Abstract
目的
探究紫草素对睾丸癌细胞I-10和精原细胞瘤TCAM-2死亡形式的影响,并从线粒体功能和糖酵解方面探讨其可能机制。
方法
I-10细胞组加入浓度分别为0 μmol/L(对照组)、1.2、1.4、1.6 μmol/L的紫草素,TCAM-2细胞组加入浓度分别为0 μmol/L(对照组)、0.5、1、1.5 μmol/L的紫草素,JC-1试剂盒和ROS试剂盒分别用来检测线粒体膜电位和活性氧的变化;乳酸试剂盒检测胞内乳酸变化;MTT法检测细胞增殖抑制;透射电镜观察细胞死亡形式;Annexin V-FITC/PI双染法检测细胞凋亡;Western blot法检测线粒体通路相关凋亡蛋白Bax、Bcl-2、cleaved caspase 3和自噬相关蛋白LC3B以及糖酵解相关蛋白PKM2、GLUT1、HK2的相对表达水平。
结果
MTT结果表明,紫草素能够随时间和剂量依赖性的抑制I-10和TCAM-2细胞的增殖(P < 0.05),I-10组24、48、72 h的IC50值分别为1.8、1.36和1.16 μmol/L,TCAM-2组24、48、72 h的IC50值分别为2.37、0.8和0.41 μmol/ L;紫草素能通过下调I-10和TCAM-2细胞线粒体膜电位和增加细胞活性氧水平而影响其线粒体功能(P < 0.05),抑制乳酸水平影响细胞糖酵解能力(P < 0.05);透射电镜和Annexin V-FITC/PI双染法检测出紫草素能够诱导I-10和TCAM-2细胞凋亡和过度自噬现象(P < 0.05);Western blot证明,紫草素可以通过下调线粒体通路相关凋亡蛋白Bax、Bcl-2、cleaved caspase 3的表达而影响I-10和TCAM-2细胞线粒体功能,下调PKM2、GLUT1、HK2影响其糖酵功能,通过上调LC3B增加细胞自噬(P < 0.05)。
结论
紫草素对睾丸癌细胞I-10和TCAM-2具有增殖抑制及诱导凋亡和增加自噬作用,其机制可能为紫草素影响I-10和TCAM-2细胞能量代谢功能。
Keywords: 睾丸癌, 精原细胞瘤, 紫草素, 糖酵解, 死亡形式
Abstract
Objective
To investigate the pattern of shikonin-induced cell death in testicular cancer cell I-10 and seminoma TCAM-2 cells and explore the possible mechanism in light of mitochondrial function and glycolysis.
Methods
I-10 cells treated with 0, 1.2, 1.4 and 1.6 μmol/L shikonin and TCAM-2 cells treated with 0, 0.5, 1 and 1.5 μmol/L shikonin were examined for mitochondrial membrane potential and production of reactive oxygen species (ROS) using JC-1 kit and ROS kit, respectively. The levels of intracellular lactic acid in the cells were detected using a lactic acid kit. The inhibitory effect of shikonin on the proliferation of the cells was assessed with MTT assay. The death patterns of the cells were observed by transmission electron microscopy, and annexin V-FITC/PI double staining was used to detect cell apoptosis. Western blotting was used to detect the relative expression levels of the apoptotic proteins Bax, Bcl-2, and cleaved caspase-3, the autophagy- related protein LC3B and glycolysis- related proteins PKM2, GLUT1 and HK2.
Results
MTT assay showed that shikonin significantly inhibited the proliferation of I-10 and TCAM-2 cells in a time- and dose-dependent manner (P < 0.05). The IC50 values of shikonin in I-10 cells at 24, 48, and 72 h were 1.8, 1.36 and 1.16 μmol/L, as compared with 2.37, 0.8 and 0.41 μmol/L in TCAM-2 cells, respectively. Shikonin treatment significantly reduced mitochondrial membrane potential, increased ROS levels and lower the level of lactic acid in both I-10 and TCAM-2 cells (P < 0.05). Transmission electron microscopy and annexin V-FITC/PI double staining demonstrated that shikonin induced apoptosis and excessive autophagy in I-10 and TCAM-2 cells (P < 0.05). In both I-10 and TCAM cells, shikonin treatment significantly down- regulated the expressions of Bax, Bcl-2, cleaved caspase-3, PKM2, GLUT1 and HK2, and up-regulated the expression of autophagy-related protein LC3B (P < 0.05).
Conclusion
Shikonin can inhibit the proliferation, induce apoptosis and increase autophagy in both I-10 and TCAM-2 cells probably by affecting energy metabolism of the cells.
