Abstract
INTRODUCTION:
Low vitamin D intake and low vitamin D circulating levels have been associated with increased risk for dementia. We aimed to examine the association between vitamin D intake and dementia in a multi-ethnic cohort.
METHODS:
A longitudinal study of 1759 non-demented older (≥65 years) participants of the Washington Heights-Inwood Columbia Aging Project with follow-up visits and completed a food frequency questionnaire. Dementia was diagnosed by consensus using DSM-IV criteria. Cox hazard regression was performed.
RESULTS:
During a mean follow up of 5.8 years, 329 participants developed dementia. Participants with the highest tertile of vitamin D intake from food sources had decreased risk (HR=0.72, 95%CI=0.54–0.97, p=0.030) for dementia compared with those with the lowest tertile, adjusting for age, sex, race/ethnicity, education, APOE-ε4, physical activity, MEDI score, income, depression, hypertension, diabetes, cardiovascular disease, and smoking.
DISCUSSION:
Higher vitamin D intake is associated with decreased risk of dementia in a multi-ethnic cohort.
Keywords: Vitamin D, vitamin D intake, dementia, Alzheimer’s dementia, Alzheimer’s disease, Alzheimer’s, dementia risk, Alzheimer’s risk, vitamin D and dementia, vitamin D and Alzheimer’s, sleep, sleep disturbance, sleep dysfunction, sleep and dementia, vitamin D and sleep, mediation, lifestyle risk factors, lifestyle and dementia, modifiable risk factors dementia, primary prevention dementia, WHICAP
1. Background
Vitamin D deficiency is highly prevalent in the general population and among older adults,1,2 often unrecognized, and is potentially a modifiable risk factor for dementia. Low vitamin D may be a risk factor for cognitive impairment and subsequent dementia via multiple potential mechanisms. Vitamin D receptors are distributed throughout the brain, with evidence from immunohistochemistry showing restriction to the cell nucleus, suggesting a “neurosteroid” paracrine/autocrine function for vitamin D.3 Vitamin D exerts broadly protective effects on the central nervous system, through regulation of neurotrophic factors, inducing beta amyloid clearance, maintenance of calcium homeostasis, modulation of oxidative stress, and regulation of the immune response.4 Multiple epidemiological studies have consistently found an association between low circulating vitamin D level5–9 and increased risk of dementia. There is also some evidence linking higher vitamin D intake10,11 and decreased risk of dementia or better cognition. Many studies have included participants who were predominantly White,12 potentially limiting the generalizability of their conclusions. One recent study in a multi-ethnic cohort found that individuals with lower serum vitamin D levels had faster rates of cognitive decline.13 This study, however, did not have dietary intake data.
Our study sought to address the limitations of previous studies. The primary objective of this study was to determine if there is an association between vitamin D intake and dementia in the Washington Heights-Inwood Columbia Aging Project (WHICAP), a multiethnic, community-based cohort in Northern Manhattan.
2. Methods
2.1. Study Population
Study subjects were selected from participants of the Washington Heights-Inwood Columbia Aging Project (WHICAP) cohort, a community-based multiethnic cohort that was recruited via random sampling of Medicare beneficiaries (ages 65 and older) living within a geographically defined area of northern Manhattan, New York. The WHICAP-II cohort includes a combination of the cohort recruited in 1999 and continuing members of the cohort recruited in 1992. The study was approved by ethics committees at Columbia University and the New York State Psychiatric Institute. All participants provided written informed consent. At study entry, each participant underwent an examination that included an assessment of health and function, a physical and neurological examination, and a neuropsychological battery of tests. Follow up visits were conducted approximately every 18 months.
The baseline visit for the primary analysis was defined as the date of study entry into the WHICAP-II cohort in 1999. Out of 2800 participants from the WHICAP-II cohort, 374 participants had dementia at baseline (prevalent dementia) and were excluded. Of the 2426 participants without dementia, 2234 had dietary information at baseline. Among the 2234, 475 participants did not have follow-up visit(s) and were excluded. The remaining 1759 non-demented participants with both diet data and follow up visit(s) were included in the study. (See Figure 1.)
Figure 1: Participant Selection.
