Abstract
Hyperprolactinemia is a prevalent endocrine disorder presented in patients with non-functional pituitary adenomas (NFPAs). However, the mechanism involved in hyperprolactinemia in NFPA is not fully illustrated. The current study aims to investigate predictors for hyperprolactinemia in NFPA via analyzing relevant clinical features. Thus, in this study, a cohort of 214 cases with integrated medical records was retrospectively analyzed concerning clinical, pathological, and endocrinological studies before and after surgery.
Hyperprolactinemia happened in 93 cases (43.5%). Women (adjust odds ratio [OR] = 3.093; P < .01), age of patients (adjust OR = 0.951; P < .01), and serum free tetraiodothyronine (FT4) level (adjust OR = 0.882; P = .02) were independent predictors for developing preoperative hyperprolactinemia. Tumor size and hypopituitarism had no impact on hyperprolactinemia. During a median follow-up of 43.5 (range, 22–80) months, 83.9% patients with preoperative hyperprolactinemia experienced prolactin (PRL) normalization. Preoperative PRL level (adjusted OR = 1.741, P = .03) was the exclusive predictor for PRL normalization after adjusting for tumor volume, preoperative serum FT4 concentration, and postoperative residual. The PRL normalization rate of patients with lower PRL level (<2.35-fold upper limit of normal range) was 95.2% and decreased to 65.5% for patients with higher PRL level.
In conclusion, our results suggest existence of potentially alternative mechanisms underlying hyperprolactinemia in NFPAs, like the discrepancy of sex and age and the negative feedback of FT4. Preoperative PRL is a predictor for postoperative PRL normalization, which is of clinically relevant for postoperative management of NFPAs.
Keywords: free tetraiodothyronine, hyperprolactinemia, non-functional pituitary adenoma, stalk effect
1. Introduction
Non-functional pituitary adenoma (NFPA) is one of the most common subtypes of pituitary adenomas. In contrast to functional pituitary adenomas, NFPAs are rarely associated with hormone hypersecretion. Nevertheless, previous reports found that approximate 40% of NFPA patients presented hyperprolactinemia, which might cause galactorrhea, amenorrhea, and erectile dysfunction.[1,2] “Pituitary stalk effect” is the most recognized hypothesis for hyperprolactinemia in NFPAs.[2] Affected by the hypothalamic dopamine from the hypophyseal portal system,[3] prolactin (PRL) is generally inhibited to a physiological level. Thus, sellar or suprasellar masses, including NFPAs, could possibly influence the pituitary stalk, and theoretically lead to hyperprolactinemia through mechanic compression or dopaminergic neuronal damage. However, tumor size shows a weak correlation with hyperprolactinemia in NFPAs. Only some large NFPAs cause hyperprolactinemia, and a significant amount of tumors with suprasellar extension have normal PRL level,[4,5] suggesting alternative mechanisms for hyperprolactinemia in NFPA. Theoretically, modulators of hypothalamic dopamine system are capable of affecting serum PRL level in NFPAs. Remarkable sexual discrepancy of activity and reactivity exists in tuberoinfundibular dopaminergic neurons (TIDA), which is one of the most crucial parts of the hypothalamic dopamine system.[6] Other factors, as neurotensin, substance P, estradiol and opioids, are likely to impact on PRL concentration by modulating the activity of TIDA.[6,7] Besides, thyrotropin-releasing hormone (TRH) can promote the PRL release and patients with primary hypothyroidism have higher prevalence of hyperprolactinemia,[8,9] which are of highly clinical relevance for NFPAs.
According to the “Pituitary stalk effect” theory, serum PRL level decreases as a consequence of pituitary stalk decompression after surgery. However, a small proportion of patients still suffer from persistent postoperative hyperprolactinemia.[10] PRL, a pleiotropic hormone predominately secreted by lactotroph cells of the anterior pituitary gland, is mainly involved in lactation and reproduction.[11] Elevated PRL concentration has markedly impact on fertility, especially for women in the child-bearing age. Furthermore, PRL is also involved in immunoregulation and autoimmune diseases.[12,13] Thus, persistent postoperative hyperprolactinemia exerts potential damage to patients with NFPAs. Additionally, recurrence of hyperprolactinemia is one of the signs for tumor relapse.[14] Also, PRL level is a predictor for postoperative neuroendocrine recovery in patients with pituitary apoplexy.[15] The natural course of PRL dynamic after surgery could facilitate further understanding of the mechanism of postoperative hyperprolactinemia.
Hence, in this study, we retrospectively reviewed a cohort of 214 patients with NFPAs and reported the pre- and postoperative evaluations of serum PRL level as well as the potential predictive factors, hoping to shed some light upon hyperprolactinemia and the natural course of PRL dynamic in NFPA.
