Abstract
Aims
S100B, a well-known damage-associated molecular pattern protein is released acutely by central and peripheral nerves and upon concomitant denervation in pulmonary vein isolation (PVI). We aimed to investigate whether the ablation technique used for PVI impacts S100B release in patients with paroxysmal atrial fibrillation (AF).
Methods and results
The study population consisted of 73 consecutive patients (age: 62.7 ± 10.9 years, 54.8% males) undergoing first-time PVI with either radiofrequency (RF; n = 30) or cryoballoon (CB; n = 43) for paroxysmal AF. S100B determined from venous plasma samples taken immediately before and after PVI increased from 33.5 ± 1.8 to 91.1 ± 5.3 pg/mL (P < 0.0001). S100B release in patients undergoing CB-PVI was 3.9 times higher compared to patients with RF-PVI (ΔS100B: 21.1 ± 2.7 vs. 83.1 ± 5.2 pg/mL, P < 0.0001). During a mean follow-up of 314 ± 186 days, AF recurrences were observed in 18/71 (25.4%) patients (RF-PVI: n = 9/28, CB-PVI: n = 9/43). Univariate Cox regression analysis indicated that an increase in S100B was associated with higher freedom from AF in follow-up (hazard ratio per 10 pg/mL release of S100B: 0.83; 95% confidence interval: 0.72–0.95; P = 0.007).
Conclusion
The ablation technique used for PVI has an impact on the release of S100B, a well-established biomarker for neural damage.
Keywords: Atrial fibrillation, Catheter ablation, Radiofrequency, Cryoballoon, Autonomic nervous system, Intracardiac nervous system, Neuromodulation
What’s new?
This is the first study to directly compare the influence of different ablation techniques used for pulmonary vein isolation (PVI) on the release of the damage-associated molecular pattern response protein S100B.
Our results show that cryoballoon-based PVI induces a higher release of S100B compared to PVI performed with radiofrequency.
Introduction
S100B is a damage-associated molecular pattern protein (DAMP) and an established surrogate marker for neuronal injury.1 Recently, we were able to demonstrate that S100B is released by cardiac glial cells during neural injury induced by pulmonary vein isolation (PVI).2 While targeted autonomic denervation in addition to PVI is feasible and has shown increased success rates for the treatment of paroxysmal atrial fibrillation (AF),3 it is also well known that PVI induces concomitant cardiac denervation.4 This disruption of autonomic nerve fibres and ganglia has been demonstrated by indirect functional parameters such as autonomic responses (e.g. vagal reactions) during PVI5 or subsequent alterations of autonomic function (heart rate, heart rate variability) after PVI, respectively.6 Even though partial denervation has been described for PVI with radiofrequency (RF)6,7 as well as the cryoballoon (CB),5,8,9 a direct comparison of the two ablation techniques has not been performed to date. Therefore, the goal of this studywas to assess the impact of the ablation technique on the release of S100B in patients with paroxysmal AF undergoing PVI.
Methods
Study protocol
In this observational study, 73 patients with symptomatic paroxysmal AF undergoing first-time PVI with either RF (n = 30) or CB (n = 43) were included. The protocol has been described in detail in the previous study, in which 113 patients with AF were enrolled 2016 and 2017 in our institution.2 This sub-study aimed to compare S100B release between the different energy forms used for PVI and excluded patients with persistent AF. Assignment to the ablation technique was performed in a mixed non-randomized order and ablation was carried out by the same operators. Venous blood samples for protein analyses were obtained after placement of femoral sheaths and after ablation. Patients were followed up in the out-patient department. Written informed consent was obtained from all patients prior to the procedure. The study protocol adheres to the principles outlined by the Declaration of Helsinki and was approved by the institutional review board of the University Heart & Vascular Center Hamburg, Germany.
Catheter ablation procedure
All patients underwent pre-procedural transoesophageal echocardiography for exclusion of thrombi. Ablation was performed under deep sedation using propofol (2 mg/mL B. Braun, Melsungen, Germany) combined with fentanyl (0.1 mg/mL Rotexmedica, Trittau, Germany). Heparin (Heparin sodium, 25 000 IU/mL, Rotexmedica) was administered intravenously after transseptal puncture to maintain an activated clotting time of >300 s. Blood pressure, surface electrocardiograms, and bipolar endocardial electrograms were monitored continuously and recorded on a digital amplifier/recorder system (LabSystem PRO, Bard Electrophysiology Inc., Lowell, MA, USA). Oesophageal temperature was measured throughout the procedure using a multipolar temperature-sensing catheter (S-CATH, oesophageal temperature probe, Circa Scientific, Englewood, CO, USA).
