Abstract
目的
探讨高丽参热水提取物预处理对N-甲基-N'-硝基-N-亚硝基胍(MNNG)诱导神经母细胞瘤(SH-SY5Y)细胞parthanatos的影响及其机制。
方法
将SH-SY5Y细胞分为空白组、MNNG诱导组及高丽参热水提取物预处理组,空白组细胞不给予任何处理,MNNG诱导组给与MNNG 250 μmol/L刺激1 h或4 h,高丽参热水提取物预处理组在给与MNNG诱导前先给与高丽参热水提取物1 mg/mL预处理。以CCK-8和细胞流式技术检测细胞存活率,Western blot检测PAR蛋白,免疫荧光检测AIF核质分布,细胞流式技术检测细胞活性氧的表达。
结果
与空白组比较,SH-SY5Y细胞的存活率随着MNNG刺激的浓度和时间的增加而逐渐降低(P < 0.05);MNNG刺激SH-SY5Y后多聚PAR蛋白表达增加(P < 0.05);高丽参热水提取物预处理可以抑制MNNG诱导的SH-SY5Y死亡,并且降低了MNNG诱导的AIF的表达和入核(P < 0.05);MNNG刺激后活性氧的表达增加,而高丽参热水提取物预处理后,活性氧的表达显著降低(P < 0.05)。
结论
高丽参热水提取物预处理SH-SY5Y细胞可以降低活性氧的产生并降低了MNNG诱导的parthanatos的发生。
Keywords: 高丽参, 热水提取物, parthanatos, 细胞活性氧, 多聚(ADP-核糖), 凋亡诱导因子
Abstract
Objective
To explore the effect of pretreatment of neuroblastoma cells with hot water extract of Korean ginseng on MNNG-induced parthanatos and its mechanism.
Methods
Neuroblastoma SH-SY5Y cells were pretreated with 1 mg/L hot water extract of Korean ginseng before induction with 250 μmol/L MNNG for 1 h or 4 h. CCK-8 and cell flow cytometry were used to detect cell survival rate. Western blotting was used to detect the changes in poly(ADP-ribose) (PAR) expression in the treated cells. Immunofluorescence assay was used to detect nuclear distribution of apoptosis-inducing factor (AIF), and flow cytometry was used to detect the level of reactive oxygen species (ROS) in the cells.
Results
Compared with the blank control cells, MNNG-treated SH-SY5Y cells showed significantly decreased survival rate as the concentration of MNNG and the stimulation time increased (P < 0.05). Stimulation with MNNG also resulted in significantly increased expression of PAR protein in the cells (P < 0.05). Pretreatment of the cells with hot water extract of Korean ginseng obviously inhibited MNNG-induced cell death and significantly reduced AIF expression and nucleation in the cells (P < 0.05). MNNG stimulation significantly increased ROS level in the cells, which was decreased significantly by pretreatment of the cells with the extract (P < 0.05).
Conclusion
Pretreatment with hot water extract of Korean ginseng reduces MNNG-induced parthanatos and ROS production in SH-SY5Y cells.
