDevicesPanel/UCM518494.pdf. Accessed January 5, 2019.
- 12.Singer AJ, Clark RA.Cutaneous wound healing. N Engl J Med. 1999;341:738–746. [DOI] [PubMed] [Google Scholar]
- 13.Edwards ML.Hyperbaric oxygen therapy. Part 1: history and principles. J Vet Emerg Crit Care (San Antonio). 2010;20:284–288. [DOI] [PubMed] [Google Scholar]
- 14.Singh S, Gambert SR.Hyperbaric oxygen therapy: a brief history and review of its benefits and indications for the older adult patient. Annals Long-Term Care: Clin Care Aging. 2014;22:37–42. [Google Scholar]
- 15.Bolton L.Hyperbaric oxygen therapy effects on chronic wounds. Wounds. 2015;27:354–355. [PubMed] [Google Scholar]
- 16.Thackham JA, McElwain DL, Long RJ.The use of hyperbaric oxygen therapy to treat chronic wounds: a review. Wound Repair Regen. 2008;16:321–330. [DOI] [PubMed] [Google Scholar]
- 17.Raman G, Kupelnick B, Chew P, et al. A Horizon Scan: Uses of Hyperbaric Oxygen Therapy. 2006Rockville, Md.: Agengy for Healthcare Research and Quality; [PubMed] [Google Scholar]
- 18.Mechem C, Manaker S.Traub SJ.Hyperbaric oxygen therapy. In: UpToDate. 2019. Waltham, Mass: Available at: https://www.uptodate.com. Accessed on June 12, 2019 [Google Scholar]
- 19.Johnston BR, Ha AY, Brea B, et al. The mechanism of hyperbaric oxygen therapy in the treatment of chronic wounds and diabetic foot ulcers. R I Med J (2013). 2016;99:26–29. [PubMed] [Google Scholar]
- 20.Camporesi EM, Bosco G.Mechanisms of action of hyperbaric oxygen therapy. Undersea Hyperb Med. 2014;41:247–252. [PubMed] [Google Scholar]
- 21.Löndahl M.Hyperbaric oxygen therapy as adjunctive treatment of diabetic foot ulcers. Med Clin North Am. 2013;97:957–980. [DOI] [PubMed] [Google Scholar]
- 22.Ishihara A.Mild hyperbaric oxygen: mechanisms and effects. J Physiol Sci. 2019;69:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmquist BM, Philipson B, Barr PO.Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol. 1984;68:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tibbles PM, Edelsberg JS.Hyperbaric-oxygen therapy. N Engl J Med. 1996;334:1642–1648. [DOI] [PubMed] [Google Scholar]
- 25.Heyboer M, 3rd, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camporesi EM.Side effects of hyperbaric oxygen therapy. Undersea Hyperb Med. 2014;41:253–257. [PubMed] [Google Scholar]
- 27.Gill AL, Bell CN.Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM. 2004;97:385–395. [DOI] [PubMed] [Google Scholar]
- 28.Stępień K, Ostrowski RP, Matyja E.Hyperbaric oxygen as an adjunctive therapy in treatment of malignancies, including brain tumours. Med Oncol. 2016;33:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett M, Heard R.Hyperbaric oxygen therapy for multiple sclerosis. CNS Neurosci Ther. 2010;16:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dulai PS, Gleeson MW, Taylor D, et al. Systematic review: the safety and efficacy of hyperbaric oxygen therapy for inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:1266–1275. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Wen Y, Shen C, et al. Hyperbaric oxygen therapy in liver diseases. Int J Med Sci. 2018;15:782–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barilaro G, Francesco Masala I, Parracchini R, et al. The role of hyperbaric oxygen therapy in orthopedics and rheumatological diseases. Isr Med Assoc J. 2017;19:429–434. [PubMed] [Google Scholar]
- 33.Gabb G, Robin ED.Hyperbaric oxygen. A therapy in search of diseases. Chest. 1987;92:1074–1082. [DOI] [PubMed] [Google Scholar]
- 34.Kranke P, Bennett M, Roeckl-Wiedmann I, et al. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2004:CD004123. [DOI] [PubMed] [Google Scholar]