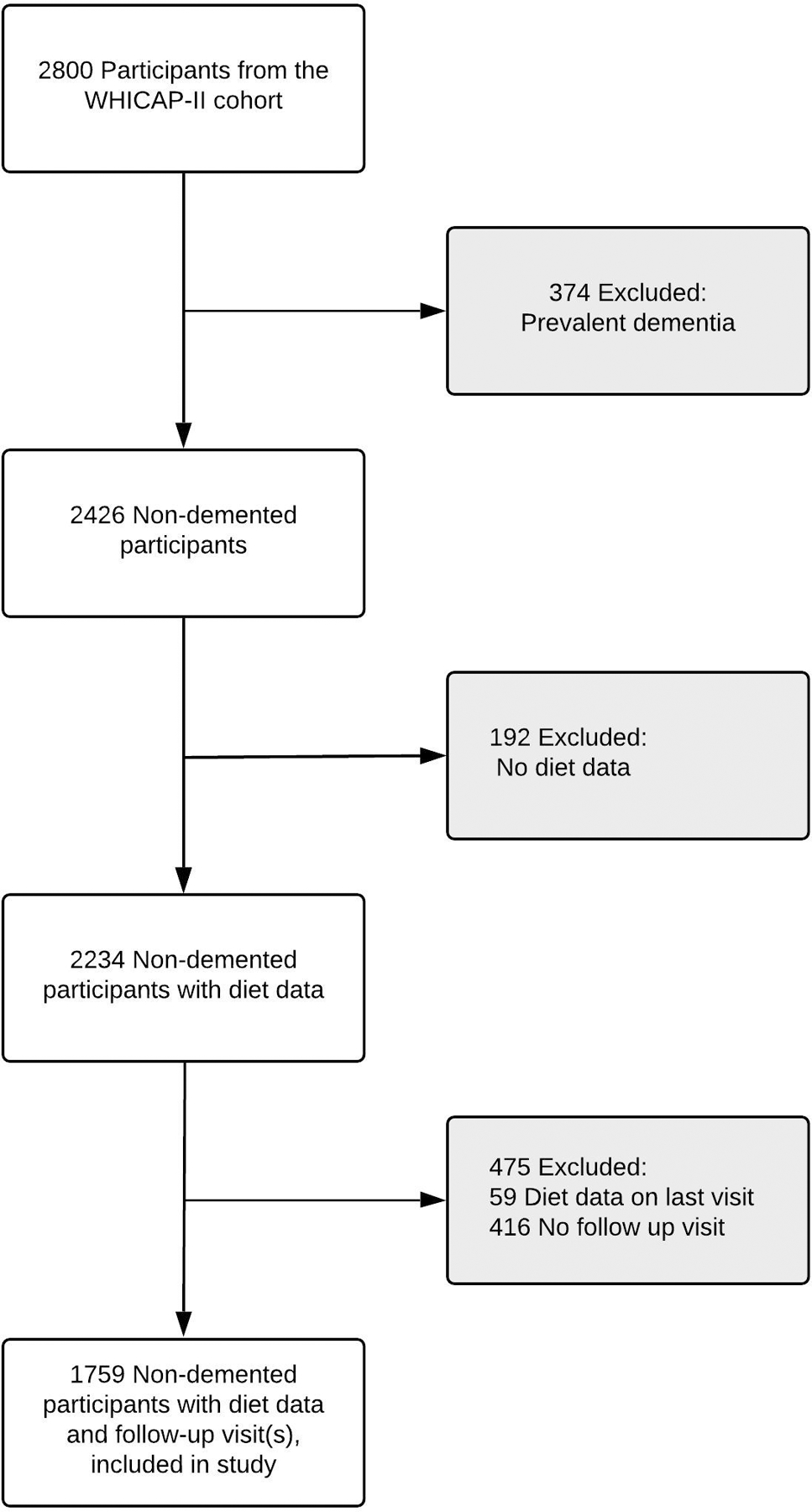
Participant Selection From participants in Washington Heights-Inwood Columbia Aging Project (WHICAP-II), we excluded 374 participants with prevalent dementia. We then excluded 192 participants without diet data. Among 2234 non-demented participants with diet data, 416 participants with no follow up visit and 59 participants with diet data at the last visit were excluded. The remaining 1759 non-demented participants with diet data and follow up visit(s) were included in the study.
2.2. Dementia Assessment
Based on information obtained at the baseline and follow-up visits, dementia diagnosis was made by consensus during diagnostic conferences that included at least one attending neurologist and at least one attending neuropsychologist. The dementia diagnosis was based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria14, which required evidence of cognitive impairment on neuropsychological testing, as well as evidence of impairment in social or occupational functioning. Diagnosis of Alzheimer’s disease (AD) was based on criteria from the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA).15 Diagnosis of mild cognitive impairment (MCI) was based on standard research criteria16 as previously described.17 Briefly, participants were considered to have MCI if they had: 1) subjective memory complaint; 2) objective impairment on neuropsychological testing in at least one of four cognitive domains (memory, executive function, language, and visuospatial function), defined based on the average scores within that domain and a 1.5-SD cutoff using corrections for age, years of education, race/ethnicity, and sex, as based on previously established norms; and 3) no diagnosis of dementia at the consensus conference. These criteria and have been previously used in analysis of data in this cohort.18
2.3. Dietary Data
Participants completed a 61-item semi-quantitative food frequency questionnaire (FFQ) by Willett et al. (Channing Laboratory, Cambridge, MA).19 The reliability and validity of the Willett FFQ in the WHICAP cohort has been reported previously.20–22 Interviewers administered the FFQ by telephone in English or Spanish at baseline. The FFQ contains 61 food items as well as additional questions regarding the frequency of use of supplements. Food items included in the FFQ covers a variety of foods found in the typical American diet, including meat, vegetable/fruit, dairy, and carbohydrate intake. For food items, frequency intervals ranged from never to six times per day (never, < 1 time / month, 1 to 3 times / month, 1 to 2 times / week, 3 to 4 times / week, 5 to 6 times / week, daily, 2 to 3 times / day, 4 to 5 times / day, 6 or more times daily.) The FFQs were sent to Channing Laboratory for calculation of daily nutrient intake, which was obtained by multiplying the frequency of consumption of each unit of food by the nutrient content of the portions.23 Intake of vitamin D was measured in international units per day, and classified as supplemental, non-supplemental, and total (supplemental plus non-supplemental). Total and non-supplemental intake of vitamin D were adjusted for total caloric intake, by calculating the residuals from linear regression models (nutrient intake regressed on total caloric intake). Residuals were standardized, then categorized into tertiles of vitamin D intake and used for all analyses.