Keywords: Korean ginseng, hot water extract, parthanatos, reactive oxygen species, poly(ADP-ribose), apoptosis-inducing factor
氧化应激涉及细胞内活性氧(ROS)水平升高,从而破坏各种生物分子,特别是核酸,蛋白质和脂质的变化,引起细胞损伤[1],最终导致细胞死亡。ROS的过量产生或消除不足可引发多种疾病,例如神经退行性变[2]、心血管疾病[3]和肝病[4]。Parthanatos是一种新的细胞程序性死亡形式,N-甲基-N'-硝基-N-亚硝基胍(MNNG)诱发的ROS产生可导致DNA损伤和聚(ADP-核糖)聚合酶-1(PARP-1)活化,PARP-1活化进一步介导了parthanatos[5]。细胞中PARP-1的激活、多聚ADP核糖(PAR)的聚集以及凋亡诱导因子(AIF)的入核[6]被认为是细胞发生parthanatos的三大典型特征[7]。Parthanatos与ROS的生成和DNA损伤有着密切联系,在脑缺血再灌注、失血性休克、帕金森氏病和阿尔茨海默病中起着重要作用[8-10]。
高丽参中含有丰富的活性物质如皂苷、多糖、蛋白、氨基酸、多肽、挥发油、维生素、无机元素等,具有极高的药用价值[11-13]。目前研究发现,高丽参具有抗癌、抗糖尿病和抗炎作用[14-15]。高丽参可通过抑制ROS的产生或清除ROS,激活抗氧化防御系统,维持氧化还原状态,有效地保护细胞免受氧化应激诱导的细胞损伤[16-17]。已有研究发现人参的主要成分人参皂苷,在神经退行性疾病、脑缺血再灌注等具有神经保护作用[18]。但高丽参是否可以通过ROS影响parthanatos起到神经保护作用,其可能的机制如何,目前尚不清楚。本研究通过MNNG诱导神经母细胞瘤细胞(SH-SY5Y)的parthanatos,观察并初步探索高丽参热水提取物对ROS引发parthanatos的影响和机制研究。
1. 材料和方法
1.1. 试剂
N-甲基-N'-硝基-N-亚硝基胍MNNG(meilunbio);CCK-8试剂盒(MCE,Cat. No.HY-K0301);Annexin V FITC Apoptosis Kit凋亡试剂盒(BD 556547);PAR-4 Antibody(CST#2328);AIF(D39D2)Rabbit mAb(CST #5318);DCFDA / H2DCFDA-Cellular ROS Assay试剂盒(ab113851);各种酶及培养基。
1.2. 分组与模型
1.2.1. SH-SY5Y细胞分成7组
空白对照组,MNNG 10 μmol/L组、50 μmol/L组、100 μmol/L组、250 μmol/L组、500 μmol/L组、1000 μmol/L组,从而确定MNNG刺激细胞的浓度。
1.2.2. SH-SY5Y细胞分成8组
MNNG 0 h组、1 h组、2 h组、3 h组、4 h组、6 h组、8 h组、10 h组,MNNG 250 μmol/L分别刺激以上不同时间,从而确定MNNG刺激细胞的有效时间。
1.2.3. SH-SY5Y细胞分成5组
MNNG 0 min组、20 min组、30 min组、45 min组、60 min组,MNNG 250 μmol/L刺激SH-SY5Y细胞检测parthanatos相关蛋白PAR。
1.2.4. SH-SY5Y细胞分成8组
空白对照组,高丽参热水提取物0 mg/mL组、0.01 mg/mL组、0.1 mg/mL组、1 mg/mL组、10 mg/mL组、50 mg/mL组、100 mg/mL组。不同浓度高丽参热水提取物预处理后,给予MNNG 250 μmol/L刺激细胞4 h,从而确定高丽参热水提取物对细胞发生parthanatos后的影响,并确定其有效浓度。
1.2.5. 高丽参热水提取物预处理SH-SY5Y细胞后产生ROS和parthanatos的相互关系
高丽参热水提取物1 mg/mL预处理SH-SY5Y细胞后,给予MNNG 250 μmol/L刺激细胞30 min或者1 h,检测空白组与实验组各组细胞ROS的含量。
1.3. 方法
1.3.1. CCK-8实验检测SH-SY5Y细胞存活率
96孔板中的SH-SY5Y细胞处理后,每孔加入10 μL CCK-8溶液,温箱内孵育2 h,酶标仪450 nm处测吸光度A450 nm,换算细胞存活率。
1.3.2. 细胞流式技术检测SH-SY5Y细胞存活率
将处理好的SH-SY5Y细胞消化离心后,先加入5 μLAnnexin V FITC混匀,室温避光孵育15 min,再加入5 μL碘化丙啶室温避光孵育5 min后,流式细胞仪检测细胞死亡率。
1.3.3. 细胞内PAR的表达检测
充分裂解处理好的SHSY5Y细胞,BCA法检测蛋白浓度,采用Western blot法检测PAR蛋白的表达。凝胶电泳后转膜,电化学发光液曝光,采用Image J软件进行灰度值分析,以目的蛋白与内参三磷酸甘油醛脱氢酶的灰度值比值表示目的蛋白表达量,并以正常组作为对照。
1.3.4. 细胞内AIF核质分布检测
通过细胞免疫荧光检测不同处理组发生parthanatos后AIF的核质分布情况。
1.3.5. 细胞内ROS检测
处理后的SH-SY5Y细胞,加入10 μmol/L的H2DCFDA混匀,37 ℃孵育30 min,激光共聚焦显微镜观察,流式细胞仪进行荧光定量检测。
1.4. 统计学处理
应用SPSS 20.0统计软件进行分析,数据以均数±标准差表示,组间比较使用单因素方差分析和LSD多重比较法,P < 0.05为差异有统计学意义。
2. 结果
2.1. MNNG刺激使SH-SY5Y细胞的死亡率升高
CCK-8实验和细胞流式结果显示:与空白对照组比较,SH-SY5Y细胞的存活率随着MNNG刺激的浓度和时间的增加而逐渐降低(P < 0.05,图 1)。
1.
