Abstract
Aims
The aim of this study was to use Mendelian randomization (MR) to determine the causality of the association between smoking and 14 different cardiovascular diseases (CVDs).
Methods and results
Our primary genetic instrument comprised 361 single-nucleotide polymorphisms (SNPs) associated with smoking initiation (ever smoked regularly) at genome-wide significance. Data on the associations between the SNPs and 14 CVDs were obtained from the UK Biobank study (N = 367 643 individuals), CARDIoGRAMplusC4D consortium (N = 184 305 individuals), Atrial Fibrillation Consortium (2017 dataset; N = 154 432 individuals), and Million Veteran Program (MVP; N = 190 266 individuals). The main analyses were conducted using the random-effects inverse-variance weighted method and complemented with multivariable MR analyses and the weighted median and MR-Egger approaches. Genetic predisposition to smoking initiation was most strongly and consistently associated with higher odds of coronary artery disease, heart failure, abdominal aortic aneurysm, ischaemic stroke, transient ischaemic attack, peripheral arterial disease, and arterial hypertension. Genetic predisposition to smoking initiation was additionally associated with higher odds of deep vein thrombosis and pulmonary embolism in the UK Biobank but not with venous thromboembolism in the MVP. There was limited evidence of causal associations of smoking initiation with atrial fibrillation, aortic valve stenosis, thoracic aortic aneurysm, and intracerebral and subarachnoid haemorrhage.
Conclusion
This MR study supports a causal association between smoking and a broad range of CVDs, in particular, coronary artery disease, heart failure, abdominal aortic aneurysm, ischaemic stroke, transient ischaemic attack, peripheral arterial disease, and arterial hypertension.
Keywords: Cardiovascular disease, Lifestyle, Mendelian randomization, Risk factors, Single-nucleotide polymorphisms, Smoking
See page 3311 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa285)
Introduction
A large body of evidence from prospective observational studies indicates that smoking is a risk factor for several cardiovascular diseases (CVDs),1–6 but the role of smoking for certain CVDs, such as aortic valve stenosis,7–9 atrial fibrillation,10 thoracic aortic aneurysm,11 , 12 and haemorrhagic stroke3 are limited or inconsistent. In contrast to prospective cohort studies,1 short-term and long-term follow-up of randomized smoking cessation studies have not revealed a significant effect on fatal coronary heart disease events.13 , 14 Given that much of available data on smoking and CVD derive from observational studies, which are unable to fully account for confounding and reverse causality, and the lack of significant effect of smoking cessation on cardiovascular events in interventional trials, the causal nature of the association between smoking and different CVDs remains to be established.
Mendelian randomization (MR) is an analytical method that uses genetic variants, generally single-nucleotide polymorphisms (SNPs), as unbiased proxy indicators for the modifiable risk factor to determine whether the risk factor is a cause of the disease.15 , 16 Given that allelic variants are randomly allocated and fixed at conception, MR studies evade reverse causality and are less susceptible to confounding compared with conventional observational studies. To the best of our knowledge, the causal association between smoking and a broad range of CVDs has not been established using MR. We, therefore, applied the MR design to determine the association between smoking and 14 CVD outcomes.
Methods
Study design
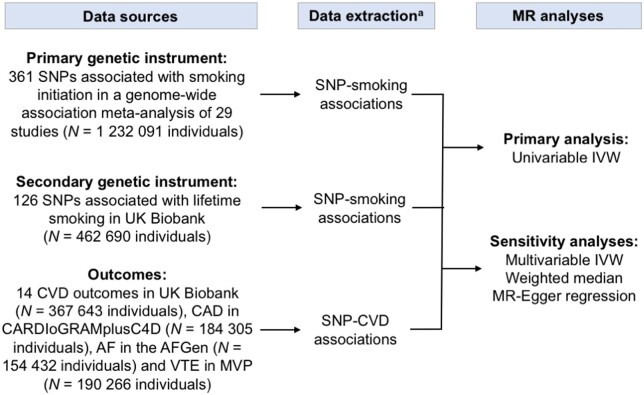
Summary-level data (i.e. beta coefficients and standard errors) of the associations between SNPs strongly associated with smoking initiation (primary exposure) and lifetime smoking (secondary exposure) were extracted from the hitherto largest genome-wide association studies for these phenotypes (Figure 1). The corresponding data for the associations of the smoking-associated SNPs with 14 CVDs were available from UK Biobank (Figure 1). In complementary analyses, we obtained summary-level data from genetic consortia of coronary artery disease17 atrial fibrillation,18 and venous thromboembolism.19 Details of the selection of instrumental variables and data sources are provided below.
Figure 1.
Summary of data sources and methods used in this study. AF, atrial fibrillation; AFGen, Atrial Fibrillation Consortium; CAD, coronary artery disease; CVD, cardiovascular disease; IVW, inverse-variance weighted; MR, Mendelian randomization; MVP, Million Veteran Program; SNP, single-nucleotide polymorphism; VTE, venous thromboembolism. aData extracted were beta coefficients with corresponding standard errors of the SNP–smoking and SNP–CVD associations.
Genetic instruments
Instrumental variables for smoking initiation were acquired from a genome-wide association meta-analysis of 29 studies, comprising a total of 1 232 091 European-descent individuals of which 383 631 individuals were part of the UK Biobank. That meta-analysis identified 378 conditionally independent genome-wide significant SNPs at 259 loci (defined as a 1MB region nearby the top P-value) associated with smoking initiation, a binary phenotype representing whether an individual had ever smoked cigarettes regularly in their life (current or former).20 Information about pipes/cigar/chew or other non-cigarette forms of tobacco use was not included.20 The smoking-associated SNPs explained 2.3% of the phenotypic variation.20 All but one of the SNPs were available in the UK Biobank. Linkage disequilibrium (defined as r 2 > 0.1 in European populations) between SNPs was evaluated using LDlink21 and was detected among 16 SNP pairs. The SNP with the largest P-value was omitted, leaving 361 SNPs as instrumental variables for smoking initiation. Genetic associations with smoking were reported in standard deviation units.20 Hence, the reported odds ratios correspond to the increase of 1 SD in prevalence of smoking initiation.