Radiofrequency-based ablation
Two long (SL0, 8.5 French, St Jude Medical Inc., St. Paul, MN, USA) and two short sheaths (Fast-Cath, 6 French and 8 French, Daig Inc., Minnetonka, MN, USA) were introduced in the right and left femoral veins, respectively. A multipolar reference catheter (Inquiry, 5 mm spacing, St. Jude Medical Inc.) was placed in the coronary sinus. Single transseptal puncture was performed using a modified Brockenbrough technique to insert both, a 3.5 mm tip mapping and ablation catheter (Thermocool SmartTouch or Carto Navistar, Biosense Webster) and a circular decapolar mapping catheter (Lasso, Biosense Webster) into the left atrium. The left atrium was reconstructed using three-dimensional electroanatomical mapping (CARTO3, Biosense Webster, Diamond Bar, CA, USA or EnSite Precision, St Jude Medical Inc.). A circumferential contiguous ablation line in point-by-point fashion was created around each pair of ipsilateral veins with adequate distance from the ostia in a temperature-controlled mode (flush rate 17–30 mL/min, max. 30 W for 30–60 s, temperature limit of 40°C, minimal contact force of 10 g). In the standard approach, RF dose was guided by ablation index. In selected patients, e.g. upon increase in oesophageal temperature, RF delivery was chosen individually guided by impedance dropping and local signal removal. Successful electrical isolation of the pulmonary veins was confirmed with the circular mapping catheter.
Cryoballoon-based ablation
An octapolar diagnostic catheter (Inquiry, 2-2-2 mm spacing, 6 French, St. Jude Medical) was placed in the coronary sinus from the right femoral vein. The 28 mm CB-G2 (Arctic Front Advance, Medtronic Inc., Minneapolis, MN, USA) was introduced into the left atrium via a long 12 French steerable sheath (FlexCath, Medtronic) after transseptal puncture. Electrograms from the pulmonary veins were mapped before, during and after CB-application with an endoluminal spiral mapping catheter (Achieve, Medtronic). Target application time in this study was 240 s, the freezing cycle was shortened to 180 s in case early isolation (within 60 s) was observed. Successful electrical isolation of the pulmonary veins was confirmed using a spiral mapping catheter (Achieve, Medtronic). During ablation of the right pulmonary vein, the phrenic nerve was electrically stimulated from the superior vena cava. The integrity of the phrenic nerve was monitored via diaphragmatic compound motor action potentials (CMAP) in conjunction with abdominal palpation. The freezing cycle was immediately terminated in cases of a CMAP reduction of 30% or less, loss of phrenic nerve capture or if temperature in the oesophagus dropped below 17°C.
Follow-up
All antiarrhythmic drugs were discontinued after the ablation procedure. Heart rate was analysed from resting ECGs in sinus rhythm performed on admission and discharge from our hospital. Patients were scheduled for follow-up in our outpatient clinic within 6 months after the procedure. Twelve-lead electrocardiogram (ECG) and/or 24-h Holter monitoring was performed when patients complained about symptoms. For longer follow-up, patients were contacted via phone to inquire for symptoms and asked to present at an outpatient clinic. Recurrence of AF was defined as an ECG-documented episode lasting at least 30 s after a 90 day blanking period.4
Measurement of S100B and high-sensitive troponin T
Venous plasma samples for protein analyses were obtained after placement of femoral sheaths before transseptal puncture and before removal of the sheaths. Samples were processed immediately and frozen at −80°C as described previously.2 S100B concentrations were assessed using a commercially available enzyme-linked immunosorbent assay (Merck Millipore, Darmstadt, Germany). Cardiac troponin I was determined using a highly sensitive immunoassay (HsTnI, Architect i2000SR, Abbott Diagnostics, USA).
Statistical analysis
Continuous variables are described as mean ± standard deviation; categorical variables as absolute numbers and percentages. Data were tested for normality using D’Agostino and Pearson omnibus test. For continuous and ordinal variables, Student’s t-test or Mann–Whitney test were used as appropriate. Categorical variables were compared using the Fisher’s exact test. P < 0.05 defined statistical significance. Statistical analysis was performed using GraphPad Prism 6.07 (GraphPad Software), Cox regression was performed using R v3.6.0 and the ‘survival’ package v2.44.–1.1.
