Supplemental Digital Content is available in the text.
Summary
We present a case report of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) that was mistaken as disseminated silicosis after multiple percutaneous biopsies. The correct diagnosis of BIA-ALCL was confirmed only after a pathologic examination of the capsulectomy specimens. A review of the literature of percutaneous biopsies of ALCL showed a diagnostic yield of only 63%. Although percutaneous biopsies may be facile to obtain and may be diagnostic, in our case, biopsies were not sufficient to exclude the diagnosis of BIA-ALCL.
Breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) is a rare, usually indolent, T-cell cancer, and remains an unusual complication of breast augmentation and reconstruction. The risk is estimated as 1:2,207 to 1:86,029, with 322 cases reported in the United States as of April 2020.1 BIA-ALCL is a subset of prosthetic-associated lymphomas and differs from the predominately B-cell metallic orthopedic and cardiac prostheses lymphomas. It was first linked to implants in 1997, and typically presents as a late seroma, mass, or rarely with lymph node metastasis.2 It is associated with textured implants, and a recent study no longer supports a link to specific bacterial biofilms.3 However, other more common complications, such as double capsule seromas and breast implant ruptures, can mimic concerning imaging findings of BIA-ALCL.4 The clinician must maintain a high level of suspicion and complete a thorough history and physical examination to determine an appropriate differential diagnosis. If BIA-ALCL is considered, it is imperative to perform total capsulectomies when removing the breast implants. Herein, we report an unusual case of BIA-ALCL, which was initially diagnosed as granulomatous silicosis on multiple core needle biopsies.
CASE REPORT
We present the case of a 62-year-old woman with a history of latent tuberculosis and rheumatoid arthritis. She underwent bilateral silicone subglandular breast augmentation in Tijuana, Mexico approximately 10 years before presentation. She also reported anterior chest wall trauma (seatbelt injury) after a rollover motor vehicle collision 5 years ago. She denied prior silicone injections. She presented with a 3-month history of “B Type” symptoms consisting of fevers, night sweats, nausea, vomiting, and a 30-pound weight loss. On initial workup with imaging, she was found to have a chest wall mass, diffuse lymphadenopathy, and a small pleural effusion. Her B symptoms were concerning for malignancy and interventional radiology was consulted for biopsy, and thoracentesis and sampling consisted of five 20-gauge core needle biopsies, 4 fine needle aspiration (FNA) passes (two 25 gauge and two 23 gauge) of the right chest wall mass, and five 25 gauge FNA passes of the left supraclavicular lymph nodes. Also, 20 mL of the right pleural effusion was obtained. Initial pathology revealed atypical cells consistent with foreign-body–induced pseudotumor, and thought to be secondary to silicosis granulomas.
A computed tomography scan with contrast showed bilateral internal mammary, right retropectoral, supraclavicular, and mediastinal lymphadenopathy with intact breast implants (Fig. 1A). This was confirmed by a dedicated breast MRI, which showed no evidence of intracapsular or extracapsular implant rupture, or periprosthetic fluid collections.
Fig. 1.
