Abstract
Aims
Natriuretic peptides are extensively studied biomarkers for atrial fibrillation (AF) and heart failure (HF). Their role in the pathogenesis of both diseases is not entirely understood and previous studies several single-nucleotide polymorphisms (SNPs) at the NPPA-NPPB locus associated with natriuretic peptides have been identified. We investigated the causal relationship between natriuretic peptides and AF as well as HF using a Mendelian randomization approach.
Methods and results
N-terminal pro B-type natriuretic peptide (NT-proBNP) (N = 6669), B-type natriuretic peptide (BNP) (N = 6674), and mid-regional pro atrial natriuretic peptide (MR-proANP) (N = 6813) were measured in the FINRISK 1997 cohort. N = 30 common SNPs related to NT-proBNP, BNP, and MR-proANP were selected from studies. We performed six Mendelian randomizations for all three natriuretic peptide biomarkers and for both outcomes, AF and HF, separately. Polygenic risk scores (PRSs) based on multiple SNPs were used as genetic instrumental variable in Mendelian randomizations. Polygenic risk scores were significantly associated with the three natriuretic peptides. Polygenic risk scores were not significantly associated with incident AF nor HF. Most cardiovascular risk factors showed significant confounding percentages, but no association with PRS. A causal relation except for small causal betas is unlikely.
Conclusion
In our Mendelian randomization approach, we confirmed an association between common genetic variation at the NPPA-NPPB locus and natriuretic peptides. A strong causal relationship between natriuretic peptides and incidence of AF as well as HF at the community-level was ruled out. Therapeutic approaches targeting natriuretic peptides will therefore very likely work through indirect mechanisms.
Keywords: Mendelian randomization, Natriuretic peptides, Atrial fibrillation, Heart failure, Epidemiology
What’s new?
The natriuretic peptides or their prohormones B-type natriuretic peptide, N-terminal pro B-type natriuretic peptide, and mid-regional pro atrial natriuretic peptide were significantly associated with the development of atrial fibrillation (AF) and heart failure (HF) in a community cohort.
Polygenic risk scores derived from the genetic loci coding for the natriuretic peptides were significantly associated with the respective protein biomarkers, but no association with disease outcomes was demonstrated.
Mendelian randomization analyses showed no causal relationship between natriuretic peptides or their prohormones and AF or HF. The proteins remain strong disease markers.
Introduction
Atrial fibrillation (AF) and heart failure (HF) are increasingly common diseases in older adults. For both conditions, natriuretic peptides as indicators of cardiac wall stress have remained among the strongest predictors of incident disease.1 They are responsible for fluid and blood pressure homeostasis through diuretic and vasodilatory effects and further affect cardiovascular remodelling. In the context of AF and HF, atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) including their pro-hormones are the most extensively studied natriuretic peptides. Both are increased in HF with preserved and reduced ejection fraction. In HF with reduced ejection fraction, N-terminal pro B-type natriuretic peptide (NT-proBNP) concentrations were higher in individuals with concomitant AF indicating an additional AF-related component of NT-proBNP.2 In AF, elevated natriuretic peptides reflect increased left atrial pressure and adverse remodelling.3 Restoration of sinus rhythm goes along with decreasing concentrations.4 Furthermore, the most prominent risk factors for AF development such as age, sex, increased body mass index (BMI), hypertension, and HF, have all been related to elevated natriuretic peptide concentrations.
Even though natriuretic peptides have been extensively investigated as biomarkers for HF and AF, their role and possible contribution in the pathogenesis of the diseases is not entirely clear. The temporal relationship of natriuretic peptide elevation and disease onset has not been elucidated. Subclinical HF may be associated with already elevated blood biomarkers and similarly, intermittent, but undiagnosed AF may result in increased natriuretic peptide concentrations due to reverse causation. Furthermore, it is not certain, if the associations between ANP and BNP/NT-proBNP to AF and HF are identical.
