Abstract
There is scarce evidence that the erythrocyte sedimentation rate (ESR) could efficiently improve the prediction accuracy of the Global Registry of Acute Coronary Events (GRACE) risk score in cases of ST-elevation myocardial infarction (STEMI).
A cohort of 1094 STEMI patients undergoing primary percutaneous coronary intervention was retrospectively recruited. Patients were categorized based on the ESR values. Final endpoints included cardiovascular death and major adverse cardiovascular event (MACE) occurrence. The predictive value of combined models with the GRACE score and ESR was assessed by receiver operating characteristic (ROC) analysis, net reclassification improvement (NRI), and integrated discrimination improvement.
During the mean follow-up of 23 months, 34 patients died and 190 experienced MACEs, of which 23 patients died in the first year; both endpoints were more frequent in the higher group. The ESR and high-sensitivity C-reactive protein (hs-CRP) were independent risk factors of 1-year cardiovascular death, together with the GRACE score (ESR: hazard ratio = 1.03, P = .006 hs-CRP: hazard ratio = 1.00, P = .001; GRACE: 1.03, P = .012). Although no statistical improvement in the area under the ROC curve was observed in either the GRACE/ESR or the GRACE/hs-CRP model (GRACE/ESR models: 0. 8073 vs GRACE: 0.7714, P = .22; GRACE/ESR models: 0. 7815 vs GRACE: 0.7714, P = .61), the GRACE score and ESR together significantly improved the NRI (0.633; P< .001) compared with the GRACE alone. Regarding the mid-term mortality, adding the ESR to the GRACE score not only improved the NRI (0.8433; P < .001), but also increased the integrated discrimination improvement (0.0509; P = .04).
The ESR is an independent risk factor of cardiovascular death and MACE in STEMI patients receiving primary percutaneous coronary intervention. The ESR comparatively enhanced the predictive values of the prognostic model, including the GRACE risk score.
Keywords: erythrocyte sedimentation rate, global registry of acute coronary events risk scores, major adverse cardiovascular events, ST-elevated myocardial infarction
1. Introduction
Patients with acute ST-segment elevated myocardial infarction (STEMI) are vulnerable to a higher risk of long-term cardiovascular mortality and adverse clinical events. Risk stratification is crucial in the management and treatment of patients with STEMI. In contrast to other risk scores used for assessment of prognosis,[1,2] the Global Registry of Acute Coronary Event (GRACE) risk score is predictive of discharge mortality and re-infarction and has been recommended as the preferred risk score in acute coronary syndrome (ACS).[3,4] However, the scant number of biological markers included in the GRACE risk score (GRS) may contribute to the insufficient discriminatory performance. Recent studies have focused on improving discrimination of GRS by integrating other biomarkers.[5,6]
Chronic inflammatory processes are related to the pathogenesis and extension of atherosclerosis in coronary artery disease.[7] Several studies show that inflammatory markers, such as C-reactive protein (CRP),[8,9] neutrophil counts,[10] mean platelet volume,[11] platelet-to-lymphocyte ratio,[12] and neutrophil-to-lymphocyte ratio,[13,14] correlate with GRS and adversely predict short- and long-term prognosis. However, none of these works demonstrates the independent predictive value of the erythrocyte sedimentation rate (ESR) on clinical adverse outcomes. A few previous studies that investigate the association of the elevated ESR with an increased risk of fatal cardiovascular events yield some positive results.[15,16] Moreover, the increased ESR in aggressive forms of coronary artery disease (CAD) could be regarded as an index to predict coronary artery disease mortality.[17] However, few studies elucidate the additional value of ESR on improving risk prediction and risk-guide therapy in the setting of STEMI.
Given the above considerations, the objective of the present study is to investigate the predictive values of the ESR for major adverse cardiovascular events (MACE) and the incremental prognostic value of the ESR combined with the GRS on cardiovascular mortality in STEMI patients undergoing primary percutaneous coronary intervention (PCI).
2. Materials and Methods
2.1. Study setting and inclusion
We retrospectively recruited consecutive patients with STEMI admitted to the Department of Cardiology at the Beijing Chaoyang Hospital in China between January 2014 and December 2018. STEMI patients received primary PCI were eligible for the study. STEMI was defined according to the 2013 American College of Cardiology Foundation/American Heart Association Guidelines. The diagnostic criteria included the following:
-
(1)
persistent symptoms of ischemia for at least 30 minutes;
-
(2)
ST-segment elevation of at least 1 mm in at least 2 adjacent limb leads, or of at least 2 mm in at least 2 contiguous precordial leads, or a new left bundle branch block in the electrocardiography; and
-
(3)
elevated serum cardiac troponin-I and creatine kinase-myocardial band more than twice the upper limit of normal level.
The excluded criteria included valvular heart disease, malignant tumors, severe liver dysfunction and end stage renal disease.