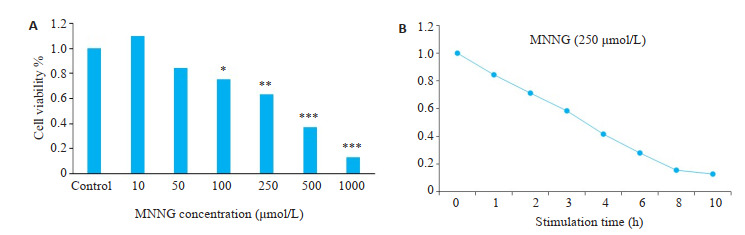
MNNG刺激使SH-SY5Y细胞的死亡率升高
MNNG stimulation increases SH-SY5Y cell mortality. A: Relationship between MNNG concentration and cell viability; B: Relationship between the stimulation time of 250 μmol/L MNNG and cell viability. *P < 0.05, **P < 0.01, ***P < 0.001 vs control
2.2. MNNG诱导SH-SY5Y细胞发生parthanatos
Western blot结果显示:与空白对照组比较,MNNG刺激后SH-SY5Y细胞内多聚PAR蛋白表达增加,且在MNNG刺激时间为20~30 min时PAR表达增加最为明显(图 2)。
2.
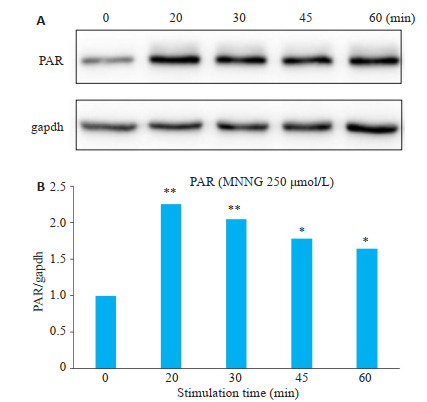
MNNG刺激后SH-SY5Y细胞内多聚PAR蛋白表达增加
Increased expression of poly(ADP-ribose) (PAR) protein in SH-SY5Y cells after MNNG stimulation. *P < 0.05, **P < 0.01 vs 0 min.
2.3. 高丽参热水提取物能降低MNNG诱导的SH-SY5Y细胞的死亡率
CCK-8实验结果显示:用250 μmol/L MNNG刺激SH-SY5Y 4 h后,与未加高丽参热水提取物预处理组比较,高丽参热水提取物预处理组的SH-SY5Y细胞的存活率提高,并且随着高丽参热水提取物预处理的浓度增高其存活率逐渐升高(P < 0.05,图 3)。
3.
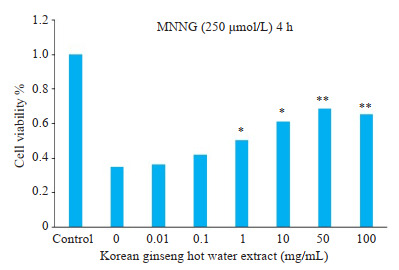
高丽参热水提取物能降低MNNG诱导的SH-SY5Y细胞的死亡率
Hot water extract of ginseng reduce the death rate of SH-SY5Y cells induced by MNNG.
2.4. 高丽参热水提取物能降低MNNG诱导的PAR蛋白的表达
Western blot结果显示:用MNNG 250 μmol/L处理SH-SY5Y细胞20或30 min,与对照组相比PAR蛋白表达升高,而用高丽参热水提取物1 mg/mL处理则可以显著减少MNNG引起的PAR蛋白的表达(P < 0.05,图 4)。
4.