Keywords: testicular cancer, seminoma, shikonin, glycolysis, cells death pattern
睾丸癌是最常见的恶性肿瘤之一,在20~35岁的成年男性中[1-2],睾丸癌的存活率很高[3]。顺铂(DDP)被认为是睾丸癌重要而有效的化学治疗剂治疗[4-5],不幸的是,耐药性随着治疗的继续[6-12],严重限制了DDP的临床使用。因此,寻找可以抑制睾丸癌新药物至关重要。
紫草素是一种天然染料和食品添加剂,具有抗病毒[13],抗氧化[14],抗炎[15],加速伤口愈合和增强免疫力的特性[16]。研究表明,紫草素可以有效抑制PKM2,但对PKM1或丙酮酸激酶L(PKL)没有抑制作用,可限制糖酵解和ATP的产生[16]。紫草素已显示出诱导多种癌细胞凋亡的作用,包括HL60人早幼粒细胞白血病细胞系,肝癌,前列腺癌,结直肠癌,口腔鳞状细胞癌,基底细胞和骨肉瘤,宫颈癌HeLa,膀胱癌细胞系T24[18-27]。目前尚未有紫草素对人睾丸癌I-10和精原细胞瘤TCAM-2细胞的实验研究,本实验主要探讨紫草素对I-10和TCAM-2细胞的死亡形式,并从能量代谢(线粒体和糖酵解)方面探讨其可能机制,为临床治疗下睾丸癌提供新的策略。
1. 材料和方法
1.1. 实验材料、试剂
人睾丸癌I-10和精原细胞瘤TCAM-2细胞购自上海细胞库,DMEM培养基、胰蛋白酶(Hyclone),胎牛血清(fetal bovine serum, FBS)(浙江天杭生物科技),MTT试剂、JC-1、ROS、乳酸、Annexin V-FITC/PI双染试剂盒(上海碧云天),Bax、Bcl-2、cleaved caspase3、LC3B、PKM2、GLUT1、HK2蛋白抗体(CST)。
1.2. 细胞培养
人睾丸癌I-10精原细胞瘤TCAM-2细胞培养在含10%胎牛血清、100 mg/L链霉素、100 U/mL青霉素的DMEM培养基中,放置于37 ℃、5% CO2饱和湿度的细胞恒温培养箱中日常培养。
1.3. JC-1染色检测细胞线粒体膜电位差
将I-10和TCAM-2细胞制成分别单细胞悬液,每孔4×105个细胞接种于六孔板中,I-10细胞组加入浓度分别为0、1.2、1.4、1.6 µmol/L的紫草素,TCAM-2细胞组加入浓度分别为0、0.5、1、1.5 µmol/L的紫草素,给药12 h时,收集细胞,每孔加入1 mL JC-1染色溶液,充分混合,然后在细胞培养箱中于37 ℃孵育20 min(避光)。1200 r/min离心5 min,弃去上清液,并用JC-1染色缓冲液(1x)洗涤2次。最后用流式细胞仪上机检测,并将实验重复3次。
1.4. 细胞内ATP水平检测
将I-10和TCAM-2细胞分别制成单细胞悬液,每孔4×105个细胞接种于六孔板中,I-10细胞组加入浓度分别为0、1.2、1.4、1.6 µmol/L的紫草素,TCAM-2细胞组加入浓度分别为0、0.5、1、1.5 µmol/L的紫草素,作用6 h,离心收集细胞,弃去上清液。每个孔加入200 μL裂解液裂解20 min,剧烈涡旋以完全裂解细胞。使用4 ℃离心机以12 000 r/min将混合物离心5 min,并将上清液(保留在冰上)用于后续分析。裂解液中的蛋白质浓度通过BCA蛋白测定试剂盒进行定量。每个样中加入100 μL ATP工作液(1:9),并在室温下放置5 min(使反应在黑暗中进行),使背景ATP全部消耗,立即向每孔添加20 μL样品,用酶标仪测量吸光度,并将实验重复3次。
1.5. 活性氧(ROS)检测
将I-10和TCAM-2细胞分别制成单细胞悬液,每孔4×105细胞接种于六孔板中,I-10细胞组加入浓度分别为0、1.2、1.4、1.6 µmol/L的紫草素,TCAM-2细胞组加入浓度分别为0、0.5、1、1.5 µmol/L的紫草素,给药6 h,收集细胞,首先用无血清培养基将DCFH-DA以1:1000比例稀释至最终浓度10 μmol/ L。每个样加入1 mL稀释的DCFH-DA,并在37 ℃的细胞培养箱中培养20 min,用PBS洗涤2次后,用流式细胞仪上机检测,并将实验重复3次。
1.6. 乳酸的测定
将I-10和TCAM-2细胞分别制成单细胞悬液,每孔4×105细胞接种于六孔板中,I-10细胞组加入浓度分别为0、1.2、1.4、1.6 µmol/L的紫草素,TCAM-2细胞组加入浓度分别为0、0.5、1、1.5 µmol/L的紫草素,给药24 h时,然后收集细胞,重悬于适量的裂解物中,并转移至1.5 mL EP管中。将细胞在-20 ℃下裂解3次,并在4 ℃下以12 000 r/min离心30 min,上清液用于结果分析。参照乳酸测定试剂盒步骤(BioVision)进行测试,并将实验重复3次。
1.7. MTT测定
将I-10和TCAM-2细胞分别制成单细胞悬液,每孔1×104细胞接种于六孔板中,I-10细胞组加入浓度分别为0、1.2、1.4、1.6 µmol/L的紫草素,TCAM-2细胞组加入浓度分别为0、0.5、1、1.5 µmol/L的紫草素,于24、48、72 h时每孔加入15 µL MTT溶液,放入培养箱中孵育4 h,小心吸去培养液,每孔加入150 µL DMSO,置于37 ℃培养箱继续孵育30 min待甲臜完全溶解,在490 nm波长处用酶联免疫检测仪测吸光度值(A490 nm)。计算细胞存活率:细胞存活率(%)=实验组A490 nm/对照组A490 nm×100%。取平均值绘制药物剂量效应曲线,计算药物的半数抑制浓度(IC50)。
1.8. 透射电子显微镜观察细胞死亡的方式