2.2. Demographic feature and biochemical measurements
Clinical and demographic characteristics of all patients were recorded and included age, sex, comorbidities, vital signs, and results of auxiliary examinations. Additionally, previous myocardial infarction (MI), previous PCI, and presence of congestive heart failure and cardiac arrest were recorded. The GRACE risk predicting model was calculated by 2 senior doctors (Dr. Chuang Li and Dr. YuXing Wang) blinded to the clinical outcomes using the web-based tool (https://www.outcomes-umassmed.org/grace). The related variables including age, heart rate, systolic blood pressure, level of creatinine, history of congestive heart failure, revascularization (primary PCI), previous MI, ST-segment depression, and elevated cardiac markers were entered into the GRS to estimate the cumulative risks of the MACE. The ESR and high-sensitivity CRP (hs-CRP) were detected using an analytical instrument (Monitor 100, Italy) method and a turbidimetric inhibition immune-assay within 24 hours of admission, respectively.
2.3. Definition of endpoint and follow-up
Patients were followed for up for major adverse outcomes. The follow-up records were completed by telephone contacts and hospital documents. The primary endpoint was defined as the cardiovascular death. The secondary endpoint was defined as a composite of the MACE consisting of cardiovascular mortality, rehospitalization due to acute heart failure, recurrent MI, revascularization due to unstable angina pectoris and non-fatal stroke. The follow-up information of all included participants was collected by 1 of the authors (Dr. Qian Zhang). This study was approved by the institutional review board of the Chaoyang Hospital and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from all patients or their legal relatives.
2.4. Statistical analysis
Continuous variables are presented as mean ± standard deviation (mean ± standard deviation) if the data distribution fits both normal distribution and homogeneity of variance. The distribution of the data was tested using the skewness and Kurtosis normality tests. The continuous variables not satisfying these criteria are presented as median (interquartile range, IQR). Categorical variables are presented as frequency (percentage). Statistical differences between the continuous variables was examined using 1-way analysis of variance or the Kruskal–Wallis rank test according to the distribution and characteristics of the data. Differences among the categorical variables were determined by the Pearson χ2 test or the Fisher exact test. The Kaplan-Meier method was used to calculate adverse clinical outcomes and the log-rank test was used to conduct intergroup comparisons. The multivariate Cox proportional hazard regression analysis was performed to identify predictors for adverse clinical outcomes.
The χ2 likelihood ratio tests were used with nested models to determine whether the logistic regression model in combination with the GRS and the ESR or hs-CRP would provide significantly better prediction probabilities of the outcomes than could the GRS would alone. Comparisons of the nested and non-nested models including the GRS or its combination with the ESR or the hs-CRP were performed using corrected Akaike information criterion (AICc), delta-AICc (Δ-AICc), and Akaike weights (wi) to estimate the “best” fitting model.[18]