In a supplementary analysis, a genetic instrument for lifetime smoking exposure that takes into account smoking status as well as smoking duration, heaviness, and cessation in ever smokers was applied.22 This genetic instrument consists of 126 genome-wide significant SNPs associated with lifetime smoking exposure in UK Biobank (N = 462 690).22
Outcome data
Summary-level data for the smoking-associated SNPs with the 14 CVDs came from the UK Biobank, a cohort study of about 500 000 adults, aged 37–73 years and enrolled between 2006 and 2010.23 We excluded non-White European participants (to minimize confounding by ancestry), those with relatedness of third degree or higher, low genotype call rate (three or more standard deviations from the mean) and excess heterozygosity, resulting in a final study sample of 367 643 individuals. In this study, participants were followed up until 31 March 2017 or the date of death (recorded until 14 February 2018). The median follow-up was 8.0 years. Outcomes were defined based on electronic health records, hospital procedure codes, and self-reported information validated by interview with a nurse (Supplementary material online, Table S1). Logistic regression with adjustment for ten genetic principal components was applied to obtain the beta coefficients and standard errors for the SNP–CVD associations. The SNPs exploited as instrumental variables and their associations with each CVD outcome are presented in Supplementary material online, Table S2.
Summary-level data for coronary artery disease, atrial fibrillation, and venous thromboembolism were additionally obtained from the Coronary ARtery DIsease Genome-wide Replication and Meta-analysis plus The Coronary Artery Disease Genetics (CARDIoGRAMplusC4D) consortium (60 801 cases and 123 504 non-cases),17 the Atrial Fibrillation Consortium (22 346 cases and 132 086 non-cases; AFGen exome chip analysis, not including UK Biobank),18 and the Million Veteran Program (MVP) (8929 cases and 181 337 non-cases), respectively.19 There was no participant overlap between those three datasets and the UK Biobank. For comparison, we also present previously reported results for ischaemic stroke based on the MEGASTROKE consortium (34 217 ischaemic stroke cases and 404 630 non-cases, not including UK Biobank).24 , 25
The UK Biobank study was approved by the North West Multicenter Research Ethics Committee. Original studies included in the consortia had been approved by a relevant review board. The present analyses were approved by the Swedish Ethical Review Authority.
Statistical analysis
The inverse-variance-weighted (IVW) method, under a multiplicative random-effects model, was used to obtain the primary causal estimates. Ratio estimates are calculated for each SNP as the beta coefficient for the SNP–CVD association divided by the beta coefficient for the SNP–smoking association. These estimates are then combined across SNPs in a random-effects IVW meta-analysis. This MR method provides the highest precision but assumes that all SNPs are valid instrumental variables. Heterogeneity among estimates based on individual SNPs was assessed with the I 2 statistic.26
Multivariable MR analyses27 were performed to adjust for potential confounders that are genetically correlated with smoking initiation, including alcohol drinking (r g = 0.36), body mass index (r g = 0.12), and years of education (r g = −0.40).20 Multivariable MR analysis was also used to assess whether the associations between genetic predisposition to smoking initiation and CVD outcomes that are comorbid or secondary to coronary artery disease28 may be driven by coronary artery disease.
The weighted median and MR-Egger regression methods were used as sensitivity analyses. The weighted median approach gives consistent estimates if at least 50% of the weight in the analysis comes from valid instrumental variables.29 The MR-Egger approach can detect and correct for directional pleiotropy but suffers from low power.29 In a further sensitivity analysis, the associations between genetic predisposition to smoking initiation and CVD outcomes were assessed in never smokers (based on self-report) in UK Biobank.
To correct for multiple testing, Bonferroni correction was applied and two-sided P-values <3.6× 10−3 (where α = 0.05/14 CVD outcomes) were deemed statistically significant and also considered strong evidence of a causal association. Findings with P-values between 0.05 and 3.6 × 10−3 were regarded as suggestive evidence of association. The analyses were performed using the mrrobust30 and MendelianRandomization31 packages.
Results
Participants
In the analytic sample of 367 643 UK Biobank participants, the mean age was 57 years (5th–95th percentile: 43–69 years) and 46% were men. The prevalence of smoking initiation (ever smokers) was 46%. Around 93% were alcohol drinkers.