While the structure of ANP is conserved among species, the primary BNP structure varies considerably and recent genome-wide association studies have revealed and validated several single-nucleotide polymorphisms (SNPs) in the NPPA-NPPB locus. Considering the inherently unidirectional association from genetics to biomarker concentrations, genetics may help to provide some evidence on potential causal relations at the population level. Therefore, we examined common polymorphisms in the genes of the two protagonist natriuretic peptides, BNP and ANP and circulating pro-hormone NT-proBNP concentrations and their association with incident AF and HF. By using Mendelian randomization, we investigated the causal relationship between natriuretic peptides and AF as well as HF.
Methods
Study population
Analyses were performed in individuals from the FINRISK 1997 cohort. The National FINRISK study enrolls individuals of five Finnish areas every 5 years. A detailed description of the study design has been published before (http://www.thl.fi/publications/morgam/cohorts/full/finland/fin-fina.htm).
On enrolment, participants underwent physical examination, filled in a questionnaire and provided blood samples. Systolic blood pressure and BMI were measured during the examination. The remaining covariates and known confounders (current smoking, antihypertensive treatment, and prevalent diabetes) were by participant history or by history and prior discharge code (prevalent AF, prevalent HF, and prevalent myocardial infarction). Total cholesterol and creatinine were measured by routine methods. The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI formula. For follow-up, the National Hospital Discharge Register, the National Causes of Death Register, the National Drug Reimbursement Register, and discharge registries of specialized ambulatory clinics were used to generate information on AF and HF outcomes either as primary disease or comorbidity.
For our analyses, we excluded all individuals with pre-existing AF (N = 165, 2.0%) and/or HF (N = 159, 1.9%). Furthermore, participants with missing covariates and/or genetic data were excluded. The number of individuals included in the analyses was initially N = 8446, but after exclusion of people with missing natriuretic peptide values there remained N = 6669 for NT-proBNP, N = 6674 for BNP, N = 6813 for mid-regional pro atrial natriuretic peptide (MR-proANP). Last follow-up was obtained 31 December 2016.
The FINRISK cohorts were approved by the respective institutional review boards. The study is in accordance with the declaration of Helsinki. All participants provided informed consent.
Natriuretic peptide measurements
We measured BNP (BNP, UniProt acc. P16860, residues 103–134), Roche Elecsys 2010 proBNP (NT-proBNP, acc. P16860 residues 27–134), and B.R.A.H.M.S. MR-proANP KRYPTOR (MR-proANP, acc. P01160) in the MORGAM Biomarker Laboratory, University of Mainz, Germany, using the Abbott Architect i2000. Inter-/intra-assay coefficients of variation have been reported (http://biomarcare.eu/) as follows: BNP 2.11%/4.28%, NT-proBNP 2.58%/1.38%, and MR-proANP 3.65%/2.33%.
Genotyping and imputation
The samples were genotyped using genotyping array Illumina OmniExpress, Illumina CoreExome, Affymetrix 6.0, Illumina 610K. Imputation reference panel was SISu v2 with 2690 hcWGS and 5092 WES Finnish genomes. Pre-imputation quality control was done per genotyping batch. Population outliers were removed based on principal components. Pre-phasing done with Eagle 2.3. Imputation with Impute2 (version 2.3.1). Post-imputation quality control consisted of checking chunk integrity (along the chromosome) and minor allele frequency for imputed variants (compared to the reference panel). Quality control for the genome-wide association analysis study (GWAS) results comprised removal of SNPs with Hardy–Weinberg equilibrium P-value ≤10−7, minor allele frequency <5%, and imputation quality (INFO-value) <0.7, proportion of missings ≥2%. Genotyping batch was used as a covariate in the analyses. Population stratification was controlled by adjusting for the first 10 principal components. Close relatives (PI_HAT > 0.2) were excluded from the analyses.