Three models that could assess and quantify the improvement of risk prediction, including increase in the area under the receiver operating characteristic curve (AUC), net reclassification improvement (NRI >0), and integrated discrimination improvement (IDI), were used to analyze the incremental predictive value of the combinations of the ESR and hs-CRP with the GRS.[19] The event NRI (NRIe) was defined as the net percentage of persons with the event of interest correctly assigned to a higher predicted risk, and non-event NRI (NRIne) was defined as the net percentage of persons without the event of interest correctly assigned to a lower predicted risk. The overall NRI was equal to the net proportion of persons with events of interest plus those without events of interest (NRI = NRIe+NRIne). The IDI was used to examine the differences between the average sensitivity and specificity for the models combined GRS with or without the ESR or the hs-CRP.[20] All statistical analyses were calculated using STATA (version 15.0).
3. Results
3.1. Study population and demographic feature
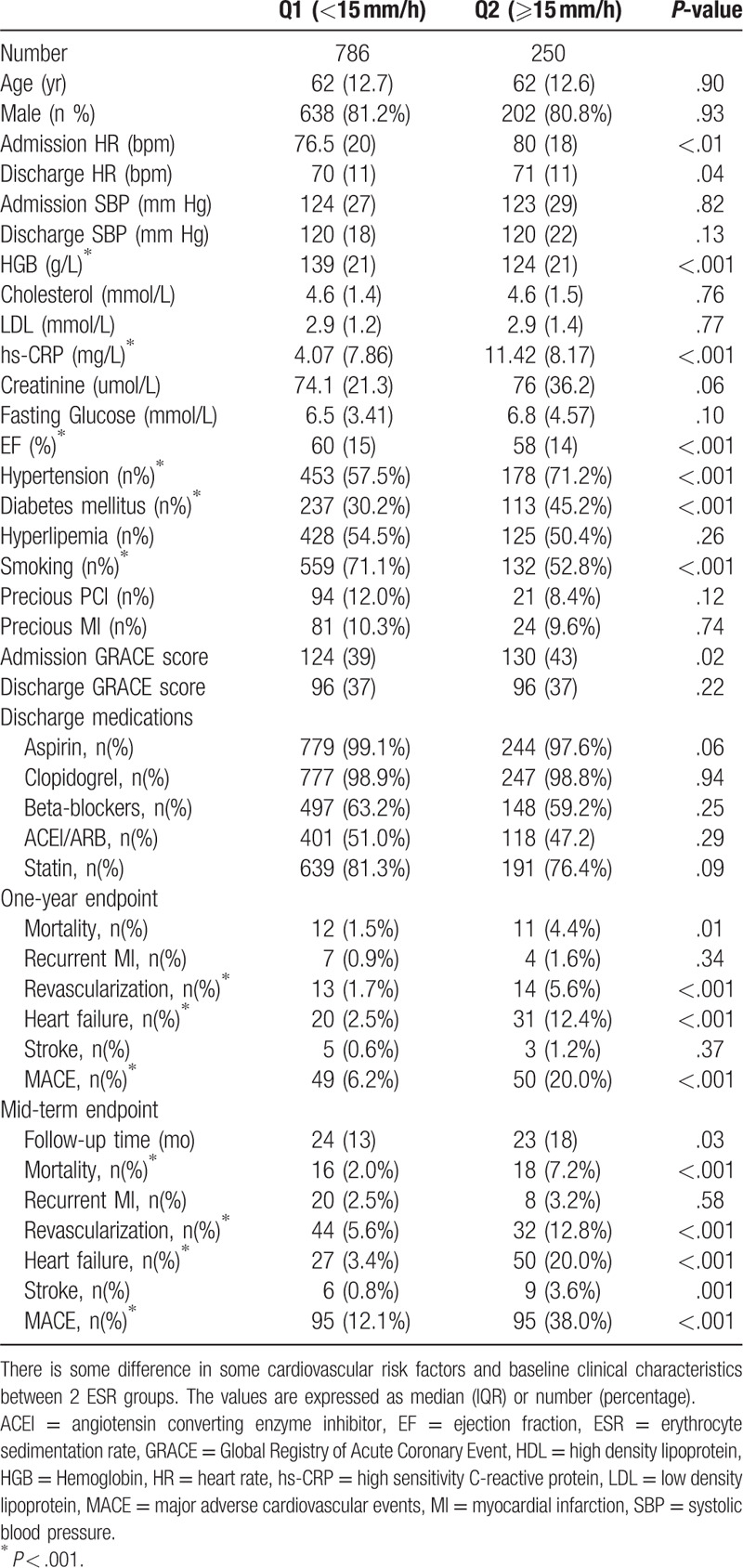
Initially, 1094 patients were enrolled in this retrospective cohort study. According to the excluded criteria, a total of 1036 patients were recruited and followed up. The final eligible population were categorized into 2 groups according to the values of ESR (Q1: < 15 mm/h; Q2 ESR≥15 mm/h). The patient baseline characteristics, history of disease, and discharge medication are listed in Table 1. As generally reported in similar cohorts of patients, patients in the higher group had a relatively lower hemoglobin count, significantly elevated level of admission heart rate, lower ejection fraction, and more frequent history of smoking, hypertension and diabetes mellitus. The value of hs-CRP was significantly higher in the higher ESR group than in the lower ESR group (P < .001), whereas no difference was seen in the discharge GRS (P = .22). There was no significant difference in other variables between the 2 groups.
Table 1.
Baseline clinical characteristics according to the ESR.
3.2. Relation of the ESR and GRS to clinical outcomes
During the average follow-up period of 23 months, a total of 99 MACE (9.6%) occurred in the first year, including 23 cardiovascular deaths (2.2%), 51 acute heart failures (4.9%), 11 recurrent MIs (1.1%), 28 revascularizations (2.7%), and 8 non-fatal strokes (0.8%), while the sum of mid-term mortality and MACE were 34 (3.3%) and 190 (18.3%), respectively (Table 1). Cardiovascular death and MACE were both more frequently presented in the higher quartiles (One-year cardiovascular death: 4.4% vs 1.5%, P = .01; One-year MACE: 20.0% vs 6.2%, P < .001; mid-term cardiovascular death: 7.2% vs 2.0%, P < .001; mid-term MACE: 38.0% vs. 12.1%, respectively). The cumulative incidence of 1-year and mid-term cardiovascular death and MACE among different ESR groups evaluated using the Kaplan-Meier survival curves are shown in Figures 1 and 2, respectively. The incident of cardiovascular death and MACE increased significantly in the higher ESR group compared with the lower 1.
Figure 1.
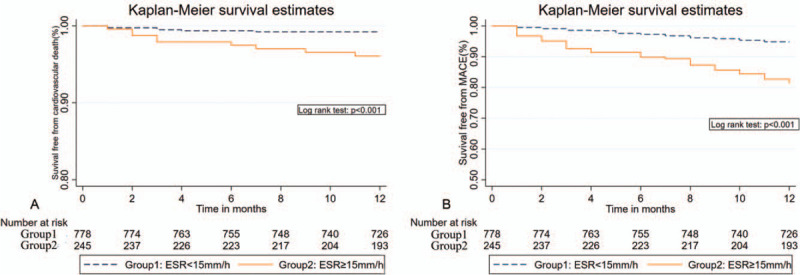
Kaplan-Meier survival curves indicating 1-year cardiovascular death and MACE according to values of the ESR.