Smoking and cardiovascular disease
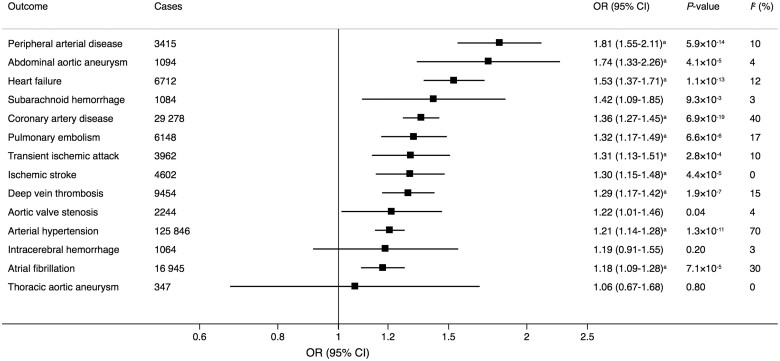
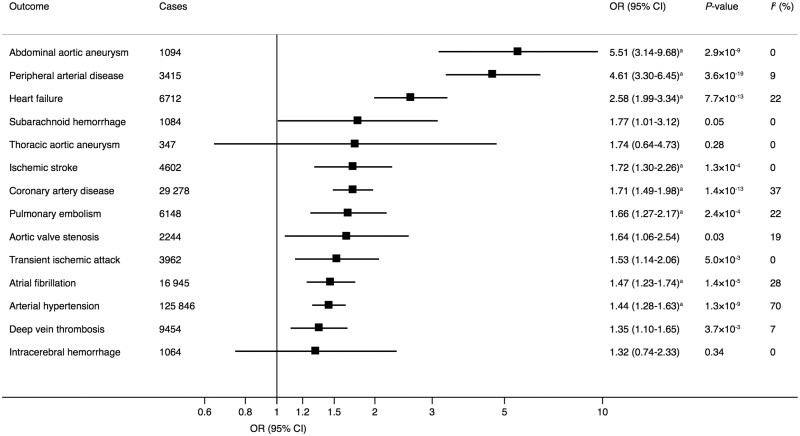
In the univariable IVW analysis, genetic predisposition to smoking initiation was statistically significantly positively associated with 10 of the 14 outcomes in UK Biobank (Figure 2). The odds ratios ranged from 1.18 (95% confidence interval 1.09–1.28; P = 7.1 × 10−5) for atrial fibrillation to 1.81 (95% confidence interval 1.55–2.11; P = 5.2 × 10−7) for peripheral arterial disease. In the multivariable IVW models adjusting for genetically correlated phenotypes (i.e. alcohol consumption, body mass index, and education), the associations remained statistically significant for coronary artery disease, heart failure, abdominal aortic aneurysm, deep vein thrombosis, pulmonary embolism, peripheral arterial disease, and arterial hypertension; suggestive evidence of associations remained for ischaemic stroke, transient ischaemic attack, and atrial fibrillation (Supplementary material online, Table S3). The association of genetic predisposition to smoking initiation with atrial fibrillation did not persist after adjustment for coronary artery disease (Supplementary material online, Table S3). The results were similar in the weighted median analysis and no evidence of directional pleiotropy was detected in the MR-Egger regression analysis (all P ≥ 0.15) (Supplementary material online, Table S3). In the analysis confined to never smokers, all associations except the association with arterial hypertension were null in the univariable or multivariable IVW models (Supplementary material online, Table S4). Genetic predisposition to smoking initiation was also positively associated with coronary artery disease and ischaemic stroke in the CARDIoGRAMplusC4D consortium and MEGASTROKE,25 respectively, but the associations were weaker than in UK Biobank (Supplementary material online, Table S3). There were no robust associations of genetic predisposition to smoking initiation with atrial fibrillation or venous thromboembolism in the AFGen and MVP, respectively (Supplementary material online, Table S3). Findings for the lifetime smoking exposure in UK Biobank (Figure 3) and the consortia and MVP (Supplementary material online, Table S5) were broadly similar to those for smoking initiation.
Figure 2.
Associations of genetic predisposition to smoking initiation with 14 cardiovascular diseases in UK Biobank. The odds ratios correspond to the increase of one standard deviation in prevalence of smoking initiation (ever smoked regularly). Estimates are from the multiplicative random-effects inverse variance-weighted method. CI, confidence interval; OR, odds ratio. The I 2 statistic quantifies the amount of heterogeneity among estimates based on individual SNPs. a Significant at the Bonferroni-corrected threshold of P < 3.6 × 10−3.
Figure 3.
Associations of genetically predicted lifetime smoking index with 14 cardiovascular diseases in UK Biobank. Odds ratios are expressed per one standard deviation increase of the lifetime smoking index. Estimates are from the multiplicative random-effects inverse variance-weighted method. CI, confidence interval; OR, odds ratio. The I 2 statistic quantifies the amount of heterogeneity among estimates based on individual SNPs individual SNPs. aSignificant at the Bonferroni-corrected threshold of P < 3.6 × 10−3.
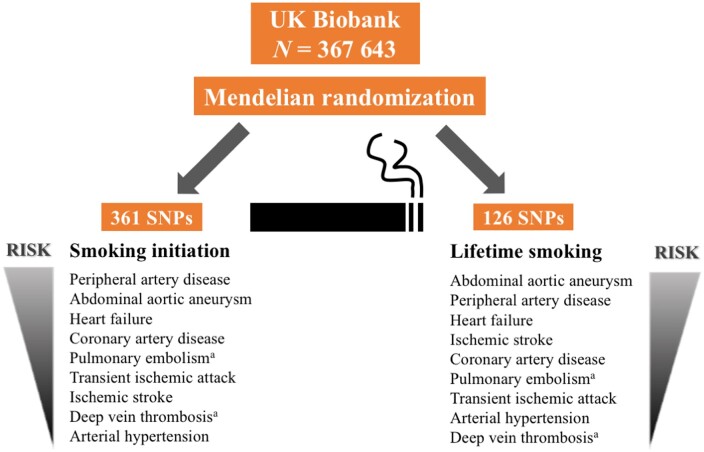
For the observed smoking initiation–CVD associations in UK Biobank (Take home figure), we estimated the predicted risk reduction that would result from a reduction in the prevalence of smoking from 46% (the observed prevalence in UK Biobank) to 20%. This corresponds to a 1.22 unit change in the log-odds of smoking, a difference similar to the difference in log odds between those in the 1st and 99th percentiles of the genetic risk score for smoking initiation. This risk reduction was 52% for peripheral arterial disease, 49% for abdominal aortic aneurysm, 41% for heart failure, 32% for coronary artery disease, 29% for pulmonary embolism, 28% for transient ischaemic attack, 27% for ischaemic stroke and deep vein thrombosis, and 21% for arterial hypertension.
Take home figure.
Observed associations of genetic predisposition to smoking initiation and lifetime smoking with cardiovascular diseases in UK Biobank. aNo robust association was observed between smoking and venous thromboembolism (pulmonary embolism and deep vein thrombosis combined) in the Million Veteran Program.