2. Patients and methods
2.1. Patients
Newly-diagnosed patients with NFPA presenting to the Department of Neurosurgery in West China Hospital of Sichuan University between January 2010 and December 2017 and operated by the senior author (SJ) were reviewed. A total of 214 cases with integrated medical archives were included in the final analyses according to the following criteria. The diagnosis of NFPA in these patients was confirmed according to preoperatively neuroradiological and endocrinological studies as well as postoperatively pathological evaluations. Blood samples for endocrinological studies were collected at the same time point (7–8 am) of the day in each patient. Exclusion criteria contained several circumstances: adenoma associated with clinical or biochemical evidence of hormonal hypersecretion; cases with other conditions affecting the serum PRL levels, such as pregnancy, psychiatric disorders, primary hypothyroidism, chronic renal failure, severe liver disease, polycystic ovary syndrome, previous hypothalamic-pituitary disease, or cranial irradiation and medication intake (metoclopramide, tricyclic antidepressants, reserpine, methyl-dopa, or oral contraceptives).[16]
After surgery, patients were recruited at 3, 6, and 12 months and annually for endocrinological and neuroradiological evaluation. Patients with postoperative hypopituitarism received hormones replacement. Postoperative Gamma Knife radiosurgery was recommended to patients with residuals.
The materials used in this study consisted only of archival specimens from this department. All procedures followed ethical standards of the Declaration of Helsinki 1975, as revised in 1983 and approved by the Biomedical Research Ethics Committee of West China Hospital of Sichuan University. For retrospective study, formal consent is not required.
2.2. Neuroradiological evaluation
Magnetic resonance (MR) images were acquired in each patient through standard 3.0-T scanner with contrast enhancement. Besides, as to evaluate the degree of surgical resection, MR scanning was likewise performed within 72 hours and 3 months after surgery. In this stage, tumor residual was identified by comparing postoperative MR images with preoperative MR scanning. Gamma knife radiosurgery was recommended to residuals at this time. Suprasellar tumor growth was considered significant in those cases with apparent compression of the optic chiasm.[17] Cavernous sinus invasion was evaluated according to the Knosp grade and considered in tumors extended beyond a line through the cross-sectional centers of the intracavernous and supracavernous internal carotid arteries (ICAs) on the coronal MRI images. Sphenoid sinus extension was verified if the lesion grew into the sphenoid sinus in preoperative MR scanning or during surgical process.[17,18] In line with tumor maximal diameter, adenomas were categorized into microadenoma (<1 cm), macroadenoma, and giant adenoma (≧4 cm). In addition, tumor volume was verified using the Region of Interest (ROI) function of MR imaging system and calculated as the sum of all tumor area measured on each tumor slice multiplied by slice thickness.[19]
2.3. Endocrinological study
For PRL evaluation, blood samples were acquired by venipuncture after revival at 7 to 8 am and maintained in anticoagulant-free vacuum tubes. Over-night fasting was not mandatory. Dynamic tests of prolactin secretion were not routinely adopted. Serum separation was performed within 2 hours and PRL levels were measured by commercial electro-chemiluminescence kits (ROCHE Diagnostics, IN) using Cobas E601 electrochemical luminescence analyzer (ROCHE Diagnostics). The intra-assay coefficients of variation (CVs) for PRL concentrations of 23.5 and 238.67 ng/mL were 1.16% and 0.95%, respectively. The sensitivity was 0.065 ng/mL with the analytic measurement range (AMR) being 0.105 to 452.88 ng/mL. The reference range was 4.6 to 21.4 ng/mL for men and 6.0 to 29.9 ng/mL for women. 10-fold or 100-fold dilution of blood samples were employed in out-of-range cases. Thus, the diagnosis of hyperprolactinemia was based on a PRL level upgrading above the upper limit of normal range.[20] Other hormones, including cortisol, adrenocorticotropic hormone (ACTH), free tetraiodothyronine (FT4), free triiodothyronine (FT3), thyroid stimulating hormone (TSH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol, testosterone, and growth hormone (GH) were measured through commercially available kits (ROCHE Diagnostics, IN). The fold change of PRL (FCP) was calculated as PRL level dividing the upper limit of normal range (ULN). Central hypothyroidism was diagnosed in accordance with low circulating FT4 within low or normal TSH level.[21,22] Central hypoadrenocorticism was verified by decreased serum cortisol.[23] The diagnosis of hypogonadotropic hypogonadism was based on reduced estradiol/testosterone level with normal or decreased FSH/LH and low or normal FSH/LH in postmenopausal women.[24]
2.4. Pathological study
Surgical specimens were fixed in formalin and embedded in paraffin. Tissue slices were evaluated by routine hematoxylin & eosin staining and further immunohistochemistry (IHC) for GH, PRL, ACTH, TSH, LH, and FSH. Pituitary transcript factors, including Pit-1, SF-1, and Tpit, were not routinely assessed during the period of January 2010 and December 2017. Therefore, histopathological classification of NFPAs was partially followed the 2017 WHO classification[25] and determined as previously described.[26,27] That is, positive immunostaining for LH and/or FSH was considered as gonadotroph adenoma. A diagnosis of null cell adenoma was made when immunostaining was negative for all studied hormones. Plurihormonal adenoma was diagnosed when immunostaining was positive for ≥2 pituitary hormones and at least one hormone was not gonadotropins. Silent corticotroph, somatotroph, and lactotroph adenomas were diagnosed as ACTH-, GH-, and PRL-positive subtypes, respectively.