Results
Baseline characteristics
Baseline characteristics of all patients are presented in Table 1. Patients in both groups did not differ in regard to baseline characteristics, as well as CHA2DS2-VASc- score and the score of the European Heart Rhythm Association. None of the patients within this study experienced a periprocedural stroke and no clinical evidence for neuronal damage was present.
Table 1.
Baseline characteristics
| RF-PVI (n = 30) | CB-PVI (n = 43) | P-value | |
|---|---|---|---|
| Characteristics | |||
| Male sex | 14 (46.7) | 26 (60.5) | 0.339 |
| Age (years) | 63.6 (8.9) | 62.1 (12.0) | 0.843 |
| BMI (kg/m²) | 25.7 (2.9) | 27.4 (4.4) | 0.078 |
| CHA2DS2-VASc score | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 0.535 |
| EHRA score | 2-5 (2.0, 3.0) | 2.0 (2.0,2.5) | 0.154 |
| Comorbidities | |||
| Hypertension | 19 (63.3) | 25 (58.2) | 0.809 |
| Coronary artery disease | 5 (16.7) | 6 (14.0) | 0.752 |
| Myocardial infarction | 5 (16.7) | 5 (11.7) | 0.731 |
| Diabetes | 0 (0.0) | 1 (2.3) | 1.000 |
| CKD | 6 (20.0) | 4 (9.3) | 0.300 |
| Medical history | |||
| Previous stroke/TIA | 2 (6.7) | 5 (11.6) | 0.693 |
| Previous cancer diagnosis | 4 (13.3) | 3 (7.0) | 0.436 |
| ICD/pacemaker | 2 (6.7) | 3 (7.0) | 1.000 |
| Medical treatment | |||
| Beta-receptor blockers | 23 (76.7) | 27 (62.8) | 0.306 |
| Class I, III antiarrhythmic drugs | 13 (43.3) | 13 (30.2) | 0.322 |
| Calcium channel blockers | 1 (3.3) | 6 (14.0) | 0.228 |
| Diuretics | 6 (20.0) | 5 (11.6) | 0.342 |
| ACE blockers | 9 (30.0) | 10 (23.3) | 0.592 |
| Sartans | 4 (13.3) | 9 (20.9) | 0.539 |
| Acetylsalicylic acid | 3 (10.0) | 3 (7.0) | 0.685 |
| Vitamin K antagonists | 4 (13.3) | 10 (23.3) | 0.372 |
| NOAC | 23 (76.7) | 24 (55.8) | 0.085 |
| Recurrences (n) | 9 (32.1.)a | 9 (20.9) | 0.403 |
Data are expressed as absolute numbers and percentages (categorical variables), quartiles (ordinary variables), or mean ± standard deviation. Differences in baseline characteristics were analysed using Student’s t-test. Categorical variables were compared using the χ2 test or Fisher’s exact test.
ACE, angiotensin converting enzyme; BMI, body mass index; CB, cryoballoon; CKD, chronic kidney disease (glomerular filtration rate <60 mL/min); EHRA, European Heart Rhythm Association; ICD, implantable cardioverter-defibrillator; NOAC, non-vitamin k oral anticoagulants; PVI, pulmonary vein isolation; RF, radiofrequency; TIA, transient ischaemic attack.
Two patients lost to follow-up.
Procedural data
Procedure duration was shorter in patients undergoing CB-PVI compared to RF-PVI (RF-PVI: 117.3 ± 28.2 min, CB-PVI: 80.6 ± 21.4 min, P < 0.0001). Dosage area product was higher in patients with CB-PVI (RF-PVI: 217.6 ± 112.2 cGycm2, CB-PVI: 414.0 ± 260.6 cGycm2, P < 0.0001).