Discussion
Principal findings
This MR study showed that genetic predisposition to smoking is associated with an increased risk of a broad range of CVDs (Take home figure). The strongest and most consistent positive associations were observed between smoking initiation and coronary artery disease, heart failure, abdominal aortic aneurysm, ischaemic stroke, transient ischaemic attack, peripheral arterial disease, and arterial hypertension. Smoking initiation was additionally associated with deep vein thrombosis and pulmonary embolism in the UK Biobank but not with venous thromboembolism in the MVP. There was limited evidence of causal associations of smoking initiation with atrial fibrillation, aortic valve stenosis, thoracic aortic aneurysm, and intracerebral and subarachnoid haemorrhage.
The results from the present MR study based on data from UK Biobank corroborate the findings of conventional prospective observational studies showing that smoking is a risk factor for coronary artery disease,1 heart failure,4 abdominal aortic aneurysm,5 ischaemic stroke,3 peripheral arterial disease,2 deep vein thrombosis,6 and pulmonary embolism6 (Supplementary material online, Figure S1). However, this and a previous MR study based on another study sample (n = 1545 cases of intracerebral haemorrhage and 1481 controls)25 found limited evidence of a causal association between smoking and intracerebral haemorrhage and subarachnoid haemorrhage. These findings are in contrast to those of a large prospective study and meta-analysis which showed that smoking was associated with increased risk of both intracerebral haemorrhage and subarachnoid haemorrhage3 (Supplementary material online, Figure S1). The present and the prior MR study25 may have been unable to observe a statistically significant association due to small number of cases of haemorrhagic stroke. With regard to ischaemic stroke subtypes, smoking has been shown to increase the risk of large artery and small vessel stroke but not cardioembolic stroke.25 Data on ischaemic stroke subtypes were not available in UK Biobank.
In contrast to abdominal aortic aneurysm, we found no evidence of a causal association between smoking and thoracic aortic aneurysm but owing to the limited number of cases, we cannot rule out that we may have overlooked a weak association. Observational studies of the association between smoking and thoracic aortic aneurysm are scarce. No association between smoking and risk of rupture or dissection of thoracic aortic aneurysm was observed in a cohort of thoracic aortic aneurysm patients without history of aortic dissection.11 However, smoking was associated with a 2.2-fold higher risk of thoracic aortic aneurysm in a cohort of 30 447 adults, including 45 thoracic aortic aneurysm cases.12 Thus, the role of smoking for thoracic aortic aneurysm requires further study.
Observational studies of smoking in relation to risk of aortic valve stenosis7–9 and atrial fibrillation10 are limited or inconsistent. One large9 and two small prospective cohort studies7 , 8 found a 30%9 to almost two-fold7 increased risk of aortic valve stenosis associated with smoking, and a meta-analysis found an overall 32% increased risk of atrial fibrillation for current vs. never smokers.10 This MR study found no association between smoking initiation and aortic valve stenosis after adjustment for body mass index, which is a strong risk factor for aortic valve stenosis.32 Likewise, no consistent association was observed between smoking initiation and atrial fibrillation.
Potential mechanisms
Cigarette smoke can damage the cardiovascular system through its content of oxidant gases (e.g. oxides of nitrogen and free radicals) and other toxic substances, which may increase the risk of CVD through lipid oxidation, endothelial dysfunction, inflammation platelet activation, thrombogenesis, and augmented coagulability.33 Furthermore, nicotine may provoke acute cardiovascular events by increasing myocardial contractility and vasoconstriction, leading to increased myocardial work and oxygen demand as well as reduced coronary and cerebral blood flow.33 Nicotine exposure may further promote aneurysmal rupture through actions on vascular smooth muscle cell nicotinic acetylcholine receptors containing α7 subunits.34 Smoking might also affect CVD risk through antioestrogenic effects, observed in both women and men.35–37
Strength and limitations
This study has several strengths. First, the MR design reduces the possibility that the observed associations were biased by reverse causality and confounding. Second, the genetic instrument for smoking initiation comprised multiple SNPs robustly associated with smoking, thereby providing a strong genetic instrument. Third, the analyses included a large sample size and generally large number of CVD events, and therefore, the statistical power was high in most analyses. Fourth, by restricting the study sample to individuals of European-descent in UK Biobank, population stratification bias was minimized.
This study also has several limitations. First, the possibility that the smoking-related SNPs affect CVD outcomes through other causal pathways than through smoking exposure cannot be entirely ruled out. Nonetheless, in never smokers the associations of genetic predisposition to smoking initiation with the CVD outcomes were generally null (except for arterial hypertension) in the crude or multivariable MR analysis. The weak associations for some CVD outcomes may be due to exposure to cigarette smoke amongst never smokers or to misclassification of smoking status (ever smokers incorrectly reported never smoked regularly) as self-reported smoking prevalence is known to be lower than directly measured values of cotinine levels.38
Second, the causal association between smoking cessation and CVD could not be assessed because identified SNPs for smoking cessation are few and explains a small proportion of the phenotypic variance (0.1%).20 To address those limitations, we conducted a complementary analysis of lifetime smoking index, which takes into account smoking duration, heaviness, and cessation,22 and observed similar results to those based on the smoking initiation phenotype.
A third limitation is that participants of the UK Biobank were included in both the exposure and outcome datasets (about 30% participant overlap) in the primary analyses. This overlap may have introduced some bias in the causal estimates in the direction of the observational association between smoking and CVD risk.39 However, the genetic variants are reasonably strongly associated with the exposure (F statistic 78.5), meaning that bias due to sample overlap is reasonably small. The associations of genetic predisposition to smoking initiation with coronary artery disease, ischaemic stroke, atrial fibrillation, and venous thromboembolism were stronger in UK Biobank than in non-overlapping sets of individuals included in the consortia. This might be related to more consistent case definitions in UK Biobank, population mixture in the consortia (as the genetic variants were chosen based on their associations with smoking status in Europeans, we may expect associations to be attenuated in a mixed ethnicity population), or differences in smoking status and prevalence of other risk factors in different populations. For example, the MVP cohort consists of over 90% men and the prevalence of ever smoking (about 75%) and CVD risk factors, such as Type 2 diabetes, hyperlipidaemia, and obesity, is much higher in the MVP cohort than in the UK Biobank.19 As environmental influences on smoking are stronger in this cohort, genetic influences on smoking are likely to be weaker.
