Summary:
Tissue expanders are known adjuncts in ventral hernia repair, used in a staged approach where tissue closure or coverage of the defect is preferred but inadequate. Placement of tissue expanders in the correct tissue plane can be difficult, especially in thin patients or with loss of domain. This case series describes a technique in which tissue expander placement is facilitated by ultrasound-guided hydro-dissection, following the placement of a transversus abdominis plane (TAP) block. In short, after induction of anesthesia, the same needle used for the ultrasound-guided TAP block can be repositioned by the anesthesiologist to instill tumescent solution into the fascial plane between the internal and external oblique muscles. This allows for identification of the fascial planes in the ensuing operation. Our technique may prove to be an alternative tool in the placement of tissue expanders for ventral hernia repair, or in other procedures requiring device placement.
INTRODUCTION
The approach to ventral hernia repair varies with complexity, location, surgeon, and defect size. Small defects may be closed primarily, but larger defects may require elaborate repair, staging, prosthetics, and/or autologous flaps.1,2 Components separation can be utilized in abdominal closure when a mesh is contraindicated or used as an adjunct to repair,3 and is associated with a recurrence rate of 18.2%, compared with that of 24% with mesh, and up to 43% with primary closure.4,5 For giant ventral hernias, components separation is preferred over prosthetic alone because it is associated with a shorter operative time and more manageable wound complications.3 Insertion of fascial tissue expanders can ensure adequate tissue before components separation.6 However, identification of correct fascial planes can be difficult in thin patients, or those with loss of domain or significant scarring.7
At our institution, through an Enhanced Recovery After Surgery (ERAS) pathway, patients undergoing complex ventral hernia repair receive a preoperative transversus abdominis plane (TAP) block for analgesia. We developed a technique in which the TAP block needle is repositioned into the fascial plane between the external and internal oblique, to hydro-dissect between the muscles. To date, there are no reports of this technique in ventral hernia repair. The following cases demonstrate that ultrasound-guided hydro-dissection facilitates intraoperative identification of the correct intermuscular plane, an alternative approach to intermuscular dissection during components separation.
DESCRIPTION OF CASES
Seven male patients underwent complex ventral hernia repair facilitated by ultrasound-guided hydro-dissection between October 2017 and February 2020. Average age was 55 years. Average body mass index (BMI) was 29.4 kg/m2. Two patients had abdominal gunshot wounds (GSWs) that required multiple surgeries. Three patients underwent exploratory laparotomies for other surgical indications. The smallest hernia was 12 × 12 cm2, the largest was 18 cm wide from xiphoid to pubis.
Six patients required staged repair with tissue expansion, followed by delayed components separation with or without biologic placement. One patient had ultrasound-guided hydro-dissection to identify the correct intermuscular plane before components separation and mesh placement. Both GSW patients underwent ostomy reversal during final repair. At the time of print, two patients have not undergone definitive repair, but have had expanders placed.
In all cases, TAP blocks were placed bilaterally using ultrasound guidance. The same needle was immediately repositioned into the plane between the external and internal oblique musculature. 100 mL of tumescent solution (1 L of saline with 10 mL of 1% lidocaine plain and 2 Amps of epinephrine per bag) was instilled into this space bilaterally (See Video [online], which displays ultrasound-guided hydro-dissection between the external and internal oblique fascial planes following transversus abdominis plane [TAP] block placement by the anesthesiologist.)
Video 1. Hydrodissection. Video 1 from “The Use of Ultrasound-guided Hydro-dissection Facilitates Tissue Expander Placement and Components Separation in Staged Complex Ventral Hernia Repair: A Case Series”.
Horizontal incisions were made above the costal margins. Dissection was carried to the external oblique fascia, which was transversely incised, and the external oblique was elevated. The intermuscular plane was identifiable with the tumescent solution. Expander pockets were created deep to the external oblique fascia in the previously injected space. Tissue expanders were inserted. Remote injection ports were placed in the subcutaneous tissues cannulated with a 21-gauge needle. Expanders were filled. The fascia and wounds were closed in layers.
