Abstract
Objectives
A hasty reopening has led to a resurgence of the novel coronavirus disease 2019 (COVID-19) in the United States (US). We aimed to quantify the impact of several public health measures including non-medical mask-wearing, shelter-in-place, and detection of silent infections to help inform COVID-19 mitigation strategies.
Methods
We extended a previously established agent-based disease transmission model and parameterized it with estimates of COVID-19 characteristics and US population demographics. We implemented non-medical mask-wearing, shelter-in-place, and case isolation as control measures, and quantified their impact on reducing the attack rate and adverse clinical outcomes.
Results
We found that non-medical mask-wearing by 75% of the population reduced infections, hospitalizations, and deaths by 37.7% (interquartile range (IQR): 36.1–39.4%), 44.2% (IQR: 42.9–45.8%), and 47.2% (IQR: 45.5–48.7%), respectively, in the absence of a shelter-in-place strategy. Sheltering individuals aged 50 to 64 years of age was the most efficient strategy, decreasing attack rate, hospitalizations, and deaths by over 82% when combined with mask-wearing. Outbreak control was achieved in the simulated scenarios and the attack rate was reduced to below 1% when at least 33% of silent pre-symptomatic and asymptomatic infections were identified and isolated.
Conclusions
Mask-wearing, even with the use of non-medical masks, has a substantial impact on outbreak control. A judicious implementation of shelter-in-place strategies remains an important public health intervention amid ongoing outbreaks.
Keywords: COVID-19, Mask-wearing, Shelter-in-place, Isolation
Introduction
The novel coronavirus disease 2019 (COVID-19) has caused the most devastating pandemic in modern times, with significant morbidity and mortality (World Health Organization, 2020). The United States (US) has recorded more cases and deaths than any other country, with over one-fifth of the global mortality as of 1 October, 2020 (Johns Hopkins University, 2020, Weinberger et al., 2020). Clinical and epidemiological studies suggest that comorbid individuals and persons older than 50 years of age have been disproportionately affected by COVID-19, in terms of both disease severity and adverse clinical outcomes (Centers for Disease Control and Prevention, 2020a, Garg, 2020a). In addition to the catastrophic toll on human health, the restrictive public health measures implemented to combat the spread of COVID-19 have led to widespread disruption of education, societal functions, and the economy (Dorn et al., 2020, Fernandes, 2020, Palumbo and Brown, 2020). Rapid easing of these measures to alleviate social and economic woes in the US during the summer of 2020 led to a resurgence of cases (Johns Hopkins University, 2020), thereby forcing many states such as Texas, California, and Oregon to restructure their reopening strategies (CNN et al., 2020a).
A capacity to identify over one-third of silent pre-symptomatic and asymptomatic infections combined with the immediate isolation of symptomatic cases is required to control the current resurgence of COVID-19 before a safe and effective vaccine becomes available (Moghadas et al., 2020a). Given that this is unlikely to be achieved with the existing testing capacity and sample-to-result timeline in the US, additional public health measures may be needed to change the trajectory of current outbreaks, such as shelter-in-place strategies for specific population segments and mask-wearing. Quantifying the effects of these measures can help to inform public health mitigation strategies to curb ongoing outbreaks.
In this study, we aimed to project the impact on adverse clinical outcomes that could be achieved by mask-wearing and implementing shelter-in-place strategies for various population segments while considering age and comorbidities associated with COVID-19 (Centers for Disease Control and Prevention, 2020b, Garg, 2020b, Stokes et al., 2020). In particular, we extended a previously established agent-based simulation model (Moghadas et al., 2020a, Shoukat et al., 2020) and simulated various outbreak scenarios while assuming 5% population immunity for our base case according to recent seroprevalence studies (SeroTracker, 2020, Bobrovitz et al., 2020). We found that the attack rate, hospitalizations, and deaths can be reduced by at least five-fold if shelter-in-place of comorbid individuals or persons aged 50–64 years is combined with mask-wearing by those not sheltered-in-place. We found that outbreak control can be achieved to bring the attack rate to below 1% if at least 33% of silent pre-symptomatic and asymptomatic infections are identified and isolated.
Methods
Model structure
We extended our agent-based transmission model (Moghadas et al., 2020a, Shoukat et al., 2020) to simulate post-reopening COVID-19 outbreak scenarios. The natural history of COVID-19 was implemented by including individual classes with epidemiological statuses: susceptible, latently infected (not yet infectious), asymptomatic, pre-symptomatic, symptomatic with either mild or severe illness, recovered, and dead (Appendix: Figure S1). The model population was stratified into five age groups comprising 0–4, 5–19, 20–49, 50–64, and ≥65 years based on US demographics (U.S. Census Bureau QuickFacts: United States, 2020). We parameterized the model (Table 1 ) and determined the proportion of individuals in each age group who had one or more comorbidities associated with COVID-19 complications based on age-specific means derived from the 2017 Behavioural Risk Factor Surveillance System (Appendix: Table S1) (Divo et al., 2014, Adams et al., 2020). In the absence of any social distancing measures, the daily number of interactions within and between different age groups were sampled from negative binomial distributions and implemented based on an empirically determined contact network (Mossong et al. 2008). When shelter-in-place was integrated as a social distancing measure, the network of interactions changed into an age-dependent contact matrix derived from a representative sample population during COVID-19 lockdown (Appendix: Table S2) (CMMID COVID-19 Working Group et al., 2020).
Table 1.