Finally, the power was insufficient for analyses of the association of genetic predisposition to smoking with CVD in individuals of non-European ancestry. Genome-wide associations studies assessing the genetics of smoking in non-Europeans, as well as large MR studies of smoking and CVD risk in other populations, are warranted.
Conclusions
This study supports a causal association between smoking and a broad range of CVDs, in particular, coronary artery disease, heart failure, abdominal aortic aneurysm, ischaemic stroke, transient ischaemic attack, peripheral arterial disease, and arterial hypertension. These findings add to the level of evidence that smoking is a causal risk factor for CVD.40
Supplementary Material
Acknowledgements
This research was conducted using the UK Biobank study under Application Number 29202.
Funding
This work was supported by funding from the Swedish Research Council (Vetenskapsrådet; 2019-00977 to S.C.L.), the Swedish Heart-Lung Foundation (Hjärt-Lungfonden; 20190247 to S.C.L.), the Swedish Research Council for Health, Working Life and Welfare (Forte; 2018-00123 to S.C.L.), and the United States Department of Veterans Affairs Office of Research and Development, Million Veteran Program Grant # MVP003 (I01-BX003362). S.M.D. was supported by the VA award IK2-CX001780. S.B. was supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (204623/Z/16/Z). This publication does not represent the views of the Department of Veterans Affairs or the United States Government.
Conflict of interest: Dr Damrauer reported receiving grants from the U.S. Department of Veterans Affairs and Renalytix AI plc outside the submitted work. No other disclosures were reported.
Contributor Information
Susanna C Larsson, Department of Surgical Sciences, Uppsala University, Uppsala 75185, Sweden; Unit of Cardiovascular and Nutritional Epidemiology, Institute of Environmental Medicine, Karolinska Institutet, Stockholm 17177, Sweden.
Amy M Mason, Department of Public Health and Primary Care, University of Cambridge, Cambridge CB1 8RN, UK.
Magnus Bäck, Department of Medicine, Center for Molecular Medicine, Karolinska Institutet, Stockholm 17177, Sweden; Division of Valvular and Coronary Disease, Heart and Vascular Theme, Karolinska University Hospital, Stockholm 14186, Sweden.
Derek Klarin, Boston VA Healthcare System, Boston, MA 02132-4927, USA; Center for Genomic Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA; Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, MA 02142, USA; Division of Vascular Surgery and Endovascular Therapy, University of Florida College of Medicine, Gainesville, FL 32610, USA.
Scott M Damrauer, Corporal Michael J. Crescenz VA Medical Center, Philadelphia, PA 19104, USA; Department of Surgery, Perlman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA.
Karl Michaëlsson, Department of Surgical Sciences, Uppsala University, Uppsala 75185, Sweden.
Stephen Burgess, Department of Public Health and Primary Care, University of Cambridge, Cambridge CB1 8RN, UK; MRC Biostatistics Unit, University of Cambridge, Cambridge CB20SR, UK.
References
- 1. Mons U, Muezzinler A, Gellert C, Schottker B, Abnet CC, Bobak M, de Groot L, Freedman ND, Jansen E, Kee F, Kromhout D, Kuulasmaa K, Laatikainen T, O'Doherty MG, Bueno-de-Mesquita B, Orfanos P, Peters A, van der Schouw YT, Wilsgaard T, Wolk A, Trichopoulou A, Boffetta P, Brenner H; CHANCES Consortium. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ 2015;350:h1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu L, Mackay DF, Pell JP. Meta-analysis of the association between cigarette smoking and peripheral arterial disease. Heart 2014;100:414–423. [DOI] [PubMed] [Google Scholar]
- 3. Price AJ, Wright FL, Green J, Balkwill A, Kan SW, Yang TO, Floud S, Kroll ME, Simpson R, Sudlow CLM, Beral V, Reeves GK. Differences in risk factors for 3 types of stroke: UK prospective study and meta-analyses. Neurology 2018;90:e298–e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aune D, Schlesinger S, Norat T, Riboli E. Tobacco smoking and the risk of heart failure: a systematic review and meta-analysis of prospective studies. Eur J Prev Cardiol 2019;26:279–288. [DOI] [PubMed] [Google Scholar]