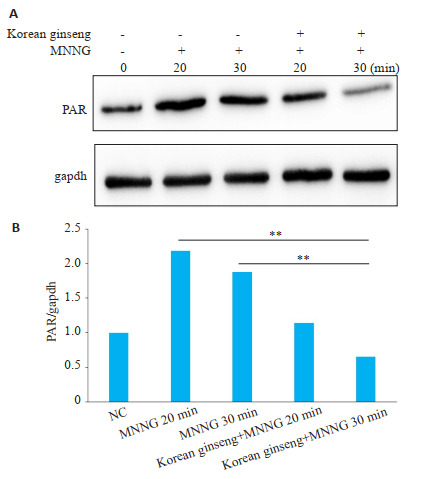
高丽参热水提取物可以降低MNNG诱导的PAR的表达
Hot water extract of ginseng reduces the expression of PAR induced by MNNG. **P < 0.05.
2.5. 高丽参热水提取物能降低MNNG诱导的AIF的表达和入核
细胞免疫荧光共聚焦结果显示:与空白对照组比较,MNNG刺激后AIF的表达以及入核荧光强度明显增强,而加入高丽参热水提取物预处理后,MNNG引起的AIF的表达减少,并且其入核荧光强度也明显减弱(图 5)。
5.
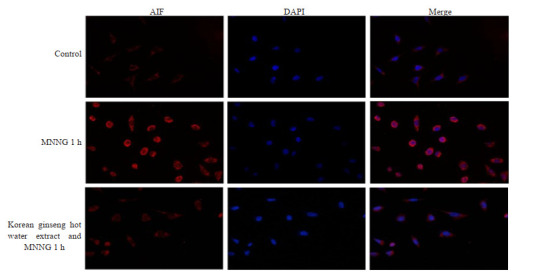
高丽参热水提取物可以抑制MNNG诱导的AIF的表达和入核
Hot water extract of ginseng inhibits the expression of AIF induced by MNN (Original magnitication: ×400).
2.6. 高丽参热水提取物通过抑制ROS的生成并减少parthanatos
ROS荧光定量细胞流式结果显示:与空白对照组相比,MNNG刺激后ROS的表达增加,但加入高丽参热水提取物预处理后,ROS的表达与单独MNNG处理组比较明显减少(图 5)。
3. 讨论
对高丽参热水提取物的抗氧化作用研究,本研究进行了体外细胞预实验结果发现,使用MNNG能够诱导细胞发生经典的parthanatos死亡,包括PAR的聚集及AIF入核等,而这些效应均能被高丽参热水提取物显著的抑制,提示了高丽参热水提取物具有抑制parthanatos发生的效应。
Parthanatos是一种新的程序性细胞死亡方式,主要以DNA损伤,PARP-1激活为主要特征,当有轻度DNA损伤时,PARP-1的活性增加至500倍,酶利用ATP的消耗产生的氧化NAD(NAD+)来合成PAR聚合物[7]。然而,当DNA损伤较深时,PARP-1过度激活,AIF从线粒体向细胞核的移位,大规模DNA片段化(约50 kb)和染色质浓缩,然后是细胞死亡[19-21]。MNNG可引起过量的DNA链断裂,导致PARP-1过度活化发生parthanatos。有研究发现MNNG诱导的parthanatos伴随着双相ROS产生和胞内钙增加,由MNNG触发的ROS产生而导致增强的DNA损伤和PARP-1的激活。此外,细胞内钙升高和ROS产生具有相互扩增作用,从而有助于PARP-1介导的parthanatos[5]。本实验结果也表明MNNG能通过增加ROS的产生和AIF核易位来诱导SH-SY5Y细胞parthanatos的发生。
氧葡萄糖剥夺触发了SH-SY5Y细胞中的parthanatos,而抗氧化剂NAC抑制ROS的过量生产,从而明显减弱了氧葡萄糖剥夺诱导的SH-SY5Y细胞中的parthanatos[22]。另有研究也同样证明了通过诱导过量ROS产生可以促进由氧化应激引起的神经胶质瘤细胞触发parthanatos[23-24]。通过细胞内活性氧ROS的检测,本研究发现高丽参热水提取物可以抑制ROS的产生,AIF核易位减弱,同时减少MNNG诱导的SH-SY5Y发生parthanatos。
有研究证明,烟酰胺腺嘌呤二核苷酸磷酸(NADPH)氧化酶具有跨膜运输电子和产生超氧化物和其他下游ROS的能力[25],尤其是能以NADPH氧化酶4依赖性的方式增加了细胞中ROS的产生[26-29]。而身体运动训练减少了NADPH氧化酶的表达,导致局部ROS生成减少[28, 30],说明NADPH氧化酶的增加或减少对ROS的生成多少有重要影响。本研究发现高丽参热水提取物可以减少MNNG诱导的ROS产生,但是其引起ROS生成减少的机制是否是通过抑制NADPH氧化酶的产生暂未进行深入探究。
有研究发现高丽参主要成分人参皂苷可通过抑制线粒体肿胀、保存线粒体膜电位和减少ROS的产生,保护分离的线粒体免受钙离子诱导的损伤,减少线粒体释放细胞色素c和AIF,从而减少缺血后线粒体介导的凋亡,起到对短暂性局灶性脑缺血的神经保护作用[29, 31]。也有文献报道参皂苷Rg1能提高H2O2损伤的SH-SY5Y细胞的存活率,减少乳酸脱氢酶漏出量,提高超氧化物歧化酶活性,能有效抑制caspase-3的免疫反应,并呈剂量依赖性参与热休克蛋白70基因的表达[30]。有研究也发现,高丽参茶可以通过降低谷胱甘肽、谷胱甘肽还原酶、过氧化氢酶、谷胱甘肽S-转移酶、谷胱甘肽过氧化物酶和超氧化物歧化酶,对低灌注或再灌注性脑损伤具有保护作用,提示高丽参茶对脑血管病(包括脑卒中)具有治疗作用[31]。那么,高丽参热水提取物中的什么成分对MNNG刺激后SH-SY5Y细胞的死亡率降低起着主导作用呢?高丽参热水提取物抑制SH-SY5Y细胞的死亡是否也存在其他机制可以解释呢?还需要更进一步的研究成果进行探究。
综上所述,高丽参热水提取物可能通过抑制MNNG诱导的ROS生成,减少DNA损伤和PAR形成这一机制来抑制SH-SY5Y细胞发生的parthanatos。这一发现对高丽参等参类在抑制氧化应激的机制方面做了补充,另外可能对中草药在氧化应激疾病的临床用药和治疗方面有一定的借鉴意义。关于高丽参热水提取物对parthanatos的具体机制接下来将会做进一步深入的研究。
Biography
郭远波,硕士,主治医师,E-mail: fengly_1314@sina.com
Funding Statement
广东省中医药局科研项目(20191006);广东省医学科研基金(A2020038);广东省基础与应用基础研究基金项目(2019A1515110063)
Contributor Information
郭 远波 (Yuanbo GUO), Email: fengly_1314@sina.com.
王 晟 (Sheng WANG), Email: shengwang_gz@163.com.
References
- 1.Kim SH, Kwon CH, Nakano I. Detoxification of oxidative stress in glioma stem cells: mechanism, clinical relevance, and therapeutic development. J Neurosci Res. 2014;92(11):1419–24. doi: 10.1002/jnr.23431. [Kim SH, Kwon CH, Nakano I. Detoxification of oxidative stress in glioma stem cells: mechanism, clinical relevance, and therapeutic development[J]. J Neurosci Res, 2014, 92(11): 1419-24.] [DOI] [PubMed] [Google Scholar]