Figure 2.
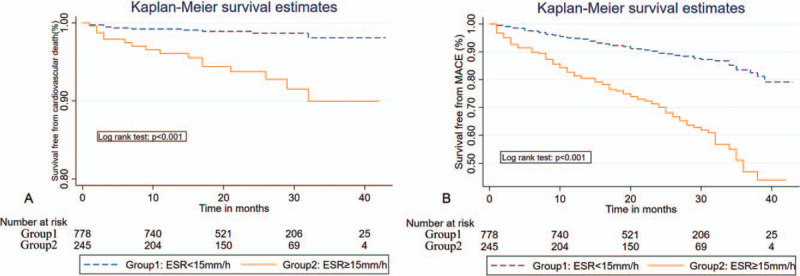
Kaplan-Meier survival curves indicating 1-year cardiovascular death and MACE during the mean follow-up time of 23 months according to values of the ESR.
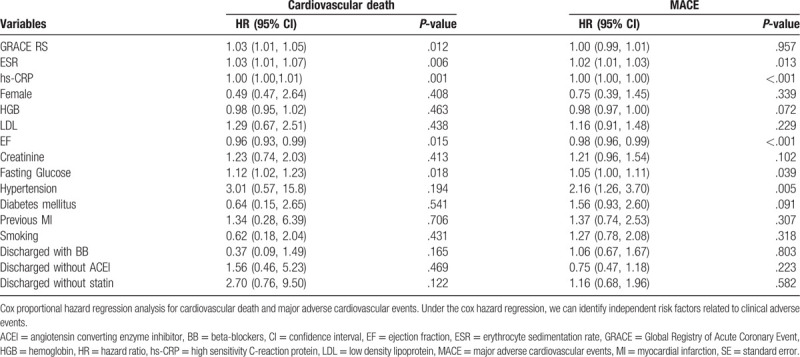
The multivariate Cox proportional hazard regression analysis suggested that the GRS 9hazard ratio [HR] = 1.03 (1.01–1.05), P = .012), ESR [HR = 1.03 (1.01–1.07), P = .006], and hs-CRP [HR = 1.00 (1.00–1.01), P < .001)] were independent predictors of the 1-year cardiovascular death after adjusting for the potential confounding factors (Table 2). When included in the same regression model, ESR [HR = 1.02 (1.01–1.03), P = .013] and hs-CRP [HR = 1.00 (1.00–1.00), P < .001], but not GRS [HR = 1.00 (0.99–1.01), P = .957], were independent predictors of the 1-year MACE at the final follow-up (Table 2).
Table 2.
Cox proportional hazard regression analysis for 1-year cardiovascular death and MACE.
3.3. Evaluating the impact of the ESR on GRS model
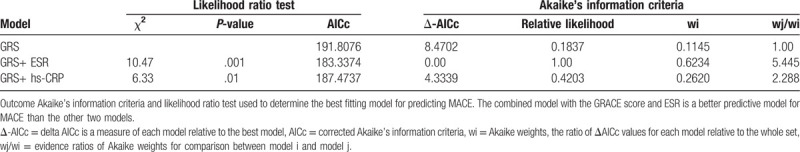
Given the negative results of GRS over MACE, we conducted the likelihood ratio tests which could provide a positively better predictive probability regarding the 1-year cardiovascular death among the 3 models. The results indicated that the models combining the GRS with the ESR, rather than with hs-CRP levels, allowed for more accurate prediction of cardiovascular death by means of quantitative comparison of the AICc, Δ-AICc, and Akaike weights (Table 3). Evidently, examination of Akaike weights revealed that models containing the GRS and the ESR is 5.445 times more likely to be superior compared with the models containing GRS alone.
Table 3.
Akaike's information criteria and likelihood ratio test to determine the best fitting model for predicting 1-year cardiovascular death.
The ROC analysis was performed to assess which of the prognostic models integrating GRS with the ESR or hs-CRP levels could better predict 1-year and mid-term mortality. There was no statistical difference in the AUC between the GRS and 2 inflammatory makers (GRS: 0.6932 vs ESR: 0.6973 vs hs-CRP: 0.6552, P = .72). The AUC of the ROC moderately increased when the GRS was coupled with the ESR (AUC = 0.8073 vs 0.7714, P = .22), but not with the hs-CRP level (AUC = 0.7815 vs 0.7714, P = .61) (Fig. 3). Regarding the mid-term mortality, the AUC of the ROC significantly elevated when the ESR was integrated into the GRS (AUC = 0.8399 vs 0.7965, P= .012) (Fig. 4).
Figure 3.
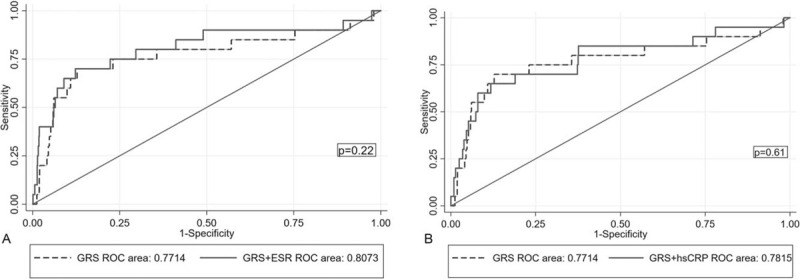
Receiver-operating characteristic (ROC) curves comparing 2 combined models and the GRS alone in predicting one-year cardiovascular mortality.