- 35.Bennett MH, Feldmeier J, Hampson NB, et al. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2016;4:CD005005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong T, Chen H, Luo R, et al. Hyperbaric oxygen therapy for people with autism spectrum disorder (ASD). Cochrane Database Syst Rev. 2016;10:CD010922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levett D, Bennett MH, Millar I.Adjunctive hyperbaric oxygen for necrotizing fasciitis. Cochrane Database Syst Rev. 2015;1:CD007937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett MH, Lehm JP, Jepson N.Hyperbaric oxygen therapy for acute coronary syndrome. Cochrane Database Syst Rev. 2015:CD004818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett MH, French C, Schnabel A, et al. Normobaric and hyperbaric oxygen therapy for the treatment and prevention of migraine and cluster headache. Cochrane Database Syst Rev. 2015:CD005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett MH, Weibel S, Wasiak J, et al. Hyperbaric oxygen therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2014:CD004954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillips JS, Jones SE.Hyperbaric oxygen as an adjuvant treatment for malignant otitis externa. Cochrane Database Syst Rev. 2013:CD004617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eskes A, Vermeulen H, Lucas C, et al. Hyperbaric oxygen therapy for treating acute surgical and traumatic wounds. Cochrane Database Syst Rev. 2013:CD008059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bennett MH, Stanford RE, Turner R.Hyperbaric oxygen therapy for promoting fracture healing and treating fracture non-union. Cochrane Database Syst Rev. 2012;11:CD004712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett MH, Kertesz T, Perleth M, et al. Hyperbaric oxygen for idiopathic sudden sensorineural hearing loss and tinnitus. Cochrane Database Syst Rev. 2012;10:CD004739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett MH, Trytko B, Jonker B.Hyperbaric oxygen therapy for the adjunctive treatment of traumatic brain injury. Cochrane Database Syst Rev. 2012;12:CD004609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao Y, Wang J, Jiang S, et al. Hyperbaric oxygen therapy for vascular dementia. Cochrane Database Syst Rev. 2012:CD009425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kranke P, Bennett MH, Martyn-St James M, et al. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2012:CD004123. [DOI] [PubMed] [Google Scholar]
- 48.Lipsky BA, Berendt AR.Hyperbaric oxygen therapy for diabetic foot wounds: has hope hurdled hype? Diabetes Care. 2010;33:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Staal SR, Ubbink DT, Lubbers MJ.Comment on: lipsky and berendt. Hyperbaric oxygen therapy for diabetic foot wounds: has hope hurdled hype? Diabetes Care. 2010;33:1143–1145. Diabetes care. 2011;34:e110; author reply e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hyperbaric oxygen therapy (HBOT) devices/equipment market analysis by product (Monoplace, Multiplace, Topical HBOT Devices), by application (wound healing, infection treatment, gas embolism), and segment forecasts. Research and Markets Report. 2017:1–80.
- 51.Lam G, Fontaine R, Ross FL, et al. Hyperbaric oxygen therapy: exploring the clinical evidence. Adv Skin Wound Care. 2017;30:181–190. [DOI] [PubMed] [Google Scholar]
- 52.Fedorko L, Bowen JM, Jones W, et al. Hyperbaric oxygen therapy does not reduce indications for amputation in patients with diabetes with nonhealing ulcers of the lower limb: A prospective, double-blind, randomized controlled clinical trial. Diabetes Care. 2016;39:392–399. [DOI] [PubMed] [Google Scholar]
- 53.Ennis WJ, Huang ET, Gordon H.Impact of hyperbaric oxygen on more advanced Wagner grades 3 and 4 diabetic foot ulcers: matching therapy to specific wound conditions. Adv Wound Care (New Rochelle). 2018;7:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Margolis DJ, Gupta J, Hoffstad O, et al. Lack of effectiveness of hyperbaric oxygen therapy for the treatment of diabetic foot ulcer and the prevention of amputation: a cohort study. Diabetes Care. 2013;36:1961–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santema KTB, Stoekenbroek RM, Koelemay MJW, et al. DAMO2CLES Study Group. Hyperbaric oxygen therapy in the treatment of ischemic lower- extremity ulcers in patients with diabetes: results of the damo2cles multicenter randomized clinical trial. Diabetes Care. 2018;41:112–119. [DOI] [PubMed] [Google Scholar]
