Abstract
OBJECTIVES
Thymus is a lymphoepithelial system in which cells responsible for the immune system are produced and directed. The aim of this study is to determine the overall survival effect of rebound thymic hyperplasia (RTH) in patients with non-small cell lung cancer (NSCLC) treated with systemic chemotherapy (CT).
MATERIALS AND METHODS
The study was designed as retrospective case series. One hundred and thirty patients who met the inclusion criteria were evaluated. Demographic data, type of tumor, and treatments administered were recorded. The frequency of RTH development and the relationship between RTH development and survival was investigated.
RESULTS
The median age of the patients was 59, and nine of 13 patients (69.4%) with RTH were iden-tified as stable disease, two patients had a partial response (15.3%), and two were evaluated as progres-sive disease (15.3%). Of the remaining 117 patients, 78 (66.6%) had stable disease, 11 (9.4%) had com-plete response, 21 (17.9%) had partial response, and seven patients were evaluated as having progressive disease (5.9%). The patients were categorized into two groups: Group 1 - without RTH and group 2 - with RTH. Thirteen (10%) of 130 patients developed RTH (group 2), while the remaining 117 (90%) patients did not have RTH (group 1). There was no difference between the two groups (59.1 years) in terms of age (p = 0.933). The RTH developed after a median time of 4.5 months (2–7 months) after CT had been administered. Overall survival was longer in patients with RTH than in patients without RTH (20.04 months) (95% CI, 4.79–35.29) vs. 10.05 months (95% CI, 8.74–11.36; p=0.049).
CONCLUSION
The developing of RTH during systemic CT may be a prognostic marker in stage 4 non-small cell lung cancer.
Keywords: Lung cancer, survival, thymus
INTRODUCTION
Thymus is a lymphoepithelial system in which cells responsible for the immune system are produced and directed. It is also considered to be the first center of cell differentiation with antibody production. T-lymphocytes responsible for cellular immunity are produced in thymus and thymus aids B-lymphocytes. Undifferentiated lymphocytes which move to the thymus via blood proliferate and differentiate in thymus [1].
Rebound thymic hyperplasia (RTH) may develop in patients receiving chemotherapy (CT) with any malignancy [2]. Increased metabolic activity caused by benign hyperplasia in the thymus has been previously described in children and adolescents receiving CT and may also be seen in young adults. Some of the research performed in this regard are case reports, while others are studies with small sample size. For example, it has been shown that RTH develops in primary synovial sarcoma after CT and pneumonectomy (2), in 3 neuroblastoma patients after high dose CT [3], in lymphoma children after CT [4].
The aim of this study is to determine the overall survival effect of RTH in patients with non-small cell lung cancer (NSCLC) treated with systemic CT.
MATERIALS AND METHODS
Of the 267 patients with NSCLC who were diagnosed and treated only with CT, 130 patients who met the inclusion criteria were retrospectively evaluated. Demographic data, type of tumor, and treatments administered were recorded. The frequency of RTH development and the relationship between RTH development and survival was investigated.
Inclusion Criteria
Those older than 18 years with cytologically or histologically diagnosed NSCLC and who received at least two cycles of systemic CT and were evaluated with contrast-enhanced chest computed tomography (CE-CT) or PET-CT before and after CT.
Exclusion Criteria
Thoracic radiotherapy in definitive doses including pre-evaluation thymus region, small cell lung cancer, radiological bronchial carcinoma cases, resection after medical treatment, and cases in which the thymus may not be evaluated due to tumor invading the upper/anterior mediastinum.
Evaluation of Thymic Gland with CE-CT
CE-CT response and thymic size assessments were evaluated by the same radiologist using axial CE-CT scans of the CE-CT obtained at baseline, after 2, 4. or 6 cycles of CT and six months after the CT was completed. According to morphology, size, and attenuation values, the thymus was classified into four grades (grades 0, 1, 2, and 3). Thymus with complete involution and homogeneous fat densities, which could not be distinguished from the mediastinal fat tissue, was classified as grade 0. The thymus, which was dominated by fat tissue and had diffuse point densities, and could not be clearly distinguished was grade 1. Thymus with approximately 50% fat and a solid density, which can be distinguished by triangular or pyramidal borders, were classified as grade 2. The thymus with solid density and triangles or pyramidal thymus was called grade 3. For detection of RTH changes in size and densities with treatment were evaluated in grade 3 thymus while changes in attenuation were evaluated in other grades [5, 6]. Cases without RTH were assigned as group 1, whereas cases that developed RTH were assigned as group 2.