将I-10和TCAM-2细胞分别制成单细胞悬液,接种于大皿中,待细胞长到70%左右时,I-10组加入浓度分别为0 µmol/L、1.4 µmol/L的紫草素,TCAM-2组加入浓度分别为0 µmol/L、1 µmol/L的紫草素,给药24 h,通过离心收集细胞,加入戊二醛固定细胞,低温保存,送至Saville公司进行后续处理,并通过透射电子显微镜观察,获取对照组和紫草素组的亚显微结构图像。
1.9. Western blot法检测蛋白表达水平
将I-10和TCAM-2细胞分别制成单细胞悬液,每孔4×105细胞接种于六孔板中,I-10细胞组加入浓度分别为0、1.2 L、1.4 L、1.6 µmol/L的紫草素,TCAM-2细胞组加入浓度分别为0、0.5、1、1.5 µmol/L的紫草素,给药48 h时收集细胞,冰上裂解30 mim,12000 r/min离心30 min,吸取上清液,每组样取60 μg蛋白进行SDS-PAGE电泳;蛋白转至聚偏氟乙烯(PVDF)膜;快速封闭液封闭15 min;4 ℃孵育一抗过夜;二抗室温下孵育2 h;ECL试剂盒暗室发光显影,凝胶成像系统获取图像。
1.10. Annexin V-FITC/PI双染法检测细胞凋亡
将I-10和TCAM-2细胞分别制成单细胞悬液,每孔4×105细胞接种于六孔板中,I-10细胞组加入浓度分别为0、1.2、1.4、1.6 µmol/L的紫草素,TCAM-2细胞组加入浓度分别为0、0.5、1、1.5 µmol/L的紫草素,给药24 h时收集细胞,每孔加入500 μL缓冲液重悬细胞,加入10 μL Annexin-V-FITC混匀,冰上15 min,之后每孔加15 μL PI染色剂,1 h内检测细胞凋亡情况。
1.11. 统计分析方法
实验数据采用SPSS21.0统计软件进行统计分析,实验数据均以均数±标准差表示,多组比较采用单因素方差分析及两因素方差分析,组间两两比较采用LSDt检验,P < 0.05认为差异具有统计学意义。
2. 结果
2.1. 紫草素对I-10和TCAM-2细胞线粒体功能的影响
I-10和TCAM-2细胞能够通过糖酵解和线粒体氧化磷酸化提供能量,首先通过评估活性氧、线粒体膜电位和胞内ATP含量,研究了紫草素对I-10和TCAM-2细胞线粒体功能的影响(图 1A、B),ROS测定的结果表明,随着紫草素浓度的增加,I-10和TCAM-2细胞活性氧水平逐渐增加。紫草素(持续24 h)显著降低了I-10和TCAM-2细胞的线粒体膜电位,也表明细胞处于凋亡的早期阶段(图 1E、F)。将紫草素作用于I-10和TCAM-2细胞6 h测量细胞内ATP的变化,与对照组相比,I-10细胞组1.2、1.4、1.6 μmol/ L的紫草素可以诱导I-10细胞中的ATP水平分别从基线的100%降至(80.1 ± 2.1)%,(60.2 ± 1.9)%和(48.7±1.34)%(图 1C)。TCAM-2细胞组0.5、1、1.5 μmol/ L的紫草素可以诱导TCAM-2细胞中的ATP水平分别从基线的100%降至(76.9± 2.6)%,(60±1.7)%和(48±1.3)%(图 1D)。
1.
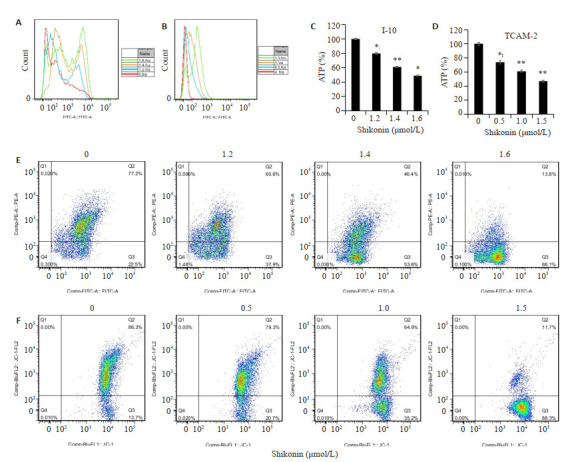
紫草素对I-10和TCAM-2细胞线粒体功能的影响
Effect of shikonin on mitochondrial function in I-10 and TCAM-2 cells. A, B: Mitochondrial function of shikonin-treated I-10 cells and TCAM-2 cells, respectively; C, D: Changes of ATP level in I-10 and TCAM-2 cells after 6 h of shikonin treatment, respectively; E, F: Effect of shikonin on the mitochondrial membrane potential of I-10 and TCAM-2 cells, respectively. n=3; *P < 0.05, **P < 0.01 vs control group.
2.2. 紫草素对I-10和TCAM-2细胞糖酵解的影响
评估紫草素对I-10和TCAM-2细胞糖酵解的影响,如图 2C、G所示,糖原蛋白PKM2,GLUT1,HK2的表达随着紫草素浓度的增加而降低,糖酵解的最终产物乳酸受到抑制(图 2A、B)。
2.
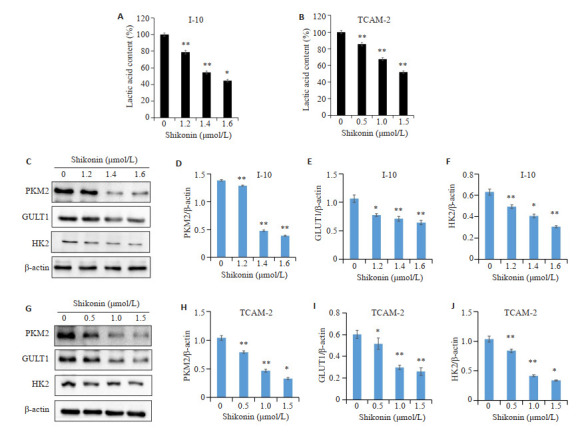
紫草素对I-10和TCAM-2细胞糖酵解的影响
Effect of shikonin on glycolysis of I-10 and TCAM-2 cells. A: Effect of shikonin on lactic acid in I-10 cell line; B: Effect of shikonin on lactic acid in TCAM-2 cell; C-F: Effect of shikonin on the expression of glycolysis-related proteins PKM2, GLUT1, HK2 in I-10 cell; G-J: The effect of shikonin on the expression of glycolysis-related proteins PKM2, GLUT1 and HK2 in TCAM-2 cells (n=3, *P < 0.05, **P < 0.01 vs control group).