Single-nucleotide selection
Common SNPs were selected from recent genome-wide association studies.5–7 The published betas were used. We selected N = 30 replicated SNPs, 27 in relation to NT-proBNP, three for BNP and MR-proANP. Due to the strong correlation of the prohormone fragment MR-proANP and ANP, we used SNPs published in relation to ANP. Only independent SNPs [distance ≥500 kbp or low linkage disequilibrium (correlation ≤0.8)] that reached genome-wide significance were selected. Dependent SNPs were removed one-by-one (based on effect size) until a set of only independent SNPs remained. For NT-proBNP, 27 SNPs were used, for both BNP and MR-proANP, three SNPs each. Details of the SNP selection and beta coefficients are outlined in the Supplementary material online, Tables S1–S3. In addition, GWAS analyses were performed for NT-proBNP, BNP, and MR-proANP, which yielded results that were in-line with the here-used effect sizes and directions.
Statistical methods
We performed six Mendelian randomizations for all three natriuretic peptide biomarkers and both outcomes, AF and HF separately. The ratio method of Mendelian randomization was used.8 Polygenic risk scores (PRSs) based on multiple SNPs were used as genetic instrumental variable (IV) in Mendelian randomizations. PRS based on multiple SNPs were used because (i) they generally are related to a stronger association between IV and biomarker (NT-proBNP, BNP, and MR-proANP) and (ii) including more SNPs in a PRS improves the normality of the IV. Weighted PRS were constructed using beta coefficients from published genome-wide association study results as weights and SNP values were the number of effect alleles (0, 1, or 2). All PRS were normalized to have zero mean and unit standard deviation.
Associations between PRSX and incident AF or HF were estimated using Cox regression (a) and associations between IV and biomarker were estimated using linear regression (b). An instrumental variable estimate of the beta coefficient for the causal association between the biomarkers NT-proBNP, BNP, and MR-proANP and AF or HF was estimated using the ratio method by dividing the beta coefficient of the Cox regression (a) by the beta coefficient of the linear regression (b) (Figure 1). The 95% confidence interval of the causal beta estimate was estimated using bootstrapping.9 Causal hazard ratios (HRs) were calculated by exponentiation of the causal beta. Observational HRs for the associations between biomarkers and AF or HF were estimated using Cox regression and are used for comparison with the causal HRs from the Mendelian randomization. A non-causal beta coefficient was calculated by subtracting the causal beta coefficient from the observational beta coefficient. The causal beta coefficient and non-causal beta coefficient were also expressed as a percentage of observational beta coefficients.
Figure 1.
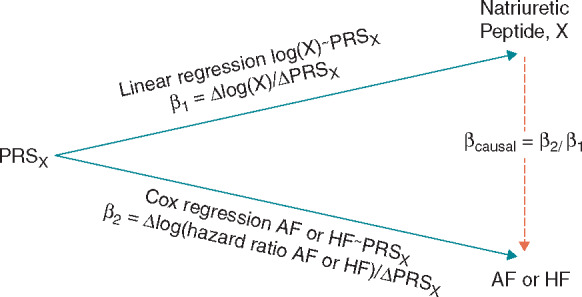
Illustration of the ratio method in Mendelian randomization of natriuretic peptides and AF and HF. Each natriuretic peptide X has its own PRS, denoted by PRSX. AF, atrial fibrillation; HF, heart failure; PRS, polygenetic risk score.
Checking assumptions underlying Mendelian randomization
The three assumptions of Mendelian randomization were checked8: Assumption 1, the association of the PRS with the natriuretic peptides; assumption 2, PRS influence on AF or HF only via the natriuretic peptides; assumption 3, PRS independent of factors that confound the association between log (X) and AF or HF (where X can be to NT-proBNP, BNP, or MR-proANP). Further details how the three assumptions were checked are provided in the Supplementary material online.
Individuals with prevalent AF and/or HF were excluded from all regressions. Bootstrapping estimates were based on 10 000 repetitions. Two-sided P-values <0.05 were considered significant.
Results
Study population
The mean age of the cohort was 48 years, 50% were men. Further baseline characteristics of the study cohort are provided in Table 1. During a mean follow-up of 16.5 years, 757 (9%) developed incident AF and 519 (6.1%) incident HF, 228 (2.7%) developed both diseases.
Table 1.