2.5. Statistical analysis
Results were displayed in the form of mean ± standard deviation (SD) or median. Student t and Mann–Whitney U tests were used for quantitative data whereas Chi-square or Fisher exact tests were used for categorical data. Pearson correlation analysis or Spearman rank correlation analysis were conducted for correlations among clinical features and endocrinological results. Receiver operating characteristics (ROC) curve was employed to excavate optimal cut-off points of FT4 for prediction of hyperprolactinemia and preoperative PRL for prediction of PRL normalization. Moreover, binary logistic regression was performed for analyzing independent predictors of hyperprolactinemia and PRL normalization. A P value of <.05 in 2-sided tests was considered significant. Data were analyzed using SPSS version 17.0 (SPSS Chicago IL).
3. Results
3.1. Preoperative hyperprolactinemia in NFPA
Total quantities of 214 patients with NFPA were enrolled in this retrospective study. With a slight female predominance, female patients accounted for 52.3% of the series (women: 112 cases; men: 102 cases). The mean age at diagnosis in this cohort was 48.86 ± 12.49 years. At presentation, the vast majority of patients (90.7%) had clinical symptoms associated with mass effect. Visual field deficit and headache were found in 164 (76.6%; 3 cases were accompanied by diplopia) and 98 (45.8%) cases, respectively. Eight women appeared irregular menstruation, in which 2 were normoprolactinemic. Eleven females (all hyperprolactinemic) noticed amenorrhea and 5 women (all hyperprolactinemic) presented galactorrhea. Laboratory examinations revealed that 7 cases suffered from hypogonadotropic hypogonadism in these patients. Additionally, detailed testing of pituitary function had revealed much higher proportions of patients with different kinds of hypopituitarism (Table 1). The prevalences of hypoadrenocorticism, hypothyroidism, and hypogonadism were 25.7%, 45.8%, and 42.5%, respectively. Panhypopituitarism was presented in 30 patients. In addition, asymptomatic macroadenoma was detected in 11 cases.
Table 1.
General characteristics of total population.
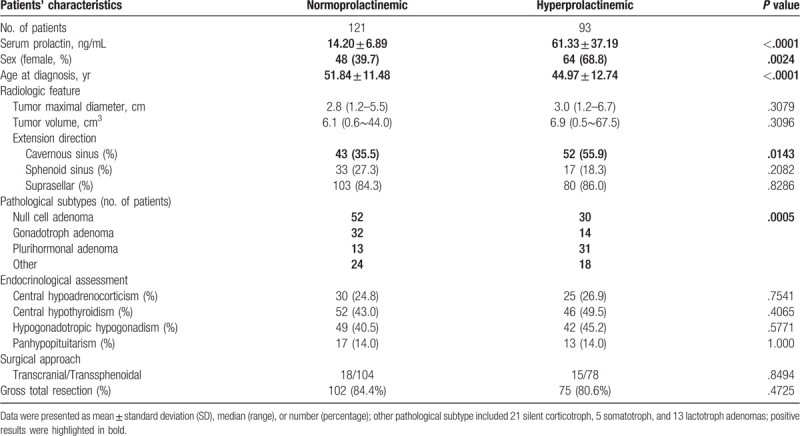
Preoperative neuroradiological assessment revealed a median maximal tumor diameter of 2.8 cm (range; 1.2–6.7 cm). Accordingly, 91.1% of cases were categorized as macroadenoma, and the remaining 19 cases were giant adenomas. What's more, 95.6% of NFPAs revealed parasellar extension, including cavernous sinus invasion in 95 cases (44.4%), sphenoid sinus extension in 50 cases (23.4%), and suprasellar extension in 182 cases (85.0%). Transsphenoidal microscopic adenoidectomy (TSA) was employed in 85.0% of patients and resulted in a gross total resection (GTR) rate of 82.7%. Postoperatively histopathological analyses demonstrated that null cell, gonadotroph, and plurihormonal adenomas accounted for 82, 46, and 44 cases, respectively. Besides, 20 cases (45.5%) coming out of the positive stained result of PRL.