2.3. 紫草素对I-10和TCAM-2细胞增殖和凋亡的抑制作用
MTT分析的结果表明,随着紫草素浓度的增加和作用时间的延长,紫草素抑制I-10和TCAM-2细胞的增殖效果增强(图 2A、B)。I-10组24、48、72 h的IC50值分别为1.8、1.36和1.16 μmol/ L,TCAM-2组24、48、72 h的IC50值分别为2.37、0.8和0.41 μmol/ L,与对照组相比,差异具有统计学意义(*P < 0.05,**P < 0.01)。通过透射电子显微镜观察到I-10和TCAM-2对照组的细胞体正常,核仁清晰、核膜边缘完整,染色质均匀地分布在细胞核中(图 3C-a和3D-a箭头所指)。紫草素组中,核中的染色质横向移动,浓缩并聚集在核膜边缘,没有核仁,即显示出经典的凋亡特征(图 3C-b和3D-b箭头所指)。对照组线粒体呈现出椭圆形或棒状,隔膜排列整齐(图 3C-c和3D-c箭头所指)。紫草素组线粒体数量的减少,肿胀,缩短和空泡化(图 3C-d和3D-d箭头所指)。Annexin V-FITC / PI双染法验证的透射电镜凋亡结果(图 3E、F),I-10对照组的总凋亡率为(8.26 ± 0.39)%,1.2、1.4、1.6 μmol/L紫草素组的总凋亡率分别为(28.26±1.8)%,(36.6±1.5)%,(65.6±1.3)%,TCAM-2对照组的总凋亡率为(6.42±0.8)%,0.5、1、1.5 μmol/L紫草素组的总凋亡率分别为(12.16 ± 2.0)%,(20.14 ± 1.6)%,(65±1.2)%差异具有统计学意义(P < 0.05)。为了进一步研究紫草素诱导的I-10和TCAM-2细胞凋亡的可能机制,我们通过蛋白质印迹评估了线粒体凋亡相关蛋白的表达,如图 3G、K所示,随着紫草素浓度的增加,线粒体凋亡相关蛋白cleaved caspase 3(caspase-3的裂解产物)和促凋亡蛋白Bax的蛋白水平增加,而抑凋亡蛋白Bcl-2减少。。
3.
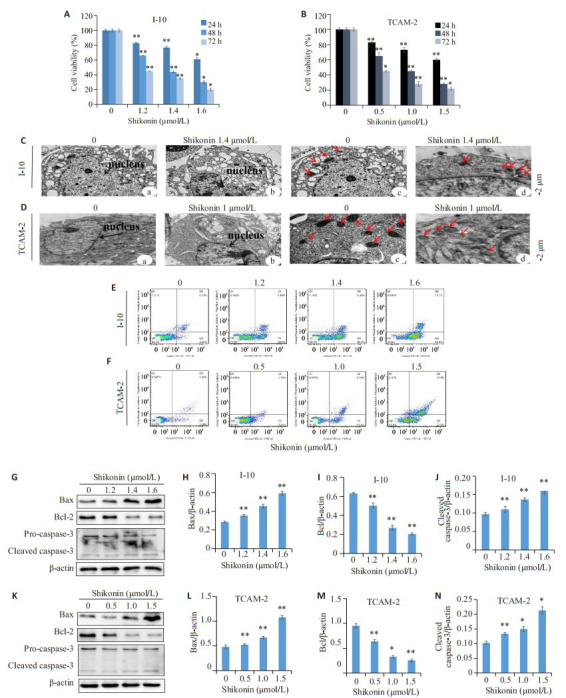
紫草素对I-10和TCAM-2细胞增殖和凋亡的抑制作用
Shikonin inhibits proliferation and apoptosis of I-10 and TCAM-2 cells. A: The effect of shikonin on the proliferation inhibition of I-10 cells; B: The effect of shikonin on the proliferation inhibition of TCAM-2 cells; C: Transmission electron microscopy was used to detect the effect of shikonin on apoptosis of I- 10 cells (The black arrow indicates the nucleus; the red arrow indicates the mitochondria); D: Transmission electron microscopey was used to detect the effect of shikonin on apoptosis of TCAM-2 cells(The black arrow indicates the nucleus; the red arrow indicates the mitochondria); E: Annexin V-FITC/PI double staining method was used to detect the effect of shikonin on apoptosis of I-10 cells; F: Annexin V-FITC/PI double staining was used to detect the effect of shikonin on apoptosis of I-10 cells; G-J: Western blot evaluation of the expression of mitochondrial apoptosis- related proteins Bax, Bcl- 2, cleaved caspase- 3 in I- 10 cells; K-N: Western blot evaluation of the expression of Bax, Bcl-2 and cleaved caspase-3 in TCAM-2 cells (n=3, *P < 0.05, **P < 0.01 vs control group.)
2.4. 紫草素诱导I-10和TCAM-2细胞增加自噬
透射电子显微镜显示,与对照组相比(图 4A-a、C-a箭头所指),紫草素组的I-10和TCAM-2细胞胞质中存在大量自噬体(图 4A-b、C-b箭头所指),并阻塞了线粒体和内质网。其特征在于细胞形成包含细胞器和胞质成分的双层或多层膜,大部分被液泡占据。为了确认自噬,进行了蛋白质印迹(图 4B、D),随着紫草素浓度的增加,自噬标志蛋白LC3B的表达增加。
4.
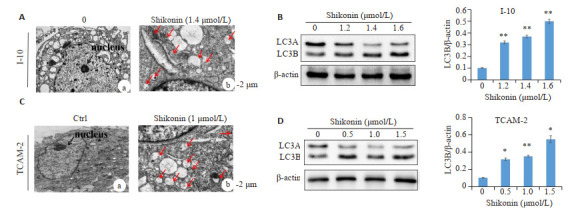
紫草素诱导I-10和TCAM-2细胞增加自噬
Shikonin enhances autophagy in I-10 and TCAM-2 cells. A, C: Transmission electron microscopy showing enhanced autophagy in shikonin-treated I-10 and TCAM-2 cells, respectively (The black arrow indicates the nucleus; the red arrow indicates the autophagosome); B, D: Effect of shikonin on the expression of LC3B in I-10 and TCAM-2 cells, respectively. n=3, *P < 0.05, **P < 0.01 vs control group.