Baseline characteristics of the study population
| Characteristic | Population (N = 8446) |
|---|---|
| Age (years) | 48.6 ± 13.6 |
| Men, N (%) | 4253 (50) |
| Prevalent myocardial infarction, N (%) | 181 (2.1) |
| Cardiovascular risk factors | |
| Body mass index (kg/m2) | 26.7 ± 4.6 |
| Systolic blood pressure (mmHg) | 136 ± 20 |
| Antihypertensive treatment, N (%) | 1103 (13) |
| Total cholesterol (mmol/L) | 5.47 (2.13–13.6) |
| Current smoking, N (%) | 1944 (23) |
| Diabetes, N (%) | 493 (5.8) |
| Biomarker | |
| Creatinine (mg/dL) | 0.90 (0.10–14.9) |
| Glomerular filtration rate (mL/min/1.73 m2) | 89.5 (2.3–244) |
| BNP (pg/mL) | 14.4 (0.1–2106) |
| NT-proBNP (pg/mL) | 44.4 (5.0–9541) |
| MR-proANP (pmol/L) | 43.0 (4.6–1240) |
For categorical variables absolute and relative frequencies are given. Continuous variables are represented as mean ± standard deviation for near to normally distributed variables or median (range).
BNP, B-type natriuretic peptide; NT-proBNP, N-terminal pro B-type natriuretic peptide; MR-proANP, mid-regional pro atrial natriuretic peptide.
Mendelian randomization
For all three natriuretic peptides, a significant association between PRS and log (natriuretic peptide) (NT-proBNP beta coefficient 0.1745, P < 0.001; BNP beta coefficient 0.1613, P < 0.001; MR-proANP beta coefficient 0.0670, P < 0.001) in univariable linear regressions was observed (Table 2).
Table 2.
Univariate linear regressions of PRSX with log (natriuretic peptide X)
| Exposure | Beta (SD) | P-value |
|---|---|---|
| Log (NT-proBNP) | 0.1745 (0.0127) | <0.001 |
| Log (BNP) | 0.1613 (0.0127) | <0.001 |
| Log (MR-proANP) | 0.0670 (0.0057) | <0.001 |
Provided are betas and SD.
BNP, B-type natriuretic peptide; MR-proANP; Mid-regional pro atrial natriuretic peptide; NT-proBNP, N-terminal pro B-type natriuretic peptide; SD, standard deviation.
In univariable Cox regressions, the PRS was not significantly associated with incident AF or HF (Table 3). For none of the natriuretic peptides, a significant causal relation with AF or HF was estimated (Table 4). For comparison, the observational HRs were all highly significant and larger (Figure 2 and Table 5). All Mendelian randomization HRs crossed the one, which indicates that no causal association may exist. The 95% confidence intervals for the HF HRs were larger compared to AF which is likely due to a smaller number of cases and the higher HRs.
Table 3.
Cox regressions for PRSX in relation to AF and HF
| AF |
HF |
|||||
|---|---|---|---|---|---|---|
| Beta (P-value) |
Beta (P-value) |
|||||
| Natriuretic peptide, X | Unadjusted | Adjusted for log (X) | Adjusted for log (Xiv-free) | Unadjusted | Adjusted for log (X) | Adjusted for log (Xiv-free) |
| NT-proBNP | 0.005942 (0.8846) | −0.1138 (0.009) | 0.02589 (0.55) | 0.07679 (0.1271) | −0.07918 (0.14) | 0.11 (0.041) |
| BNP | 0.02353 (0.5616) | −0.1131 (0.009) | 0.04215 (0.32) | 0.06647 (0.1797) | −0.06002 (0.26) | 0.1053 (0.044) |
| MR-proANP | −0.01275 (0.7572) | −0.1468 (<0.001) | −0.02836 (0.51) | 0.07036 (0.1456) | −0.05683 (0.26) | 0.0736 (0.14) |
Provided are beta coefficients of the PRSX in the Cox regressions AF/HF ∼ PRSX, AF/HF ∼ PRSX + log (X), and AF/HF ∼ PRSX + log (Xiv-free).
AF, atrial fibrillation; BNP, B-type natriuretic peptide; HF, heart failure; MR-proANP; Mid-regional pro atrial natriuretic peptide; NT-proBNP, N-terminal pro B-type natriuretic peptide.
