Abstract
Purpose of review
The purpose of this review is to describe the variability of obstructive sleep apnea (OSA), both from a standpoint of underlying mechanisms and in terms of clinical manifestations.
Recent findings
Recent data suggest that not all patients with sleep apnea get their disease for the same reason. As such, no one variable is effective at defining which patients do or do not have sleep apnea. Identifying the mechanism(s) underlying OSA for an individual is helpful as it can help to determine whether personalized therapy could be developed based on an individual’s characteristics. In addition, these underlying mechanisms may be helpful in predicting response to therapy and prognosticating regarding future complications.
Summary
OSA is a heterogeneous disease with highly varying underlying mechanisms. OSA has variable clinical manifestations with definable subsets having risk of particular complications. Future studies will be helpful to identify mechanisms underlying OSA using clinically accessible tools and then using these data to focus individualized treatment approaches.
Keywords: airway, apnea, lung, sleep, ventilation
INTRODUCTION
Obstructive sleep apnea (OSA) affects nearly one billion people worldwide, with substantial associated morbidity, mortality, and financial and social costs [1▀ ▀]. OSA is a disease characterized by repetitive pharyngeal collapse during sleep [2]. This intermittent collapse results in reduced airflow which has two sets of consequences: arousal from sleep to resume airflow, and gas exchange disturbances from hypopneas (reductions in breathing) or apneas (cessations of breathing) [3]. The recurrent arousals in OSA lead to neurocognitive consequences including diminished memory consolidation, daytime sleepiness, and reduced quality of life [4]. Hypoxemia and hypercapnia with resulting catecholamine surges, oxidative stress, and low-grade inflammation are thought to underlie the cardio-metabolic consequences of OSA [5]. However, the mechanisms contributing to these adverse health outcomes of OSA are complex and incompletely understood.
Recent studies have suggested that OSA is a heterogeneous disease both from the standpoint of underlying mechanisms (endotypes) and in its clinical expression (phenotypes) [6–8]. Disease heterogeneity has been long recognized in the cancer field, but more recently has been discussed in other respiratory disorders such as asthma (e.g. allergic, occupational, exercise-induced, cough-variant), chronic obstructive pulmonary disease (e.g. pink puffers and blue bloaters, but also small airways fibrosis and other manifestations), acute respiratory distress syndrome (hypo-inflammatory, hyper inflammatory, variable lung compliance, and response to positive pressure ventilation) [9]. The recognition of this heterogeneity in disease states is potentially helpful because therapy can sometimes be personalized based on individual characteristics [6]. Other biological insights such as predictive models and genetic susceptibility are more likely to be fruitful if homogeneous patient groups can be identified rather than searching in broad groups of disease e.g. analogous to searching for a single ‘cancer’ gene for all types of cancers.
Although individuals with OSA are generally categorized under the same universal diagnosis, the diagnosis of OSA carries with it variable and complex underlying mechanisms and various clinical and polysomnographic features. The OSA diagnosis is usually defined by a single data point captured by polysomnography or polygraphy, the apnea-hypopnea index, but recent studies have demonstrated that this simplistic approach has the potential to underestimate the complex heterogeneity of OSA. Determining individual risks and anticipating treatment responses (both in routine practice and for designing clinical trials) requires a better characterization of the disease complexity. There is growing evidence that a new delineation of specific OSA subgroups is the prerequisite for the development of precision and personalized medicine in this field. [10▀ ▀,11,12,13]
Obstructive sleep apnea endotypes
The pathogenesis of OSA is generally accepted to be highly variable. The underlying endotypes include anatomical compromise, impaired pharyngeal dilator muscle function, unstable ventilatory control (elevated loop gain), and low arousal threshold (being predisposed to wake up with respiratory disturbances) [14,15]. Other factors such as end expiratory lung volume, arousal intensity, and redistribution of body fluid, are also likely important [16–18]. The studies on anatomical compromise have consisted of imaging and sophisticated measures of mechanics under passive conditions [19,20▀]. In general, patients with OSA have anatomical compromise as compared to matched controls, but considerable overlap exists since pharyngeal anatomy explains only a portion of the variance in the occurrence and severity of OSA [21,22]. Thus, anatomy is not the whole story, perhaps explaining the variable benefits seen with surgical interventions directed primarily at anatomical compromise.
Pharyngeal dilator muscle function is also important, because patients with OSA have been shown to have increased activity in the major dilator muscles during wakefulness as compared to matched controls [23]. Compensatory reflexes exist during wakefulness which increase the activity of these muscles; however, with the onset of sleep there is a loss of protective reflexes which yields a fall in pharyngeal dilator muscle activity, resulting in collapse in those who are anatomically susceptible [24]. The negative pressure reflex (NPR) refers to the robust activation of the pharyngeal dilator muscles in response to a collapsing (subatmospheric) perturbation. The NPR has been shown to be highly variable both during wakefulness and during sleep, implying that a therapy to augment this reflex would also be predicted to have variable efficacy [25]. Of note, muscle activation, as assessed by EMG, is an important component of muscle activity but abnormalities of electrical–mechanical coupling have also been reported in some OSA studies [26–28]. The importance of these upper airway muscles and reflexes is emphasized by the observation that these muscles are both necessary and sufficient to stabilize breathing if adequate respiratory stimuli (negative pressure, carbon dioxide) can accumulate with sufficient magnitude and for adequate duration [29]. For example, some very obese patients do not have OSA, because of robust upper airway muscle responses [30].