Statistical Analysis
The Statistical Package for Social Sciences program version 22.0 was used (IBM SPSS Corp., Armonk, NY, USA), and statistical analysis was also performed using the same program. The mean, standard deviation, median, min, and max values of the continuous variables, frequencies, percentages of the class variables were determined. It was accepted that normal distribution with continuous variables did not fit with sample size and normal distribution tests. The Mann-Whitney U test (non-parametric) was used to compare these variables, and the Kaplan-Meier method was used to evaluate the comparison between groups in terms of survival. Comparisons of survival times were performed by log-rank test. For all the statistical comparison tests, the probability of a type 1 error was α=0.05 and two sided. A p-value of <0.05 was considered statistically significant.
RESULTS
Between 2015 and 2017, 130 patients (121 men) with stage 4 NSCLC who underwent CT were included in the study. Median age was 59 (min-max: 40–79). Adenocarcinoma was 45.4%, squamous cell carcinoma 33.8%, and NSCLC without subtyping 20.8%; 124 patients included in the study died and the mean follow-up period was 13.99±11.61 months.
All the patients received the first-line platinum-based combination CT [(median cycles: 4 (2–6)]. Given treatments were cisplatin plus gemcitabine (n=59), cisplatin plus vinorelbine (n=38), cisplatin plus taxan (n=31), cisplatin plus etoposide (n=2). Fifty-two (n=52) patients received second-line CT. These were cisplatin plus docetaxel (n=19), cisplatin plus pemetrexed (n = 15), docetaxel (n=8), vinorelbine (n=4), cisplatin plus gemcitabine (n=6) [(median 3 (1–11)]. Eighteen patients (14%) underwent third-line systemic CT. These were vinorelbine (n=9), gemcitabine (n=7), and docetaxel (n=2) [(median 3 (1–6)], each 3 patients received four cycles of cisplatin plus vinorelbine, cisplatin plus docetaxel and cisplatin plus pemetrexed. Erlotinib was given to two patients. None of the patients received immunotherapy (Table 1).
Table 1.
Demographic data of the patients included in this study
| Variables | |
|---|---|
| Age, Med (Min, Max) | 59 (40, 79) |
| Follow-up period, Mean±SD (months) | 13.99±11.61 |
| Gender, number of females | 130 (9) |
| Diagnosis frequency (%) | |
| Adenocarcinoma | 45.4 |
| Squamous cell carcinoma | 33.8 |
| Non-small cell carcinoma | 20,8 |
| First-line CT, n | |
| Cisplatin plus gemcitabine | 59 |
| Cisplatin plus vinorelbine | 38 |
| Cisplatin plus taxan | 31 |
| Cisplatin plus etoposide | 2 |
| Second-line CT | |
| Cisplatin plus docetaxel | 19 |
| Cisplatin plus pemetrexed | 15 |
| Docetaxel | 8 |
| Vinorelbine | 4 |
| Cisplatin plus gemcitabine | 6 |
| Third-line CT | |
| Vinorelbine | 9 |
| Gemcitabine | 7 |
| Docetaxel | 2 |
CT: chemotherapy; SD: standard deviation
Nine of 13 patients with RTH were detected to have stable disease (69.4%), two patients partial response (15.3%), and two patients were evaluated as having progressive disease (15.3%). Of the remaining 117 patients, 78 (66.6%) had stable disease, 11 (9.4%) had complete response, 21 (17.9%) had partial response, and seven were evaluated as having progressive disease (5.9%).
The patients were categorized into two groups; group 1 without RTH and group 2 with RTH. Thirteen (10%) of the 130 patients developed RTH (group 2), while the remaining 117 (90%) patients were without RTH (group 1). There was no difference between the groups in terms of age (p=0.933). RTH developed after the first-line systemic CT in 11 out of 13 (84.6%) patients, while it developed in two patients after the second cycle of the second-line CT. RTH developed after a median time of 4.5 months (2–7 months) (Table 2).
Table 2.