- 56.Elraiyah T, Tsapas A, Prutsky G, et al. A systematic review and meta-analysis of adjunctive therapies in diabetic foot ulcers. J Vasc Surg. 2016;63:46S-58S e41-42. [DOI] [PubMed] [Google Scholar]
- 57.Löndahl M, Katzman P, Nilsson A, et al. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care. 2010;33:998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao D, Luo S, Xu W, et al. Efficacy and safety of hyperbaric oxygen therapy used in patients with diabetic foot: a meta-analysis of randomized clinical trials. Clin Ther. 2017;39:2088–2094.e2. [DOI] [PubMed] [Google Scholar]
- 59.Golledge J, Singh TP.Systematic review and meta-analysis of clinical trials examining the effect of hyperbaric oxygen therapy in people with diabetes-related lower limb ulcers. Diabet Med. 2019;36:813–826. [DOI] [PubMed] [Google Scholar]
- 60.Löndahl M, Fagher K, Katzman P.Comment on Fedorko, et al. Hyperbaric oxygen therapy does not reduce indications for amputation in patients with diabetes with nonhealing ulcers of the lower limb: a prospective, double-blind, randomized controlled clinical trial. Diabetes Care 2016;39:392–399. Diabetes Care. 2016;39:e131–e132. [DOI] [PubMed] [Google Scholar]
- 61.Huang ET.Comment on Fedorko, et al. Hyperbaric oxygen therapy does not reduce indications for amputation in patients with diabetes with nonhealing ulcers of the lower limb: a prospective, double-blind, randomized controlled clinical trial. Diabetes care 2016;39:392-399. Diabetes Care. 2016;39:e133–e134. [DOI] [PubMed] [Google Scholar]
- 62.Murad MH.Comment on Fedorko, et al. hyperbaric oxygen therapy does not reduce indications for amputation in patients with diabetes with nonhealing ulcers of the lower limb: a prospective, double-blind, randomized controlled clinical trial. Diabetes Care. 2016;39:392–399. Diabetes Care. 2016;39:e135. [DOI] [PubMed] [Google Scholar]
- 63.Organization BI. Clinical Development Success Rates 2006-2015. Available at: https://www.bio.org/sites/default/files/Clinical%20Development%20Success%20Rates%202006-2015%20-%20BIO,%20Biomedtracker,%20Amplion%202016.pdf. Accessed January 5, 2019.
- 64.Harrison RK.Phase II and phase III failures: 2013-2015. Nat Rev Drug Discov. 2016;15:817–818. [DOI] [PubMed] [Google Scholar]
- 65.Angell M.Industry-sponsored clinical research: a broken system. JAMA. 2008;300:1069–1071. [DOI] [PubMed] [Google Scholar]
- 66.DeMets DL, Califf RM.A historical perspective on clinical trials innovation and leadership: where have the academics gone? JAMA. 2011;305:713–714. [DOI] [PubMed] [Google Scholar]
- 67.Rogers LC, Lavery LA, Armstrong DG.The right to bear legs–an amendment to healthcare: how preventing amputations can save billions for the US Health-care System. J Am Podiatr Med Assoc. 2008;98:166–168. [PubMed] [Google Scholar]
- 68.Chuck AW, Hailey D, Jacobs P, et al. Cost-effectiveness and budget impact of adjunctive hyperbaric oxygen therapy for diabetic foot ulcers. Int J Technol Assess Health Care. 2008;24:178–183. [DOI] [PubMed] [Google Scholar]
- 69.Health Quality O. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1–142. [PMC free article] [PubMed] [Google Scholar]
- 70.Glauser W.Unregulated hyperbaric oxygen therapy clinics assailed. CMAJ. 2010;182:1950–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang CC, Ho CH, Chen YC, et al. Hyperbaric oxygen therapy is associated with lower short- and long-term mortality in patients with carbon monoxide poisoning. Chest. 2017;152:943–953. [DOI] [PubMed] [Google Scholar]