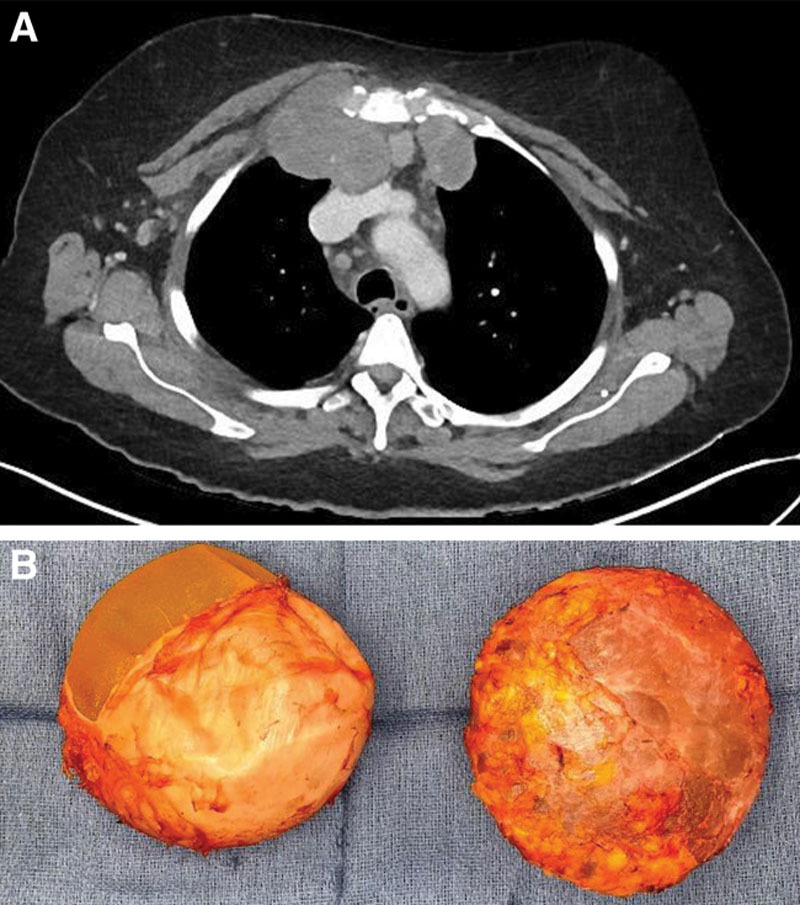
The patient underwent: A, Contrast enhanced CT scan demonstrating retrosternal and right clavicular lymphadenopathy, followed by B, Left and right en-bloc capsulotomies. The capsule was incised to reveal textured implants.
The patient underwent left supraclavicular lymph node excisional biopsy and en-bloc bilateral breast implant removal with total capsulectomies (Fig. 1B). The textured breast implants (CUI 340 cc) were intact by gross examination. Both the breast and lymphatic specimens were sent to pathology for analysis and revealed large atypical CD30-positive T-cells in both the breast implant capsule and within the lymph node, concerning for BIA-ALCL. Given the prior diagnosis of granulomatous silicosis, the lymph node and implant capsules were subsequently sent out for extramural expert consultation, and the diagnosis of BIA-ALCL was confirmed. The patient was enrolled in a clinical trial of EPOCH and Nivolumab chemotherapy. She had a good response to chemotherapy, with no evidence of disease on imaging approximately 7 months after her surgery.
DISCUSSION
Diagnosis of BIA-ALCL requires a strong understanding of the disease process, diagnostic pathway, and, in this specific case, an understanding of the generations of breast implants and illicit silicone injections. The patient in this case report was admitted to the hospital unwell. She spent the last 3 months needing assistance in her activities of daily living and lost a substantial amount of weight. This presentation was an unusually aggressive form of BIA-ALCL, which typically has a more benign presentation and indolent course.5 However, the initial diagnostic results of FNA and core needle biopsies suggested the findings were a foreign body reaction consistent with silicosis granulomas. The medical and surgical consulting teams encountered an anchoring effect bias, and excluded BIA-ALCL based on the negative existing biopsy results. However, the diagnosis was discordant with her clinical vignette, and en-bloc capsulectomies were performed with an excisional biopsy of a palpable lymph node. In fact, although the surgical pathology was in process, the patient was discharged from the hospital with the presumed diagnosis of silicosis.
The gold standard for lymphoma diagnosis is excisional lymph node biopsy, although core needle biopsy is an excellent cost-saving, minimally invasive alternative, and has substituted excisional lymph node biopsy for the diagnosis of many lymphomas.6 However, given the rarity of BIA-ALCL and requirement for comprehensive evaluation, which includes histology, immunophenotyping, and molecular genetics, the disease should not necessarily be excluded on the basis of a negative FNA or core biopsy. According to a large systematic review by Frederiksen et al6 in 2015, 14% of FNA and core needle biopsy samples were inadequate or inconclusive to diagnose lymphomas. The authors also concluded that 25%–35% of FNAs or core needle biopsies must be followed by an excisional lymph node biopsy to fully classify lymphomas. Specifically, for BIA-ALCL, the diagnostic yield for FNA and core needle biopsy was 63% (12/19)6. In our case, the FNA and core biopsies were non-diagnostic because the neoplastic T-cells were thought to be reactive histiocytes, which may demonstrate overlapping morphologic features. Additionally, the large neoplastic T-cells in BIA-ALCL tend to fragment during flow cytometric analysis, thus precluding adequate immunophenotypic evaluation. However, re-review of the cytology specimens by a hematopathologist and application of additional immunohistochemical studies revealed that the large atypical cells were CD30-positive, T-cells (as opposed to reactive histiocytes), compatible with BIA-ALCL. [See figure, Supplemental Digital Content 1, which displays (A and B) Aspirate smears of chest wall mass showing large lymphoma cells in a background of mixed inflammatory cells (Romanowsky stain; A-low power; B-high power). (C and D) Thickened breast implant capsule showing dense fibrosis and sheets of lymphoma cells (hematoxylin and eosin [H&E]; C-low power; D-high power). (E) CD30 immunohistochemical study highlighting numerous lymphoma cells within the breast capsule. (F) Low power, lymph node excisional biopsy showing distorted lymph node architecture (H&E stain). (G) High power, lymph node excisional biopsy showing lymphoma cells clustered within the lymph node sinuses (H&E stain). (H) CD30 immunohistochemical study highlighting lymphoma cells within the lymph node sinus. http://links.lww.com/PRSGO/B482.] This underscores the importance of expert hematopathology consultation in cases of potential BIA-ALCL.
