Objective
An enigmatic epidemiologic feature of the ongoing coronavirus disease 2019 pandemic is the high rate of asymptomatic infection in pregnant women.1 This is puzzling because systemic immune changes predispose pregnant women to increased severity of respiratory viral infections, especially influenza A.2 A major roadblock in understanding this atypical clinical presentation is the poor characterization of cellular entry factors for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—angiotensin-converting enzyme 2 (ACE2) and the androgen-sensitive transmembrane protease serine 2 (TMPRSS2)—in the respiratory tract during pregnancy. Motivated by a recent report showing an estradiol-mediated down-regulation of ACE2 in the airway epithelium,3 we hypothesized that the hormonal changes of pregnancy will decrease the expression of SARS-CoV-2 cell entry factors. Here, we compare their expression and examine the innate immune system in the nasal epithelium of term pregnant (20 days’ gestation) vs nonpregnant 2-month-old female rats.
Study Design
All experiments were conducted after an appropriate institutional approval (protocol ID: 19-1071) and comply with Animal Research: Reporting of In Vivo Experiments guidelines. Briefly, the nasal epithelia from euthanized rats (n=9 each) were dissected according to the protocol described by Dunston et al4 with modifications. Collected samples were assayed for the expression of SARS-CoV-2 entry factors (ACE2, TMPRSS2), innate antiviral immune genes that are highly coexpressed with ACE2 (TNFSF10, MX1, nitric oxide synthase 2 [NOS2]),5 and genes involved in SARS-CoV-2 detection and defense RIG-1, TLR7, MYD88, interferon regulatory factor 7 [IRF7]) with TaqMan quantitative polymerase chain reaction (PCR) (Thermo Fisher Scientific, Waltham, MA). In addition, we determined the expression of ACE2 (LS-c763699, 1:1000 dilution; Lifespan Biosciences, Seattle, WA) and TMPRSS2 (sc-515727, 1:250 dilution; Santa Cruz Biotechnology, Inc, Dallas, TX) protein with immunoblots. Finally, we assayed ACE2 enzyme activity with a fluorometric assay (K897-100, BioVision Inc, Milpitas, CA).
Results
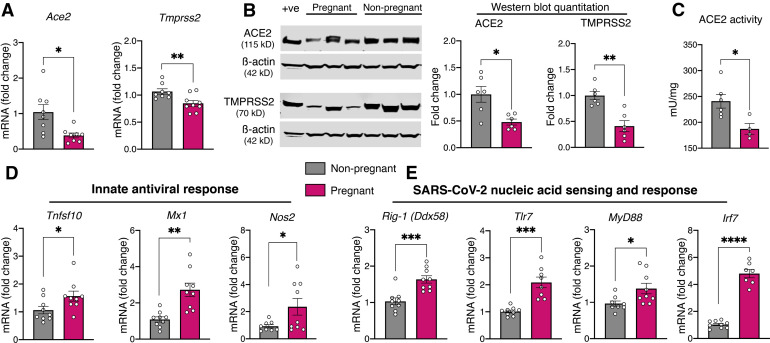
We observed a marked down-regulation of the expression of ACE2 and TMPRSS2 genes (Figure , A) along with concomitant changes in protein expression (Figure, B) and a marked decrease in ACE2 enzyme activity in the nasal epithelium during pregnancy (Figure, C). Innate immune genes with antiviral function that are highly coexpressed with ACE2 (TNFSF10, MX1, NOS2) were markedly elevated in the nasal epithelium from pregnant rats (Figure, D). Similarly, the expression of cytoplasmic (RIG-1) and endosomal viral sensors (TLR7, MYD88, and IRF7) involved in the detection of SARS-CoV-2 was substantially up-regulated with pregnancy (Figure, E). Collectively, our results show a decrease in cell entry factors for SARS-CoV-2 and a surprisingly robust expression of innate immune response genes in the nasal epithelium of pregnant rats.
Figure.