Patients followed up biweekly to increase tissue expander volumes (Figs. 1, 2). Definitive repair was performed 3–11 months following expander placement. The costal margin incisions were used to remove the implants and remote injection ports. Capsulectomies were performed. The back musculature fascia was incised laterally for component release of the external oblique. The muscles were advanced to close the defect. Biologic mesh was placed only if necessary, in 2 patients, in an underlaying fashion as a buttress to support primary fascial edge closure. Otherwise the fascial edges were used as the primary means of hernia repair.
Fig. 1.
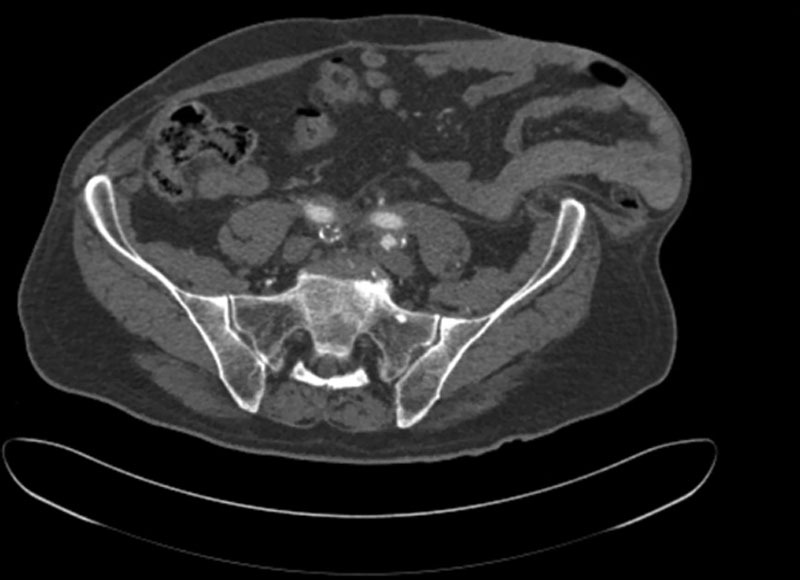
Representative image of a preoperative computed tomographic scan. Note the loss of domain in the patient’s left lower quadrant, with bowel adjacent to skin.
Fig. 2.
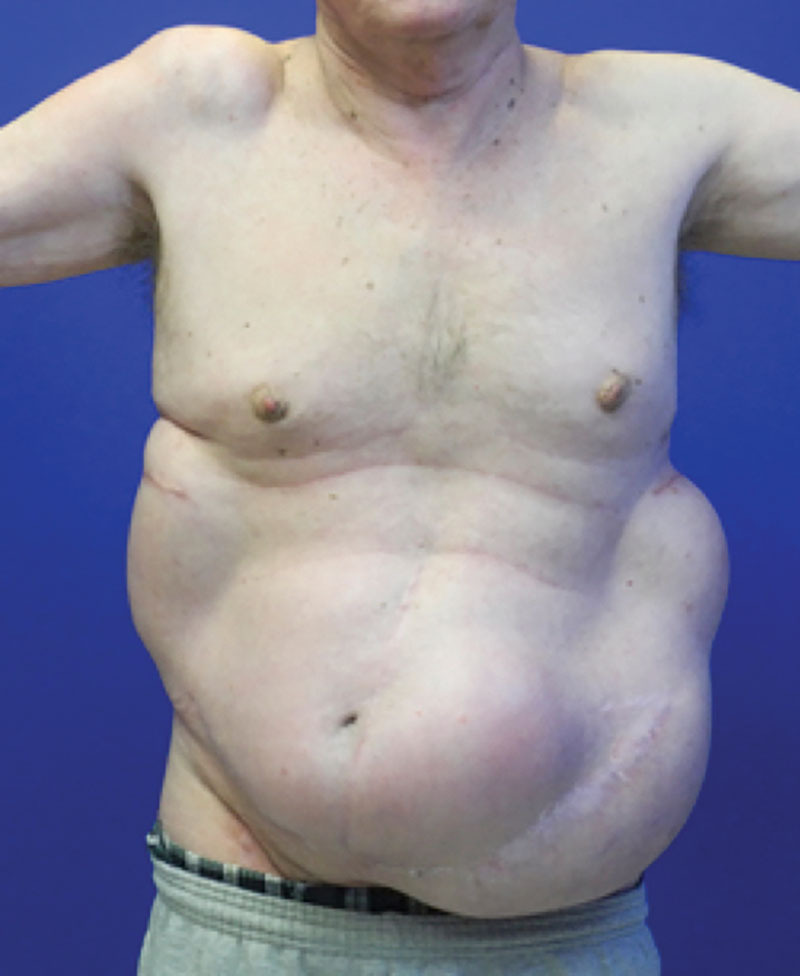
Tissue expanders in place in the bilateral flanks. Note that the ventral wall defect is present in the left lower quadrant.