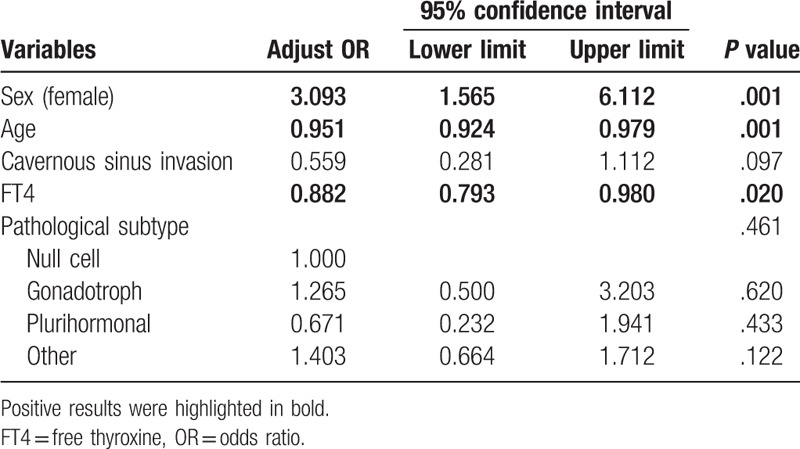
Preoperative hyperprolactinemia was found in 93 cases (43.5%) and median serum PRL concentration was 34.68 ng/mL (range, 0.23–213.90 ng/mL). Spearman rank correlation analyses disclosed that serum PRL level was correlated with sex, age at diagnosis, and cavernous sinus invasion rather than tumor size, sphenoid sinus, or suprasellar extension (data not shown). Further detailed univariate analyses (Table 1) revealed that hyperprolactinemic patients were more likely to be women (68.8% vs 39.7%; P < .01) or younger (44.97 ± 12.74 vs 51.84 ± 11.48 years; P < .01). Hyperprolactinemic cases also tended to invade cavernous sinus (55.9% vs 35.5%; P = .01). Pathological subtype of NFPAs had potential influences on preoperative PRL level (P < .01). However, there was no statistic difference between hyperprolactinemic and normoprolactinemic patients when it came to tumor size and preoperative endocrinological assessments. Surgical approach and GTR rate were consistent in 2 groups of patients. Additionally, median serum PRL concentration of women was relatively higher than that of men (35.33 vs 16.34 ng/mL; P < .01). Allowing for the discrepant ULN of PRL, PRL concentration was transformed as the fold change (FCP). Median FCP of women was also higher than that of men (1.181- vs 0.764-fold; P < .01). Further binary logistic regression (Table 2) indicated that sex (female) (adjust OR = 3.093; P < .01) was a risk factor for developing preoperative hyperprolactinemia. Furthermore, elder patients (adjust OR = 0.951; P < .01) or cases with higher serum FT4 level (adjust OR = 0.882; P = .02) were less likely to have hyperprolactinemia. Cavernous sinus invasion and pathological subtype were not independent predictors for preoperative hyperprolactinemia.
Table 2.
Binary logistic regression of variables associated with hyperprolactinemia.
Although the prevalence of hypothyroidism was consistent in normoprolactinemic and hyperprolactinemic cases, serum FT4 level had a significant impact on hyperprolactinemia in binary logistic regression. Preoperatively, the mean serum FT4 concentration was 12.30 ± 3.40 pmol/L. Thus, ROC curve (Fig. 1) was performed to excavate a better cutoff point for prediction of hyperprolactinemia. The area under ROC curve (AUC) was 0.5829 (P = .04) with the optimal cutoff point of FT4 being 6.445 pmol/L.
Figure 1.
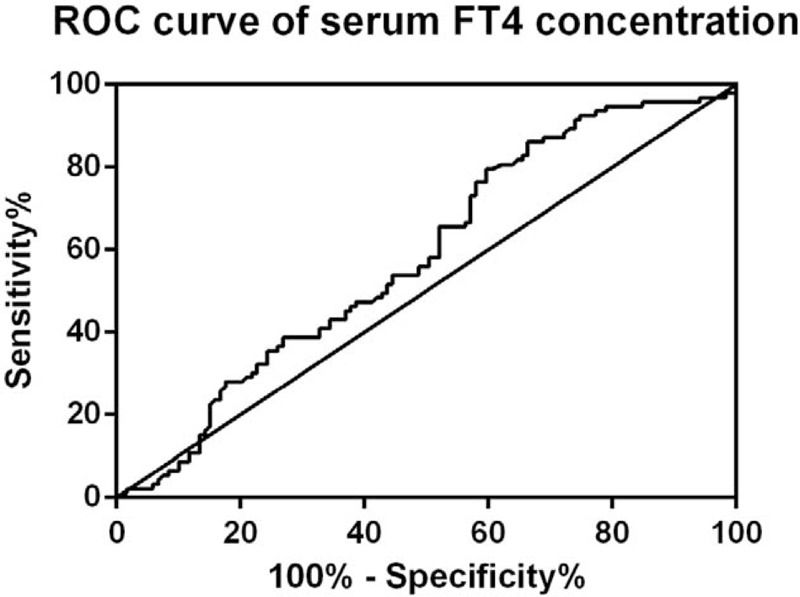
ROC curve of serum FT4 concentration. ROC curve revealed that FT4 level of 6.445 pmol/L was the optimal cutoff point for prediction of hyperprolactinemia (AUC, 0.5829; P = .03849). AUC = area under ROC curve; FT4 = free tetraiodothyronine; ROC = receiver operating characteristics.