Protein measurements
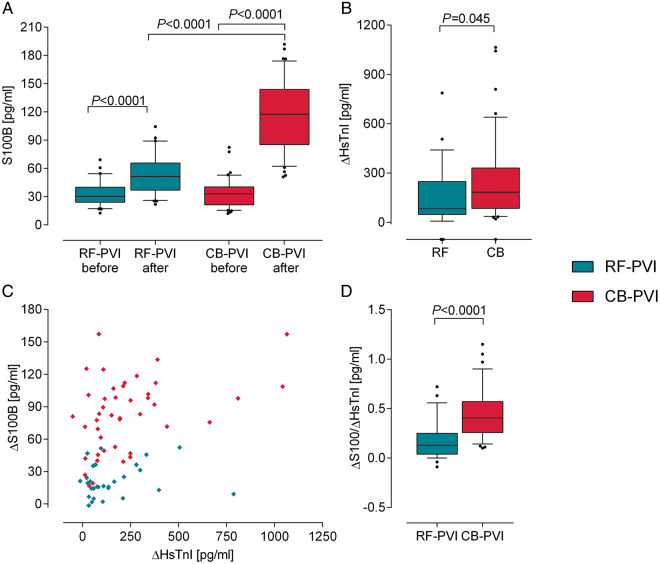
Biomarker release is presented in Table 2. Baseline S100B plasma concentrations did not differ between the groups (RF-PVI: 33.2 ± 13.9 pg/mL, CB-PVI: 33.6 ± 15.8 pg/mL, P = 0.984). Overall, S100B increased from 33.5 ± 15.0 to 91.1 ± 45.0 pg/mL (P < 0.0001) following PVI. S100B was released in both groups of patients after PVI (RF-PVI: 33.2 ± 13.9 to 54.3 ± 22.5 pg/mL, P < 0.0001; CB-PVI: 33.6 ± 15.8 to 116.7 ± 38.5 pg/mL, P < 0.0001, Figure 1A), but ΔS100B in patients undergoing CB-PVI was 3.9 times higher compared to patients with RF-PVI (RF-PVI: 21.1 ± 14.8 pg/mL, CB-PVI: 83.1 ± 33.9 pg/mL, P < 0.0001).
Table 2.
Pre- to postprocedural measurements
| RF-PVI (n = 30) |
CB-PVI (n = 43) |
|||||
|---|---|---|---|---|---|---|
| Preprocedural | Postprocedural | P-value | Preprocedural | Postprocedural | P-value | |
| S100B (pg/mL) | 33.2 ± 13.9 | 54.3 ± 22.5 | <0.0001 | 33.6 ± 15.8 | 116.7 ± 38.5 | <0.0001 |
| HsTnI (pg/mL) | 41.3 ± 134.2 | 196 ± 209.1 | <0.0001 | 12.6 ± 31.0 | 261.8 ± 252.1 | <0.0001 |
| Heart rate (b.p.m.) | 59.9 ± 11.4 | 70.7 ± 11.4 | <0.0001 | 59.6 ± 8.0 | 68.9 ± 10.5 | <0.0001 |
Data are expressed as mean ± standard deviation and were compared using Wilcoxon test or paired t-test as appropriate.
CB, cryoballoon; HsTnI, high-sensitive troponin T; PVI, pulmonary vein isolation; RF, radiofrequency.
Figure 1.
Effect of the ablation technique on biomarker release. (A) Comparison of S100B release after cryoballoon-based (CB)-PVI compared to radiofrequency (RF)-PVI. (B) Comparison of S100B release normalized to release of HsTnI to account for differences in myocardial damage during PVI. (C) ΔS100B plotted against ΔHsTnI concentrations for both groups of patients show that S100B release is independent of myocardial damage. (D) HsTnI release indicates a slightly higher amount of myocardial damage in patients undergoing CB-PVI. CB, cryoballoon; HsTnI, high-sensitive troponin T; PVI, pulmonary vein isolation; RF, radiofrequency.
High-sensitive troponin T (HsTnI) plasma concentrations increased from 23.6 ± 86.8 to 236.4 ± 237.0 pg/mL after PVI (P < 0.0001), but ΔhsTnI concentrations (before to after the procedure) showed a higher increase in patients undergoing CB-PVI (RF-PVI: 154.4 ± 196.0 pg/mL, CB-PVI: 249.2 ± 252.0 pg/mL, P < 0.04, Figure 1B). Plotting ΔS100B and ΔhsTnI release (Figure 1C) visualized this. Normalization of S100B to hsTnI was performed to account for the amount of myocardial damage of the particular procedure (Figure 1D). The S100B/hsTnI ratio was higher in patients undergoing CB-PVI, indicating that the higher amount of S100B is not explained by more myocardial damage.
Follow-up
Heart rate measured in patients with sinus rhythm increased after the procedure (59.7 ± 9.5 to 69.7 ± 10.8 b.p.m., P < 0.0001, Table 2). No differences in heart rate increase (ΔHR) were detectable between the groups (RF-PVI: 10.5 ± 10.8 b.p.m., CB-PVI: 9.8 ± 8.8, P = 0.79) or between patients with and without recurrences (ΔHR in patients with recurrences: 8.1 ± 11.9 b.p.m. vs. ΔHR in patients without recurrences: 10.8 ± 9.0 b.p.m., P = 0.381). There was no correlation of ΔHR and ΔS100B (data not shown).