3. 讨论
在癌变和肿瘤发展过程中,癌细胞将能量代谢重新规划成糖酵解表型,这是癌症的一个新的特征。肿瘤发生的最初原因是线粒体呼吸功能障碍,为了维持细胞生存和满足大分子合成的需要,细胞选择激活另一种能量代谢方式-有氧糖酵解[28]。已有文献表明睾丸癌细胞的糖酵解活性增加[29],在本课题的研究中,我们从紫草素抑制I-10和TCAM-2细胞糖酵解和线粒体功能进而诱导其凋亡方面入手,并探寻其凋亡的可能机制,实验结果表明紫草素可以抑制I-10和TCAM-2细胞中胞内乳酸和糖酵解相关酶(PKM2,GLUT1和HK2)的表达。同时,紫草素会导致I-10和TCAM-2细胞内ATP和线粒体膜电位下降,而ROS的水平增加。这些早期的变化随后是线粒体结构破坏,其中包括释放小分子,例如细胞色素c,细胞凋亡诱导因子以及caspase-3和caspase-9反应[30]。线粒体是细胞ROS的主要来源[31],ROS的异常积累可能引起氧化应激并导致DNA修饰和破坏[32]。与此同时,紫草素组的I-10和TCAM-2细胞透射电子显微镜观察更明显地显示了线粒体的肿胀,缩短和空泡化。这些数据表明,紫草素可对I-10和TCAM-2细胞的线粒体造成重大损害。以上结果表明,紫草素可通过影响I-10和TCAM-2细胞的线粒体功能和抑制其糖酵解来抑制I-10和TCAM-2细胞的能量代谢。
MTT分析的结果表明,随着紫草素浓度的增加和作用时间的延长,紫草素抑制I-10和TCAM-2细胞的增殖效果增强,为了检测紫草素抑制I-10和TCAM-2细胞糖酵解后是否会细胞凋亡,通过透射电镜观察到紫草素处理后I-10和TCAM-2细胞亚显微结构显示出细胞凋亡,这也被Annexin V-FITC双染法检测细胞凋亡所证实,与对照组相比,紫草素组的I-10和TCAM-2细胞凋亡明显增加。凋亡的机制似乎是通过下调线粒体通路相关凋亡蛋白Bax、Bcl-2、cleaved caspase 3的相对表达。自噬是一种进化上保守的机制适应微环境条件,例如代谢抑制,它确保了在缺氧和能量饥饿的情况下癌细胞的存活和增殖[33]。糖酵解抑制会产生能量剥夺并进一步触发自噬,最终导致自噬细胞死亡[34-36],这有可能是抑制了肿瘤细胞中Akt/mTOR信号通路启动了自噬细胞的死亡[37]。通过透射电子显微镜我们发现紫草素组的I-10和TCAM-2细胞出现大量自噬溶酶体,自噬标记物LC3B蛋白的表达高于对照组,提示紫草素可促进I-10和TCAM-2细胞自噬的增加。以上结果表明,紫草素对睾丸癌细胞I-10和TCAM-2具有增殖抑制及诱导凋亡和增加自噬作用,其机制可能为紫草素影响I-10和TCAM-2细胞糖酵解和线粒体功能。
Biography
姚越,硕士,E-mail: 568243114@qq.com
Funding Statement
国家自然科学基金(81903142);安徽省自然科学基金(1808085MH293);安徽省高校自然科学研究重点项目(KJ2019A0293, KJ2019A0400);蚌埠医学院科技发展基金项目(BYKF1763)
Supported by National Natural Science Foundation of China (81903142)
Contributor Information
姚 越 (Yue YAO), Email: 568243114@qq.com.
汪 盛 (Sheng WANG), Email: bydoctorw@163.com.