3.2. Postoperative evaluation of PRL in NFPA
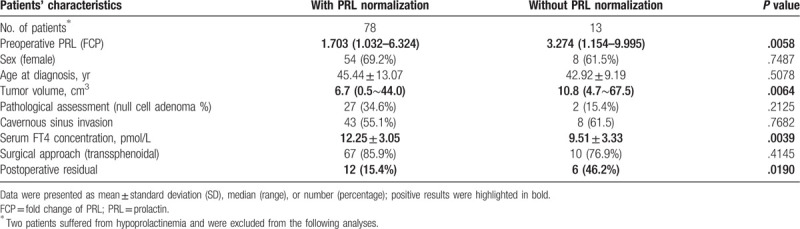
After surgery, 93.0% of patients experienced decreased serum PRL concentration (normalized in 72.0% of patients). The median postoperative PRL level was 9.87 ng/mL (range, 1.86–55.73 ng/mL). Meanwhile, the prevalences of postoperative hypothyroidism and hypogonadism were 25.7% (43/167) and 43.8% (60/137), respectively. After surgery, the mean serum FT4 level was 14.90 ± 4.25 pmol/L, which showed no correlation with postoperative PRL concentration. As expected, the decreasing extent of PRL in preoperatively hyperprolactinemic patients was higher than that in normoprolactinemic cases (median, 71.24% vs 41.14%, P < .01). Moreover, in preoperative hyperprolactinemic patients, sex and tumor volume were not related with the percentage of PRL decrease. With a median follow-up of 43.5 (range, 22–80) months for preoperatively hyperprolactinemic patients, 97.8% of patients experienced the decrease in serum PRL concentration, in which 78 cases (83.9%) showed normalization of their serum PRL at last visit (Table 3). The menstrual cycle of 8 female patients with irregular menstruation (included 2 normoprolactinemic cases) got back to normal. Galactorrhea remitted in all 5 patients who initially presented. Meanwhile, amenorrhea was relieved in 4 patients and 4 women reconstructed normal menstrual cycle. Compared with cases with PRL normalization, patients with persistent postoperative hyperprolactinemia had higher preoperative PRL level (median FCP, 3.274- vs 1.703-fold, P < .01), larger tumor volume (median, 10.8 vs 6.7 cm3, P < .01), lower preoperative serum FT4 concentration (9.51 ± 3.33 vs 12.25 ± 3.05 pmol/L), and were more likely to have tumor residuals (46.2% vs 15.4%, P = .02).
Table 3.
Factors affected PRL normalization.
However, preoperative PRL level (FCP, adjusted OR = 1.741, P = .03) was the only independent predictive factor for PRL normalization in binary logistic regression. Further analyses confirmed that the optimal cutoff point of FCP was 2.35-fold with the AUC being 0.7357 (P < .01, Fig. 2). The PRL normalization rate of patients with lower FCP (<2.35-fold) was 95.2% and decreased to 65.5% for patients with higher FCP.
Figure 2.
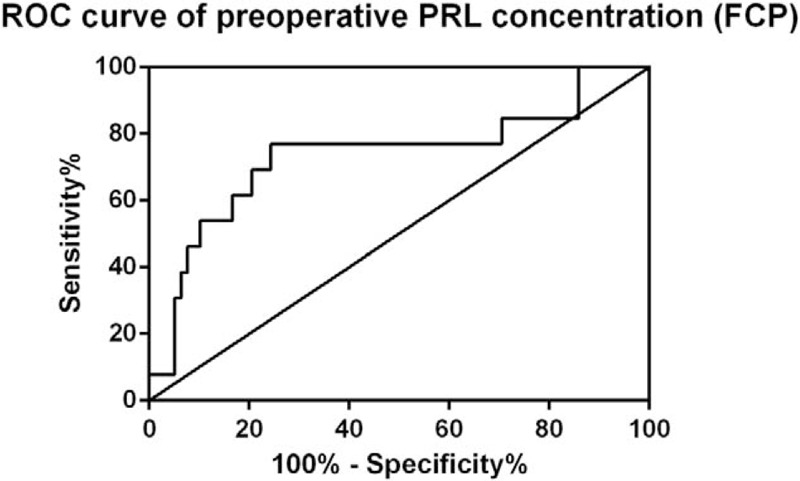
ROC curve of preoperative PRL concentration (FCP). ROC curve revealed that preoperative PRL concentration (FCP) of 2.35-fold was the optimal cutoff point for prediction of postoperative PRL normalization (AUC, 0.7357; P = .00673). AUC = area under ROC curve; FCP = fold change of PRL; PRL = prolactin; ROC = receiver operating characteristics.
4. Discussion
In this study, 93 (43.5%) of 214 patients with NFPAs exhibited preoperative hyperprolactinemia. Young female patients and patients with lower serum FT4 concentration were more likely to develop hyperprolactinemia. During the follow-up, 83.9% patients bearing preoperative hyperprolactinemia experienced PRL normalization. Preoperative PRL level was associated with PRL normalization after adjustment for tumor volume, preoperative serum FT4 concentration, and postoperative residual.