Treatments of patients with thymic hyperplasia
| Patient # | First line treatment | First line treatment | Second line treatment | Cycles |
|---|---|---|---|---|
|
| ||||
| Cycles | ||||
| 1 | Cisplatine+Docetaxel | 2 | ||
| 2 | Carboplatine+Docetaxel | 4 | ||
| 3 | Cisplatine+ Vinorelbine | 6 | ||
| 4 | Cisplatine+ Vinorelbine | 6 | ||
| 5 | Cisplatine+Docetaxel | 6 | ||
| 6 | Cisplatine+Vinorelbine | 4 | ||
| 7 | Carboplatine+Gemcitabine | 4 | ||
| 8 | Carboplatine+Gemcitabine | 4 | ||
| 9 | Cisplatine+Navelbine | 2 | ||
| 10 | Cisplatine+ Vinorelbine | Pemetrexed | 2 | |
| 11 | Cisplatine+Gemcitabine | 2 | ||
| 12 | Cisplatine+Docetaxel | 2 | ||
| 13 | Cisplatine+ Vinorelbine | Pemetrexed | 2 |
Overall survival was 20.04 months (95% CI, 4.79–35.29) in patients with RTH and 10.05 months (95% CI, 8.74–11.36) in patients without RTH (p=0.049) (Figure 1).
Figure 1.
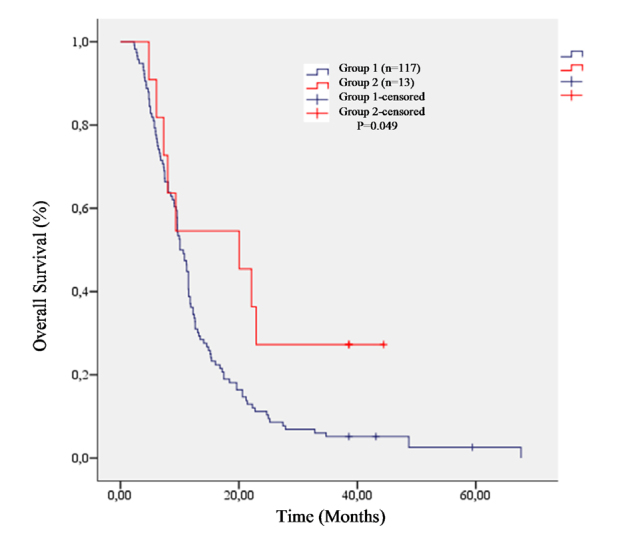
Comparison of life span between groups
DISCUSSION
Our study showed that RTH, which developed in patients with stage 4 NSCLC treated with CT, may have a positive effect on survival. RTH developed in 13 (10%) patients (11 patients after first-line CT and two patients after the second cycles in the second-line CT).
RTH has been reported in relatively small sample-sized studies and case reports in different cancer types [2–4]. Histologically, thymic hyperplasia can be divided into 2 distinct types; true hyperplasia and lymphoid (follicular) hyperplasia. Clinically, patients with true thymic hyperplasia can be divided into three groups: those without a related preexisting condition; those recovering from a recent stress event such as pneumonia, corticosteroid therapy, radiation therapy, chemotherapy surgery, or burns; and those with other disorders such as hyperthyroidism, sarcoidosis, or red blood cell aplasia [7]. In our study, patients with RTH underwent systemic CT and those can be classified into the second group of true thymic hyperplasia.
In a study of 54 patients with lymphoma who had thymic growth (age 33; 18–53), initial thymic volume was inversely correlated with age (Spearman test r=−0.707, p<0.001). In addition, in 30% (16/54) of the patients, treatment reduced the amount of thymic soft tissue mass; and 69% (37/54) had no change in the thymus volume. Only one patient had an increase in the thymus volume. After treatment was completed, 44% of 54 patients were found to develop thymic enlargement. This study confirms that thymus regeneration capacity is preserved after CT, and thymic volume and output function increase in young adults. Researchers concluded that following CT, the adult thymus may be crucial for the reconstitution of T lymphocyte immunity and may be the target of therapeutic advances [8]. In our study, RTH developed in 10% of the patients with a median age of 59 years who had stage 4 NSCLC and underwent CT. This rate was lower than the study. It was seen that RTH did not develop after three and more serial CTs. Twenty-four patients (44%) with lymphoma developed RTH after treatment was completed. This difference can be explained by involution of thymus with age. Although the thymic function decreases with age, some residual activities can be found even in the older adults [9].