2.4. Covariates Assessment
Demographic information including age, sex, ethnicity, and education was obtained by self-report from baseline interviews. Age (in years), sex (male sex as the referent group), race/ethnicity (White, Black, or Hispanic/Other), and education (in years) were input into basic adjusted models. Full models further adjusted for apolipoprotein E (APOE-ϵ4) status (absence or presence (either 1 or 2) of ϵ4alleles), physical activity, Mediterranean diet score (MeDI, a measure of healthy diet), income, depression (history of major depression by DSM-IV criteria), hypertension, diabetes, cardiovascular disease, and history of smoking. History of hypertension, diabetes, or cardiovascular disease were obtained by self-report or by use of disease-specific medications, as previously described.24 Physical activity was assessed via the Godin leisure time exercise questionnaire25, and a summary physical activity score (total metabolic equivalent minutes in 2 weeks) was calculated, as previously described,26 then divided into tertiles. MeDI is a proxy measure of overall diet quality, and was calculated as previously described.27
2.5. Statistical Analysis
To evaluate differences in demographic characteristics for participants with different tertiles of vitamin D intake, Chi-square tests were conducted for categorical variables (sex, race/ethnicity, APOE-ε4 status, physical activity), and ANOVA tests were conducted for continuous variables (age, education). Cox hazard regression models were conducted with tertiles of total vitamin D intake and vitamin D intake without supplements as the exposures of interest, incident all-cause dementia as the outcome, and time-to-event as the time metric. Martingale residuals were plotted and the Supremum test was conducted to assess for proportional hazards. The proportional hazards assumption was met (p > 0.1). For the primary analysis (n=1759), the time-to-event variable was the time from baseline to the first visit of dementia diagnosis for incident cases, or the time of the last follow-up for non-cases.
The basic model adjusted for age, sex, race/ethnicity, and level of education. Fully-adjusted models additionally controlled for APOE-ε4 status, physical activity, MeDI, income, depression, hypertension, diabetes, cardiovascular disease, and smoking. We assessed for interaction between vitamin D and APOE-ε4, sex, race/ethnicity, education, as well as smoking. In addition, we conducted the following sensitivity analyses: 1) restricting analysis to AD participants, 2) excluding participants with MCI on or before the first visit (prevalent MCI), and 3) excluding participants with limited follow-up (less than 2 years) SPSS version 25 and SAS version 9.4 were used for data preparation. SAS version 9.4 was used for all statistical analyses.
3. Results
During a mean follow up of 5.8 years, 329 participants developed dementia. Age, APOE-ε4 status, physical activity, MeDI score, depression, hypertension, cardiovascular disease,did not differ across total vitamin D intake tertiles. Tertiles of total vitamin D intake did differ by sex, race/ethnicity, education, income, smoking, and diabetes (See Table 1a). Those with the highest total vitamin D intake tended to be women, white, more highly educated, non-smokers, with healthier overall diet (higher MeDI score). A greater proportion of participants with diabetes was observed in the middle tertile of vitamin D intake. Analyzing vitamin D intake from food sources only, age, sex, APOE-ε4 status, physical activity, depression, hypertension, diabetes, cardiovascular disease did not differ across tertiles of vitamin D intake without supplements. Tertiles of vitamin D intake without supplements did differ by race/ethnicity, education, MeDI score, income, smoking (see Table 1b). Those with the highest vitamin D intake from food sources tended to be non-blacks who were more highly educated and had a higher income, non-smokers, with a healthier overall diet (higher MeDI score).
Table 1a.
Comparison of Demographic Characteristics by Tertiles of Total Vitamin D Intake
| Vitamin D Intake | ||||||||
|---|---|---|---|---|---|---|---|---|
| N | Tertile 1 | Tertile 2 | Tertile 3 | Total N (%) | P-value^ | P-value (T1 vs. T2) | P-value (T1 vs. T3) | P-value (T2 vs. T3) |
| Vitamin D (IU, median, IQR) | 95.4 (58.7–140.0) | 203.3 (159.4–292.8) | 570.7 (514.0–681.2) | 1759 (100) | N/A | < 0.001 | < 0.001 | < 0.001 |
| Age (y, mean, std) | 76.7 (6.5) | 77.3 (6.3) | 76.7 (6.7) | 1759 (100) | 0.12 | 0.075 | 0.998 | 0.074 |
| Female sex (%) | 378 (64.6) | 391 (66.6) | 435 (74.2) | 1204 (68.5) | < 0.001 | 0.47 | < 0.001 | 0.0042 |
| Ethnicity, n (%) | < 0.001 | 0.33 | < 0.001 | 0.0047 | ||||
| White | 146 (25.0) | 169 (28.8) | 211 (36.0) | 526 (29.9) | ||||
| Black | 190 (32.5) | 183 (31.2) | 190 (32.4) | 563 (32.0) | ||||
| Other | 249 (42.6) | 235 (40.0) | 185 (31.6) | 669 (38.1) | ||||
| Education (y, mean, std) | 9.83 (4.7) | 10.35 (4.9) | 11.38 (4.7) | 1759 (100) | < 0.001 | 0.060 | < 0.001 | < 0.001 |
| Positive APOE ε4, n (%) | 149 (26.9) | 133 (23.8) | 142 (25.7) | 424 (25.4) | 0.48 | 0.23 | 0.65 | 0.46 |
| Physical activity, n (%) | 0.26 | 0.61 | 0.07 | 0.42 | ||||
| Lowest tertile | 214 (38.6) | 202 (36.0) | 187 (33.1) | 603 (35.9) | ||||
| Middle tertile | 166 (30.0) | 181 (32.3) | 202 (35.8) | 549 (32.7) | ||||
| Highest tertile | 174 (31.4) | 178 (31.7) | 176 (31.2) | 528 (31.4) | ||||
| MeDI score (mean, std) | 3.9 (1.6) | 4.1 (1.7) | 4.1 (1.6) | 1759 (100) | 0.074 | 0.043 | 0.054 | 0.92 |
| Income (monthly), n (%) | < 0.001 | 0.04 | < 0.001 | 0.12 | ||||
| $1000 or less | 251 (45.6) | 219 (40.0) | 182 (34.0) | 652 (39.9) | ||||
| $1001 to $2000 | 153 (27.8) | 144 (26.3) | 153 (28.6) | 450 (27.6) | ||||
| $2000 or more | 147 (26.7) | 184 (33.6) | 200 (37.4) | 531 (32.5) | ||||
| Smoking, n (%) | 282 (48.1) | 235 (40.0) | 223 (38.1) | 740 (42.1) | 0.0011 | 0.01 | < 0.001 | 0.49 |
| Depression, n (%) | 192 (32.8) | 193 (32.9) | 203 (34.6) | 588 (33.4) | 0.75 | 0.97 | 0.50 | 0.52 |
| Hypertension, n (%) | 500 (85.3) | 506 (86.2) | 495 (84.5) | 1501 (85.3) | 0.70 | 0.67 | 0.68 | 0.40 |
| Diabetes, n (%) | 167 (28.5) | 180 (30.7) | 138 (23.6) | 485 (27.6) | 0.020 | 0.42 | 0.054 | 0.006 |
| Cardiovascular disease, n (%) | 280 (47.8) | 276 (47.0) | 287 (49.0) | 843 (47.9) | 0.80 | 0.79 | 0.68 | 0.50 |
| Incident Dementia, n (%) | 136 (23.2) | 105 (17.9) | 88 (15.0) | 329 (18.7) | 0.0013 | 0.024 | < 0.001 | 0.18 |
IU = international units; y= years; std = standard deviation; IQR = interquartile range; n=sample size; MeDI = Mediterranean diet score
Chi-Square tests conducted for sex, race/ethnicity, APOE-E4 status, physical activity, income, smoking, depression, hypertension, diabetes, cardiovascular disease, incident dementia; ANOVA tests were conducted for age, education, MeDi
Table 1b.