This case was also unique, as the differential diagnosis included silicosis. Illicit injection of silicone, which is banned by the FDA, is associated with catastrophic complications, including blindness, pulmonary embolism, and death.7 Silicone breast implants were first invented by Cronin in the 1960s, and first- and second-generation implants were used until the FDA moratorium in 1992.7 These initial generations used lightly cross-linked silicone and extracapsular leaking of silicone, termed “silicone bleeding,” which was an uncommon complication.7,8 This silicone differs from the highly cross-linked cohesive silicone in third generation that garnered FDA approval in 2006 and is not prone to the same complication.8 In this case, the patient’s breast implants were approximately 10 years old and would have been third or subsequent generation. Although this patient did not present with the typical delayed seroma, her newer implants combined with the patient’s B symptoms, the negative computed tomography and MRI for rupture, and a negative history of illicit silicone injection kept BIA-ALCL high on the clinical differential diagnosis. The initial lymph node core needle biopsies also could have been confirmed with electron microscopy or infrared spectroscopy for the presence of silicone.
CONCLUSIONS
BIA-ALCL is an uncommon T-cell lymphoma that is typically indolent but can present in a more aggressive form with “B type” symptoms and diffuse lymphadenopathy. Clinicians must maintain a high level of suspicion in patients with prior breast augmentation with implants, especially when forming a surgical plan for implant removal as total capsulectomies should be performed. Biopsy of lymphadenopathy while useful for conformational diagnosis should not be used to exclude BIA-ALCL in the case of a negative result. In addition, all biopsy results should be reviewed by an expert hematopathologist and tested for CD30. While the diagnosis of silicosis may be considered, it is not common in third and subsequent generations of breast implants.
Supplementary Material
Footnotes
Published online 28 September 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Clemens M. BIA-ALCL Resources: By the numbers, and what they mean. Available at: https://www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-physician-resources/by-the-numbers. Accessed June 3, 2020.
- 2.Brody GS, Deapen D, Taylor CR, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg. 2015;135:695–705. [DOI] [PubMed] [Google Scholar]
- 3.Walker JN, Hanson BM, Pinkner CL, et al. Insights into the microbiome of breast implants and periprosthetic tissue in breast implant-associated anaplastic large cell lymphoma. Sci Rep. 2019;9:10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemens MW, Nava MB, Rocco N, et al. Understanding rare adverse sequelae of breast implants: anaplastic large-cell lymphoma, late seromas, and double capsules. Gland Surg. 2017;6:169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rupani A, Frame JD, Kamel D.Lymphomas associated with breast implants: a review of the literature. Aesthet Surg J. 2015;35:533–544. [DOI] [PubMed] [Google Scholar]
- 6.Frederiksen JK, Sharma M, Casulo C, et al. Systematic review of the effectiveness of fine-needle aspiration and/or core needle biopsy for subclassifying lymphoma. Arch Pathol Lab Med. 2015;139:245–251. [DOI] [PubMed] [Google Scholar]
- 7.Samreen N, Glazebrook KN, Bhatt A, et al. Imaging findings of mammary and systemic silicone deposition secondary to breast implants. Br J Radiol. 2018;91:20180098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillard C, Fowler JD, Barta R, et al. Silicone breast implant rupture: a review. Gland Surg. 2017;6:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