Among patients with NFPAs, the majority of patients showed mass effect-related symptoms. Only a small proportion (10.3%) of patients manifested as irregular menstruation, amenorrhea and galactorrhea, even if 43.5% of patients exhibited hyperprolactinemia. Therefore, mild hyperprolactinemia was not likely to cause noteworthy clinical symptoms associated with elevated PRL concentration in NFPAs. Meanwhile, we found that hyperprolactinemia frequently happened in young and female patients with NFPAs. Two facts should be focused, which enables to account for this phenomenon. On the one hand, we believe that age and sex influences something on this issue. Similar to previously reports,[28,29] most young women initially experience amenorrhea or galactorrhea whereas men and postmenopausal women do not present these hyperprolactinemia-associated symptoms, which represents a similar phenomenon observed in microprolactinomas.[30] On the other hand, sex- and age-related differences of gonadal hormones may prompt younger women to be vulnerable to the disturbance of hypothalamic-pituitary dopaminergic system and thereby to develop hyperprolactinemia. Several shreds of evidences support this hypothesis. Firstly, PRL secretion is tonically inhibited by hypothalamic dopaminergic neurons.[3] It is reported that basal dopaminergic neuronal activity is higher in women than in men.[31] Therefore, the basal secretory activity of pituitary lactotrophs, which is regulated by dopaminergic neurons, could also be higher in women under physiological condition. Secondly, hypothalamic tyrosine hydroxylase (TH), which is involved in the biosynthesis of dopamine, could be inactivated by estrogens.[32] Lastly, PRL secretion is also stimulated by estrogens in animal experiments[33] and estrogen has the ability to promote PRL secretion by inhibiting dopamine incorporation into PRL secretory granules.[34] Therefore, there might be some differences between NFPAs and prolactinoma with respect to the female preponderance.
Generally, hyperprolactinemia in NFPAs is attributed to the pituitary stalk effect. However, investigations of neuroradiological features of NFPAs report controversial results. Suprasellar growth and higher width/anteroposterior diameter ratio are associated with hyperprolactinemia.[2,28] However, the degree of pituitary stalk compression, stalk deviation, or tumor size are not predictors for PRL level in a more detailed neuroradiological study.[4] Instead, increased intrasellar pressure presents a positive correlation with hyperprolactinemia.[35] Consistent with these studies, tumor size and extension direction of tumor were not related to hyperprolactinemia in NFPAs in this study. Recently, a new theory about hyperprolactinemia in NFPAs has been proposed, which considers hyperprolactinemia is the consequence of pituitary stalk effect and lactotroph failure.[29] Pituitary stalk effect and lactotroph deficiency, which is accompanied by other hypopituitarism, gradually develop with the growth of NFPAs. In the early stage, the pituitary stalk effect causes significant hyperprolactinemia with intact lactotroph function. On the contrary, in the late stage of NFPAs, lactotroph failure may lead to normal or even low serum PRL concentration. Thus, hyperprolactinemia, which represents normal lactotroph function, is an indication of normal pituitary function. However, in this study, there was no statistical difference in the prevalence of hypopituitarism between normoprolactinemic and hyperprolactinemic patients.
Furthermore, we found that lower serum FT4 concentration was an independent risk factor for hyperprolactinemia, though a diagnosis of central hypothyroidism did not correlate with hyperprolactinemic status. Further analysis proved that the optimal cutoff point of FT4 for predicting hyperprolactinemia was 6.445 pmol/L, rather than the lower limit of normal reference range. For the mechanism, primary hypothyroidism is a well-known cause of hyperprolactinemia for reason that elevated thyrotropin-releasing hormone (TRH) is a physiologic stimulus on the release of PRL from lactotrophs.[36] It might also fit for central hypothyroidism for increased TRH secondary to negative feedback of low thyrotropin or thyroxine, though whether TRH could reach pituitary gland under the stalk effect remained to be solved. Additionally, reduced sensitivity to tonic inhibition of dopamine on PRL release among hypothyroid patient is also thought to be a contributor to hyperprolactinemia.[8] As a result, for patients with NFPAs, remarkable central hypothyroidism might aggravate hyperprolactinemia.
Increased pressure on pituitary stalk is one of the mechanisms for hyperprolactinemia in NFPAs. Thus, surgical decompressing can normalize the stalk effect-induced hyperprolactinemia.[28] Surgical disruption of the hypothalamic-pituitary transport can lead to an increased postoperative PRL level. In the current study, the vast majority of patients experienced a decrease of PRL, and 72.0% of patients had normalization of PRL after tumor debulking, suggesting that iatrogenic damage of the pituitary stalk had limited impact on postoperative PRL concentration. At the last visit, 83.9% of patients exhibited normalization of PRL. In binary logistic regression, preoperative PRL concentration (FCP) was the sole factor for PRL normalization. More importantly, patients with <2.35-fold preoperative FCP were more likely to normalize their PRL levels after surgery. This fact is meaningful for postoperative surveillance for possible tumor recurrence. Elevated PRL level is not a convincing indication for tumor progression in cases with obviously preoperative hyperprolactinemia.
This study has several limitations. Firstly, this study contained all drawbacks of retrospective investigation. Secondly, we included clinical NFPAs in this study. However, some of them are silent GH/PRL/ACTH adenomas which perhaps differ from null cell and gonadotroph adenomas in pathological aspect.[25] Thirdly, pituitary transcript factors recommended in the 2017 WHO classification were not tested during this period, this may show some impacts on the diagnostic accuracy of null cell and gonadotroph adenoma. Lastly, macroprolactinemia is a relevant issue for this study.[13] However, macroprolactin was not routinely assessed in our institution. As a consequence, the information of macroprolactinemia could not be analyzed in this study.
5. Conclusion
In summary, this study confirms that younger females with NFPAs are more likely to develop hyperprolactinemia. It also reveals a potential association between preoperative PRL level and central hypothyroidism which requires further studies to illuminate the detailed mechanism. Preoperative PRL is a predictor for postoperative PRL normalization which is of clinical relevance for postoperative management of NFPAs.