Model parameters, values, and distributions.
| Description | 0–4 | 5–19 | 20–49 | 50–64 | ≥ 65 | Source | |
|---|---|---|---|---|---|---|---|
| Transmission probability per contact during the pre-symptomatic stage | 0.08 | Calibrated to R0 = 2.5 (Li et al., 2020a, Wu et al., 2020) |
|||||
| Incubation period (days) | Log-Normal (mean: 5.2, SD: 0.1) | (Lauer et al., 2020, Li et al., 2020a) | |||||
| Asymptomatic period (days) | Gamma (shape: 5, scale: 1) | Derived from (Gatto et al., 2020, Li et al., 2020b) |
|||||
| Pre-symptomatic period (days) | Gamma (shape: 1.058, scale: 2.174) | (He et al., 2020) | |||||
| Infectious period from onset of symptoms (days) | Gamma (shape: 2.768, scale: 1.1563) | Derived from (Li et al., 2020b) |
|||||
| Proportion of infections that are asymptomatic | 0.3 | 0.31 | 0.29 | 0.29 | 0.18 | (Mizumoto et al., 2020, Nishiura et al., 2020) | |
| Proportion of symptomatic cases that exhibit mild symptoms | 0.95 | 0.9 | 0.85 | 0.60 | 0.20 | (Moghadas et al., 2020b, Shoukat et al., 2020) | |
| Relative transmissibility of severe symptomatic compared to pre-symptomatic | 0.89 | (Moghadas et al., 2020a) | |||||
| Relative transmissibility of mild symptomatic compared to pre-symptomatic | 0.44 | (Moghadas et al., 2020a) | |||||
| Relative transmissibility of asymptomatic compared to pre-symptomatic | 0.11 | (Moghadas et al., 2020a) | |||||
| Proportion of individuals with one or more comorbidities | 0.05 | 0.1 | 0.28 | 0.55 | 0.76 | (Adams et al., 2020) | |
| Proportion of cases hospitalized with one or more comorbidities | 40% | (Centers for Disease Control and Prevention, 2020b, Garg, 2020b) | |||||
| Proportion of cases hospitalized with one or more comorbidities – by type of bed occupied | Non-ICU | 67% | |||||
| ICU | 33% | ||||||
| Proportion of cases hospitalized without any comorbidities | 9% | (Centers for Disease Control and Prevention, 2020b, Garg, 2020b) | |||||
| Proportion of cases hospitalized without any comorbidities – by type of bed occupied | Non-ICU | 75% | |||||
| ICU | 25% | ||||||
| Length of non-ICU stay | Gamma (shape: 4.5, scale: 2.75) | Derived from (Sanche et al., 2020, Yang et al., 2020) |
|||||
| Length of ICU stay | Gamma (shape: 4.5, scale: 2.75) | Derived from (Sanche et al., 2020, Yang et al., 2020) |
|||||
| Efficacy of mask in preventing transmission (eM) | 20% | (Davies et al., 2013, MacIntyre et al., 2015, Konda et al., 2020, Mondal et al., 2020) | |||||
Infection dynamics
Disease transmission occurred probabilistically when susceptible individuals interacted with infectious individuals in asymptomatic, pre-symptomatic, or symptomatic stages of the disease. For each newly infected individual, we sampled an incubation period from a log-normal distribution with an average of 5.2 days (Lauer et al., 2020, Li et al., 2020a). A proportion of infected individuals develop symptoms after a highly infectious pre-symptomatic stage as part of their incubation period (He et al., 2020). The pre-symptomatic period was sampled from a gamma distribution with a mean of 2.3 days (He et al., 2020). The infectious period following symptom onset was also sampled from a gamma distribution with a mean of 3.2 days (Li et al., 2020b). We used age-dependent estimates to determine the probability of developing mild, severe, or critical illness during the symptomatic infection period (Moghadas et al., 2020b, Shoukat et al., 2020). Infected individuals who were not pre-symptomatic after the latent period became asymptomatic until recovery, with an infectious period sampled from a gamma distribution with a mean of 5 days (Gatto et al., 2020, Li et al., 2020b). Recovery from infection was assumed to provide adequate immunity for the duration of the outbreak and prevent re-infection.
The infectivity was parameterized for individuals in asymptomatic, mild symptomatic, and severe symptomatic stages relative to the infectivity during the pre-symptomatic stage. These parameters were based on the proportion of secondary cases resulting from disease transmission during each stage of infection (Ferretti et al., 2020, Moghadas et al., 2020a). The pre-symptomatic stage accounts for the highest proportion of secondary infections (Ferretti et al., 2020, Moghadas et al., 2020a), so we determined the relative infectivity in the asymptomatic, mild symptomatic, and severe symptomatic stages to be 11%, 44%, and 89%, respectively (Moghadas et al., 2020a).
Infection outcomes
We assumed that mild cases recover without the need for hospitalization. The probabilities of hospitalization and intensive care unit (ICU) admissions for severe and critical cases were informed by estimates from COVID-19 outbreaks in the US, and further classification of individuals with and without comorbidities (Centers for Disease Control and Prevention, 2020b, Garg, 2020b). Hospitalized patients were isolated and did not contribute to further transmission in the population. The average time from symptom onset to hospital admission was sampled in the range of 2 to 5 days (Moghadas et al., 2020b, Shoukat et al., 2020). The lengths of non-ICU and ICU stays for hospitalized patients were sampled from gamma distributions with means of 12.4 and 14.4 days, respectively (Sanche et al., 2020, Yang et al., 2020). We assumed that severe symptomatic cases who were not hospitalized self-isolated immediately upon symptom onset and they limited their daily contacts to a maximum of three until recovery.