Discharge was an average 12.8 days postoperatively (range, 4–36 days), with longer lengths of stay associated with recovery from concomitant procedures. Four patients who underwent definitive repair have no apparent recurrent hernia up to 24 months postoperatively (Fig. 3). One patient was lost to follow-up.
Fig. 3.
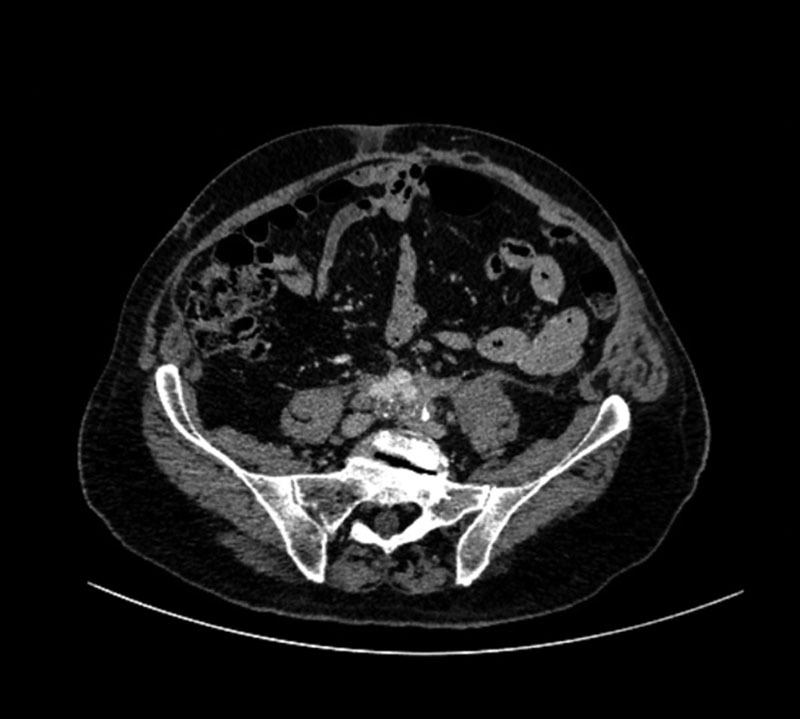
Representative image of a patient’s postoperative computed tomographic scan.
DISCUSSION
This case series describes ultrasound-guided fascial plane hydro-dissection following TAP block to facilitate tissue expander placement and/or components separation in the repair of complex ventral hernias. After the block, hydro-dissection was achieved by partially withdrawing the anesthetic needle and injecting tumescent solution in the desired intermuscular plane. As TAP blocks are part of our institution’s ERAS protocol for ventral hernia repairs due to improved postoperative pain,8,9 the implementation of the hydro-dissection technique was an obvious adjunct and was met with full cooperation from the anesthesia team.