Table 4.
Mendelian Randomization HRs
| Atrial fibrillation |
Heart failure |
|||
|---|---|---|---|---|
| Exposure | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Log (NT-proBNP) | 1.03 (0.67–1.58) | 0.90 | 1.55 (0.90–2.67) | 0.12 |
| Log (BNP) | 1.16 (0.72–1.81) | 0.56 | 1.51 (0.82–2.75) | 0.18 |
| Log (MR-proANP) | 0.83 (0.24–2.62) | 0.70 | 2.86 (0.65–11.47) | 0.16 |
Provided are HRs and 95% CIs.
BNP, B-type natriuretic peptide; CI, confidence interval; HR, hazard ratio; MR-proANP; mid-regional pro atrial natriuretic peptide; NT-proBNP, N-terminal pro B-type natriuretic peptide.
Figure 2.
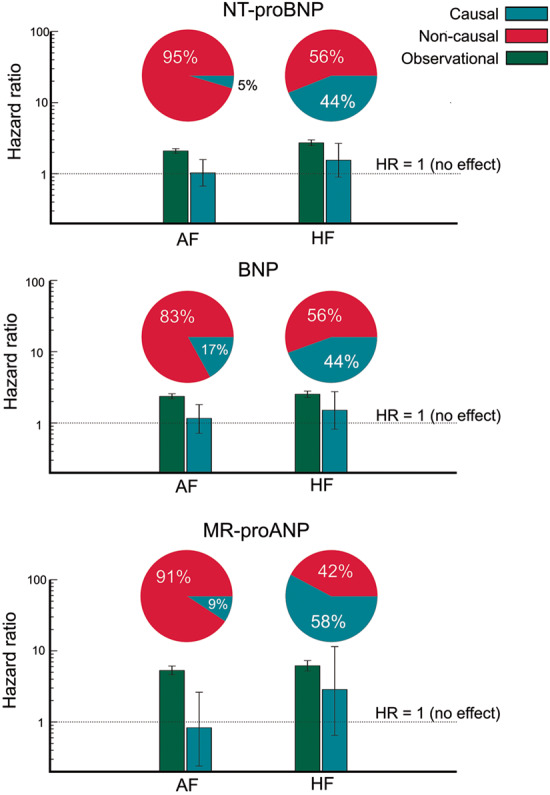
Comparison of observational HRs, causal HRs, and non-causal HRs. HRs and 95% confidence intervals are provided. The pie charts illustrate the percentage of non-causal and causal beta coefficients of the observational beta coefficient. In the AF/MR-proANP case there is a negative causal beta coefficient. In order to avoid negative percentages or percentages larger than 100, the absolute values of the causal and non-causal beta coefficients were expressed as a percentage of their sum. AF, atrial fibrillation; BNP, B-type natriuretic peptide; HF, heart failure; HR, hazard ratio; MR-proANP, mid-regional pro atrial natriuretic peptide.
Table 5.
Observational HRs
| Atrial fibrillation |
Heart failure |
|||
|---|---|---|---|---|
| Exposure | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Log (NT-proBNP) | 2.09 (1.94–2.25) | <0.001 | 2.72 (2.48–2.99) | <0.001 |
| Log (BNP) | 2.36 (2.18–2.57) | <0.001 | 2.53 (2.28–2.80) | <0.001 |
| Log (MR-proANP) | 5.30 (4.60–6.11) | <0.001 | 6.16 (5.19–7.31) | <0.001 |
Provided are HRs and 95% CIs.
BNP, B-type natriuretic peptide; CI, confidence interval; HR, hazard ratio; MR-proANP, mid-regional pro atrial natriuretic peptide; NT-proBNP, N-terminal pro B-type natriuretic peptide.
Assumption checking
F-statistics for the relation between PRS and natriuretic peptide were all >10 (Supplementary material online, Table S4), indicating that assumption 1 was met. When, for natriuretic peptide X, PRSX is adjusted for log (Xiv-free) or log (X), the beta coefficient of PRSX in the Cox regression with AF or HF as outcomes loses significance (Table 3). Assumption 2 was not violated.