References
- 1.Kamel MH, Elfaramawi M, Jadhav S, et al. Insurance status and differences in treatment and survival of testicular cancer patients. Urology. 2016;87:140–5. doi: 10.1016/j.urology.2015.06.059. [Kamel MH, Elfaramawi M, Jadhav S, et al. Insurance status and differences in treatment and survival of testicular cancer patients[J]. Urology, 2016, 87: 140-5.] [DOI] [PubMed] [Google Scholar]
- 2.Kurobe M, Kawai K, Suetomi T, et al. High prevalence of hypogonadism determined by serum free testosterone level in Japanese testicular cancer survivors. Int J Urol. 2018;25:457–62. doi: 10.1111/iju.13537. [Kurobe M, Kawai K, Suetomi T, et al. High prevalence of hypogonadism determined by serum free testosterone level in Japanese testicular cancer survivors[J]. Int J Urol, 2018, 25: 457-62.] [DOI] [PubMed] [Google Scholar]
- 3.Leveridge MJ, Siemens DR, Brennan K, et al. Temporal trends in management and outcomes of testicular cancer: A population-based study. Cancer. 2018;124:2724–32. doi: 10.1002/cncr.31390. [Leveridge MJ, Siemens DR, Brennan K, et al. Temporal trends in management and outcomes of testicular cancer: A population-based study[J]. Cancer, 2018, 124: 2724-32.] [DOI] [PubMed] [Google Scholar]
- 4.Apps MG, Choi EH, Wheate NJ. The state-of-play and future of platinum drugs. Endocr Relat Cancer. 2015;22(4):R219–33. doi: 10.1530/ERC-15-0237. [Apps MG, Choi EH, Wheate NJ. The state-of-play and future of platinum drugs[J]. Endocr Relat Cancer, 2015, 22(4): R219-33.] [DOI] [PubMed] [Google Scholar]
- 5.Matsuzaki S, Serada S, Morimoto A, et al. Annexin A4 is a promising therapeutic target for the treatment of platinum-resistant cancers. Expert Opin Ther Targets. 2014;18(4):403–14. doi: 10.1517/14728222.2014.882323. [Matsuzaki S, Serada S, Morimoto A, et al. Annexin A4 is a promising therapeutic target for the treatment of platinum-resistant cancers[J]. Expert Opin Ther Targets, 2014, 18(4): 403-14.] [DOI] [PubMed] [Google Scholar]
- 6.Ghosh S. Cisplatin: The first metal based anticancer drug. Bioorg Chem. 2019;88:102925. doi: 10.1016/j.bioorg.2019.102925. [Ghosh S. Cisplatin: The first metal based anticancer drug[J]. Bioorg Chem, 2019, 88: 102925.] [DOI] [PubMed] [Google Scholar]
- 7.Wu D, Li B, Liu H, et al. In vitro inhibited effect of gap junction composed of Cx43 in the invasion and metastasis of testicular cancer resistanced to cisplatin. Biomed Pharmacother. 2018;98:826–33. doi: 10.1016/j.biopha.2018.01.016. [Wu D, Li B, Liu H, et al. In vitro inhibited effect of gap junction composed of Cx43 in the invasion and metastasis of testicular cancer resistanced to cisplatin[J]. Biomed Pharmacother, 2018, 98: 826-33.] [DOI] [PubMed] [Google Scholar]
- 8.Oechsle K, Honecker F, Cheng T, et al. Preclinical and clinical activity of sunitinib in patients with cisplatin-refractory or multiply relapsed germ cell tumors: a canadian urologic oncology group/ german testicular cancer study group cooperative study. Ann Oncol. 2011;22:2654–60. doi: 10.1093/annonc/mdr026. [Oechsle K, Honecker F, Cheng T, et al. Preclinical and clinical activity of sunitinib in patients with cisplatin-refractory or multiply relapsed germ cell tumors: a canadian urologic oncology group/ german testicular cancer study group cooperative study[J]. Ann Oncol, 2011, 22: 2654-60.] [DOI] [PubMed] [Google Scholar]
- 9.Koster R, van Vugt MA, Timmer-Bosscha H, et al. Unravelling mechanisms of cisplatin sensitivity and resistance in testicular cancer. Expert Rev Mol Med. 2013;15:e12. doi: 10.1017/erm.2013.13. [Koster R, van Vugt MA, Timmer-Bosscha H, et al. Unravelling mechanisms of cisplatin sensitivity and resistance in testicular cancer[J]. Expert Rev Mol Med, 2013, 15: e12.] [DOI] [PubMed] [Google Scholar]
- 10.Cheng L, Albers P, Berney DM, et al. Testicular cancer. Nat Rev Dis Primers. 2018;4:29. doi: 10.1038/s41572-018-0029-0. [Cheng L, Albers P, Berney DM, et al. Testicular cancer[J]. Nat Rev Dis Primers, 2018, 4: 29.] [DOI] [PubMed] [Google Scholar]
- 11.Mao P, Hever MP, Niemaszyk L, et al. Serine/threonine kinase 17A is a novel p53 target gene and modulator of cisplatin toxicity and reactive oxygen species in testicular cancer cells. J Biol Chem. 2011;286:19381–91. doi: 10.1074/jbc.M111.218040. [Mao P, Hever MP, Niemaszyk L, et al. Serine/threonine kinase 17A is a novel p53 target gene and modulator of cisplatin toxicity and reactive oxygen species in testicular cancer cells[J]. J Biol Chem, 2011, 286: 19381-91.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen JF, Andersen JL, Adamsen L, et al. Progressive resistance training and cancer testis (PROTRACT)-efficacy of resistance training on muscle function, morphology and inflammatory profile in testicular cancer patients undergoing chemotherapy: design of a randomized controlled trial. BMC Cancer. 2011;11:326. doi: 10.1186/1471-2407-11-326. [Christensen JF, Andersen JL, Adamsen L, et al. Progressive resistance training and cancer testis (PROTRACT)-efficacy of resistance training on muscle function, morphology and inflammatory profile in testicular cancer patients undergoing chemotherapy: design of a randomized controlled trial[J]. BMC Cancer, 2011, 11: 326.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao H, Liu L, Qu Z, et al. Anti-adenovirus activities of shikonin, a component of Chinese herbal medicine in vitro. Biol Pharm Bull. 2011;34:197–202. doi: 10.1248/bpb.34.197. [Gao H, Liu L, Qu Z, et al. Anti-adenovirus activities of shikonin, a component of Chinese herbal medicine in vitro[J]. Biol Pharm Bull, 2011, 34: 197-202.] [DOI] [PubMed] [Google Scholar]