Acknowledgments
The authors thank the financial support from funding (Grant No. 2016SZ0015) owned by Dr. Feng Ye from People's Hospital of Deyang City.
Author contributions
Conceptualization: Liang Lyu.
Data curation: Liang Lyu, Senlin Yin, Cheng Chen, Yong Jiang, Weichao Ma.
Formal analysis: Liang Lyu, Yu Hu, Zeming Wang.
Funding acquisition: Shu Jiang.
Software: Liang Lyu, Senlin Yin.
Supervision: Shu Jiang, Peizhi Zhou.
Validation: Peizhi Zhou.
Writing – original draft: Liang Lyu.
Writing – review & editing: Yu Hu, Yang Yu, Shu Jiang.
Glossary
Abbreviations: ACTH = adrenocorticotropic hormone, AMR = analytic measurement range, AUC = area under ROC curve, CV = coefficient of variation, FCP = fold change of PRL, FSH = follicle-stimulating hormone, FT3 = free triiodothyronine, FT4 = free tetraiodothyronine, GH = growth hormone, GTR = gross total resection, ICA = internal carotid artery, IHC = immunohistochemistry, LH = luteinizing hormone, MR = magnetic resonance, NFPA = non-functional pituitary adenomas, OR = odds ratio, PRL = prolactin, ROC = receiver operating characteristics, ROI = Region of Interest, SD = standard deviation, TH = tyrosine hydroxylase, TIDA = tuberoinfundibular dopaminergic neurons, TRH = thyrotropin-releasing hormone.
References
- [1].Karavitaki N, Thanabalasingham G, Shore HC, et al. Do the limits of serum prolactin in disconnection hyperprolactinaemia need re-definition? A study of 226 patients with histologically verified non-functioning pituitary macroadenoma. Clin Endocrinol (Oxf) 2006;65:524–9. [DOI] [PubMed] [Google Scholar]
- [2].Behan LA, O'Sullivan EP, Glynn N, et al. Serum prolactin concentration at presentation of non-functioning pituitary macroadenomas. J Endocrinol Invest 2013;36:508–14. [DOI] [PubMed] [Google Scholar]
- [3].Melmed S. Mechanisms for pituitary tumorigenesis: the plastic pituitary. J Clin Invest 2003;112:1603–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Smith MV, Laws ER. Magnetic resonance imaging measurements of pituitary stalk compression and deviation in patients with nonprolactin-secreting intrasellar and parasellar tumors: lack of correlation with serum prolactin levels. Neurosurgery 1994;34:834–9. discussion 839. [DOI] [PubMed] [Google Scholar]
- [5].Kruse A, Astrup J, Gyldensted C, et al. Hyperprolactinaemia in patients with pituitary adenomas. The pituitary stalk compression syndrome. Br J Neurosurg 1995;9:453–8. [DOI] [PubMed] [Google Scholar]
- [6].Benjonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev 2001;22:724–63. [DOI] [PubMed] [Google Scholar]
- [7].Duvilanski BH, Castrillon PO, Cano P, et al. Changes in substance P content at the hypothalamic-pituitary axis during the Wallerian degeneration of peripheral sympathetic neurons after superior cervical ganglionectomy in male rats: effect of hyperprolactinemia. Exp Biol Med (Maywood) 2001;226:612–7. [DOI] [PubMed] [Google Scholar]
- [8].Sharma LK, Sharma N, Gadpayle AK, et al. Prevalence and predictors of hyperprolactinemia in subclinical hypothyroidism. Eur J Intern Med 2016;35:106–10. [DOI] [PubMed] [Google Scholar]
- [9].Raber W, Gessl A, Nowotny P, et al. Hyperprolactinaemia in hypothyroidism: clinical significance and impact of TSH normalization. Clin Endocrinol (Oxf) 2003;58:185–91. [DOI] [PubMed] [Google Scholar]
- [10].Zaidi HA, Cote DJ, Castlen JP, et al. Time course of resolution of hyperprolactinemia after transsphenoidal surgery among patients presenting with pituitary stalk compression. World Neurosurg 2017;97:2–7. [DOI] [PubMed] [Google Scholar]
- [11].Bernard V, Young J, Chanson P, et al. New insights in prolactin: pathological implications. Nat Rev Endocrinol 2015;11:265–75. [DOI] [PubMed] [Google Scholar]
- [12].Köller M, Kotzmann H, Clodi M, et al. Effect of elevated serum prolactin concentrations on the immunophenotype of human lymphocytes, mitogen-induced proliferation and phagocytic activity of polymorphonuclear cells. Eur J Immunol 1983;13:78–82. [DOI] [PubMed] [Google Scholar]
- [13].Larouche V, Correa JA, Cassidy P, et al. Prevalence of autoimmune disease in patients with prolactinomas and non-functioning pituitary adenomas. Pituitary 2016;19:202–9. [DOI] [PubMed] [Google Scholar]