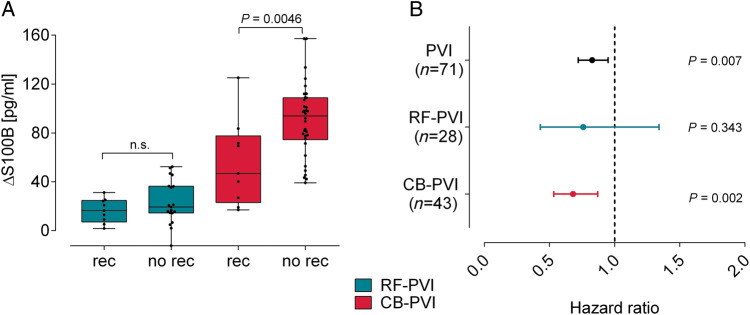
During a follow-up of 314 ± 186 days, 18/71 (25.4%) patients experienced a recurrence of AF [RF-PVI: 9/28 patients (32.1%), CB-PVI: 9/43 patients (20.9%)]. No significant differences in recurrences were detectable between the groups (P = 0.4). In the Cox regression analysis, higher release of S100B was associated with freedom from AF during follow-up (hazard ratio per 10 pg/mL increase of S100B: 0.83; 95% confidence interval: 0.72–0.95; P = 0.007), however, increase in HsTnI or other variables (Table 3) did not. Hazard ratios for AF recurrence in both subgroups are similar (CB-PVI: 0.68; 95% confidence interval: 0.53–0.87; P = 0.002; RF-PVI: 0.76; 95% confidence interval: 0.43–1.3; P = 0.35, Figure 2B) with larger confidence intervals in the RF group. Therefore, we additionally performed an adjustment to the subgroup which results in a reduction of the hazard ratio by 9% per 10 pg/mL S100B increase (hazard ratio: 0.74; 95% confidence interval: 0.61–0.89; P = 0.002).
Table 3.
Cox regression analysis for predictors of recurrences
| Predictor | HR | CI95.low | CI95.high | P-value |
|---|---|---|---|---|
| Univariate analysis | ||||
| 10 pg/mL ΔS100B | 0.8279 | 0.7216 | 0.9498 | 0.007 |
| 10 pg/mL ΔHsTnI | 0.9998 | 0.9977 | 1.002 | 0.844 |
| ΔHeart rate | 0.9753 | 0.9194 | 1.035 | 0.407 |
| Group | 1.797 | 0.7099 | 4.551 | 0.216 |
| Age | 0.996 | 0.9499 | 1.044 | 0.868 |
| BMI | 0.9808 | 0.8838 | 1.088 | 0.715 |
| Male sex | 0.1195 | 0.4684 | 3.05 | 0.709 |
| ERAF | 1.981 | 0.7751 | 5.064 | 0.153 |
| Multivariate analysis | ||||
| 10 pg/mL ΔS100B adjusted for group | 0.7364 | 0.60710 | 0.8932 | 0.002 |
AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; ERAF, early recurrence of AF (within the first 90 days after ablation); HR, hazard ratio; HsTnI, high-sensitive troponin T.
Figure 2.
Correlation of S100B release and recurrences of AF. (A) Comparison of S100B concentration for patients with (rec) and without (no rec) recurrences of AF in both groups. (B) Hazard ratios of Cox regression analysis per 10 pg/mL increased S100B release. AF, atrial fibrillation; CB, cryoballoon; PVI, pulmonary vein isolation; RF, radiofrequency.
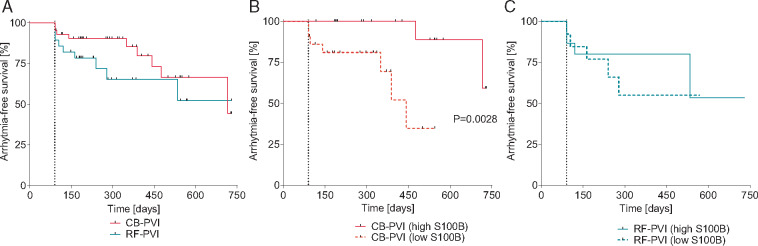
Arrhythmia-free survival is depicted as Kaplan–Meier plot (Figure 3). Patients in the RF-PVI group do not differ in regard to AF recurrences to the CB-PVI group (Figure 3A). When patients are stratified in high and low amounts of S100B release (Figure3B and C), differences are only detectable in the CB-PVI group.