- 2.Islam MT. Oxidative stress and mitochondrial dysfunction- linked neurodegenerative disorders. Neurol Res. 2017;39(1):73–82. doi: 10.1080/01616412.2016.1251711. [Islam MT. Oxidative stress and mitochondrial dysfunction- linked neurodegenerative disorders[J]. Neurol Res, 2017, 39(1): 73-82.] [DOI] [PubMed] [Google Scholar]
- 3.Mondal NK, Roychoudhury S, Mukherjee S, et al. Increased risk of cardiovascular disease in premenopausal female ragpickers of Eastern India: involvement of inflammation, oxidative stress, and platelet hyperactivity. Mol Cell Biochem. 2016;419(1/2):193–203. doi: 10.1007/s11010-016-2773-3. [Mondal NK, Roychoudhury S, Mukherjee S, et al. Increased risk of cardiovascular disease in premenopausal female ragpickers of Eastern India: involvement of inflammation, oxidative stress, and platelet hyperactivity[J]. Mol Cell Biochem, 2016, 419(1/2): 193- 203.] [DOI] [PubMed] [Google Scholar]
- 4.Mohamed J, Nazratun AH, Zariyantey AH, et al. Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Univ Med J. 2016;16(2):e132–41. doi: 10.18295/squmj.2016.16.02.002. doi: 10.18295/squmj.2016.16.02.002. [Mohamed J, Nazratun AH, Zariyantey AH, et al. Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation[J]. Sultan Qaboos Univ Med J, 2016, 16(2): e132-41.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu LY, Ho FM, Shiah SG, et al. Oxidative stress initiates DNA damager MNNG-induced poly (ADP-ribose) polymerase-1- dependent parthanatos cell death. Biochem Pharmacol. 2011;81(3):459–70. doi: 10.1016/j.bcp.2010.10.016. [Chiu LY, Ho FM, Shiah SG, et al. Oxidative stress initiates DNA damager MNNG-induced poly (ADP-ribose) polymerase-1- dependent parthanatos cell death[J]. Biochem Pharmacol, 2011, 81 (3): 459-70.] [DOI] [PubMed] [Google Scholar]
- 6.Wang YF, Kim NS, Li XL, et al. Calpain activation is not required for AIF translocation in PARP-1-dependent cell death (parthanatos) J Neurochem. 2009;110(2):687–96. doi: 10.1111/j.1471-4159.2009.06167.x. [Wang YF, Kim NS, Li XL, et al. Calpain activation is not required for AIF translocation in PARP-1-dependent cell death (parthanatos)[J]. J Neurochem, 2009, 110(2): 687-96.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fatokun AA, Dawson VL, Dawson TM. Parthanatos: mitochondriallinked mechanisms and therapeutic opportunities. Br J Pharmacol. 2014;171(8):2000–16. doi: 10.1111/bph.12416. [Fatokun AA, Dawson VL, Dawson TM. Parthanatos: mitochondriallinked mechanisms and therapeutic opportunities[J]. Br J Pharmacol, 2014, 171(8): 2000-16.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang YF, Kim NS, Haince JF, et al. Poly (ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase- 1- dependent cell death (parthanatos) Sci Signal. 2011;4(167):ra20–8. doi: 10.1126/scisignal.2000902. [Wang YF, Kim NS, Haince JF, et al. Poly (ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase- 1- dependent cell death (parthanatos)[J]. Sci Signal, 2011, 4(167): ra20-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrabi SA, Kim NS, Yu SW, et al. Poly (ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci USA. 2006;103(48):18308–13. doi: 10.1073/pnas.0606526103. [Andrabi SA, Kim NS, Yu SW, et al. Poly (ADP-ribose) (PAR) polymer is a death signal[J]. Proc Natl Acad Sci USA, 2006, 103 (48): 18308-13.