In another study, 26 patients with lymphoma were diagnosed with RTH at an average of four months after the last CT (1–12 months) [10]. Despite being a variable group of primary diagnoses, RTH developed after 4.5 months in our study, and RTH in all patients developed during CT treatment.
Patients with a diagnosis of breast cancer (7) or lymphoma (1) were reviewed from the medical records of 8 consecutive patients seen at two institutions. All patients were female with a median age of 41 years and an age range from 35 to 44. Each patient presented with benign thymic hyperplasia following the completion of chemotherapy treatments. The median time for diagnosis of thymic hyperplasia (demonstration of a new anterior mediastinal mass on follow-up CT scan) was 7 months following completion of chemotherapy, with a range of 1 to 11 months [11]. In spite of the small sample size and patients with breast cancer, the results were similar to our study. RTH development time was moderately longer than our study. Similarly, in this study the effect of RTH on survival was not examined.
RTH was detected in 51.9% of the patients with lymphoma in a study (14/27). It developed a median of 2.5 months after the completion of CT. Patients with RTH had significantly shorter treatment durations, and it was found there were no significant differences between patients with and without RTH with regard to sex, age at diagnosis, type of lymphoma, or type of treatment received. All the patients with RTH were asymptomatic, and routine laboratory tests did not detect any abnormalities in them. None of the patients with RTH had a recurrence, and RTH resolved spontaneously within a median of 6 months (range 4.0–11.0 months) [12]. Compared to our study, even if the primary diagnosis is different, the duration of RTH development (last study 2.5, previous 7 months) is close to our study and the two studies above. While in our study, the median age of the patients was 59, in the last study it was 6, and similarly, there was no difference between patients with or without RTH in terms of age. The last study examined recurrence of disease, and patients with RTH did not have a recurrence; however, RTH regressed in six months. In our study, patients with RTH had longer survival rates than those without it. Actually, thymic regrowth is not likely associated with tumor types and treatment. It can occur following treatment for various malignancies, of which malignant lymphoma is the most common [10]. Besides, RTH after cessation of CT does not appear to be influenced by the degree of lymphocyte depletion, but appears to be a common response to the withdrawal of CT [13].
In general, RTH may indicate a good prognosis, but may be misdiagnosed as a progressive disease or recurrence sign [14, 15]. In our study, it was determined that patients developing RTH had a better prognosis. The mean age of the patients in our study was 59 years. According to the World Health Organization, patients below 65 years of age are considered young, and we have identified that RTH can be seen in this age group. In this group, it has been determined that the thymus still has regeneration capacity [16]. Some authors suggest that RTH development after CT indicates a good prognosis. RTH mostly happens within the first year following CT. It is usually observed in children but may also occur in young adults [17].
Although case-based publications, especially in hematologic and childhood malignancies, are generally available, there was not any article that examined patients with lung cancer. There are some limitations to our study. In addition to being a retrospective analysis, low RTH prevalence disrupts the numerical harmony between the groups. However, this may be due to the fact that the study population is composed of patients with a solid organ tumor. This study is the first of its kind in literature because it was carried out with patients with solid organ tumors, lung cancer, and a relatively large sample size.
As a result, the developing of RTH during systemic CT may be a prognostic marker in stage 4 non-small cell lung cancer.
MAIN POINTS.
Rebound thymic hyperplasia (RTH) may develop in patients receiving chemotherapy (CT) with any malignancy.
The developing of RTH during systemic CT may be a prognostic marker in stage 4 non-small cell lung cancer.
It could positively effect on survival in patients with RTH in malignancy
Footnotes
Ethics Committee Approval: Ethics Committee approval for the study was obtained from the Suat Seren Chest Diseases and Thoracic Surgery Research and Education Hospital.