Figure 3.
Kaplan–Meier analysis of arrhythmia-free survival. Patients are stratified according to the energy form (A) or S100B release (B and C). Three months blanking period is depicted as dashed line. CB, cryoballoon; PVI, pulmonary vein isolation; RF, radiofrequency.
Discussion
In the present study, we demonstrate that the ablation technique used for PVI has an impact on release of the neuronal injury marker and DAMP S100B.
Concomitant cardiac denervation during pulmonary vein isolation
The autonomic nervous system is well known to play a crucial role in the pathophysiology of AF.10 Concomitant cardiac denervation in patients undergoing PVI is proposed to affect procedural outcome.6 It is important to note that sympathetic and parasympathetic structures cannot be considered separately: even though the intracardiac nervous system and its ganglionated plexi are classically considered as ‘parasympathetic’, they also contain sympathetic cell bodies and fibres.10 As both types of cardiac nerves and neurons within ganglionated plexi are surrounded by S100B-expressing glial cells,2 differentiating between the type of denervation would not be possible using S100B and was not the goal of this study.
Previous studies used either autonomic reflexes during PVI5 or indirect functional measurements such as changes in heart rate and heart rate variability to investigate cardiac denervation.7,8 These methods are well established but are often prone to artefacts, environmental factors and not applicable in the clinical routine in each electrophysiological laboratory. With these endpoints, partial cardiac denervation has been reported for RF-PVI.6,7 A recent study has shown that CB-PVI leads to a markedly decreased vagal response to high-frequency stimulation of the major atrial ganglionated plexi, indicating a relevant modification of the cardiac autonomic nervous system.9 This is confirmed by other studies,5,8 but until now, the two ablation techniques were not directly compared regarding the extent of concomitant denervation.
We recently demonstrated that the measurement of S100B release is a feasible blood-based method for the investigation of acute neural damage during ablation procedures.2 In line with these findings, the current study presents evidence that the ablation technique used for PVI impacts S100B release in patients with paroxysmal AF undergoing first-time PVI.
S100B release and myocardial damage during pulmonary vein isolation
Different ablation techniques for PVI induce different lesions, but studies with a direct comparison of lesion characteristics are rare and partially conflicting: a recent case–control study based on late-enhancement cardiac magnetic resonance imaging found no differences in lesion size between the ablation techniques in a cohort of 60 patients,11 while it was also reported that the isolated area after cryoablation is smaller than after RF ablation.12 A case report described cryogenic lesions histologically as more homogenous and more demarcated compared to RF.13 Evaluation of the lesion after ablation was beyond the scope of our study, but hsTnI measurements indicated that more myocardial damage was created with the CB. Still, normalization of S100B to hsTnI release indicates that the higher amount of S100B in CB-PVI cannot be explained by a larger ablated area alone as the S100B/hsTnI ratio is still higher in the CB-PVI group. While differences in S100B release might be explained by atrial remodelling or hyperinnervation, this seems not likely as patients in this study did not differ in regard to baseline characteristics and patients with persistent AF were not included. Also, only few patients had comorbidities related to structural and functional changes within the intracardiac nervous system, such as arterial hypertension or ischaemic heart disease.
Assessing denervation induced by pulmonary vein isolation
Assessing denervation induced by PVI is challenging. Acute vagal reactions such as bradycardia, asystole, atrioventricular block, or hypotension have been the most widely used markers for acute autonomic modulation. Whether or not these autonomic reflexes are associated with improved procedural outcomes varies amongst studies.4 Some studies describe a reduction of AF recurrences when vagal reactions were present in patients undergoing PVI,6 in others, vagal reactions were not predictive for recurrences.14
Even though transient increases in heart rate for up to one year after PVI due to reduced vagal innervation of the sinus node have traditionally been used as marker for denervation,6,7 limitations of this approach are well known: the main pathway for vagal innervation of the sinus node converges through ganglia at the right pulmonary veins.15 Therefore, changes in heart rate do reflect autonomic modulation to some degree but cannot reflect autonomic modulation in the left atrial neural network, which has repeatedly been demonstrated to be of high importance for the pathophysiology of AF and its triggers from the pulmonary veins.3 This might explain why we do not observe difference in heart rate increases between the ablation techniques. A different explanation could be the possibility that a particular amount of autonomic disruption induces heart rate changes in an all-or-nothing fashion instead of a gradual effect. This is endorsed by the fact that a similar change in heart rate is observed after targeted ablation of ganglionated plexi in patients with PAF16 and that the heart rate increase we observed is in line with others and the current consensus statement on catheter ablation of AF (10–20 b.p.m.).4 It highlights the need for novel markers to assess the impact of acute cardiac denervation and indicates that a higher release of S100B can represent more neuronal damage without a stronger increase in heart rate.