Comparison of Demographic Characteristics by Tertiles of Vitamin D Intake without Supplements
| Vitamin D Intake | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Tertile 1 | Tertile 2 | Tertile 3 | Total N (%) | P-value^ | P-value (T1 vs. T2) | P-value (T1 vs. T3) | P-value (T2 vs. T3) | |
| Vitamin D (IU, median, IQR) | 108.4 (58.7–435.7) | 174.0 (133.3–521.9) | 340.3 (212.8–605.1) | 1759 (100) | N/A | < 0.001 | < 0.001 | 0.540 | |
| Age (y, mean, std) | 76.4 (6.5) | 77.1 (6.6) | 77.2 (6.3) | 1759 (100) | 0.087 | 0.082 | 0.040 | 0.76 | |
| Female sex (%) | 404 (69.1) | 393 (67.0) | 407 (69.5) | 1204 (68.5) | 0.61 | 0.44 | 0.88 | 0.36 | |
| Ethnicity, n (%) | 0.0081 | 0.18 | 0.0014 | 0.15 | |||||
| White | 152 (26.0) | 172 (29.3) | 202 (34.5) | 526 (29.9) | |||||
| Black | 213 (36.4) | 185 (31.5) | 165 (28.2) | 563 (32.0) | |||||
| Other | 220 (37.6) | 230 (39.2) | 219 (37.4) | 669 (38.1) | |||||
| Education (y, mean, std) | 10.3 (4.6) | 10.3 (4.9) | 11.0 (4.8) | 1759 (100) | 0.013 | 0.76 | 0.007 | 0.017 | |
| Positive APOE ε4, n (%) | 150 (27.2) | 138 (24.6) | 136 (24.5) | 424 (25.4) | 0.52 | 0.34 | 0.31 | 0.96 | |
| Physical activity, n (%) | 0.20 | 0.18 | 0.48 | 0.12 | |||||
| Lowest tertile | 200 (36.0) | 215 (38.5) | 188 (33.3) | 603 (35.9) | |||||
| Middle tertile | 192 (34.5) | 164 (29.3) | 193 (34.2) | 549 (32.7) | |||||
| Highest tertile | 164 (29.5) | 180 (32.2) | 184 (32.6) | 528 (31.4) | |||||
| MeDI score (mean, std) | 4.0 (1.6) | 4.0 (1.6) | 4.2 (1.7) | 1759 (100) | 0.040 | 0.93 | 0.031 | 0.025 | |
| Income (monthly), n (%) | 0.013 | 0.059 | 0.0025 | 0.46 | |||||
| $1000 or less | 239 (44.3) | 212 (38.5) | 201 (37.1) | 652 (39.9) | |||||
| $1001 to $2000 | 154 (28.5) | 155 (28.1) | 141 (26.0) | 450 (27.6) | |||||
| $2000 or more | 147 (27.2) | 184 (33.4) | 200 (36.9) | 531 (32.5) | |||||
| Smoking, n (%) | 274 (46.8) | 248 (42.3) | 218 (37.2) | 740 (42.1) | 0.0041 | 0.12 | < 0.001 | 0.077 | |
| Depression, n (%) | 205 (35.0) | 187 (31.9) | 196 (33.5) | 588 (33.4) | 0.53 | 0.26 | 0.58 | 0.56 | |
| Hypertension, n (%) | 511 (87.2) | 489 (83.3) | 501 (85.5) | 1501 (85.3) | 0.17 | 0.060 | 0.40 | 0.30 | |
| Diabetes, n (%) | 147 (25.1) | 168 (28.6) | 170 (29.0) | 485 (27.6) | 0.25 | 0.17 | 0.13 | 0.880 | |
| Cardiovascular disease, n (%) | 284 (48.5) | 286 (48.7) | 273 (46.6) | 843 (47.9) | 0.73 | 0.93 | 0.52 | 0.46 | |
| Incident Dementia, n (%) | 132 (22.5) | 96 (16.4) | 101 (17.2) | 329 (18.7) | 0.0140 | 0.0076 | 0.0230 | 0.69 | |
IU = international units; y= years; std = standard deviation; IQR = interquartile range; n=sample size; MeDi = Mediterranean diet score
Chi-Square tests conducted for sex, race/ethnicity, APOE-E4 status, physical activity, income, smoking, depression, hypertension, diabetes, cardiovascular disease, incident dementia; ANOVA tests were conducted for age, education, MeDi
Participants in the highest tertile had a median total vitamin D intake of 571 IU (IQR 514 to 681 IU), and a median vitamin D intake from food sources of 340 IU (IQR 213 to 605 IU). Participants in the middle tertile had a median total vitamin D intake of 203 IU (IQR 159 to 293 IU) and a median vitamin D intake from food sources of 174 IU (IQR 133 to 522 IU). Participants in the lowest tertile had a median total vitamin D intake of 95 IU (IQR 59 to 140 IU), and a median vitamin D intake from food sources of 108 IU (IQR 59 to 436 IU).