Informed consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - H.A.U., B.M., O.K.; Design - H.A.U., B.M., O.K.; Supervision - H.A.U., B.M., O.K.; Resources - H.A.U.; Materials - H.A.U.; Data Collection and/or Processing - H.A.U., B.M.; Analysis and/or Interpretation - H.A.U., B.M.; Literature Search - H.A.U., B.M.; Writing Manuscript - H.A.U., B.M., O.K.; Critical Review - H.A.U., B.M., O.K.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Junqueira LC, Carneiro JT. Text and Atlas. Thirteenth Edition. 2009. Junqueira's Basic Histology; pp. 266–8. [Google Scholar]
- 2.Ford ME, Stevens R, Rosado-de-Christenson ML, et al. Rebound thymic hyperplasia after pneumonectomy and chemotherapy for primary synovial sarcoma. J Thorac Imaging. 2008;23:178–81. doi: 10.1097/RTI.0b013e3181620e61. [DOI] [PubMed] [Google Scholar]
- 3.Sokol E, Huang E, Pytel P, et al. Rebound thymic hyperplasia following high dose chemotherapy and stem cell transplant in three neuroblastoma patients. Pediatr Blood Cancer. 2017;64 doi: 10.1002/pbc.26226. doi: 10.1002/pbc.26226. Epub 2016 Sep 24. [DOI] [PubMed] [Google Scholar]
- 4.Chen CH, Hsiao CC, Chen YC, et al. Rebound Thymic Hyperplasia after Chemotherapy in Children with Lymphoma. Pediatr Neonatol. 2017;58:151–7. doi: 10.1016/j.pedneo.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Ackman JB, Kovacina B, Carter BW, et al. Sex difference in normal thymic appearance in adults 20–30 years of age. Radiology. 2013;268:245–53. doi: 10.1148/radiol.13121104. [DOI] [PubMed] [Google Scholar]
- 6.Araki T, Nishino M, Gao W, et al. Normal Thymus in Adults: Appearance on CT and Associations with Age, Sex, BMI and Smoking. Eur Radiol. 2016;26:15–24. doi: 10.1007/s00330-015-3796-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasseri F, Eftekhari F. Clinical and radiologic review of the normal and abnormal thymus: pearls and pitfalls. Radiographics. 2010;30:413–28. doi: 10.1148/rg.302095131. [DOI] [PubMed] [Google Scholar]
- 8.Sun DP, Jin H, Ding CY, et al. Thymic hyperplasia after chemotherapy in adults with mature B cell lymphoma and its influence on thymic output and CD4(+) T cells repopulation. Oncoimmunology. 2016;5:e1137417. doi: 10.1080/2162402X.2015.1137417. doi: 10.1080/2162402X.2015.1137417. eCollection 2016 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhen Z, Sun X, Xia Y, et al. Clinical analysis of thymic regrowth following chemotherapy in children and adolescents with malignant lymphoma. Jpn J Clin Oncol. 2010;40:1128–34. doi: 10.1093/jjco/hyq149. [DOI] [PubMed] [Google Scholar]
- 11.Pamela DP, Maxey N, Mohammadi Z, et al. Thymic Hyperplasia after Chemotherapy: Combined Experience of Two Centers. Blood. 2015;126:4633. doi: 10.1182/blood.V126.23.4633.4633. [DOI] [Google Scholar]
- 12.Fouda A, kandil S, Hamid G, et al. Rebound (reactive) thymic hyperplasia after chemotherapy in children with lymphoma. An Pediatr (Barc) 2019. pii: S1695-4033(18)30551-4. [Epub ahead of print] [DOI] [PubMed]
- 13.Sun DP, Wang L, Ding CY, et al. Investigating Factors Associated with Thymic Regeneration after Chemotherapy in Patients with Lymphoma. Front Immunol. 2016;27(7):654. doi: 10.3389/fimmu.2016.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gawande RS, Khurana A, Messing S, et al. Differentiation of normal thymus from anterior mediastinal lymphoma and lymphoma recurrence at pediatric PET/CT. Radiology. 2012;262:613–22. doi: 10.1148/radiol.11110715. [DOI] [PubMed] [Google Scholar]
- 15.Jerushalmi J, Frenkel A, Bar-Shalom R, et al. Physiologic thymic uptake of 18F-FDG in children and young adults: a PET/CT evaluation of incidence, patterns, and relationship to treatment. J Nucl Med. 2009;50:849–53. doi: 10.2967/jnumed.108.058586. [DOI] [PubMed] [Google Scholar]
- 16.Sfikakis PP, Gourgoulis GM, Moulopoulos LA, et al. Age related thymic activity in adults following chemotherapy-induced lymphopenia. Eur J Clin Invest. 2005;35:380–7. doi: 10.1111/j.1365-2362.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- 17.Küpeli S. Rebound thymic hyperplasia. Cukurova Med J. 2017;42:799–800. doi: 10.17826/cumj.326915. [DOI] [Google Scholar]