Figure 4.
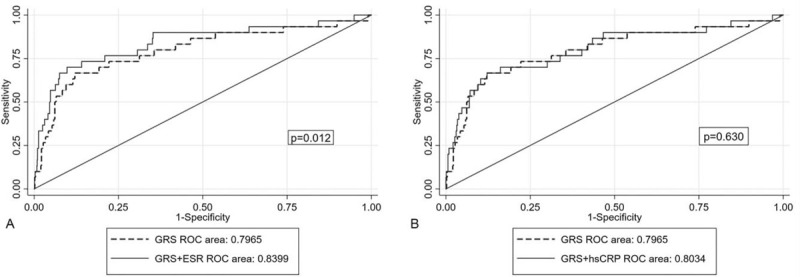
Receiver-operating characteristic (ROC) curves comparing 2 combined models and the GRS alone in predicting mid-term cardiovascular mortality.
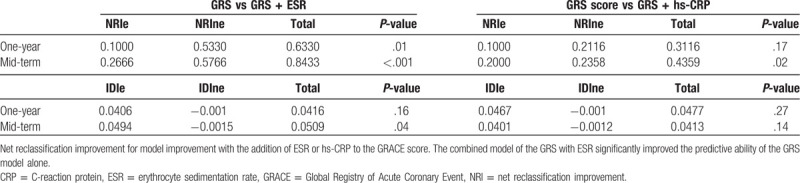
When incorporated into a logistic regression model containing the GRS, the ESR could enhance the net reclassification of the new model in predicting the 1-year cardiovascular death during follow-up (Table 4). In the analysis of NRI, the ESR could improve reclassification by 10.0% for those with events of interest and by 53.3% for those with events of not of interest, respectively. Finally, under the equation mentioned above, the total NRI was 0.633 (P = .01). However, the integrated discrimination during follow-up was seldomly improved by the addition of the ESR into the GRACE scoring system (IDI: 4.16%, P = .16). In contrast, models combining GRACE with hs-CRP couldn’t improve reclassification and discrimination compared with models containing GRACE alone (NRI = 0.3116, p = 0.17; IDI = 4.77%, P = .27). However, in the mid-term duration, while the ESR could progressively improve the net reclassification by 84.33% and integrated discrimination by 5.09% for the GRS (NRI: 0.8433, P < .001; IDI: 0.0509, P = .04).
Table 4.
Net reclassification improvement for model improvement with the addition of ESR or hs-CRP into GRS on cardiovascular mortality.
4. Discussion and Conclusions
This retrospective cohort study of STEMI patients demonstrated that
-
(1)
GRS remains accurate for the prediction of 1-year and mid-term cardiovascular mortality under the current management of patients with STEMI, similar to a previous study[21];
-
(2)
the ESR as well as hs-CRP collected during the first 24-hour after admission are independent risk factors for cardiovascular mortality and adverse clinical events; and
-
(3)
the additive value of the ESR on models based on the GRS could efficiently enhance the predictive probability of cardiovascular death.
To the best of our knowledge, the present study is the first to demonstrate that the ESR could enhance the predictive probability of the GRACE risk model in STEMI patients undergoing primary PCI.
The ESR is a simple and inexpensive test and influenced by plasma constituents, such as fibrinogen, globulins, and hemoglobin, but generally varies with age and sex.[17,22,23] In the present study, after adjusting for gender and conventional risk factors, the independent association we observed between the ESR and cardiovascular death remained, as has been previously suggested.[15–17,24] Destabilization and rupture of plaque in the pathophysiology of STEMI may primarily be presented as an intimal hemorrhage. In this circumstance, the accumulation of erythrocytes, which participate in the necrotic-core enlargement within the lesions, may be the proinflammatory factor that increases the risk of plaque rupture.[25] On the other hand, increased ESR correlates with increased red blood cell adhesiveness and aggregation. A previous study by Sargento et al[26] demonstrated that lower erythrocyte aggregation is associated with a lower incidence of long-term recurrent cardiovascular events in survivors with STEMI. Moreover, the recent study by Yunoki et al indicates that hyper-aggregability induced by severe oxidative stress elicits erythrocyte-rich thrombus, which is proportional to the extent of myocardial necrosis and deterioration of ventricular function and remodels from the acute to recovery phase in the setting of re-perfused STEMI.[27] Our findings provide the cumulative evidence that increased ESR can lead to worse prognosis in the course of STEMI.