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu SW, Wang HM, Poitras MF, et al. Mediation of poly (ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297(5579):259–63. doi: 10.1126/science.1072221. [Yu SW, Wang HM, Poitras MF, et al. Mediation of poly (ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor[J]. Science, 2002, 297(5579): 259-63.] [DOI] [PubMed] [Google Scholar]
- 11.Shin KK, Yi YS, Kim JK, et al. Korean red ginseng plays an antiaging role by modulating expression of aging-related genes and immune cell subsets. Molecules. 2020;25(7):E1492–501. doi: 10.3390/molecules25071492. [Shin KK, Yi YS, Kim JK, et al. Korean red ginseng plays an antiaging role by modulating expression of aging-related genes and immune cell subsets[J]. Molecules, 2020, 25(7): E1492-501.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YY, Seo HW, Kyung JS, et al. Proteomic studies of putative molecular signatures for biological effects by Korean Red Ginseng. J Ginseng Res. 2019;43(4):666–75. doi: 10.1016/j.jgr.2019.05.001. [Lee YY, Seo HW, Kyung JS, et al. Proteomic studies of putative molecular signatures for biological effects by Korean Red Ginseng[J]. J Ginseng Res, 2019, 43(4): 666-75.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MG, Kim Y, Jeon JY, et al. Effect of fermented red ginseng on cytochrome P450 and P-glycoprotein activity in healthy subjects, as evaluated using the cocktail approach. Br J Clin Pharmacol. 2016;82(6):1580–90. doi: 10.1111/bcp.13080. [Kim MG, Kim Y, Jeon JY, et al. Effect of fermented red ginseng on cytochrome P450 and P-glycoprotein activity in healthy subjects, as evaluated using the cocktail approach[J]. Br J Clin Pharmacol, 2016, 82(6): 1580-90.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung SY, Kim C, Kim WS, et al. Korean red ginseng extract enhances the anticancer effects of imatinib mesylate through abrogation p38 and STAT5 activation in KBM-5 cells. Phytother Res. 2015;29(7):1062–72. doi: 10.1002/ptr.5347. [Jung SY, Kim C, Kim WS, et al. Korean red ginseng extract enhances the anticancer effects of imatinib mesylate through abrogation p38 and STAT5 activation in KBM-5 cells[J]. Phytother Res, 2015, 29 (7): 1062-72.] [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Cho JY, Kim WK. Anti-inflammation effect of Exercise and Korean red ginseng in aging model rats with diet-induced atherosclerosis. Nutr Res Pract. 2014;8(3):284–91. doi: 10.4162/nrp.2014.8.3.284. [Lee J, Cho JY, Kim WK. Anti-inflammation effect of Exercise and Korean red ginseng in aging model rats with diet-induced atherosclerosis[J]. Nutr Res Pract, 2014, 8(3): 284-91.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choudhry QN, Kim JH, Cho HT, et al. Ameliorative effect of black ginseng extract against oxidative stress-induced cellular damages in mouse hepatocytes. J Ginseng Res. 2019;43(2):179–85. doi: 10.1016/j.jgr.2017.10.003. [Choudhry QN, Kim JH, Cho HT, et al. Ameliorative effect of black ginseng extract against oxidative stress-induced cellular damages in mouse hepatocytes[J]. J Ginseng Res, 2019, 43(2): 179-85.