Participants in the lowest and middle tertiles of total vitamin D intake did not meet the estimated average requirement of vitamin D intake (400 IU for individuals over 1 years old28). Participants in the highest tertile of total vitamin D intake met the estimated average requirement and were close to meeting the recommended dietary allowance (600 IU for ages 1 to 70, 800 IU for older adults over 7028). Median intake of vitamin D from food only appeared to be insufficient to meet the recommended dietary allowance.
Participants in the highest tertile of total vitamin D intake had decreased risk of dementia compared to participants in the lowest tertile in the unadjusted (HR 0.62, 95% CI 0.48–0.81, p=0.0005) and basic (HR 0.74, 95% CI 0.55–0.98, p=0.037) models. There was a significant p-trend in the unadjusted and basic models (p-trend < 0.05). Participants in the highest tertile of vitamin D intake from food sources only (without supplements) had decreased risk of dementia compared to participants in the lowest tertile in the unadjusted (HR 0.76, 95% CI 0.59–0.99, p=0.042), basic (HR 0.69, 95% CI 0.52–0.91, p=0.0087), and full (HR 0.72, 95% CI 0.54–0.97, p=0.030) models There was significant p-trend in all three models (p-trend < 0.05) (See Table 2b.)
Table 2b.
Hazard Ratios and 95% Confidence Intervals for All-Cause Dementia for Subjects in Each Tertile of Vitamin D Intake without Supplements, using the Lowest Tertile as a Reference
| Vitamin D Intake | Unadjusted | Basic Model* | Full Model^ |
|---|---|---|---|
| Tertile 1 | |||
| Tertile 2 | 0.71 (0.0.54–0.92, p=0.0097) | 0.65 (0.49–0.85, p=0.0018) | 0.66 (0.49–0.88, p=0.0049) |
| Tertile 3 | 0.76 (0.59–0.99, p=0.042) | 0.69 (0.52–0.91, p=0.0087) | 0.72 (0.54–0.97, p=0.030) |
| p-trend | 0.035 | 0.0065 | 0.018 |
Adjusted for age, sex, ethnicity, education
Additionally adjusted for APOE-E4 allele status, physical activity, MeDI score, income, depression, hypertension, diabetes, cardiovascular disease, smoking
Note: n=1591 subjects were included in the basic model, n=1477 subjects included in the full model
No significant interaction was found between total vitamin D intake and APOE-ε4, sex, race/ethnicity, education, or smoking. A significant interaction was found between vitamin D intake without supplements and APOE-ε4 (p=0.044), and the interaction between vitamin D and smoking bordered on significance (p=0.092). Stratifying by APOE-ε4, we found a significant association between vitamin D intake and dementia among non- ε4 carriers in the unadjusted (HR 0.73, 95% CI 0.53–0.99, p=0.042) model, and associations bordering on significance in the basic (HR 0.74, 95% CI 0.53–1.029, p=0.073) and full (HR 0.72, 95% CI 0.52–1.017, p=0.063) models. No association between vitamin D intake without supplements and dementia were found among APOE-ε4 carriers. No significant interaction was found between vitamin D intake without supplements and sex, race, education (all p > 0.1).
Out of 329 demented participants, 285 participants had AD. Sensitivity analyses restricted to AD as the outcome showed similar results for vitamin D intake from food sources Participants in the highest tertile of vitamin D intake without supplements had approximately 30% decreased risk of AD in the full model (HR 0.70, 95% CI 0.51–0.96, p=0.027), compared to participants in the lowest tertile of intake. For total vitamin D intake, the association between vitamin D and AD was not significant in the fully adjusted model (HR 0.81, 95% CI 0.59–1.12, p=0.20).
Excluding 384 participants with MCI at baseline and limiting the analyses to 1375 cognitively normal subjects, we found those in the highest tertile of total vitamin D intake had decreased risk of dementia compared to those in the lowest tertile in the unadjusted (HR 0.54, 95% CI 0.39–0.75, p=0.0003, p-trend=0.0002), basic (HR 0.68, 95% CI 0.49–0.95, p=0.025, p-trend=0.020, n=1371), and full (HR 0.68, 95% CI 0.47–0.99, p=0.044, p-trend=0.036, n=1159 )models. For food-source only vitamin D intake, participants in the highest tertile also had decreased risk of dementia compared to those in the lowest tertile in the unadjusted (HR=0.67, 95% CI 0.49–0.93, p=0.015, p-trend=0.014), basic (HR 0.66, 95% CI 0.48–0.92, p=0.013, p-trend=0.011, n=1371), and full (HR=0.58, 95% CI 0.41–0.84, p=0.0034, p-trend=0.0027, n=1159 ) models.
Cognitively normal participants in the second tertile of total vitamin D intake had decreased risk in the unadjusted (HR 0.69, 95% CI 0.51–0.95, p=0.021), basic (HR 0.73, 95% CI 0.53–1.00, p=0.051), and full (HR 0.75, 95% CI 0.53–1.054, p=0.098) models. Similarly, cognitively normal participants in the second tertile of vitamin D intake without supplements had decreased risk in the unadjusted (HR=0.72, 95% CI 0.52–0.99, p=0.043), basic (HR=0.66, 95% CI 0.48–0.91, p=0.011), and full (HR=0.65, 95% CI 0.46–0.91, p=0.014) models.