Interventions
We considered non-medical cloth masks (referred to as masks in the following) as an intervention measure for the general population, with compliance rates of 0%, 25%, 50%, and 75% for individuals aged 2 and older based on the Centers for Disease Control and Prevention guidelines (CDC, 2020). This range of compliance is based on recent polling results, which suggest that the rate of compliance remains below 75% despite mandatory mask-wearing in some states (CNN, 2020b). We chose a conservative non-medical mask efficacy, i.e., eM = 20%, within the estimated range (Davies et al., 2013, MacIntyre et al., 2015, Konda et al., 2020, Mondal et al., 2020) for reducing disease transmission during interactions between susceptible and infected individuals. The probability of disease transmission was then reduced by a factor of (1 – eM) or (1 – eM)2 depending on whether only one or both interacting individuals wore masks, respectively. We also implemented shelter-in-place strategies based on age and comorbidities by considering eight scenarios, as described in Table 2. Under any of these scenarios, the daily interactions of individuals who were sheltered-in-place were parameterized from an age-dependent contact matrix (Appendix: Table S2). The total number of contacts was sampled from a negative binomial distribution, with parameters derived from a recent study of contact patterns during COVID-19 lockdown ( CMMID COVID-19 Working Group et al., 2020). When both shelter-in-place and mask-wearing interventions were applied, only individuals who were not sheltered-in-place wore masks during daily interactions. In our model, the baseline scenario corresponded to the simulation of outbreaks without these interventions. For all other scenarios, we measured the effect of shelter-in-place and mask-wearing on reductions in the attack rate, hospitalizations, and deaths throughout the outbreak. In order to compare scenarios for shelter-in-place, we estimated the strategy efficiency (S e) as: S e = L a /Ns, where La is the cumulative number of the outcome of interest averted (i.e., infections, hospitalizations, or deaths) compared to the no shelter-in-place intervention, and Ns is the number of sheltered-in-place individuals. Using S e, we determined the most efficient strategy for shelter-in-place among the simulated scenarios.
Table 2.
Shelter-in-place strategies implemented in the model.
| Scenario | Who is sheltered-in-place? | % of the population |
|---|---|---|
| S1 | Children aged 5 to 19 (school closures) | 18.9 |
| S2 | All individuals with comorbidities associated with COVID-19 | 36.3 |
| S3 | All individuals between 50 and 64 years of age | 18.9 |
| S4 | All individuals aged 65 and older | 16.6 |
| S5 | All individuals aged 50 and older | 35.6 |
| S6 | All individuals with comorbidities associated with COVID-19 or aged 50 to 64 | 44.9 |
| S7 | All individuals with comorbidities associated with COVID-19 or 65 and older | 40.3 |
| S8 | All individuals with comorbidities associated with COVID-19 or 50 and older | 48.9 |
Model implementation
We calibrated the model to a baseline transmission probability per contact to obtain a reproduction number R 0 = 2.5 (defined as the average number of secondary cases generated by a primary case), as estimated for the initial COVID-19 outbreaks (Li et al., 2020a, Wu et al., 2020). This reproduction number corresponds to an attack rate of 60% in an entirely susceptible population. Recent seroprevalence studies suggest that initial outbreaks generated population level immunity of about 3.35% at the global scale (SeroTracker, 2020). This level of immunity varies in the US population (95% confidence interval: 3.59–9.36%) (SeroTracker, 2020), and thus we considered scenarios with 5%. To account for the age distribution of pre-existing population immunity, we first simulated the model in an entirely susceptible population and replicated the scenario for initial outbreaks. We then used the infection rates in different age groups and initialized our model with a population that included immune individuals according to the age-specific distribution of pre-existing immunity (Appendix: Table S3). To evaluate the intervention measures, we seeded simulations with one initial infection in the latent stage of the disease in a population of 10,000 individuals. We averaged the results over 1000 independent Monte Carlo realizations in each scenario. The model was coded in Julia language and it is available at: https://github.com/thomasvilches/covid-shelterin.
Results
Impact of mask-wearing
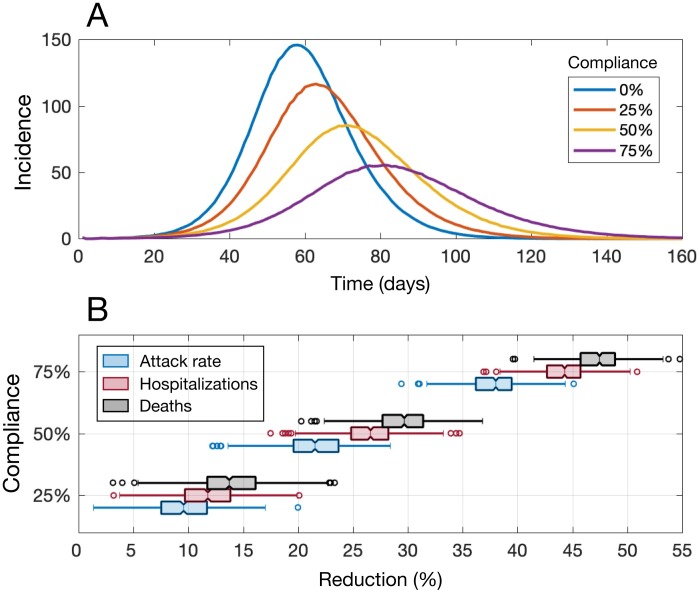
We evaluated the impact of mask-wearing in the absence of a shelter-in-place strategy by assuming population immunity of 5%. As the proportion of the population wearing masks increased from 0% to 75%, the peak incidence was delayed and its magnitude decreased (Figure 1A). For instance, with no mask-wearing (0% compliance) and no shelter-in-place intervention, a mean peak incidence of 146 per 10,000 population was observed 58 days into the outbreak. However, with 75% mask-wearing compliance, the peak incidence was reduced to 55 per 10,000 population (62.3% reduction) and it occurred with a 3-week delay on day 81 of the outbreak. For a mask-wearing compliance rate of 25%, we projected a median reduction of 9.6% (interquartile range (IQR): 7.3–11.7%) for the attack rate, 11.9% (IQR: 9.8–14.3%) for hospitalizations, and 14.0% (IQR: 11.9–16.1%) for deaths compared to no mask-wearing ( Figure 1B). When we increased the mask-wearing compliance rate to 75%, the median reductions in the attack rate, hospitalizations, and deaths were substantially higher at 37.7% (IQR: 36.1–39.4%), 44.2% (IQR: 42.9–45.8%), and 47.2% (IQR: 45.5–48.7%), respectively (Figure 1B).