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YH, Dong JH, Liu P, et al. Ginsenoside Rb3 attenuates oxidative stress and preserves endothelial function in renal arteries from hypertensive rats. Br J Pharmacol. 2014;171(13):3171–81. doi: 10.1111/bph.12660. [Wang YH, Dong JH, Liu P, et al. Ginsenoside Rb3 attenuates oxidative stress and preserves endothelial function in renal arteries from hypertensive rats[J]. Br J Pharmacol, 2014, 171(13): 3171-81.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, Li N, Pu YQ, et al. Neuroprotective effects of ginseng phytochemicals: recent perspectives. Molecules. 2019;24(16):E2939–50. doi: 10.3390/molecules24162939. [Huang X, Li N, Pu YQ, et al. Neuroprotective effects of ginseng phytochemicals: recent perspectives[J]. Molecules, 2019, 24(16): E2939-50.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrabi SA, Dawson TM, Dawson VL. Mitochondrial and nuclear cross talk in cell death: parthanatos. Ann N Y Acad Sci. 2008;1147:233–41. doi: 10.1196/annals.1427.014. [Andrabi SA, Dawson TM, Dawson VL. Mitochondrial and nuclear cross talk in cell death: parthanatos[J]. Ann N Y Acad Sci, 2008, 1147: 233-41.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.David KK, Andrabi SA, Dawson TM, et al. Parthanatos, a messenger of death. Front Biosci (Landmark Ed) 2009;14:1116–28. doi: 10.2741/3297. [David KK, Andrabi SA, Dawson TM, et al. Parthanatos, a messenger of death[J]. Front Biosci (Landmark Ed), 2009, 14: 1116-28.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang RX, Li CS, Qiao P, et al. OGG1-initiated base excision repair exacerbates oxidative stress-induced parthanatos. Cell Death Dis. 2018;9(6):628–37. doi: 10.1038/s41419-018-0680-0. [Wang RX, Li CS, Qiao P, et al. OGG1-initiated base excision repair exacerbates oxidative stress-induced parthanatos[J]. Cell Death Dis, 2018, 9(6): 628-37.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang HF, Wang ZQ, Ding Y, et al. Endoplasmic Reticulum stress regulates oxygen-glucose deprivation-induced parthanatos in human SH-SY5Y cells via improvement of intracellular ROS. CNS Neurosci Ther. 2018;24(1):29–38. doi: 10.1111/cns.12771. [Wang HF, Wang ZQ, Ding Y, et al. Endoplasmic Reticulum stress regulates oxygen-glucose deprivation-induced parthanatos in human SH-SY5Y cells via improvement of intracellular ROS[J]. CNS Neurosci Ther, 2018, 24(1): 29-38.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma DD, Lu B, Feng C, et al. Deoxypodophyllotoxin triggers parthanatos in glioma cells via induction of excessive ROS. Cancer Lett. 2016;371(2):194–204. doi: 10.1016/j.canlet.2015.11.044. [Ma DD, Lu B, Feng C, et al. Deoxypodophyllotoxin triggers parthanatos in glioma cells via induction of excessive ROS[J]. Cancer Lett, 2016, 371(2): 194-204.] [DOI] [PubMed] [Google Scholar]