Excluding 197 participants with less than 2 years follow-up, among 1562 participants with follow up, we found those in the highest tertile of total vitamin D intake had decreased risk of dementia compared to those in the lowest tertile in the unadjusted (HR 0.65, 95% CI 0.48–0.87, p=0.0034, p-trend=0.0031), basic (HR 0.79, 95% CI 0.59–1.068, p=0.13, p-trend=0.12, n=1559), and full models (HR 0.85, 95% CI 0.61–1.16, p=0.30, p-trend=0.26, n=1385). For food-source only vitamin D intake, participants in the highest tertile also had decreased risk of dementia compared to those in the lowest tertile in the unadjusted (HR 0.74, 95% CI 0.55–0.98, p=0.036, p-trend 0.029), basic (HR 0.73, 95% CI 0.55–0.97, p=0.032, p-trend=0.026, n=1559), and full (HR 0.73, 95% CI 0.53–1.00, p=0.051, p-trend=0.040, n=1385) models.
Participants with follow up in the second tertile of total vitamin D intake also had decreased risk in the unadjusted (HR=0.77, 95% CI 0.58–1.022, p=0.070), basic (HR 0.83, 95% CI 0.62–1.11, p=0.20), and full (HR 0.81, 95% CI 0.59–1.10, p=0.17) models. Similarly, participants with follow up in the second tertile of vitamin D intake without supplements had decreased risk in the unadjusted (HR 0.69, 95% CI 0.52–0.93, p=0.013), basic (0.65, 95% CI 0.49–0.87, p=0.0042), and full (HR 0.67, 95% CI 0.49–0.92, p=0.014) models.
4. Discussion
Higher total vitamin D intake and higher vitamin D intake from food sources were associated with decreased risk for dementia in this multiethnic, community-based cohort. Specifically, individuals in the highest tertile of vitamin D intake from food sources had decreased risk for all-cause dementia and AD, as compared to individuals in the lowest tertile of intake, while adjusting for age, sex, race/ethnicity, education, APOE-ε4, physical activity, MeDi score, income, depression, hypertension, diabetes, cardiovascular disease, and smoking.
There were no significant interactions between race/ethnicity or sex on the association between vitamin D intake and dementia. A significant interaction was found between APOE-ε4 and vitamin D intake from food sources on risk for dementia. Specifically, among non-ε4 carriers, a significant association was observed between vitamin D intake from food sources and dementia. APOE-ε4 is associated with dementia in late-life; however, it remains prevalent in certain regions across the globe, particularly in regions with diminished sun exposure or individuals with melanin-rich skin. Thus, it been previously hypothesized APOE-ε4 may exert a protective role against vitamin D deficiency.29 Our study is limited by lack of serologic vitamin D data. Further research is necessary to clarify the relationship between APOE-ε4 and vitamin D level in vivo.
Sensitivity analyses excluding participants with MCI at baseline, and excluding participants with limited follow-up (less than 2 years) showed similar or an even stronger association between vitamin D and dementia. Overall, our results replicate findings from prior epidemiological studies.
A systematic review and meta-analysis found that low vitamin D was associated with increased dementia risk, but that the majority of participants included in studies were White.12 There is evidence that individuals with darker skin pigment require more sun exposure to generate similar amounts of vitamin D as individuals with less skin pigment.30 Darker-skinned individuals have higher prevalence of low vitamin D levels1, which potentially makes vitamin D intake from food even more important for non-Whites. Our findings add to the literature by replicating prior findings in a diverse, multi-ethnic, cohort, which increases the generalizability (external validity) of our findings for the general population.
Whereas multiple prior studies have examined the relationship between serum vitamin D levels and dementia risk, our study adds to the literature by specifically examining the role of vitamin D intake. Our findings may also be particularly relevant for women from certain minority groups, who have chronic reduced sun exposure due to cultural clothing habits (veiling) and might benefit from higher vitamin D intake from food.31 While our findings suggest an important role for vitamin D intake, further studies are needed before definitive conclusions can be drawn. Specifically, future studies should assess both vitamin D intake and serum vitamin D level (reflective of vitamin D from both food sources and sun exposure), to disentangle the effects of vitamin D from food sources and vitamin D from sun exposure.
Low vitamin D has been broadly linked to many negative health outcomes aside from dementia, including increased risk for cardiovascular disease, type 1 diabetes, cancer, and autoimmune diseases.2 Increasing vitamin D intake likely has benefits beyond primary prevention of dementia, including the prevention of other poor health outcomes as evidenced in previous studies.
One major strength of our study is the study design, which excluded prevalent dementia in the primary analysis, as well as participants with MCI, and participants with limited follow-up in additional sensitivity analyses. By restricting our analysis to participants without MCI, we minimized the possibility of recall bias (increased in participants with baseline cognitive impairment) as well as reverse causation (participants with cognitive impairment eating a more poor diet), thus improving internal validity. By excluding participants with limited follow-up, we also minimize the possibility of reverse causation. Another major strength of our study is the use of cohort data from WHICAP, a well-established multi-ethnic cohort. All measurements made in WHICAP were made prospectively as part of a longitudinal study of dementia and its risk factors. Furthermore, dementia and MCI diagnosis was made in a standardized fashion by consensus, according to accepted criteria. Lastly, we were able to control for a number of potential confounders, including sociodemographic, lifestyle, and vascular risk factors for dementia.