Figure 1.
(A) Projected incidence of COVID-19 infections per 10,000 population at different mask-wearing compliance rates and with 5% level of pre-existing immunity. (B) Reductions in attack rate, hospitalizations, and deaths at different mask-wearing compliance rates compared to no mask-wearing. Simulations correspond to mask-wearing scenarios in the absence of shelter-in-place strategies.
We also estimated the reduction in secondary infections that could be achieved by mask-wearing. Compared to the no-intervention scenario (corresponding to model calibration with R0 = 2.5), mask-wearing compliance rates of 25%, 50%, and 75% reduced the reproduction number to 2.30, 2.04, and 1.79, respectively.
Impact of shelter-in-place
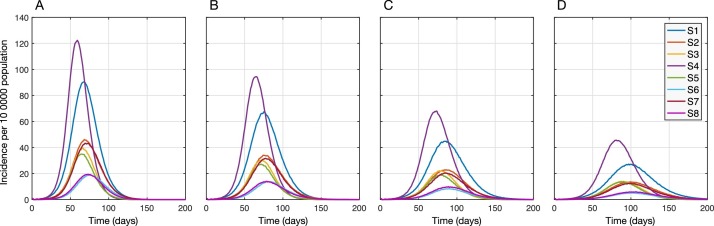
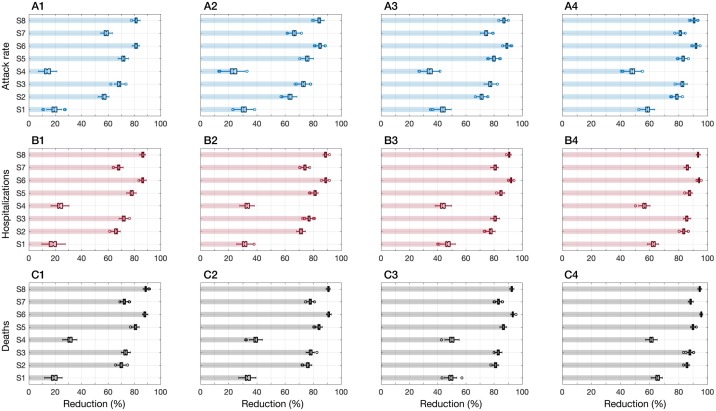
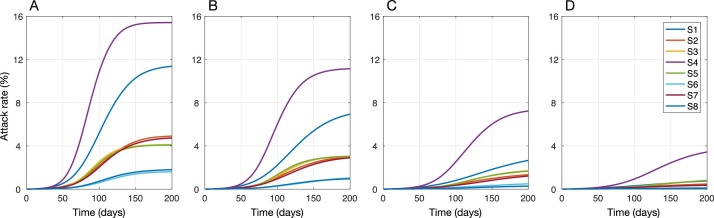
When shelter-in-place was implemented, the timing of peak incidence was delayed and its magnitude was reduced for each scenario (Figure 2). Assuming population immunity of 5% and in the absence of mask-wearing, the lowest effect on reducing the attack rate (median: 13.9%; IQR: 11.6–16.2%) was associated with sheltering individuals aged 65 years and older (S4). S4 also led to the highest and earliest peak of incidence ( Figure 2A). Sheltering children aged 5–19 years (S1) was slightly more effective in reducing the attack rate (median: 19.7%; IQR: 17.5–21.4%) as well as delaying and lowering the peak incidence. However, S1 underperformed in terms of reducing hospitalizations and deaths compared to S4 (Figure 3 – A1, B1, C1). We observed the greatest effects on reducing the attack rate (over 80%), hospitalizations (over 85%), and deaths (over 87%) with strategies S6 and S8 ( Figure 3 – A1, B1, C1), where comorbid individuals combined with those aged 50–64 (S6) or 50 and older (S8) were sheltered-in-place. We also found that despite a significantly lower number of individuals (i.e., 18.9% of the population) being sheltered-in-place with strategy S3 (i.e., those aged 50–64 years), S3 outperformed strategy S7 where over 40% of the population (i.e., all individuals with comorbidities or aged 65 and older) were sheltered-in-place (Figure 3).
Figure 2.
Projected incidence of COVID-19 with different shelter-in-place strategies combined with mask-wearing compliance rates of 0% (A), 25% (B), 50% (C), and 75% (D) among those not sheltered-in-place. The level of pre-existing immunity in the population was assumed to be 5% for all scenarios.
Figure 3.
Reductions achieved in attack rate, hospitalizations, and deaths with each shelter-in-place strategy combined with mask-wearing compliance rates of 0% (A1, B1, C1), 25% (A2, B2, C2), 50% (A3, B3, C3), and 75% (A4, B4, C4). The level of pre-existing immunity in the population was assumed to be 5% for all scenarios.