- 5. Aune D, Schlesinger S, Norat T, Riboli E. Tobacco smoking and the risk of abdominal aortic aneurysm: a systematic review and meta-analysis of prospective studies. Sci Rep 2018;8:14786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gregson J, Kaptoge S, Bolton T, Pennells L, Willeit P, Burgess S, Bell S, Sweeting M, Rimm EB, Kabrhel C, Zoller B, Assmann G, Gudnason V, Folsom AR, Arndt V, Fletcher A, Norman PE, Nordestgaard BG, Kitamura A, Mahmoodi BK, Whincup PH, Knuiman M, Salomaa V, Meisinger C, Koenig W, Kavousi M, Volzke H, Cooper JA, Ninomiya T, Casiglia E, Rodriguez B, Ben-Shlomo Y, Despres JP, Simons L, Barrett-Connor E, Bjorkelund C, Notdurfter M, Kromhout D, Price J, Sutherland SE, Sundstrom J, Kauhanen J, Gallacher J, Beulens JWJ, Dankner R, Cooper C, Giampaoli S, Deen JF, Gomez de la Camara A, Kuller LH, Rosengren A, Svensson PJ, Nagel D, Crespo CJ, Brenner H, Albertorio-Diaz JR, Atkins R, Brunner EJ, Shipley M, Njolstad I, Lawlor DA, van der Schouw YT, Selmer RM, Trevisan M, Verschuren WMM, Greenland P, Wassertheil-Smoller S, Lowe GDO, Wood AM, Butterworth AS, Thompson SG, Danesh J, Di Angelantonio E, Meade T, Emerging Risk Factors Collaboration. Cardiovascular risk factors associated with venous thromboembolism. JAMA Cardiol 2019;4:163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martinsson A, Ostling G, Persson M, Sundquist K, Andersson C, Melander O, Engstrom G, Hedblad B, Smith JG. Carotid plaque, intima-media thickness, and incident aortic stenosis: a prospective cohort study. Arterioscler Thromb Vasc Biol 2014;34:2343–2348. [DOI] [PubMed] [Google Scholar]
- 8. Eveborn GW, Schirmer H, Lunde P, Heggelund G, Hansen JB, Rasmussen K. Assessment of risk factors for developing incident aortic stenosis: the Tromso Study. Eur J Epidemiol 2014;29:567–575. [DOI] [PubMed] [Google Scholar]
- 9. Larsson SC, Wolk A, Back M. Alcohol consumption, cigarette smoking and incidence of aortic valve stenosis. J Intern Med 2017;282:332–339. [DOI] [PubMed] [Google Scholar]
- 10. Aune D, Schlesinger S, Norat T, Riboli E. Tobacco smoking and the risk of atrial fibrillation: a systematic review and meta-analysis of prospective studies. Eur J Prev Cardiol 2018;25:1437–1451. [DOI] [PubMed] [Google Scholar]
- 11. Kim JB, Kim K, Lindsay ME, MacGillivray T, Isselbacher EM, Cambria RP, Sundt TM 3rd. Risk of rupture or dissection in descending thoracic aortic aneurysm. Circulation 2015;132:1620–1629. [DOI] [PubMed] [Google Scholar]
- 12. Landenhed M, Engstrom G, Gottsater A, Caulfield MP, Hedblad B, Newton-Cheh C, Melander O, Smith JG. Risk profiles for aortic dissection and ruptured or surgically treated aneurysms: a prospective cohort study. J Am Heart Assoc 2015;4:e001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rose G, Colwell L. Randomised controlled trial of anti-smoking advice: final (20 year) results. J Epidemiol Community Health 1992;46:75–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Multiple risk factor intervention trial. Risk factor changes and mortality results. Multiple Risk Factor Intervention Trial Research Group. JAMA 1982;248:1465–1477. [PubMed] [Google Scholar]
- 15. Davey Smith G, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003;32:1–22. [DOI] [PubMed] [Google Scholar]
- 16. Burgess S, Thompson SG. Mendelian Randomization: Methods for Using Genetic Variants in Causal Estimation. London, UK: Chapman and Hall/CRC Press; 2015. [Google Scholar]
- 17. Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell JC, Webb TR, Zeng L, Dehghan A, Alver M, Armasu SM, Auro K, Bjonnes A, Chasman DI, Chen S, Ford I, Franceschini N, Gieger C, Grace C, Gustafsson S, Huang J, Hwang SJ, Kim YK, Kleber ME, Lau KW, Lu X, Lu Y, Lyytikainen LP, Mihailov E, Morrison AC, Pervjakova N, Qu L, Rose LM, Salfati E, Saxena R, Scholz M, Smith AV, Tikkanen E, Uitterlinden A, Yang X, Zhang W, Zhao W, de Andrade M, de Vries PS, van Zuydam NR, Anand SS, Bertram L, Beutner F, Dedoussis G, Frossard P, Gauguier D, Goodall AH, Gottesman O, Haber M, Han BG, Huang J, Jalilzadeh S, Kessler T, Konig IR, Lannfelt L, Lieb W, Lind L, Lindgren CM, Lokki ML, Magnusson PK, Mallick NH, Mehra N, Meitinger T, Memon FU, Morris AP, Nieminen MS, Pedersen NL, Peters A, Rallidis LS, Rasheed A, Samuel M, Shah SH, Sinisalo J, Stirrups KE, Trompet S, Wang L, Zaman KS, Ardissino D, Boerwinkle E, Borecki IB, Bottinger EP, Buring JE, Chambers JC, Collins R, Cupples LA, Danesh J, Demuth I, Elosua R, Epstein SE, Esko T, Feitosa MF, Franco OH, Franzosi MG, Granger CB, Gu D, Gudnason V, Hall AS, Hamsten A, Harris TB, Hazen SL, Hengstenberg C, Hofman A, Ingelsson E, Iribarren C, Jukema JW, Karhunen PJ, Kim BJ, Kooner JS, Kullo IJ, Lehtimaki T, Loos RJ, Melander O, Metspalu A, Marz W, Palmer CN, Perola M, Quertermous T, Rader DJ, Ridker PM, Ripatti S, Roberts R, Salomaa V, Sanghera DK, Schwartz SM, Seedorf U, Stewart AF, Stott DJ, Thiery J, Zalloua PA, O'Donnell CJ, Reilly MP, Assimes TL, Thompson JR, Erdmann J, Clarke R, Watkins H, Kathiresan S, McPherson R, Deloukas P, Schunkert H, Samani NJ, Farrall M. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet 2015;47:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Christophersen IE, Rienstra M, Roselli C, Yin X, Geelhoed B, Barnard J, Lin H, Arking DE, Smith AV, Albert CM, Chaffin M, Tucker NR, Li M, Klarin D, Bihlmeyer NA, Low S-K, Weeke PE, Müller-Nurasyid M, Smith JG, Brody JA, Niemeijer MN, Dörr M, Trompet S, Huffman J, Gustafsson S, Schurmann C, Kleber ME, Lyytikäinen L-P, Seppälä I, Malik R, Horimoto ARVR, Perez M, Sinisalo J, Aeschbacher S, Thériault S, Yao J, Radmanesh F, Weiss S, Teumer A, Choi SH, Weng L-C, Clauss S, Deo R, Rader DJ, Shah SH, Sun A, Hopewell JC, Debette S, Chauhan G, Yang Q, Worrall BB, Paré G, Kamatani Y, Hagemeijer YP, Verweij N, Siland JE, Kubo M, Smith JD, Van Wagoner DR, Bis JC, Perz S, Psaty BM, Ridker PM, Magnani JW, Harris TB, Launer LJ, Shoemaker MB, Padmanabhan S, Haessler J, Bartz TM, Waldenberger M, Lichtner P, Arendt M, Krieger JE, Kähönen M, Risch L, Mansur AJ, Peters A, Smith BH, Lind L, Scott SA, Lu Y, Bottinger EB, Hernesniemi J, Lindgren CM, Wong JA, Huang J, Eskola M, Morris AP, Ford I, Reiner AP, Delgado G, Chen LY, Chen Y-DI, Sandhu RK, Li M, Boerwinkle E, Eisele L, Lannfelt L, Rost N, Anderson CD, Taylor KD, Campbell A, Magnusson PK, Porteous D, Hocking LJ, Vlachopoulou E, Pedersen NL, Nikus K, Orho-Melander M, Hamsten A, Heeringa J, Denny JC, Kriebel J, Darbar D, Newton-Cheh C, Shaffer C, Macfarlane PW, Heilmann-Heimbach S, Almgren P, Huang PL, Sotoodehnia N, Soliman EZ, Uitterlinden AG, Hofman A, Franco OH, Völker U, Jöckel K-H, Sinner MF, Lin HJ, Guo X, Dichgans M, Ingelsson E, Kooperberg C, Melander O, Loos RJF, Laurikka J, Conen D, Rosand J, van der Harst P, Lokki M-L, Kathiresan S, Pereira A, Jukema JW, Hayward C, Rotter JI, März W, Lehtimäki T, Stricker BH, Chung MK, Felix SB, Gudnason V, Alonso A, Roden DM, Kääb S, Chasman DI, Heckbert SR, Benjamin EJ, Tanaka T, Lunetta KL, Lubitz SA, Ellinor PT; AFGen Consortium. Large-scale analyses of common and rare variants identify 12 new loci associated with atrial fibrillation. Nat Genet 2017;49:946–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klarin D, Busenkell E, Judy R, Lynch J, Levin M, Haessler J, Aragam K, Chaffin M, Haas M, Lindstrom S, Assimes TL, Huang J, Min Lee K, Shao Q, Huffman JE, Kabrhel C, Huang Y, Sun YV, Vujkovic M, Saleheen D, Miller DR, Reaven P, DuVall S, Boden WE, Pyarajan S, Reiner AP, Tregouet DA, Henke P, Kooperberg C, Gaziano JM, Concato J, Rader DJ, Cho K, Chang KM, Wilson PWF, Smith NL, O'Donnell CJ, Tsao PS, Kathiresan S, Obi A, Damrauer SM, Natarajan P; INVENT Consortium; Veterans Affairs’ Million Veteran Program. Genome-wide association analysis of venous thromboembolism identifies new risk loci and genetic overlap with arterial vascular disease. Nat Genet 2019;51:1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, Datta G, Davila-Velderrain J, McGuire D, Tian C, Zhan X23andMe Research Team; HUNT All-In PsychiatryChoquet H, Docherty AR, Faul JD, Foerster JR, Fritsche LG, Gabrielsen ME, Gordon SD, Haessler J, Hottenga JJ, Huang H, Jang SK, Jansen PR, Ling Y, Magi R, Matoba N, McMahon G, Mulas A, Orru V, Palviainen T, Pandit A, Reginsson GW, Skogholt AH, Smith JA, Taylor AE, Turman C, Willemsen G, Young H, Young KA, Zajac GJM, Zhao W, Zhou W, Bjornsdottir G, Boardman JD, Boehnke M, Boomsma DI, Chen C, Cucca F, Davies GE, Eaton CB, Ehringer MA, Esko T, Fiorillo E, Gillespie NA, Gudbjartsson DF, Haller T, Harris KM, Heath AC, Hewitt JK, Hickie IB, Hokanson JE, Hopfer CJ, Hunter DJ, Iacono WG, Johnson EO, Kamatani Y, Kardia SLR, Keller MC, Kellis M, Kooperberg C, Kraft P, Krauter KS, Laakso M, Lind PA, Loukola A, Lutz SM, Madden PAF, Martin NG, McGue M, McQueen MB, Medland SE, Metspalu A, Mohlke KL, Nielsen JB, Okada Y, Peters U, Polderman TJC, Posthuma D, Reiner AP, Rice JP, Rimm E, Rose RJ, Runarsdottir V, Stallings MC, Stancakova A, Stefansson H, Thai KK, Tindle HA, Tyrfingsson T, Wall TL, Weir DR, Weisner C, Whitfield JB, Winsvold BS, Yin J, Zuccolo L, Bierut LJ, Hveem K, Lee JJ, Munafo MR, Saccone NL, Willer CJ, Cornelis MC, David SP, Hinds DA, Jorgenson E, Kaprio J, Stitzel JA, Stefansson K, Thorgeirsson TE, Abecasis G, Liu DJ, Vrieze S. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet 2019;51:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 2015;31:3555–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wootton RE, Richmond RC, Stuijfzand BG, Lawn RB, Sallis HM, Taylor GMJ, Jones HJ, Zammit S, Smith Munafo G, Munafò MR. Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. Psychol Med 2019;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, Rutten-Jacobs L, Giese AK, van der Laan SW, Gretarsdottir S, Anderson CD, Chong M, Adams HHH, Ago T, Almgren P, Amouyel P, Ay H, Bartz TM, Benavente OR, Bevan S, Boncoraglio GB, Brown RD Jr, Butterworth AS, Carrera C, Carty CL, Chasman DI, Chen WM, Cole JW, Correa A, Cotlarciuc I, Cruchaga C, Danesh J, de Bakker PIW, DeStefano AL, den Hoed M, Duan Q, Engelter ST, Falcone GJ, Gottesman RF, Grewal RP, Gudnason V, Gustafsson S, Haessler J, Harris TB, Hassan A, Havulinna AS, Heckbert SR, Holliday EG, Howard G, Hsu FC, Hyacinth HI, Ikram MA, Ingelsson E, Irvin MR, Jian X, Jiménez-Conde J, Johnson JA, Jukema JW, Kanai M, Keene KL, Kissela BM, Kleindorfer DO, Kooperberg C, Kubo M, Lange LA, Langefeld CD, Langenberg C, Launer LJ, Lee JM, Lemmens R, Leys D, Lewis CM, Lin WY, Lindgren AG, Lorentzen E, Magnusson PK, Maguire J, Manichaikul A, McArdle PF, Meschia JF, Mitchell BD, Mosley TH, Nalls MA, Ninomiya T, O'Donnell MJ, Psaty BM, Pulit SL, Rannikmäe K, Reiner AP, Rexrode KM, Rice K, Rich SS, Ridker PM, Rost NS, Rothwell PM, Rotter JI, Rundek T, Sacco RL, Sakaue S, Sale MM, Salomaa V, Sapkota BR, Schmidt R, Schmidt CO, Schminke U, Sharma P, Slowik A, Sudlow CLM, Tanislav C, Tatlisumak T, Taylor KD, Thijs VNS, Thorleifsson G, Thorsteinsdottir U, Tiedt S, Trompet S, Tzourio C, van Duijn CM, Walters M, Wareham NJ, Wassertheil-Smoller S, Wilson JG, Wiggins KL, Yang Q, Yusuf SAFGen Consortium; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium; International Genomics of Blood Pressure (iGEN-BP) Consortium; INVENT Consortium; STARNETBis JC, Pastinen T, Ruusalepp A, Schadt EE, Koplev S, Björkegren JLM, Codoni V, Civelek M, Smith NL, Trégouët DA, Christophersen IE, Roselli C, Lubitz SA, Ellinor PT, Tai ES, Kooner JS, Kato N, He J, van der Harst P, Elliott P, Chambers JC, Takeuchi F, Johnson ADBioBank Japan Cooperative Hospital Group; COMPASS Consortium; EPIC-CVD Consortium; EPIC-InterAct Consortium; International Stroke Genetics Consortium (ISGC); METASTROKE Consortium; Neurology Working Group of the CHARGE Consortium; NINDS Stroke Genetics Network (SiGN); UK Young Lacunar DNA Study; MEGASTROKE ConsortiumSanghera DK, Melander O, Jern C, Strbian D, Fernandez-Cadenas I, Longstreth WT Jr, Rolfs A, Hata J, Woo D, Rosand J, Pare G, Hopewell JC, Saleheen D, Stefansson K, Worrall BB, Kittner SJ, Seshadri S, Fornage M, Markus HS, Howson JMM, Kamatani Y, Debette S, Dichgans M. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet 2018;50:524–537.29531354 [Google Scholar]
- 25. Larsson SC, Burgess S, Michaëlsson K. Smoking and stroke: a Mendelian randomization study. Ann Neurol 2019;86:468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 27. Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol 2015;181:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ntalla I, Kanoni S, Zeng L, Giannakopoulou O, Danesh J, Watkins H, Samani NJ, Deloukas P, Schunkert H, Group U. Genetic risk score for coronary disease identifies predispositions to cardiovascular and noncardiovascular diseases. J Am Coll Cardiol 2019;73:2932–2942. [DOI] [PubMed] [Google Scholar]
- 29. Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology 2017;28:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spiller W, Davies NM, Palmer TM. Software application profile: mrrobust—a tool for performing two-sample summary Mendelian randomization analyses. Int J Epidemiol 2019;48:684–690. [Google Scholar]
- 31. Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol 2017;46:1734–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Larsson SC, Bäck M, Rees JMB, Mason AM, Burgess S. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: a Mendelian randomization study. Eur Heart J 2020;41:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rigotti NA, Clair C. Managing tobacco use: the neglected cardiovascular disease risk factor. Eur Heart J 2013;34:3259–3267. [DOI] [PubMed] [Google Scholar]
- 34. Kamio Y, Miyamoto T, Kimura T, Mitsui K, Furukawa H, Zhang D, Yokosuka K, Korai M, Kudo D, Lukas RJ, Lawton MT, Hashimoto T. Roles of nicotine in the development of intracranial aneurysm rupture. Stroke 2018;49:2445–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baron JA, La Vecchia C, Levi F. The antiestrogenic effect of cigarette smoking in women. Am J Obstet Gynecol 1990;162:502–514. [DOI] [PubMed] [Google Scholar]
- 36. Michnovicz JJ, Hershcopf RJ, Haley NJ, Bradlow HL, Fishman J. Cigarette smoking alters hepatic estrogen metabolism in men: implications for atherosclerosis. Metabolism 1989;38:537–541. [DOI] [PubMed] [Google Scholar]
- 37. Michnovicz JJ, Hershcopf RJ, Naganuma H, Bradlow HL, Fishman J. Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med 1986;315:1305–1309. [DOI] [PubMed] [Google Scholar]
- 38. Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res 2009;11:12–24. [DOI] [PubMed] [Google Scholar]
- 39. Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol 2016;40:597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.