- [14].Dallapiazza RF, Grober Y, Starke RM, et al. Long-term results of endonasal endoscopic transsphenoidal resection of nonfunctioning pituitary macroadenomas. Neurosurgery 2015;76:42–52. discussion 52–3. [DOI] [PubMed] [Google Scholar]
- [15].Lammert A, Walter MS, Giordano FA, et al. Neuro-endocrine recovery after pituitary apoplexy: prolactin as a predictive factor. Exp Clin Endocrinol Diabetes 2018;128:283–9. [DOI] [PubMed] [Google Scholar]
- [16].Arafah BM, Nasrallah M P. Pituitary tumors: pathophysiology, clinical manifestations and management. Endocr Relat Cancer 2001;8:287–305. [DOI] [PubMed] [Google Scholar]
- [17].Andujar-Plata P, Villar-Taibo R, Ballesteros-Pomar MD, et al. Long-term outcome of multimodal therapy for giant prolactinomas. Endocrine 2017;55:231–8. [DOI] [PubMed] [Google Scholar]
- [18].Moraes AB, Silva CM, Vieira Neto L, et al. Giant prolactinomas: the therapeutic approach. Clin Endocrinol (Oxf) 2013;79:447–56. [DOI] [PubMed] [Google Scholar]
- [19].Hsu CY, Guo WY, Chien CP, et al. MIB-1 labeling index correlated with magnetic resonance imaging detected tumor volume doubling time in pituitary adenoma. Eur J Endocrinol 2010;162:1027–33. [DOI] [PubMed] [Google Scholar]
- [20].Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:273–88. [DOI] [PubMed] [Google Scholar]
- [21].Beck-Peccoz P, Rodari G, Giavoli C, et al. Central hypothyroidism - a neglected thyroid disorder. Nat Rev Endocrinol 2017;13:588–98. [DOI] [PubMed] [Google Scholar]
- [22].Persani L. Clinical review: central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab 2012;97:3068–78. [DOI] [PubMed] [Google Scholar]
- [23].Reimondo G, Bovio S, Allasino B, et al. Secondary hypoadrenalism. Pituitary 2008;11:147–54. [DOI] [PubMed] [Google Scholar]
- [24].Boehm U, Bouloux PM, Dattani MT, et al. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism--pathogenesis, diagnosis and treatment. Nat Rev Endocrinol 2015;11:547–64. [DOI] [PubMed] [Google Scholar]
- [25].Mete O, Lopes MB. Overview of the 2017 WHO Classification of pituitary tumors. Endocr Pathol 2017;28:228–43. [DOI] [PubMed] [Google Scholar]
- [26].Chanson P, Daujat F, Young J, et al. Normal pituitary hypertrophy as a frequent cause of pituitary incidentaloma: a follow-up study. J Clin Endocrinol Metab 2001;86:3009–15. [DOI] [PubMed] [Google Scholar]
- [27].Brochier S, Galland F, Kujas M, et al. Factors predicting relapse of nonfunctioning pituitary macroadenomas after neurosurgery: a study of 142 patients. Eur J Endocrinol 2010;163:193–200. [DOI] [PubMed] [Google Scholar]
- [28].Park SS, Kim JH, Kim YH, et al. Clinical and radiographic characteristics related to hyperprolactinemia in nonfunctioning pituitary adenomas. World Neurosurg 2018;119:e1035–40. [DOI] [PubMed] [Google Scholar]
- [29].Bergsneider M, Mirsadraei L, Yong WH, et al. The pituitary stalk effect: is it a passing phenomenon? J Neurooncol 2014;117:477–84. [DOI] [PubMed] [Google Scholar]
- [30].Colao A, Savastano S. Medical treatment of prolactinomas. Nat Rev Endocrinol 2011;7:267–78. [DOI] [PubMed] [Google Scholar]
- [31].Freeman ME, Kanyicska B, Lerant A, et al. Prolactin: structure, function, and regulation of secretion. Physiol Rev 2000;80:1523–631. [DOI] [PubMed] [Google Scholar]
- [32].Arbogast LA, Voogt JL. Mechanisms of tyrosine hydroxylase regulation during pregnancy: evidence for protein dephosphorylation during the prolactin surges. Endocrinology 1991;129:2575–82. [DOI] [PubMed] [Google Scholar]
- [33].Kohama SG, Bethea CL. Steroid regulation of tyrosine hydroxylase messenger ribonucleic acid in dopaminergic subpopulations of monkey hypothalamus. Endocrinology 1995;136:1790–800. [DOI] [PubMed] [Google Scholar]
- [34].Gudelsky GA, Nansel DD, Porter JC. Role of estrogen in the dopaminergic control of prolactin secretion. Endocrinology 1981;108:440–4. [DOI] [PubMed] [Google Scholar]
- [35].Arafah BM, Prunty D, Ybarra J, et al. The dominant role of increased intrasellar pressure in the pathogenesis of hypopituitarism, hyperprolactinemia, and headaches in patients with pituitary adenomas. J Clin Endocrinol Metab 2000;85:1789–93. [DOI] [PubMed] [Google Scholar]
- [36].Asa SL, Ezzat S. The pathogenesis of pituitary tumours. Nat Rev Cancer 2002;2:836–49. [DOI] [PubMed] [Google Scholar]