When mask-wearing was implemented in combination with shelter-in-place interventions, the peak incidence was further delayed (Figure 2) and the performance of all strategies improved proportionally in reducing the attack rate, hospitalizations, and deaths (Figure 3). We found that when the mask-wearing compliance was 75%, sheltering individuals aged 50–64 years (S3; 18.9% of the population) reduced the attack rate, hospitalizations, and deaths, with comparable rates to those obtained when all comorbid individuals or those aged 50 and older were sheltered-in-place (S8; 48.9% of the population) in the absence of mask-wearing (Figure 3). These results suggest that mask-wearing can reduce the burden of disease and improve the performance of shelter-in-place strategies without increasing the number of individuals sheltered-in-place (Appendix: Tables S4–S6). With 75% mask-wearing, the median reduction in the attack rate under S3 was projected as 82.5% (IQR: 81.6–83.5%) and the median reductions in hospitalizations and deaths exceeded 86% and 87%, respectively.
Efficiency of shelter-in-place strategies
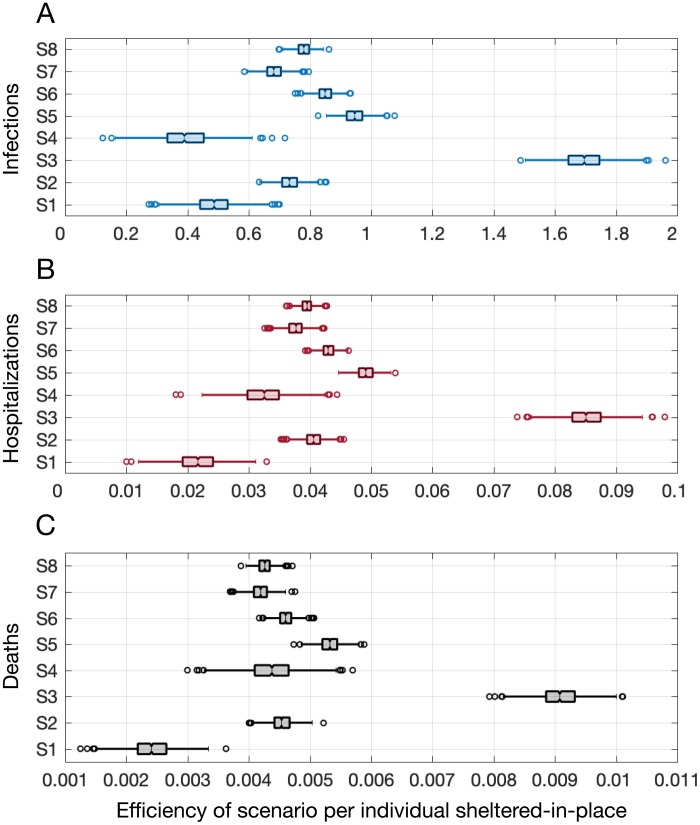
The number of individuals affected by each shelter-in-place strategy was variable due to the population distribution in the US and the various combinations of age groups and comorbidities modeled in each scenario. Based on the number of averted infections, hospitalizations, and deaths per person sheltered-in-place, we found that S3 was the most efficient strategy, where all individuals between 50 and 64 years of age were sheltered-in-place (Figure 4). In the absence of mask-wearing, S3 resulted in the highest numbers of averted infections (median: 1.69; IQR: 1.65–1.75), hospitalizations (median: 0.085; IQR: 0.083–0.087), and deaths (median: 0.0091; IQR: 0.0088–0.0093) per person sheltered-in-place, as shown in Figure 4. By contrast, as shown in Figure 3, strategies S6 and S8 provided the highest reductions in adverse clinical outcomes but they had relatively low efficiency because a high proportion of the population was sheltered-in-place. Strategy S1 (children aged 5 to 19) required sheltering-in-place for the same proportion of the population as strategy S3, but S1 was the least efficient strategy in terms of averting hospitalizations (0.021, IQR: 0.019–0.024) and deaths (0.0024, IQR: 0.0022–0.0027) per person sheltered-in-place, mainly due to a high proportion of daily contacts occurring within their own age group (5–19 years), as well as better clinical outcomes when children are infected with COVID-19 compared to older age groups (Shekerdemian et al., 2020).
Figure 4.
Efficiency of various shelter-in-place strategies as the ratio of the number of adverse outcomes (infections, hospitalizations, and deaths) averted relative to the number of individuals sheltered-in-place in the absence of mask-wearing.
Outbreak control
Shelter-in-place and mask-wearing strategies can substantially reduce the magnitude of outbreaks and adverse clinical outcomes. However, since we excluded healthy individuals between 20 and 49 years of age from our shelter-in-place strategies, effective control of COVID-19 outbreaks will need to include the rapid identification of silent pre-symptomatic and asymptomatic infections (Moghadas et al., 2020a). Therefore, we conducted simulations to identify the level of non-symptomatic case detection required with testing to bring the attack rate below 1% of the population when a highly efficient strategy of sheltering individuals aged 50–64 years (S3) was combined with mask-wearing by those not sheltered-in-place. Our results showed that if testing capacity and contact tracing allowed for 33% of silent infections to be identified, outbreak control could be achieved by implementing S3 with 75% mask-wearing (Figure 5D). When the mask-wearing compliance rate was ≤50%, the attack rate under most strategies remained above 2% even with a 33% detection rate for silent infections ( Figure 5A,B,C).
Figure 5.
Projected attack rates under different shelter-in-place strategies with a detection rate of 33% for silent (i.e., pre-symptomatic and asymptomatic) infections and mask-wearing compliance rates of 0% (A), 25% (B), 50% (C), and 75% (D).