Clinical studies show that both ESR and hs-CRP are associated with incremental MI and stroke risk in the general population,[15,24,28,29] particularly in patients with chronic inflammation disorders.[30,31] Notably, a large amount of published data indicate that hs-CRP is significantly associated with the extent of coronary stenosis and prognosis in patients with ACS and may even improve some risk stratifications.[5,32] In a previous meta-analysis by He et al., CRP is correlated with the long-term risk of recurrent cardiovascular events or death in patients with ACS.[33] However, this meta-analysis does not clarify the association between hs-CRP and the clinical prognosis in patients with acute MI. Our present study also found that there is no significant increase in the C-statistics of the GRS when hs-CRP is incorporated into the routine assessment of patients with STEMI, similar to 2 previous studies.[9,34] In recent genetic studies and meta-analyses,[35,36] published data cannot confer any evidence of a causal association between CRP and atherogenesis and indicates that it is likely to be a bystander rather than a true risk factor. In another study by Van Toorenburg et al[5] the addition of other biomarkers, including hs-CRP, to the GRS improves the AUC over GRS alone; however, the final model is excessively complicated and it is not certain if the relationship between hs-CRP and mortality contributes to this increment. Meanwhile, investigations by Sumayya et al find that the GRS of MI patients nearly correlates with hs-CRP and inflammation markers.[8] This intimate interaction between the hs-CRP and GRS can provide some rational explanation about the negative consequences of the new model, which combines the hs-CRP and GRS, in our study.
In this study, we excluded patients, who had tumors, but not those with inflammatory or metabolic diseases. There is a possibility that other potential disorders can influence the ESR and confound its association with mortality. In the HDDRISC study, Gosland et al show that elevated ESR could predict death from cardiovascular disease during a period of 18 to 20 years, independent of metabolic syndrome.[37] Meanwhile, prior studies indicate that patients with chronic inflammation disorders, such as rheumatoid arthritis, psoriasis, and chronic obstructive pulmonary disease, are vulnerable to a higher risk of MACE.[38–40] Moreover, in this circumstance, increased ESR is associated with an increased prevalence of future MACE risk. Therefore, we assume that the fluctuation in rheology could offer a possible mechanism to mediate the higher prevalence of cardiovascular morbidity and mortality in patients with inflammatory disorders.
Finally, our findings of this retrospective study indicate that the GRS is only an independent risk factor for mortality, but not for MACE. A number of previous studies show that the GRS has a predictable probability for MACE composited of death and MI.[5,10,14,41] Although the study by Fan et al of Chinese STEMI patients finds some association between the GRS and complex cardiovascular events,[42] our study finds the predictive ability of the GRS on multiple major adverse events to remain inadequate and further work is required. Nevertheless, the disparity between our and Fan et al results could be due to the lower rate of cardiovascular death and MACE in our center, which is owed by the timely reperfusion and complete revascularization in accordance with current guidelines and management of STEMI.
Several limitations exist in this study and should be considered. First, our study did not clarify the status and extent of chronic inflammation disorders, which may influence the ESR and confounded the association with clinical adverse endpoints. From the viewpoint of prior studies, severe diseases, which have inflammatory properties, do not significantly influence the association between ESR and MACE.[28] However, the severity and classification of different chronic inflammation disorders may ultimately interfere with the final results of this study. Secondly, it must be stated that the number of eligible patients with STEMI from only 1 cardiac center is relatively small, and this low prevalence of 1-year mortality could also contribute to the negative result of the models containing the ESR and GRS seen here. Accordingly, in cases of higher cardiovascular deaths, the improvement of integrated discrimination was seen in the models combining the ESR and GRS. Thirdly, in the mean period of 23 months, we did not report the details of patients, who could not be followed up or did not consent to the study. This could inevitably result in selection bias. While we propose that the ESR to be used as a predictor of clinical prognosis, future study with multiple centers and larger numbers of patients should be conducted to support our results.
In conclusion, the present study showed that the ESR is a strong predictor of cardiovascular death and adverse clinical outcomes in STEMI patients undergoing primary PCI after adjustment for traditional cardiovascular risk factors. Moreover, the ESR enhanced the predictability of the GRS with regard to the mortality of the STEMI patients. This study provides support for future investigations aiming to evaluate the value of ESR on risk prediction and risk-guided therapy.
Author contributions
Chuang Li: Conceptualization, Software, Validation, Writing-Original Draft; YuXing Wang: Methodology, Investigation, Data Curation, Writing-Original Draft; Qian Zhang: Software, Writing-Original Draft, Visualization; LeFeng Wang: Resources, Supervision, Funding acquisition; KuiBao Li: Formal analysis, Writing-Review and Editing; XinChun Yang: Writing-Review and Editing, Visualization, Project administration.
Glossary
Abbreviations: ACS = acute coronary syndrome, AIC = Akaike information criterion, AUC = area under receiver operating characteristic curve, ESR = erythrocyte sedimentation rate, GRACE = global registry of acute coronary event, HR = hazard ratio, Hs-CRP = high sensitivity C-reactive protein, IDI = integrated discrimination improvement, MACE = major adverse cardiovascular events, NRI = net reclassification improvement, PCI = percutaneous coronary intervention, STEMI = acute ST-elevated myocardial infarction.