One limitation is potential unmeasured confounders. It is possible that comorbid conditions and/or medication use may confound the relationship between vitamin D intake and cognitive status. We sought to minimize this possibility by controlling for vascular risk factors. However, it should be noted that we lacked data on severity of disease among participants (i.e. adequate vs. inadequate control of blood glucose, among diabetics), and we did not specifically adjust for medication use. Future studies would ideally account for disease severity as well as specific medication use as potential confounders. It is also possible that high vitamin D intake may be a marker of overall healthy diet and/or lifestyle. We minimized this possibility by including physical activity, MEDI score (a measure of healthy diet), income, and smoking status as covariates in the full model. Another limitation of our study is the lack of vitamin D serologic data. We relied on a semi-quantitative FFQ to ascertain vitamin D intake. FFQs are subject to recall bias and may have underestimated vitamin D intake due to limited number of food items included in the questionnaire. Nevertheless, a FFQ has previously been validated against a 24-day weighted food record, which does not rely on memory and is not limited by food items/categories or portion sizes.32 Furthermore, FFQs have been widely used in prior epidemiological studies.19,21,33,34
In addition, higher vitamin D intake was associated with higher serum vitamin D level in a population-based study, even after adjusting for confounders including sun exposure and use of vitamin/mineral supplements. Of note, however, this association reached statistical significance for participants < 50 years old, but not for older participants, which may have been due to the overall low dietary vitamin D intake among elderly participants.35 These findings suggest that food sources of vitamin D are particularly important to maintain higher serum vitamin D levels in the elderly, who are already at higher risk for dementia.
Future studies should employ both objective measures (serologic levels) and subjective measures (FFQ) of vitamin D, to distinguish between vitamin D intake from food and vitamin D from sun exposure. Further studies are needed, which employ more nuanced approaches to the study of lifestyle factors in both the design and analytic phases. In conclusion, we found that in an elderly, diverse population, higher intake of vitamin D is associated with reduced risk of dementia.
Table 2a.
Hazard Ratios and 95% Confidence Intervals for All-Cause Dementia for Subjects in Each Tertile of Total Vitamin D Intake, using the Lowest Tertile as a Reference
| Vitamin D Intake | Unadjusted | Basic Model* | Full Model^ |
|---|---|---|---|
| Tertile 1 | |||
| Tertile 2 | 0.78 (0.61–1.01, p=0.057) | 0.81 (0.62–1.056, p=0.12) | 0.80 (0.61–1.070, p=0.13) |
| Tertile 3 | 0.62 (0.48–0.81, p=0.0005) | 0.74 (0.55–0.98, p=0.037) | 0.79 (0.58–1.061, p=0.12) |
| p-trend | 0.0004 | 0.031 | 0.098 |
Adjusted for age, sex, ethnicity, education
Additionally adjusted for APOE-E4 allele status, physical activity, MeDi score, income, depression, hypertension, diabetes, cardiovascular disease, smoking
Note: n=1591 subjects were included in the basic model, n=1477 subjects included in the full model
Acknowledgments
Author contributions: Study concept and design: CZ and YG. Acquisition of data: YG. Analysis and interpretation of data: all authors. Statistical analysis: CZ and YG. Drafting of the manuscript: CZ. Critical revision of the manuscript for important intellectual content: all authors. Administrative, technical, or material support: YG. Study supervision: YG. This manuscript has been reviewed by WHICAP investigators for scientific content and consistency of data interpretation with previous WHICAP Study publications. We acknowledge the WHICAP study participants and the WHICAP research and support staff for their contributions to this study. We additionally acknowledge Dr. Karen Marder for providing assistance in critical revision of the manuscript for intellectual content. This work was supported by the National Institute on Aging (NIA) through grant numbers PO1AG007232, R01AG037212, RF1AG054023, R00AG042483, and R01AG059013, National Institute of Neurological Disorders and Stroke (NINDS) through T32NS07153, and by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001873, as well as the Charles and Ann Lee Brown Fellowship Fund.
Footnotes
Financial Disclosures:
The authors report no disclosures.
References
- 1.Cashman KD, Dowling KG, Škrabáková Z, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103(4):1033–1044. doi: 10.3945/ajcn.115.120873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–373. doi: 10.4065/81.3.353 [DOI] [PubMed] [Google Scholar]
- 3.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the Vitamin D receptor and 1α-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. doi: 10.1016/j.jchemneu.2004.08.006 [DOI] [PubMed] [Google Scholar]
- 4.Gezen-Ak D, Yılmazer S, Dursun E. Why vitamin D in Alzheimer’s disease? The hypothesis. J Alzheimers Dis JAD. 2014;40(2):257–269. doi: 10.3233/JAD-131970 [DOI] [PubMed] [Google Scholar]
- 5.Afzal S, Bojesen SE, Nordestgaard BG. Reduced 25-hydroxyvitamin D and risk of Alzheimer’s disease and vascular dementia. Alzheimers Dement J Alzheimers Assoc. 2014;10(3):296–302. doi: 10.1016/j.jalz.2013.05.1765 [DOI] [PubMed] [Google Scholar]
- 6.Littlejohns TJ, Henley WE, Lang IA, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83(10):920–928. doi: 10.1212/WNL.0000000000000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17(11):1006–1015. doi: 10.1016/S1474-4422(18)30338-7 [DOI] [PubMed] [Google Scholar]