Discussion
In the absence of a COVID-19 vaccine, mitigation measures to curb initial outbreaks have included strict social distancing and movement restrictions (Flaxman et al., 2020, Khosrawipour et al., 2020, Lau et al., 2020, The Lancet, 2020). The resulting societal and economic repercussions have led to a hasty reopening, thereby causing a resurgence of cases in many US states. However, curtailing these outbreaks is unlikely in the presence of insufficient testing, inadequate social distancing, and the ongoing debate (Feng et al., 2020) over the use of masks in public. In this study, we aimed to quantify the effects of mask-wearing and shelter-in-place to identify the optimal strategies for effective control of ongoing and future COVID-19 outbreaks. A strategic approach to lifting restrictive public health measures will help to facilitate a safe economic recovery.
Our results showed that the greatest reductions in the attack rate, hospitalizations, and deaths were achieved when nearly half of the population was sheltered-in-place (Figure 3). However, a similar impact was obtained by sheltering a substantially smaller proportion (∼19%) of the population (S3: individuals aged 50–65) when 75% of those who were not sheltered-in-place wore masks. When considering a similar proportion of the population in S4, however, the efficiency of the strategy decreased substantially despite a high proportion of comorbid individuals because persons aged 65 and older have the lowest number of daily contacts of any age group. On a population level, our results demonstrated that school closures (sheltering children aged 5–19 years) were comparatively less effective in terms of reducing hospitalizations and mortality, which can mainly be explained by empirical observations that over 60% of daily contacts among school children occur in their own age group (Mossong et al., 2008) (Appendix: Table S2), and that they are more likely to exhibit milder COVID-19 outcomes with lower hospitalization rates (Shekerdemian et al., 2020).
The use of face coverings has been recommended by the Centers for Disease Control and Prevention to help reduce the spread of COVID-19 (CDC, 2020). However, there has been a widespread debate on the effectiveness of mask-wearing despite preliminary evidence suggesting that it can help reduce transmission (MacIntyre et al., 2015, CDC, 2020, Konda et al., 2020, Mondal et al., 2020). Our results demonstrate the benefits of mask-wearing for reducing the spread of infection and adverse clinical outcomes, particularly when combined with shelter-in-place strategies for vulnerable populations. The assumed non-medical mask efficacy in our analysis (20%) is likely to be conservative given the range of estimates (20–80%) used in previous studies (Eikenberry et al., 2020, Ngonghala et al., 2020). However, factors such as the materials used to make cloth masks (Konda et al., 2020), imperfect use (Mondal et al., 2020), and the behavior of the mask-wearer could reduce their effectiveness (Stutt et al., 2020). If non-medical masks are more effective than assumed in this study, our results would be conservative and a greater impact on reducing disease burden may be expected.
Our study has important implications for COVID-19 mitigation strategies. First, a strategic and coordinated response is necessary to suppress the ongoing resurgence of cases in the US. Second, mask-wearing and shelter-in-place continue to be important measures for reducing the disease burden, and enhancing the capacity for testing and contact tracing remains a critical pathway toward curbing the trajectory of developing outbreaks. Given that the majority of COVID-19 transmission is attributable to shedding from pre-symptomatic and asymptomatic individuals (Moghadas et al., 2020a), outbreak control cannot be achieved without detecting and isolating at least one-third of silent infections. In this context, mask-wearing can help to reduce the risk of silent transmission. Finally, in the absence of a vaccine, a judicious implementation of shelter-in-place strategies could have a large impact on the control of ongoing and future outbreaks, while minimizing socioeconomic repercussions in the coming months.
Availability of data and materials
Details of the model are provided in the Appendix. The computational model is available at https://github.com/thomasvilches/covid-shelterin.
Competing interests
None declared.
Funding
SMM gratefully acknowledges support from the Canadian Institutes of Health Research COVID-19 Rapid Research OV4-170643, and the Natural Sciences and Engineering Research Council of Canada. APG gratefully acknowledges funding from the NSF RAPID - 2027755.
Authors’ contributions
KZ, APG, and SMM conceived the study; SMM designed the model framework and parameterized it; TNV developed the computational code and performed simulations; TNV, KZ, MT, and SMM analyzed the data; KZ, TNV, MT, APG, and SMM wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval was not required because all data are publicly available.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.10.002.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Adams M.L., Katz D.L., Grandpre J. Population based estimates of comorbidities affecting risk for complications from COVID-19 in the US. medRxiv. 2020;(April) doi: 10.3201/eid2608.200679. Available from: https://www.medrxiv.org/content/10.1101/2020.03.30.20043919v1. [cited 2020 June 10];2020.03.30.20043919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrovitz N., Arora R.K., Yan T., Rahim H., Duarte N., Boucher E. Lessons from a rapid systematic review of early SARS-CoV-2 serosurveys. medRxiv. 2020;(May) Available from: https://www.medrxiv.org/content/10.1101/2020.05.10.20097451v1. [cited 2020 June 10];2020.05.10.20097451. [Google Scholar]
- CDC . Centers for Disease Control and Prevention; 2020. Considerations for wearing cloth face coverings. Available from: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html. [cited 2020 July 9] [Google Scholar]