Assessment of viral cell entry factors and innate immune response genes in the nasal epithelium during pregnancy
A, Scatter plots showing markedly decreased expression of ACE2 and TMPRSS2 in the nasal epithelium during pregnancy. B, Representative immunoblots showing markedly reduced ACE2 and TMPRSS2 protein expression along with quantification as scatter plots. Rat small intestinal lysate was used as positive control and β-actin as the loading control. C, Scatter plot demonstrating a marked reduction in enzymatic ACE2 activity in the nasal epithelium of pregnant rats. D, Scatter plots showing substantial up-regulation of innate immune genes highly coexpressed with ACE2 (TNFSF10, MX1, NOS2). E, Scatter plots showing up-regulation of genes involved with SARS-CoV-2 detection (RIG-1, TLR7, MYD88, IRF7) in pregnant nasal epithelial samples suggesting the possibility of heightened innate immune surveillance at baseline. Expression levels of genes of interest were assayed in duplicate along with 2 endogenous housekeeping control genes (EEF2 and ACTB). All TaqMan primers were acquired from Thermo Fisher Scientific, and thermal cycling was performed in 7500 Fast Real-Time PCR System (Applied Biosystems: Makrogiannis Phil, Foster City, CA). Relative mRNA expression, normalized to the geometric mean of EEF2 and ACTB, was calculated using the 2-ΔΔCT method. Data outliers were eliminated using robust regression and outlier analysis with Q set to 10% and normality of residuals was assessed with D’Agostino-Pearson omnibus test. Normally and nonnormally distributed data were analyzed with Welch’s t test and Mann-Whitney U test, respectively, with P≤.05 accorded statistical significance. Data were analyzed with Prism 8 for macOS (version 8.2.1; GraphPad Software Inc, San Diego, CA) and presented as mean±SEM; aP≤.05; bP≤.01; cP≤.001; and dP≤.0001 (n=9 each for all experiments except western blot where n=6 per group).
ACE2, angiotensin-converting enzyme 2; mRNA, messenger RNA; MYD88, myeloid differentiation primary response 88; NOS2, nitric oxide synthase 2; RIG-1, retinoic acid-inducible gene I; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SEM, standard error of mean; TLR7, toll-like receptor 7; TMPRSS2, transmembrane protease serine 2; TNFSF10, tumor necrosis factor ligand superfamily member 10.
Palanisamy. Reduced SARS-CoV-2 entry factors and innate immune gene expression in the epithelium of pregnant rats. Am J Obstet Gynecol 2021.
Conclusion
Based on our preclinical findings, we surmise that the high rate of asymptomatic infection in pregnant women is likely caused by decreased SARS-CoV-2 tropism secondary to reduced expression of cell entry factors. Our observation of up-regulated innate immune defense in the nasal epithelium, in contrast to the immunologic indolence at the placental-fetal interface, was unexpected and novel. Considering the exquisite vulnerability of pregnant women to influenza A virus, another single-stranded RNA virus, but not SARS-CoV-2, our findings set the stage for comprehensive characterization of respiratory mucosal immunology in pregnant women to better understand host-pathogen interaction in this unique demographic subset.
Footnotes
The authors report no conflict of interest.
Departmental startup funds were given to A.P.
References
- 1.Sutton D., Fuchs K., D’Alton M., Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kourtis A.P., Read J.S., Jamieson D.J. Pregnancy and infection. N Engl J Med. 2014;370:2211–2218. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stelzig K.E., Canepa-Escaro F., Schiliro M., Berdnikovs S., Prakash Y.S., Chiarella S.E. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2020;318:L1280–L1281. doi: 10.1152/ajplung.00153.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunston D., Ashby S., Krosnowski K., Ogura T., Lin W. An effective manual deboning method to prepare intact mouse nasal tissue with preserved anatomical organization. J Vis Exp. 2013:50538. doi: 10.3791/50538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sungnak W., Huang N., Bécavin C. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]