Most cardiovascular risk factors showed significant confounding percentages, with some exceptions as outlined in Supplementary material online, Tables S5–S7. For each possible observed confounder Q, we assessed violations of assumption 3 by the linear or logistic regression Q ∼ PRSX and checking if the beta coefficients of PRSX in these regressions were non-significant. Beta coefficients with associated P-values for X = NT-proBNP, BNP, and MR-proANP are provided in Supplementary material online, Table S8. We could not show significant associations. Assumption 3 was not violated.
Discussion
In our Mendelian randomization approach in a prospective community study, natriuretic peptides were not directly causally related to AF or HF incidence. Whereas the known associations of SNPs and circulating biomarkers with AF and HF outcomes were robust, no association with common genetic variation at the NPPA-NPPB locus was shown. Our observations remained stable after carefully accounting for known, common AF and HF confounders including age, sex, BMI, systolic blood pressure, antihypertensive treatment, total cholesterol, current smoking, diabetes, prevalent myocardial infarction, and eGFR.
Natriuretic peptides play a counter-regulatory role in cardiac stress mediated through modulation of haemodynamics and beneficial cardiovascular effects on inflammatory activity, proliferation, hypertrophy, and fibrosis.10 Medical inhibition of the membrane metalloproteinase neprilysin increases the concentration of natriuretic peptides and has proven to reduce cardiovascular outcomes in HF in combination with angiotensin receptor blockade.11 But the enzyme has pleiotropic actions and it also increases the concentration of other endogenous vasoactive peptides such as bradykinin and adrenomedullin. Therefore, the modulation of natriuretic peptides may not be the main mechanism for its beneficial effects. Convincing data on therapeutic benefits and improved outcomes of increasing natriuretic peptide concentrations with natriuretic peptide substitution have not been shown in clinical trials.12 In this context, our data support the observation that therapies addressing natriuretic peptides only may not be efficient.
A prior Mendelian randomization analysis revealed an association of SNPs in the NPPA-NPPB locus with natriuretic peptides and hypertension,5 one of the strongest risk factors for AF and HF with a high population attributable risk. In our cohort, the PRS was not related to blood pressure. Furthermore, genetic variation of rs5068 among other NPPA-NPPB locus SNPs was associated with a favourable cardiometabolic profile, lower prevalence of metabolic syndrome and hypertension, lower blood pressure, and BMI in Whites of European ancestry and African Americans.13,14 However, common SNPs at the NPPA-NPPB locus have not yet been shown to be associated with AF or HF. None of the recent genome-wide association studies for AF and HF demonstrated strong signals in the NPPA-NPPB genes.15,16 No association of rs198389, rs5068, and rs198358 with incident HF could be observed in the prospective population-based EPIC-Norfolk cohort after stratification for hypertension, obesity, and coronary heart disease.17 For AF common non-synonymous genetic variants within NPPA in two cohorts of European ancestry could not be demonstrated either. Other SNPs outside the NPPA-NPPB locus such as a missense variant LC39A8 (rs13107325) on chromosome four have been related to NT-proBNP concentrations and cardiovascular death.7 The association may again be through alternate pathways because the variant has been identified in relation to blood pressure and BMI.18,19
In accordance, our candidate gene study did not reveal a significant association of the selected SNPs with incident AF or HF. Lack of direct causality of natriuretic peptide concentrations and incident AF or HF in our examination of common polymorphisms does not preclude causal mechanisms in rare mutations of the NPPA-NPPB locus. For example, a frameshift mutation in the NPPA gene results in a mutant peptide with normal circulating concentrations with shortening of the action potential duration and the effective refractory period in familial AF.20 Furthermore, there are multiple indirect causal pathways which involve genetic variation in the genes coding for the cardiac hormones and may underlie AF and/or HF onset. We examined eleven classical risk factors which are frequent confounders of the association of natriuretic peptides and incident AF or HF and share pathophysiological pathways.8 A certain vertical pleiotropy for these risk factors, i.e. downstream risk factors related with the biomarkers, may exist and improve our understanding of the pathways from natriuretic peptides to risk factors. Our conclusions are not changed in the absence of a significant association with our outcomes of interest.