- Centers for Disease Control and Prevention Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 — United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020:69. doi: 10.15585/mmwr.mm6913e2. Available from: https://www.cdc.gov/mmwr/volumes/69/wr/mm6913e2.htm. [cited 2020 June 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 — United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020:69. doi: 10.15585/mmwr.mm6913e2. Available from: https://www.cdc.gov/mmwr/volumes/69/wr/mm6913e2.htm. [cited 2020 June 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- CMMID COVID-19 working group, Jarvis C.I., Van Zandvoort K., Gimma A., Prem K., Klepac P. Quantifying the impact of physical distance measures on the transmission of COVID-19 in the UK. BMC Med. 2020;18(December (1)):124. doi: 10.1186/s12916-020-01597-8. Available from: https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-020-01597-8. [cited 2020 August 9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- CNN C.M., Levenson Eric, Waldrop Theresa. Some US states return to previous restrictions to slow surge of coronavirus cases. CNN. 2020 Available from: https://www.cnn.com/2020/06/29/health/us-coronavirus-monday/index.html. [cited 2020 July 9] [Google Scholar]
- CNN H.E. The Northeast leads the country in mask-wearing. CNN. 2020 Available from: https://www.cnn.com/2020/06/26/politics/maskwearing-coronavirus-analysis/index.html. [cited 2020 August 5] [Google Scholar]
- Davies A., Thompson K.-A., Giri K., Kafatos G., Walker J., Bennett A. Testing the efficacy of homemade masks: would they protect in an influenza pandemic? Disaster Med Public Health Prep. 2013;7(August (4)):413–418. doi: 10.1017/dmp.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divo M.J., Martinez C.H., Mannino D.M. Ageing and the epidemiology of multimorbidity. Eur Respir J. 2014;44(October (4)):1055–1068. doi: 10.1183/09031936.00059814. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4918092/. [cited 2020 June 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Hancock B., Sarakatsannis J., Viruleg E. 2020. Achievement gap and coronavirus | McKinsey. Available from: https://www.mckinsey.com/industries/public-sector/our-insights/covid-19-and-student-learning-in-the-united-states-the-hurt-could-last-a-lifetime. [cited 2020 July 9] [Google Scholar]
- Eikenberry S.E., Mancuso M., Iboi E., Phan T., Eikenberry K., Kuang Y. To mask or not to mask: modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. Infect Dis Model. 2020;5(January):293–308. doi: 10.1016/j.idm.2020.04.001. Available from: http://www.sciencedirect.com/science/article/pii/S2468042720300117. [cited 2020 July 9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Shen C., Xia N., Song W., Fan M., Cowling B.J. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020;8(May (5)):434–436. doi: 10.1016/S2213-2600(20)30134-X. Available from: https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30134-X/abstract. [cited 2020 July 15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes N. Social Science Research Network; Rochester, NY: 2020. Economic effects of coronavirus outbreak (COVID-19) on the world economy.https://papers.ssrn.com/abstract=3557504 March. Report No.: ID 3557504. Available from: [Google Scholar]
- Ferretti L., Wymant C., Kendall M., Zhao L., Nurtay A., Abeler-Dörner L. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;(March) doi: 10.1126/science.abb6936. Available from: https://www.sciencemag.org/lookup/doi/10.1126/science.abb6936. [cited 2020 April 16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaxman S., Mishra S., Gandy A., Unwin H.J.T., Mellan T.A., Coupland H. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584(August (7820)):257–261. doi: 10.1038/s41586-020-2405-7. Available from: https://www.nature.com/articles/s41586-020-2405-7. [cited 2020 August 24] [DOI] [PubMed] [Google Scholar]
- Garg S. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020:69. doi: 10.15585/mmwr.mm6915e3. Available from: https://www.cdc.gov/mmwr/volumes/69/wr/mm6915e3.htm. [cited 2020 June 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020:69. doi: 10.15585/mmwr.mm6915e3. Available from: https://www.cdc.gov/mmwr/volumes/69/wr/mm6915e3.htm. [cited 2020 June 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto M., Bertuzzo E., Mari L., Miccoli S., Carraro L., Casagrandi R. Spread and dynamics of the COVID-19 epidemic in Italy: effects of emergency containment measures. Proc Natl Acad Sci U S A. 2020;117(May (19)):10484–10491. doi: 10.1073/pnas.2004978117. Available from: http://www.pnas.org/lookup/doi/10.1073/pnas.2004978117. [cited 2020 June 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(May (5)):672–675. doi: 10.1038/s41591-020-0869-5. Available from: http://www.nature.com/articles/s41591-020-0869-5. [cited 2020 June 10] [DOI] [PubMed] [Google Scholar]
- Johns Hopkins University . 2020. COVID-19 map. Johns Hopkins Coronavirus Resource Center. Available from: https://coronavirus.jhu.edu/map.html. [cited 2020 July 9] [Google Scholar]
- Khosrawipour V., Lau H., Khosrawipour T., Kocbach P., Ichii H., Bania J. Failure in initial stage containment of global COVID-19 epicenters. J Med Virol. 2020;92(7):863–867. doi: 10.1002/jmv.25883. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/jmv.25883. [cited 2020 August 24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konda A., Prakash A., Moss G.A., Schmoldt M., Grant G.D., Guha S. Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano. 2020;14(May (5)):6339–6347. doi: 10.1021/acsnano.0c03252. [DOI] [PubMed] [Google Scholar]