- 24.Zheng LJ, Wang C, Luo TF, et al. JNK activation contributes to oxidative stress-induced parthanatos in glioma cells via increase of intracellular ROS production. Mol Neurobiol. 2017;54(5):3492–505. doi: 10.1007/s12035-016-9926-y. [Zheng LJ, Wang C, Luo TF, et al. JNK activation contributes to oxidative stress-induced parthanatos in glioma cells via increase of intracellular ROS production[J]. Mol Neurobiol, 2017, 54(5): 3492- 505.] [DOI] [PubMed] [Google Scholar]
- 25.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology[J]. Physiol Rev, 2007, 87 (1): 245-313.] [DOI] [PubMed] [Google Scholar]
- 26.Sciarretta S, Zhai PY, Shao D, et al. Activation of NADPH oxidase 4 in the endoplasmic Reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNAactivated-like endoplasmic Reticulum kinase/eukaryotic initiation factor 2α/activating transcription factor 4 pathway. Circ Res. 2013;113(11):1253–64. doi: 10.1161/CIRCRESAHA.113.301787. [Sciarretta S, Zhai PY, Shao D, et al. Activation of NADPH oxidase 4 in the endoplasmic Reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNAactivated-like endoplasmic Reticulum kinase/eukaryotic initiation factor 2α/activating transcription factor 4 pathway[J]. Circ Res, 2013, 113(11): 1253-64.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CF, Qiao M, Schröder K, et al. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circ Res. 2010;106(9):1489–97. doi: 10.1161/CIRCRESAHA.109.215392. [Lee CF, Qiao M, Schröder K, et al. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death[J]. Circ Res, 2010, 106(9): 1489-97.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams V, Linke A, Kränkel N, et al. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation. 2005;111(5):555–62. doi: 10.1161/01.CIR.0000154560.88933.7E. [Adams V, Linke A, Kränkel N, et al. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease[J]. Circulation, 2005, 111(5): 555-62.] [DOI] [PubMed] [Google Scholar]
- 29.Ye R, Zhang X, Kong X, et al. Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia. Neuroscience. 2011;178:169–80. doi: 10.1016/j.neuroscience.2011.01.007. [Ye R, Zhang X, Kong X, et al. Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia[J]. Neuroscience, 2011, 178: 169-80.] [DOI] [PubMed] [Google Scholar]
- 30.Sun ZG, Chen LP, Wang FW, et al. Protective effects of ginsenoside Rg1 against hydrogen peroxide-induced injury in human neuroblastoma cells. Neural Regen Res. 2016;11(7):1159–64. doi: 10.4103/1673-5374.187057. [Sun ZG, Chen LP, Wang FW, et al. Protective effects of ginsenoside Rg1 against hydrogen peroxide-induced injury in human neuroblastoma cells[J]. Neural Regen Res, 2016, 11(7): 1159-64.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah ZA, Gilani RA, Sharma P, et al. Cerebroprotective effect of Korean ginseng tea against global and focal models of ischemia in rats. J Ethnopharmacol. 2005;101(1/2/3):299–307. doi: 10.1016/j.jep.2005.05.002. [Shah ZA, Gilani RA, Sharma P, et al. Cerebroprotective effect of Korean ginseng tea against global and focal models of ischemia in rats[J]. J Ethnopharmacol, 2005, 101(1/2/3): 299-307.] [DOI] [PubMed] [Google Scholar]