- 14.Tong Y, Bai L, Gong R, et al. Shikonin protects PC12 cells against β- amyloid peptide-induced cell injury through antioxidant and antiapoptotic activities. Sci Rep. 2018;8:26. doi: 10.1038/s41598-017-18058-7. [Tong Y, Bai L, Gong R, et al. Shikonin protects PC12 cells against β- amyloid peptide-induced cell injury through antioxidant and antiapoptotic activities[J]. Sci Rep, 2018, 8: 26.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao P, Lin C, Li C, et al. Anti-inflammatory properties of shikonin contribute to improved early-stage diabetic retinopathy. Sci Rep. 2017;7:44985. doi: 10.1038/srep44985. [Liao P, Lin C, Li C, et al. Anti-inflammatory properties of shikonin contribute to improved early-stage diabetic retinopathy[J]. Sci Rep, 2017, 7: 44985.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Lu HL, Gu YC, et al. Enhancement of NK cells proliferation and function by Shikonin. Immunopharmacol Immunotoxicol. 2017;39(3):124–30. doi: 10.1080/08923973.2017.1299174. [Li Y, Lu HL, Gu YC, et al. Enhancement of NK cells proliferation and function by Shikonin[J]. Immunopharmacol Immunotoxicol, 2017, 39(3): 124-30.] [DOI] [PubMed] [Google Scholar]
- 17.Lu B, Wang Z, Ding Y, et al. RIP1 and RIP3 contribute to shikonininduced glycolysis suppression in glioma cells via increase of intracellular hydrogen peroxide. Cancer Lett. 2018;425:31–42. doi: 10.1016/j.canlet.2018.03.046. [Lu B, Wang Z, Ding Y, et al. RIP1 and RIP3 contribute to shikonininduced glycolysis suppression in glioma cells via increase of intracellular hydrogen peroxide[J]. Cancer Lett, 2018, 425: 31-42.] [DOI] [PubMed] [Google Scholar]
- 18.Trivedi R, Müller G, Rathore M S, et al. Anti-leukemic activity of shikonin: Role of ERP57 in shikonin induced apoptosis in acute myeloid leukemia. Cell Physiol Biochem. 2016;39:604–16. doi: 10.1159/000445652. [Trivedi R, Müller G, Rathore M S, et al. Anti-leukemic activity of shikonin: Role of ERP57 in shikonin induced apoptosis in acute myeloid leukemia[J]. Cell Physiol Biochem, 2016, 39: 604-16.] [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto S, Xu M, Masuda Y, et al. Beta-hydroxyisovalerylshikonin inhibits the cell growth of various cancer cell lines and induces apoptosis in leukemia HL-60 cells through a mechanism different from those of Fas and etoposide. J Biochem. 1999;125(1):17–23. doi: 10.1093/oxfordjournals.jbchem.a022255. [Hashimoto S, Xu M, Masuda Y, et al. Beta-hydroxyisovalerylshikonin inhibits the cell growth of various cancer cell lines and induces apoptosis in leukemia HL-60 cells through a mechanism different from those of Fas and etoposide[J]. J Biochem, 1999, 125 (1): 17-23.] [DOI] [PubMed] [Google Scholar]
- 20.Gara R, Srivastava V, Duggal S, et al. Shikonin selectively induces apoptosis in human prostate cancer cells through the endoplasmic reticulum stress and mitochondrial apoptotic pathway. J Biomed Sci. 2015;22:26. doi: 10.1186/s12929-015-0127-1. [Gara R, Srivastava V, Duggal S, et al. Shikonin selectively induces apoptosis in human prostate cancer cells through the endoplasmic reticulum stress and mitochondrial apoptotic pathway[J]. J Biomed Sci, 2015, 22: 26.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gara RK, Srivastava VK, Duggal S, et al. Shikonin selectively induces apoptosis in human prostate cancer cells through the endoplasmic reticulum stress and mitochondrial apoptotic pathway. J Biomed Sci. 2015;22:26. doi: 10.1186/s12929-015-0127-1. [Gara RK, Srivastava VK, Duggal S, et al. Shikonin selectively induces apoptosis in human prostate cancer cells through the endoplasmic reticulum stress and mitochondrial apoptotic pathway[J]. J Biomed Sci, 2015, 22: 26.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruan M, Yan M, Yang WJ, et al. Role of NF-kappaB pathway in shikonin induced apoptosis in oral squamous cell carcinoma Tca- 8113 cells. Shanghai J Stomatol. 2010;19(1):66–71. [Ruan M, Yan M, Yang WJ, et al. Role of NF-kappaB pathway in shikonin induced apoptosis in oral squamous cell carcinoma Tca- 8113 cells[J]. Shanghai J Stomatol, 2010, 19(1): 66-71.] [PubMed] [Google Scholar]
- 23.Andújar I, Martí-Rodrigo A, Giner RM, et al. Shikonin prevents early phase inflammation associated with azoxymethane/dextran sulfate sodium-induced colon cancer and induces apoptosis in human colon cancer cells. Planta Med. 2018;84(9/10):674–83. doi: 10.1055/a-0599-1145. [Andújar I, Martí-RodrigoA, Giner RM, et al. Shikonin prevents early phase inflammation associated with azoxymethane/dextran sulfate sodium-induced colon cancer and induces apoptosis in human colon cancer cells[J]. Planta Med, 2018, 84(9/10): 674-83.] [DOI] [PubMed] [Google Scholar]