In the current study, higher S100B values were associated with freedom from AF. Still, we did not detect significant differences regarding freedom from AF between patients undergoing RF- or CB-PVI. This is puzzling, as S100B concentrations are continuously higher in the CB-PVI group, also in patients with recurrences. A possible explanation is that released S100B induces negative effects, such as increased nerve sprouting, thus contributing to a new pro-arrhythmic substrate. It is known that PVI also leads to the release of nerve growth factor, which exerts nerve sprouting and pro-arrhythmic effects.17
Limitations
This study has some limitations: Most importantly, intra-procedural autonomic reactions were not systematically assessed. Therefore, no conclusion can be drawn whether autonomic responses go hand in hand with an increased release of S100B. This has to be verified in future studies.
Second, this was an observational study and not a randomized control trial. Still, patients were assigned to the ablation technique in a mixed, non-randomized order and it is therefore unlikely that S100B release is affected. Additionally, patients in both groups did not differ in regard to baseline characteristics and outcome which confirms our approach.
Third, is it important to note that silent cerebral events, which have been observed frequently after PVI (see Supplementary material online, Table S1 for an overview on this topic), might present a potential additional source for released S100B in both groups of patients, even though no stroke or indication for subclinical central damage was observed in this study. Brain magnetic resonance imaging for detection of silent cerebral events would have been ideal but was beyond the scope of this study. Studies report silent cerebral events occurring over a range of 11.1–33% for patients undergoing CB-PVI with the second generation CB18 and 1.7–38%19,20 for patients undergoing RF-PVI, indicating that no clear conclusion can be drawn on which technique induces more silent cerebral events. Also, cerebral events are regularly observed in AF patients (see Supplementary material online, Table S2). In contrast, S100B is released in more than 98% of patients after PVI.2
It should be noted that the higher release of S100B in the CB-PVI group could potentially derive at least in part from injury of extra-cardiac nerves, such as the phrenic nerve.2 To reduce this risk, phrenic nerve activity is continuously monitored throughout CB-based PVI in our centre. Still, we cannot exclude that subclinical damage occurs in at least some patients undergoing CB-PVI leading to an increased release of S100B.
Conclusion
We show here that the ablation technique used for PVI has an impact on the acute release of the neural injury marker S100B. More specifically, CB-PVI leads to a higher S100B release than RF-PVI. Whether measurement of S100B release can help to characterize the role of autonomic modulation in the treatment of patients with arrhythmias has to be shown in future studies.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
The authors kindly thank Prof. Dr. Tanja Zeller and her team for troponin measurements, Hartwig Wieboldt for excellent technical support and the team of the electrophysiology lab for assistance with blood samples.
Funding
This work was supported by the German Centre for Cardiovascular Research (DZHK; FKZ 81Z2710104 and 81Z0710112).
Conflict of interest: none declared.
Data availability
Data available on request.