- 8.Annweiler C, Milea D, Whitson HE, et al. Vitamin D insufficiency and cognitive impairment in Asians: a multi-ethnic population-based study and meta-analysis. J Intern Med. 2016;280(3):300–311. doi: 10.1111/joim.12491 [DOI] [PubMed] [Google Scholar]
- 9.Feart C, Helmer C, Merle B, et al. Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer’s disease in older adults. Alzheimers Dement J Alzheimers Assoc. 2017;13(11):1207–1216. doi: 10.1016/j.jalz.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 10.Annweiler C, Rolland Y, Schott AM, et al. Higher vitamin D dietary intake is associated with lower risk of alzheimer’s disease: a 7-year follow-up. J Gerontol A Biol Sci Med Sci. 2012;67(11):1205–1211. doi: 10.1093/gerona/gls107 [DOI] [PubMed] [Google Scholar]
- 11.Gu Y, Manly JJ, Mayeux RP, Brickman AM. An Inflammation-related Nutrient Pattern is Associated with Both Brain and Cognitive Measures in a Multiethnic Elderly Population. Curr Alzheimer Res. 2018;15(5):493–501. doi: 10.2174/1567205015666180101145619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sommer I, Griebler U, Kien C, et al. Vitamin D deficiency as a risk factor for dementia: a systematic review and meta-analysis. BMC Geriatr. 2017;17(1):16. doi: 10.1186/s12877-016-0405-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller JW, Harvey DJ, Beckett LA, et al. Vitamin D Status and Rates of Cognitive Decline in a Multiethnic Cohort of Older Adults. JAMA Neurol. 2015;72(11):1295–1303. doi: 10.1001/jamaneurol.2015.2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Psychiatric Association, ed. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV ; Includes ICD-9-CM Codes Effective 1. Oct. 96. 4. ed., 7. print. Washington, DC; 1998. [Google Scholar]
- 15.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. [DOI] [PubMed] [Google Scholar]
- 17.Manly JJ, Tang M-X, Schupf N, Stern Y, Vonsattel J-PG, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63(4):494–506. doi: 10.1002/ana.21326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsapanou A, Gu Y, Manly J, et al. Daytime Sleepiness and Sleep Inadequacy as Risk Factors for Dementia. Dement Geriatr Cogn Disord Extra. 2015;5(2):286–295. doi: 10.1159/000431311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 20.Luchsinger JA, Tang M-X, Shea S, Mayeux R. Caloric Intake and the Risk of Alzheimer Disease. Arch Neurol. 2002;59(8):1258. doi: 10.1001/archneur.59.8.1258 [DOI] [PubMed] [Google Scholar]
- 21.Luchsinger JA, Tang M-X, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60(2):203–208. [DOI] [PubMed] [Google Scholar]
- 22.Luchsinger JA, Tang M-X, Siddiqui M, Shea S, Mayeux R. Alcohol Intake and Risk of Dementia: ALCOHOL INTAKE AND RISK OF DEMENTIA. J Am Geriatr Soc. 2004;52(4):540–546. doi: 10.1111/j.1532-5415.2004.52159.x [DOI] [PubMed] [Google Scholar]
- 23.Willett W Nutritional Epidemiology. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 24.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65(4):545–551. doi: 10.1212/01.wnl.0000172914.08967.dc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci J Can Sci Appl Au Sport. 1985;10(3):141–146. [PubMed] [Google Scholar]
- 26.Scarmeas N Physical Activity, Diet, and Risk of Alzheimer Disease. JAMA. 2009;302(6):627. doi: 10.1001/jama.2009.1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scarmeas N, Stern Y, Tang M-X, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59(6):912–921. doi: 10.1002/ana.20854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross C, Taylor C, Yaktine A, Del Valle H, eds. Dietary Reference Intakes for Calcium and Vitamin D. Washington, D.C.: National Academies Press; 2011. doi: 10.17226/13050 [DOI] [PubMed] [Google Scholar]
- 29.Huebbe P, Nebel A, Siegert S, et al. APOE ε4 is associated with higher vitamin D levels in targeted replacement mice and humans. FASEB J Off Publ Fed Am Soc Exp Biol. 2011;25(9):3262–3270. doi: 10.1096/fj.11-180935 [DOI] [PubMed] [Google Scholar]
- 30.Chen TC, Chimeh F, Lu Z, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460(2):213–217. doi: 10.1016/j.abb.2006.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holvik K, Meyer HE, Haug E, Brunvand L. Prevalence and predictors of vitamin D deficiency in five immigrant groups living in Oslo, Norway: the Oslo Immigrant Health Study. Eur J Clin Nutr. 2005;59(1):57–63. doi: 10.1038/sj.ejcn.1602033 [DOI] [PubMed] [Google Scholar]
- 32.Pietinen P, Hartman AM, Haapa E, et al. Reproducibility and validity of dietary assessment instruments. I. A self-administered food use questionnaire with a portion size picture booklet. Am J Epidemiol. 1988;128(3):655–666. doi: 10.1093/oxfordjournals.aje.a115013 [DOI] [PubMed] [Google Scholar]
- 33.Munger RG, Folsom AR, Kushi LH, Kaye SA, Sellers TA. Dietary assessment of older Iowa women with a food frequency questionnaire: nutrient intake, reproducibility, and comparison with 24-hour dietary recall interviews. Am J Epidemiol. 1992;136(2):192–200. doi: 10.1093/oxfordjournals.aje.a116485 [DOI] [PubMed] [Google Scholar]
- 34.Longnecker MP, Lissner L, Holden JM, et al. The reproducibility and validity of a self-administered semiquantitative food frequency questionnaire in subjects from South Dakota and Wyoming. Epidemiol Camb Mass. 1993;4(4):356–365. doi: 10.1097/00001648-199307000-00012 [DOI] [PubMed] [Google Scholar]
- 35.Yoo K, Cho J, Ly S. Vitamin D Intake and Serum 25-Hydroxyvitamin D Levels in Korean Adults: Analysis of the 2009 Korea National Health and Nutrition Examination Survey (KNHANES IV-3) Using a Newly Established Vitamin D Database. Nutrients. 2016;8(10). doi: 10.3390/nu8100610 [DOI] [PMC free article] [PubMed] [Google Scholar]