References
- [1].Littnerova S, Kala P, Jarkovsky J, et al. GRACE score among six risk scoring systems (CADILLAC, PAMI, TIMI, Dynamic TIMI, Zwolle) demonstrated the best predictive value for prediction of long-term mortality in patients with ST-elevation myocardial infarction. PloS One 2015;10:e0123215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Raposeiras-Roubin S, Abu-Assi E, Cabanas-Grandio P, et al. Walking beyond the GRACE (Global Registry of Acute Coronary Events) model in the death risk stratification during hospitalization in patients with acute coronary syndrome: what do the AR-G (ACTION [Acute Coronary Treatment and Intervention Outcomes Network] Registry and GWTG [Get With the Guidelines] Database), NCDR (National Cardiovascular Data Registry), and EuroHeart Risk Scores Provide? JACC Cardiovas Intervent 2012;5:1117–25. [DOI] [PubMed] [Google Scholar]
- [3].Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation of the european society of cardiology (ESC). European Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- [4].Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction (Updating the 2007 Guideline and Replacing the 2011 Focused Update) A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012;60:645–81. [DOI] [PubMed] [Google Scholar]
- [5].van Toorenburg M, van den Berg VJ, van der Ploeg T, et al. Addition of routinely measured blood biomarkers significantly improves GRACE risk stratification in patients with myocardial infarction. Int J Cardiol 2018;273:237–42. [DOI] [PubMed] [Google Scholar]
- [6].Liu XJ, Wan ZF, Zhao N, et al. Adjustment of the GRACE score by HemoglobinA1c enables a more accurate prediction of long-term major adverse cardiac events in acute coronary syndrome without diabetes undergoing percutaneous coronary intervention. Cardiovascular Diabetol 2015;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci 2018;132:1243–52. [DOI] [PubMed] [Google Scholar]
- [8].Shahzad S, Mateen S, Hasan A, et al. GRACE score of myocardial infarction patients correlates with oxidative stress index, hsCRP and inflammation. Immunobiology 2019. [DOI] [PubMed] [Google Scholar]
- [9].Correia LC, Vasconcelos I, Garcia G, et al. Does C-reactive protein add prognostic value to GRACE score in acute coronary syndromes? Arquivos brasileiros de cardiologia 2014;102:449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang S, Wan Z, Zhang Y, et al. Neutrophil count improves the GRACE risk score prediction of clinical outcomes in patients with ST-elevation myocardial infarction. Atherosclerosis 2015;241:723–8. [DOI] [PubMed] [Google Scholar]
- [11].Wan ZF, Zhou D, Xue JH, et al. Combination of mean platelet volume and the GRACE risk score better predicts future cardiovascular events in patients with acute coronary syndrome. Platelets 2014;25:447–51. [DOI] [PubMed] [Google Scholar]
- [12].Zhou D, Fan Y, Wan Z, et al. Platelet-to-lymphocyte ratio improves the predictive power of GRACE risk score for long-term cardiovascular events in patients with acute coronary syndrome. Cardiology 2016;134:39–46. [DOI] [PubMed] [Google Scholar]
- [13].Zhou D, Wan Z, Fan Y, et al. A combination of the neutrophil-to-lymphocyte ratio and the GRACE risk score better predicts PCI outcomes in Chinese Han patients with acute coronary syndrome. Anatolian J Cardiol 2015;15:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oncel RC, Ucar M, Karakas MS, et al. Relation of neutrophil-to-lymphocyte ratio with GRACE risk score to in-hospital cardiac events in patients with ST-segment elevated myocardial infarction. Clin Appl Thromb-Hem 2015;21:383–8. [DOI] [PubMed] [Google Scholar]
- [15].Erikssen G, Liestol K, Bjornholt JV, et al. Erythrocyte sedimentation rate: a possible marker of atherosclerosis and a strong predictor of coronary heart disease mortality. European Heart J 2000;21:1614–20. [DOI] [PubMed] [Google Scholar]
- [16].Timmer JR, Ottervanger JP, Hoorntje JC, et al. Prognostic value of erythrocyte sedimentation rate in ST segment elevation myocardial infarction: interaction with hyperglycaemia. J Intern Med 2005;257:423–9. [DOI] [PubMed] [Google Scholar]
- [17].Natali A, L’Abbate A, Ferrannini E. Erythrocyte sedimentation rate, coronary atherosclerosis, and cardiac mortality. European Heart J 2003;24:639–48. [DOI] [PubMed] [Google Scholar]
- [18].Wagenmakers EJ, Farrell S. AIC model selection using Akaike weights. Psychon B Rev 2004;11:192–6. [DOI] [PubMed] [Google Scholar]
- [19].Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–72. [DOI] [PubMed] [Google Scholar]
- [20].Kerr KF, McClelland RL, Brown ER, et al. Evaluating the incremental value of new biomarkers with integrated discrimination improvement. Am J Epidemiol 2011;174:364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shuvy M, Beeri G, Klein E, et al. Accuracy of the global registry of acute coronary events (GRACE) risk score in contemporary treatment of patients with acute coronary syndrome. Can J Cardiol 2018;34:1613–7. [DOI] [PubMed] [Google Scholar]