- Lau H., Khosrawipour V., Kocbach P., Mikolajczyk A., Schubert J., Bania J. The positive impact of lockdown in Wuhan on containing the COVID-19 outbreak in China. J Travel Med. 2020;27(May (3)) doi: 10.1093/jtm/taaa037. Available from: https://academic.oup.com/jtm/article/27/3/taaa037/5808003. [cited 2020 August 24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(March (9)):577–582. doi: 10.7326/M20-0504. Available from: https://www.acpjournals.org/doi/10.7326/M20-0504. [cited 2020 June 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(March (13)):1199–1207. doi: 10.1056/NEJMoa2001316. Available from: http://www.nejm.org/doi/10.1056/NEJMoa2001316. [cited 2020 June 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Pei S., Chen B., Song Y., Zhang T., Yang W. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368(May (6490)):489–493. doi: 10.1126/science.abb3221. Available from: https://science.sciencemag.org/content/368/6490/489. [cited 2020 June 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre C.R., Seale H., Dung T.C., Hien N.T., Nga P.T., Chughtai A.A. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open. 2015;5(April (4)) doi: 10.1136/bmjopen-2014-006577. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4420971/. [cited 2020 July 9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(March (10)) doi: 10.2807/1560-7917.ES.2020.25.10.2000180. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7078829/. [cited 2020 June 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadas S.M., Fitzpatrick M.C., Sah P., Pandey A., Shoukat A., Singer B.H. The implications of silent transmission for the control of COVID-19 outbreaks. PNAS. 2020;(July) doi: 10.1073/pnas.2008373117. Available from: https://www.pnas.org/content/early/2020/07/02/2008373117. [cited 2020 July 9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadas S.M., Shoukat A., Fitzpatrick M.C., Wells C.R., Sah P., Pandey A. Projecting hospital utilization during the COVID-19 outbreaks in the United States. PNAS. 2020;117(April (16)):9122–9126. doi: 10.1073/pnas.2004064117. Available from: https://www.pnas.org/content/117/16/9122. [cited 2020 June 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal A., Das A., Goswami R.P. Utility of cloth masks in preventing respiratory infections: a systematic review. medRxiv. 2020;(May) Available from: https://www.medrxiv.org/content/10.1101/2020.05.07.20093864v1. [cited 2020 July 9];2020.05.07.20093864. [Google Scholar]
- Mossong J., Hens N., Jit M., Beutels P., Auranen K., Mikolajczyk R. Social contacts and mixing patterns relevant to the spread of infectious diseases. Riley S, editor. PLoS Med. 2008;5(March (3)):e74. doi: 10.1371/journal.pmed.0050074. Available from: https://dx.plos.org/10.1371/journal.pmed.0050074. [cited 2020 May 22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngonghala C.N., Iboi E., Eikenberry S., Scotch M., MacIntyre C.R., Bonds M.H. Mathematical assessment of the impact of non-pharmaceutical interventions on curtailing the 2019 novel Coronavirus. Math Biosci. 2020;325(July) doi: 10.1016/j.mbs.2020.108364. Available from: http://www.sciencedirect.com/science/article/pii/S0025556420300560. [cited 2020 July 9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H., Kobayashi T., Miyama T., Suzuki A., Jung S.-M., Hayashi K. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020;94:154–155. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo LJ Daniele, Brown D. Coronavirus: a visual guide to the economic impact. BBC News. 2020;(June) Available from: https://www.bbc.com/news/business-51706225. [cited 2020 July 9] [Google Scholar]
- Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N., Ke R. The novel coronavirus, 2019-nCoV, is highly contagious and more infectious than initially estimated. medRxiv. 2020;(February) Available from: https://www.medrxiv.org/content/10.1101/2020.02.07.20021154v1. [cited 2020 June 10];2020.02.07.20021154. [Google Scholar]
- SeroTracker . 2020. COVID-19 seroprevalence. Available from: https://serotracker.com/Dashboard. [cited 2020 July 9] [Google Scholar]
- Shekerdemian L.S., Mahmood N.R., Wolfe K.K., Riggs B.J., Ross C.E., McKiernan C.A. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;(May) doi: 10.1001/jamapediatrics.2020.1948. Available from: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2766037. [cited 2020 July 9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoukat A., Wells C.R., Langley J.M., Singer B.H., Galvani A.P., Moghadas S.M. Projecting demand for critical care beds during COVID-19 outbreaks in Canada. CMAJ. 2020;192(May (19)):E489–496. doi: 10.1503/cmaj.200457. Available from: http://www.cmaj.ca/lookup/doi/10.1503/cmaj.200457. [cited 2020 June 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes E.K., Zambrano L.D., Anderson K.N., Marder E.P., Raz K.M., El Burai Felix S. Coronavirus disease 2019 case surveillance — United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(June (24)):759–765. doi: 10.15585/mmwr.mm6924e2. Available from: http://www.cdc.gov/mmwr/volumes/69/wr/mm6924e2.htm?s_cid=mm6924e2_w. [cited 2020 June 23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutt R.O.J.H., Retkute R., Bradley M., Gilligan C.A., Colvin J. A modelling framework to assess the likely effectiveness of facemasks in combination with ‘lock-down’ in managing the COVID-19 pandemic. Proc R Soc A Math Phys Eng Sci. 2020;476(June (2238)) doi: 10.1098/rspa.2020.0376. Available from: https://royalsocietypublishing.org/doi/10.1098/rspa.2020.0376. [cited 2020 July 9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lancet India under COVID-19 lockdown. Lancet. 2020;395(10233):1315. doi: 10.1016/S0140-6736(20)30938-7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7180023/. [cited 2020 August 24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau QuickFacts: United States . 2020. Population demographics. Available from: https://www.census.gov/quickfacts/fact/table/US/PST045219. [cited 2020 April 16] [Google Scholar]
- Weinberger D.M., Chen J., Cohen T., Crawford F.W., Mostashari F., Olson D. Estimation of excess deaths associated with the COVID-19 pandemic in the United States, March to May 2020. JAMA Intern Med. 2020;(July) doi: 10.1001/jamainternmed.2020.3391. Available from: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2767980. [cited 2020 July 9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. WHO coronavirus disease (COVID-19) dashboard. Available from: https://covid19.who.int/?gclid=EAIaIQobChMI-pzkyub56QIVGKSzCh3W_wE2EAAYASAAEgKUPvD_BwE. [cited 2020 June 11] [Google Scholar]
- Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(February (10225)):689–697. doi: 10.1016/S0140-6736(20)30260-9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673620302609. [cited 2020 June 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(May (5)):475–481. doi: 10.1016/S2213-2600(20)30079-5. Available from: https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30079-5/abstract. [cited 2020 June 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Details of the model are provided in the Appendix. The computational model is available at https://github.com/thomasvilches/covid-shelterin.