Complicated ventral hernia repair poses unique challenges. Recruiting enough tissue to close the defect primarily, or even place an adequate mesh, can be difficult with extensive scarring or loss of domain. These factors can also hinder tissue expander placement, or even proper components separation. Tissue expanders effectively promote tissue growth for staged pediatric and adult ventral hernia repairs.6,7 Placement of expanders in the correct plane is especially prudent in patients with comorbidities, such as low BMI or hypertension, which can increase the risk of tissue expander-associated necrosis.10 Thus, our ultrasound-guided hydro-dissection technique, which facilitates intraoperative identification of intermuscular tissue planes, could aid expander placement or components separation in complex ventral hernia repair for high-risk patients. Moreover, while staged repair incurs the cost of an additional operating room trip, hydro-dissection with or without tissue expander placement reduced the use of biologic meshes to only two of our patients, decreasing cost overall.
Our limitations include small sample size, lack of measurable outcomes, and absence of a control group. More formal investigation is needed to objectively compare ultrasound-guided hydro-dissection to standard ventral hernia repair techniques and determine effects on ease of dissection, operative times, and outcomes.
These cases demonstrate identification and dissection of tissue planes facilitated by ultrasound-guided fascial plane hydro-dissection. Ultrasound provides radiographic evidence of the correct intermuscular plane and aids dissection in patients with scarring and/or loss of domain. Furthermore, performing hydro-dissection in patients already undergoing ultrasound-guided TAP block does not substantially complicate the procedure, as the plane would otherwise still be traversed by the needle. This technique may be valuable in tissue expander placement for ventral hernia repair, or in other procedures requiring intermuscular or sub-fascial device placement.
ACKNOWLEDGEMENT
Publication of this article was funded in part by the Temple University Libraries Open Access Publishing Fund.
Footnotes
Published online 23 September 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
J. Maroney and Dr. Taylor contributed equally to the writing of this case series and will thus share first authorship.
REFERENCES
- 1.Mathes SJ, Steinwald PM, Foster RD, et al. Complex abdominal wall reconstruction: a comparison of flap and mesh closure. Ann Surg. 2000;232:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elango S, Perumalsamy S, Ramachandran K, et al. Mesh materials and hernia repair. Biomedicine (Taipei). 2017;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vries Reilingh TS, van Goor H, Charbon JA, et al. Repair of giant midline abdominal wall hernias: “components separation technique” versus prosthetic repair: interim analysis of a randomized controlled trial. World J Surg. 2007;31:756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Vries Reilingh TS, Bodegom ME, van Goor H, et al. Autologous tissue repair of large abdominal wall defects. Br J Surg. 2007;94:791–803. [DOI] [PubMed] [Google Scholar]
- 5.Luijendijk RW, Hop WC, van den Tol MP, et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med. 2000;343:392–398. [DOI] [PubMed] [Google Scholar]
- 6.Clifton MS, Heiss KF, Keating JJ, et al. Use of tissue expanders in the repair of complex abdominal wall defects. J Pediatr Surg. 2011;46:372–377. [DOI] [PubMed] [Google Scholar]
- 7.Jacobsen WM, Petty PM, Bite U, et al. Massive abdominal-wall hernia reconstruction with expanded external/internal oblique and transversalis musculofascia. Plast Reconstr Surg. 1997;100:326–335. [DOI] [PubMed] [Google Scholar]
- 8.Chesov I, Belîi A.Postoperative analgesic efficiency of transversus abdominis plane block after ventral hernia repair: a prospective, randomized, controlled clinical trial. Rom J Anaesth Intensive Care. 2017;24:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colvin J, Rosen M, Prabhu A, et al. Enhanced recovery after surgery pathway for patients undergoing abdominal wall reconstruction. Surgery. 2019;166:849–853. [DOI] [PubMed] [Google Scholar]
- 10.Smolle C, Tuca A, Wurzer P, et al. Complications in tissue expansion: a logistic regression analysis for risk factors. Burns. 2017;43:1195–1202. [DOI] [PubMed] [Google Scholar]