References
- 1. Undén J, Ingebrigtsen T, Romner B; the Scandinavian Neurotrauma Committee (SNC). Scandinavian guidelines for initial management of minimal, mild and moderate head injuries in adults: an evidence and consensus-based update. BMC Med 2013;11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scherschel K, Hedenus K, Jungen C, Lemoine MD, Rübsamen N, Veldkamp MW. et al. Cardiac glial cells release neurotrophic S100B upon catheter-based treatment of atrial fibrillation. Sci Transl Med 2019;11:1–12. [DOI] [PubMed] [Google Scholar]
- 3. Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GCM, Po SS. et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol 2013;62:2318–25. [DOI] [PubMed] [Google Scholar]
- 4. Calkins H, Hindricks G, Cappato R, Kim Y-H, Saad EB, Aguinaga L. et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20:e1–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yorgun H, Aytemir K, Canpolat U, Ahiner L, Kaya EB, Oto A.. Additional benefit of cryoballoon-based atrial fibrillation ablation beyond pulmonary vein isolation: modification of ganglionated plexi. Europace 2014;16:645–51. [DOI] [PubMed] [Google Scholar]
- 6. Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G. et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 2004;109:327–34. [DOI] [PubMed] [Google Scholar]
- 7. Hsieh MH, Chiou CW, Wen ZC, Wu CH, Tai CT, Tsai CF. et al. Alterations of heart rate variability after radiofrequency catheter ablation of focal atrial fibrillation originating from pulmonary veins. Circulation 1999;100:2237–43. [DOI] [PubMed] [Google Scholar]
- 8. Oswald H, Klein G, Koenig T, Luesebrink U, Duncker D, Gardiwal A.. Cryoballoon pulmonary vein isolation temporarily modulates the intrinsic cardiac autonomic nervous system. J Interv Card Electrophysiol 2010;29:57–62. [DOI] [PubMed] [Google Scholar]
- 9. Garabelli P, Stavrakis S, Kenney JFA, Po SS.. Effect of 28-mm cryoballoon ablation on major atrial ganglionated plexi. JACC Clin Electrophysiol 2018;4:831–8. [DOI] [PubMed] [Google Scholar]
- 10. Katritsis GD, Katritsis DG.. Cardiac autonomic denervation for ablation of atrial fibrillation. Arrhythmia Electrophysiol Rev 2014;3:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alarco F, Cabanelas N, Izquierdo M, Benito E, Figueras R, Guasch E. et al. Cryoballoon vs. radiofrequency lesions as detected by late-enhancement cardiac magnetic resonance after ablation of paroxysmal atrial fibrillation: a case–control study. Europace 2019;22:382–7. [DOI] [PubMed] [Google Scholar]
- 12. Miyazaki S, Taniguchi H, Hachiya H, Nakamura H, Takagi T, Iwasawa J. et al. Quantitative analysis of the isolation area during the chronic phase after a 28-mm second-generation cryoballoon ablation demarcated by high-resolution electroanatomic mapping. Circ Arrhythmia Electrophysiol 2016;9:1–9. [DOI] [PubMed] [Google Scholar]
- 13. Hirao T, Nitta J, Adachi A, Takahashi Y, Goya M, Hirao K.. First confirmation of histologic changes in the human heart after cryoballoon ablation. Heart Case Rep 2019;5:93–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mikhaylov E, Kanidieva A, Sviridova N, Abramov M, Gureev S, Szili-Torok T. et al. Outcome of anatomic ganglionated plexi ablation to treat paroxysmal atrial fibrillation: a 3-year follow-up study. Europace 2011;13:362–70. [DOI] [PubMed] [Google Scholar]
- 15. Hou Y, Scherlag BJ, Lin J, Zhang Y, Lu Z, Truong K. et al. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input. Effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol 2007;50:61–8. [DOI] [PubMed] [Google Scholar]
- 16. Pokushalov E, Romanov A, Shugayev P, Artyomenko S, Shirokova N, Turov A. et al. Selective ganglionated plexi ablation for paroxysmal atrial fibrillation. Heart Rhythm 2009;6:1257–64. [DOI] [PubMed] [Google Scholar]
- 17. Shen MJ, Choi E-K, Tan AY, Lin S-F, Fishbein MC, Chen LS. et al. Neural mechanisms of atrial arrhythmias. Nat Rev Cardiol 2012;9:30–9. [DOI] [PubMed] [Google Scholar]
- 18. Okishige K, Nakamura T, Aoyagi H, Kawaguchi N, Yamashita M, Kurabayashi M. et al. Comparative study of hemorrhagic and ischemic complications among anticoagulants in patients undergoing cryoballoon ablation for atrial fibrillation. J Cardiol 2017;69:11–5. [DOI] [PubMed] [Google Scholar]
- 19. Schmidt B, Széplaki G, Merkely B, Kautzner J, Driel V. V, Bourier F. et al. Silent cerebral lesions and cognitive function after pulmonary vein isolation with an irrigated gold-tip catheter: REDUCE-TE Pilot study. J Cardiovasc Electrophysiol 2019;30:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deneke T, Shin D-I, Balta O, Bünz K, Fassbender F, Mügge A. et al. Postablation asymptomatic cerebral lesions: long-term follow-up using magnetic resonance imaging. Heart Rhythm 2011;8:1705–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request.