- 24.Li S, Zhang T, Xu W, et al. Sarcoma-targeting peptide-decorated polypeptide nanogel intracellularly delivers shikonin for upregulated osteosarcoma necroptosis and diminished pulmonary metastasis. Theranostics. 2018;8:1361–75. doi: 10.7150/thno.18299. [Li S, Zhang T, Xu W, et al. Sarcoma-targeting peptide-decorated polypeptide nanogel intracellularly delivers shikonin for upregulated osteosarcoma necroptosis and diminished pulmonary metastasis[J]. Theranostics, 2018, 8: 1361-75.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu D, Qian J, Li Wei, et al. β-hydroxyisovaleryl-shikonin induces human cervical cancer cell apoptosis via PI3K/AKT/mTOR signaling. Oncol Lett. 2015;10:3434–42. doi: 10.3892/ol.2015.3769. [Lu D, Qian J, Li Wei, et al. β-hydroxyisovaleryl-shikonin induces human cervical cancer cell apoptosis via PI3K/AKT/mTOR signaling[J]. Oncol Lett, 2015, 10: 3434-42.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Zhang FL, Wu XR. Inhibition of pyruvate kinase M2 markedly reduces chemoresistance of advanced bladder cancer to cisplatin. Sci Rep. 2017;7:45983. doi: 10.1038/srep45983. [Wang X, Zhang FL, Wu XR. Inhibition of pyruvate kinase M2 markedly reduces chemoresistance of advanced bladder cancer to cisplatin[J]. Sci Rep, 2017, 7: 45983.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oronsky BT, Oronsky N, Fanger GR, et al. Follow the ATP: tumor energy production: a perspective. Anticancer Agents Med Chem. 2014;14(9):1187–98. doi: 10.2174/1871520614666140804224637. [Oronsky BT, Oronsky N, Fanger GR, et al. Follow the ATP: tumor energy production: a perspective[J]. Anticancer Agents Med Chem, 2014, 14(9): 1187-98.] [DOI] [PubMed] [Google Scholar]
- 28.Zhou S, Min Z, Sun K, et al. MiR-199a-3p/Sp1/LDHA axis controls aerobic glycolysis in testicular tumor cells. Int J Mol Med. 2018;42:2163–74. doi: 10.3892/ijmm.2018.3771. [Zhou S, Min Z, Sun K, et al. MiR-199a-3p/Sp1/LDHA axis controls aerobic glycolysis in testicular tumor cells[J]. Int J Mol Med, 2018, 42: 2163-74.] [DOI] [PubMed] [Google Scholar]
- 29.Bonatelli M, Silva E, Cárcano F, et al. The warburg effect is associated with tumor aggressiveness in testicular germ cell tumors. Front Endocrinol (Lausanne) 2019;10:417. doi: 10.3389/fendo.2019.00417. [Bonatelli M, Silva E, Cárcano F, et al. The warburg effect is associated with tumor aggressiveness in testicular germ cell tumors[J]. Front Endocrinol (Lausanne), 2019, 10: 417.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duan D, Zhang B, Yao J, et al. Shikonin targets cytosolic thioredoxin reductase to induce ROS-mediated apoptosis in human promyelocytic leukemia HL- 60 cells. Free Radic Biol Med. 2014;70:182–93. doi: 10.1016/j.freeradbiomed.2014.02.016. [Duan D, Zhang B, Yao J, et al. Shikonin targets cytosolic thioredoxin reductase to induce ROS-mediated apoptosis in human promyelocytic leukemia HL- 60 cells[J]. Free Radic Biol Med, 2014, 70: 182-93.] [DOI] [PubMed] [Google Scholar]
- 31.Holzerová E, Prokisch H. Mitochondria: Much ado about nothing? How dangerous is reactive oxygen species production? Int J Biochem. Cell Biol. 2015;63:16–20. doi: 10.1016/j.biocel.2015.01.021. [Holzerová E, Prokisch H, Mitochondria: Much ado about nothing? How dangerous is reactive oxygen species production[J]? Int J Biochem. Cell Biol, 2015, 63: 16-20.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi YH. Schisandrin A prevents oxidative stress-induced DNA damage and apoptosis by attenuating ROS generation in C2C12 cells. Biomedecine Pharmacother. 2018;106:902–9. doi: 10.1016/j.biopha.2018.07.035. [Choi YH. Schisandrin A prevents oxidative stress-induced DNA damage and apoptosis by attenuating ROS generation in C2C12 cells[J]. Biomedecine Pharmacother, 2018, 106: 902-9.] [DOI] [PubMed] [Google Scholar]
- 33.Holzerová E, Prokisch H. Mitochondria: Much ado about nothing? How dangerous is reactive oxygen species production? Int J Biochem Cell Biol. 2015;63:16–20. doi: 10.1016/j.biocel.2015.01.021. [Holzerová E, Prokisch H. Mitochondria: Much ado about nothing? How dangerous is reactive oxygen species production[J]? Int J Biochem Cell Biol, 2015, 63: 16-20.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J, Nam B, Choi Y, et al. Enhanced glycolysis supports cell survival in EGFR-mutant lung adenocarcinoma by inhibiting autophagy-mediated EGFR degradation. Cancer Res. 2018;78:4482–96. doi: 10.1158/0008-5472.CAN-18-0117. [Kim J, Nam B, Choi Y, et al. Enhanced glycolysis supports cell survival in EGFR-mutant lung adenocarcinoma by inhibiting autophagy-mediated EGFR degradation[J]. Cancer Res, 2018, 78: 4482-96.] [DOI] [PubMed] [Google Scholar]
- 35.Shun C, Lin S, Hong C, et al. Sirtuin 6 modulates hypoxia-induced autophagy in nasal polyp fibroblasts via inhibition of glycolysis. Am J RhinolAllergy. 2016;30:179–85. doi: 10.2500/ajra.2016.30.4282. [Shun C, Lin S, Hong C, et al. Sirtuin 6 modulates hypoxia-induced autophagy in nasal polyp fibroblasts via inhibition of glycolysis[J]. Am J RhinolAllergy, 2016, 30: 179-85.] [DOI] [PubMed] [Google Scholar]
- 36.Shi D, Zhao D, Niu P, et al. Glycolysis inhibition via mTOR suppression is a key step in cardamonin-induced autophagy in SKOV3 cells. BMC ComplementAltern Med. 2018;18:317. doi: 10.1186/s12906-018-2380-9. [Shi D, Zhao D, Niu P, et al. Glycolysis inhibition via mTOR suppression is a key step in cardamonin-induced autophagy in SKOV3 cells[J]. BMC ComplementAltern Med, 2018, 18: 317.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dey P, Kundu A, Sachan R, et al. PKM2 knockdown induces autophagic cell death via AKT/mTOR pathway in human prostate cancer cells. Cell Physiol Biochem. 2019;52:1535–52. doi: 10.33594/000000107. [Dey P, Kundu A, Sachan R, et al. PKM2 knockdown induces autophagic cell death via AKT/mTOR pathway in human prostate cancer cells[J]. Cell Physiol Biochem, 2019, 52: 1535-52.] [DOI] [PubMed] [Google Scholar]