- [22].Fest J, Ruiter R, Mooijaart SP, et al. Erythrocyte sedimentation rate as an independent prognostic marker for mortality: a prospective population-based cohort study. J Intern Med 2019;285:341–8. [DOI] [PubMed] [Google Scholar]
- [23].Danesh J, Collins R, Peto R, et al. Haematocrit, viscosity, erythrocyte sedimentation rate: meta-analyses of prospective studies of coronary heart disease. European Heart J 2000;21:515–20. [DOI] [PubMed] [Google Scholar]
- [24].Godsland IF, Bruce R, Jeffs JA, et al. Inflammation markers and erythrocyte sedimentation rate but not metabolic syndrome factor score predict coronary heart disease in high socioeconomic class males: the HDDRISC study. Int J Cardiol 2004;97:543–50. [DOI] [PubMed] [Google Scholar]
- [25].Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 2003;349:2316–25. [DOI] [PubMed] [Google Scholar]
- [26].Sargento L, Saldanha C, Monteiro J, et al. Long-term prognostic value of protein C activity, erythrocyte aggregation and membrane fluidity in transmural myocardial infarction. Thrombosis and Haemostasis 2005;94:380–8. [DOI] [PubMed] [Google Scholar]
- [27].Yunoki K, Naruko T, Sugioka K, et al. Erythrocyte-rich thrombus aspirated from patients with ST-elevation myocardial infarction: association with oxidative stress and its impact on myocardial reperfusion. European Heart J 2012;33:1480–90. [DOI] [PubMed] [Google Scholar]
- [28].Toss F, Nordstrom A, Nordstrom P. Inflammation in young adulthood is associated with myocardial infarction later in life. Am Heart J 2013;165:164–9. [DOI] [PubMed] [Google Scholar]
- [29].Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004;350:1387–97. [DOI] [PubMed] [Google Scholar]
- [30].Navarro-Millan I, Yang S, DuVall SL, et al. Association of hyperlipidaemia, inflammation and serological status and coronary heart disease among patients with rheumatoid arthritis: data from the National Veterans Health Administration. Ann Rheum 2016;75:341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang J, Chen L, Delzell E, et al. The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Ann Rheum 2014;73:1301–8. [DOI] [PubMed] [Google Scholar]
- [32].Musikhina NA, Petelina TI, Kostousova AI, et al. Inflammatory markers in patients with acute coronary syndrome. European Heart J 2018;7:262. [Google Scholar]
- [33].He LP, Tang XY, Ling WH, et al. Early C-reactive protein in the prediction of long-term outcomes after acute coronary syndromes: a meta-analysis of longitudinal studies. Heart (British Cardiac Society) 2010;96:339–46. [DOI] [PubMed] [Google Scholar]
- [34].Raposeiras Roubin S, Barreiro Pardal C, Roubin-Camina F, et al. High-sensitivity C-reactive protein predicts adverse outcomes after non-ST-segment elevation acute coronary syndrome regardless of GRACE risk score, but not after ST-segment elevation myocardial infarction. Revista portuguesa de cardiologia 2013;32:117–22. [DOI] [PubMed] [Google Scholar]
- [35].Dehghan A, Dupuis J, Barbalic M, et al. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation 2011;123:731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Elliott P, Chambers JC, Zhang W, et al. Genetic Loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA 2009;302:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Godsland IF, North BV, Johnston DG. Simple indices of inflammation as predictors of death from cancer or cardiovascular disease in a prospective cohort after two decades of follow-up. Qjm-an International Journal of Medicine 2011;104:387–94. [DOI] [PubMed] [Google Scholar]
- [38].Charles-Schoeman C, DeMasi R, Valdez H, et al. Risk factors for major adverse cardiovascular events in phase III and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis & rheumatology (Hoboken, NJ) 2019;71:1450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lazzeri C, Valente S, Attana P, et al. The prognostic role of chronic obstructive pulmonary disease in ST-elevation myocardial infarction after primary angioplasty. European journal of preventive cardiology 2013;20:392–8. [DOI] [PubMed] [Google Scholar]
- [40].Cooksey R, Brophy S, Kennedy J, et al. Cardiovascular risk factors predicting cardiac events are different in patients with rheumatoid arthritis, psoriatic arthritis, and psoriasis. Seminars in arthritis and rheumatism 2018;48:367–73. [DOI] [PubMed] [Google Scholar]
- [41].Moady G, Iakobishvili Z, Beigel R, et al. The predictive value of low admission hemoglobin over the GRACE score in patients with acute coronary syndrome. J Cardiol 2019;73:271–5. [DOI] [PubMed] [Google Scholar]
- [42].Fan Y, Wang J, Zhang S, et al. Homocysteine enhances the predictive value of the GRACE risk score in patients with ST-elevation myocardial infarction. Anatolian J Cardiol 2017;18:182–93. [DOI] [PMC free article] [PubMed] [Google Scholar]