Limitations
Our analysis was based on incident AF and HF using national hospital-based databases. However, especially AF is often an outpatient diagnosis and underdiagnosed in asymptomatic patients. Thus, our results may not be generalizable to all AF cases. Likewise, in our study population, no distinction was made between HF with preserved and reduced ejection fraction, which may have different relations with natriuretic peptides. Furthermore, progression of both AF and HF was not studied here and may still be causally related to natriuretic peptides. Although we carefully checked for violations of the assumptions underlying Mendelian randomization, it cannot be excluded that such violations would have been detected with more statistical power and/or information on possible confounders that were not measured. Studies with more power are required to obtain more precise estimates of the percentage of the association that can be called causal and more precise assumption validation. The second and third assumptions of Mendelian randomization, might then also be tested at the level of individual SNPs and SNPs that violate these assumptions can then be left out of the genetic risk score. We combined replicated variants into a polygenic risk instrument to increase power. However, as known for Mendelian randomization studies using common variants, power was limited for small effects. We calculated that if the causal effect of the natriuretic peptides on the two examined outcomes were very small, i.e. 10% of the observational beta estimate, we would have needed up to 800 000 individuals based on the data in the FINRISK study. Strengths of the study are the homogenous sample structure, uniform risk factor, confounder, and outcome ascertainment and the availability of information on all three commonly measured natriuretic peptide biomarkers.
Conclusion
Based on a Mendelian randomization approach comprising common genetic variation at the NPPA-NPPB locus and all three commonly determined natriuretic peptides, we were not able to show a causal relationship between the peptides to either AF or HF in the community. The observed strong associations of the blood biomarkers with incident disease may be explained by genetic determination of the cardiac hormones and their relationship with common risk factors of both diseases such as obesity and hypertension. Therapeutic approaches targeting natriuretic peptides will therefore very likely work through indirect mechanisms, in particular AF and HF risk factor modulation.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
The samples and data used for the research were obtained from THL biobank. We thank all study participants for their generous participation in the FINRISK study.
Funding
This work was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 648131); the European Union’s Horizon 2020 research and innovation programme under the grant agreement No 847770 (AFFECT-EU); the German Center for Cardiovascular Research (DZHK e.V.) (81Z1710103); the German Ministry of Research and Education (BMBF 01ZX1408A) and ERACoSysMed3 (031L0239). The BiomarCaRE Project is supported by the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no. HEALTH-F2-2011 to 278913. The activities of the MORGAM Data Centre have been sustained by recent funding from European Union FP 7 project CHANCES (HEALTHF3-2010–242244). A part of the biomarker determinations in the population cohorts was supported by the Medical Research Council London (G0601463, identification no. 80983: Biomarkers in the MORGAM Populations). The FINRISK surveys were mainly supported by budgetary funds of the Finnish Institute for Health and Welfare, Helsinki. Additional support has been obtained from numerous non-profit foundations. V.S. has been supported by the Finnish Foundation for Cardiovascular Research. T.Z. and S.B. are PIs of the German Centre for Cardiovascular Research (DZHK).
Conflict of interest: V.S. has received honoraria for consulting from Novo Nordisk and Sanofi. He also has ongoing research collaboration with Bayer Ltd. (All unrelated to the present study). R.B.S. has received lecture fees and advisory board fees from BMS/Pfizer outside this work.
Data availability
The data underlying this article cannot be shared publicly to protect the privacy of the individuals that participated in the study. The data are available from the THL Biobank upon submission of a research plan and signing a data transfer Agreement (https://thl.fi/en/web/thl-biobank/for-researchers/application-process).
References
- 1. Willeit P, Kaptoge S, Welsh P, Butterworth AS, Chowdhury R, Spackman SA. et al. Natriuretic peptides and integrated risk assessment for cardiovascular disease: an individual-participant-data meta-analysis. Lancet Diabetes Endocrinol 2016;4:840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kristensen SL, Jhund PS, Mogensen UM, Rorth R, Abraham WT, Desai A. et al. Prognostic value of N-terminal pro-B-type natriuretic peptide levels in heart failure patients with and without atrial fibrillation. Circ Heart Fail 2017;10:e004409. [DOI] [PubMed] [Google Scholar]
- 3. Sramko M, Melenovsky V, Wichterle D, Franekova J, Clemens M, Kautzner J.. Impact of atrial fibrillation on natriuretic peptides: an invasive atrial hemodynamic study. JACC Clin Electrophysiol 2018;4:153–4. [DOI] [PubMed] [Google Scholar]
- 4. Freynhofer MK, Jarai R, Hochtl T, Bruno V, Vogel B, Aydinkoc K. et al. Predictive value of plasma Nt-proBNP and body mass index for recurrence of atrial fibrillation after cardioversion. Int J Cardiol 2011;149:257–9. [DOI] [PubMed] [Google Scholar]
- 5. Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A. et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet 2009;41:348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Del Greco MF, Pattaro C, Luchner A, Pichler I, Winkler T, Hicks AA. et al. Genome-wide association analysis and fine mapping of NT-proBNP level provide novel insight into the role of the MTHFR-CLCN6-NPPA-NPPB gene cluster. Hum Mol Genet 2011;20:1660–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johansson A, Eriksson N, Lindholm D, Varenhorst C, James S, Syvanen AC. et al. Genome-wide association and Mendelian randomization study of NT-proBNP in patients with acute coronary syndrome. Hum Mol Genet 2016;25:1447–56. [DOI] [PubMed] [Google Scholar]
- 8. Teumer A. Common methods for performing Mendelian randomization. Front Cardiovasc Med 2018;5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burgess S, Small DS, Thompson SG.. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res 2017;26:2333–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kerkela R, Ulvila J, Magga J.. Natriuretic peptides in the regulation of cardiovascular physiology and metabolic events. J Am Heart Assoc 2015;4:e002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR. et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 12. Packer M, O’Connor C, McMurray JJV, Wittes J, Abraham WT, Anker SD. et al. Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med 2017;376:1956–64. [DOI] [PubMed] [Google Scholar]
- 13. Cannone V, Scott CG, Decker PA, Larson NB, Palmas W, Taylor KD. et al. A favorable cardiometabolic profile is associated with the G allele of the genetic variant rs5068 in African Americans: the Multi-Ethnic Study of Atherosclerosis (MESA). PLoS One 2017;12:e0189858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cannone V, Cefalu AB, Noto D, Scott CG, Bailey KR, Cavera G. et al. The atrial natriuretic peptide genetic variant rs5068 is associated with a favorable cardiometabolic phenotype in a Mediterranean population. Diabetes Care 2013;36:2850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roselli C, Chaffin MD, Weng LC, Aeschbacher S, Ahlberg G, Albert CM. et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet 2018;50:1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith NL, Felix JF, Morrison AC, Demissie S, Glazer NL, Loehr LR. et al. Association of genome-wide variation with the risk of incident heart failure in adults of European and African ancestry: a prospective meta-analysis from the cohorts for heart and aging research in genomic epidemiology (CHARGE) consortium. Circ Cardiovasc Genet 2010;3:256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pfister R, Luben RN, Khaw KT, Wareham NJ.. Common genetic variants of the natriuretic peptide gene locus are not associated with heart failure risk in participants in the EPIC-Norfolk study. EurJ Heart Fail 2013;15:624–7. [DOI] [PubMed] [Google Scholar]
- 18. Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU. et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010;42:937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI. et al. ; CHARGE-HF consortium. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011;478:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Menon A, Hong L, Savio-Galimberti E, Sridhar A, Youn SW, Zhang M. et al. Electrophysiologic and molecular mechanisms of a frameshift NPPA mutation linked with familial atrial fibrillation. J Mol Cell Cardiol 2019;132:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly to protect the privacy of the individuals that participated in the study. The data are available from the THL Biobank upon submission of a research plan and signing a data transfer Agreement (https://thl.fi/en/web/thl-biobank/for-researchers/application-process).