Regarding unstable ventilatory control, engineers use the concept of ‘loop gain’, which is a term that has been adapted into the medical literature. An elevated loop gain refers to a system which is unstable with perturbation, whereas a low loop gain system describes one that is intrinsically stable [17,31]. An analogy to room temperature is often helpful when considering the factors that can lead to instability in negative feedback control. Although the human body generally tries to maintain a PaCO2of roughly 40 mmHg, the temperature in a room is generally set to roughly 208C. The temperature in a room might be prone to oscillation if the thermostat were too sensitive. That is, if a change to 19.998C were enough to turn on a furnace, the temperature in the room would likely overshoot and the result would be fluctuations in room temperature [32]. By analogy, if an individual had robust chemosensitivity (high loop gain), such that for a minor change in PaCO2there resulted a major change in ventilation, there would similarly be fluctuations in PaCO2[33]. Another analogy would be a furnace that was too powerful, such that a temperature fall to 198C led to a major output of the furnace to 708C, there would clearly be risk of major fluctuations in room temperature. By analogy, if an individual were to wake up from sleep with a PaCO2of 45 mmHg, and the ventilatory response led to a fall in a PaCO2down to 10 mmHg (below the chemical apnea threshold), there would clearly be an unstable control system and marked fluctuations in PaCO2would be predictable [34].
The classic breathing pattern defined by high loop gain is Cheyne–Stokes breathing, or periodic breathing, as seen at high altitude [35]. Elevated loop gain, however, is also thought to be important in OSA, and several classic studies have showed an important role of high loop gain in OSA [17,31]. Fluctuations in output from the central pattern generator in the brainstem affect the activity of both the diaphragm and the upper airway dilator muscles. As a result, the upper airway may be vulnerable to collapse when output to the upper airway dilator muscles is at its nadir, particularly in those who are anatomically susceptible. High loop gain is important particularly in a subset of individuals in whom anatomical factors are not the predominant mechanism underlying the presence or absence of OSA [36]. High loop gain has also been shown to predict failure of upper airway surgery, presumably because the surgery does not address the underlying abnormalities in control of breathing [37]. Efforts to lower loop gain using either oxygen or acetazolamide have been helpful, although these studies have also emphasized the complexity of the situation given the multiple interacting variables [38].
Some patients with OSA have a propensity to wake up readily in response to respiratory disturbance; this inclination to easy arousal from sleep is termed low arousal threshold. Several points deserve emphasis:
A low arousal threshold has been demonstrated in roughly one-third of patients with OSA [15].
Premature awakening from sleep, as occurs with low arousal threshold, might lead to insufficient accumulation of respiratory stimuli to activate the pharyngeal dilator muscles. As such, repetitive apnea may result [39].
Agents which raise the arousal threshold, such as hypnotic agents like trazodone or eszopiclone, may have a role to allow more accumulation of respiratory stimuli and thus activate dilator muscles which could stabilize breathing. Some physiological studies have shown potential benefits although hard outcome data are lacking [40].
The arousal threshold interacts in complex ways with other pathophysiological traits, because the stimulus to arouse from sleep is thought to be negative intrathoracic pressure. Thus, factors that affect control of breathing can impact the time to arousal, depending on ventilatory drive and prevailing mechanics [16].
OSA endotypes are worth consideration for several reasons (see Fig. 1). First, as stated, the endo types may guide individualized therapies to allow interventions to be guided based on underlying mechanism. In some cases, with multiple underlying mechanisms, combination therapies may be needed to yield an acceptable result [38]. Second, underlying endotypes of OSA may be related to risk factors for disease. For example, posttraumatic stress disorder is a known risk factor for OSA, which may reflect a low arousal threshold seen in some of these patients [41]. Moreover, patients with neuro muscular disease commonly have OSA, perhaps as a result of underlying upper airway dilator muscle dysfunction. Third, the endotypes of OSA may be important predictors of therapeutic response to clinical interventions. For instance, a high loop gain is predictive of treatment-emergent central sleep apnea (complex sleep apnea). High loop gain and low arousal threshold can also predict failure of uvulopalatopharyngoplasty surgery. Moreover, patients with low arousal threshold are reported to have decreased adherence to positive airway pressure (PAP) therapy compared to those with normal arousal threshold [42]. Fourth, the recognition for the variability in clinical expression of OSA (phenotypes) has led to the assertion that OSA endotypes may predict disease complications. These findings amplify the potential importance of OSA pathogenesis in guiding clinical management. Nonetheless, these concepts have not been widely adopted into clinical practice because of rigorous outcome data remain sparse, and simplified methods to identify the various pathophysiological traits clinically are still evolving. [43].
FIGURE 1.
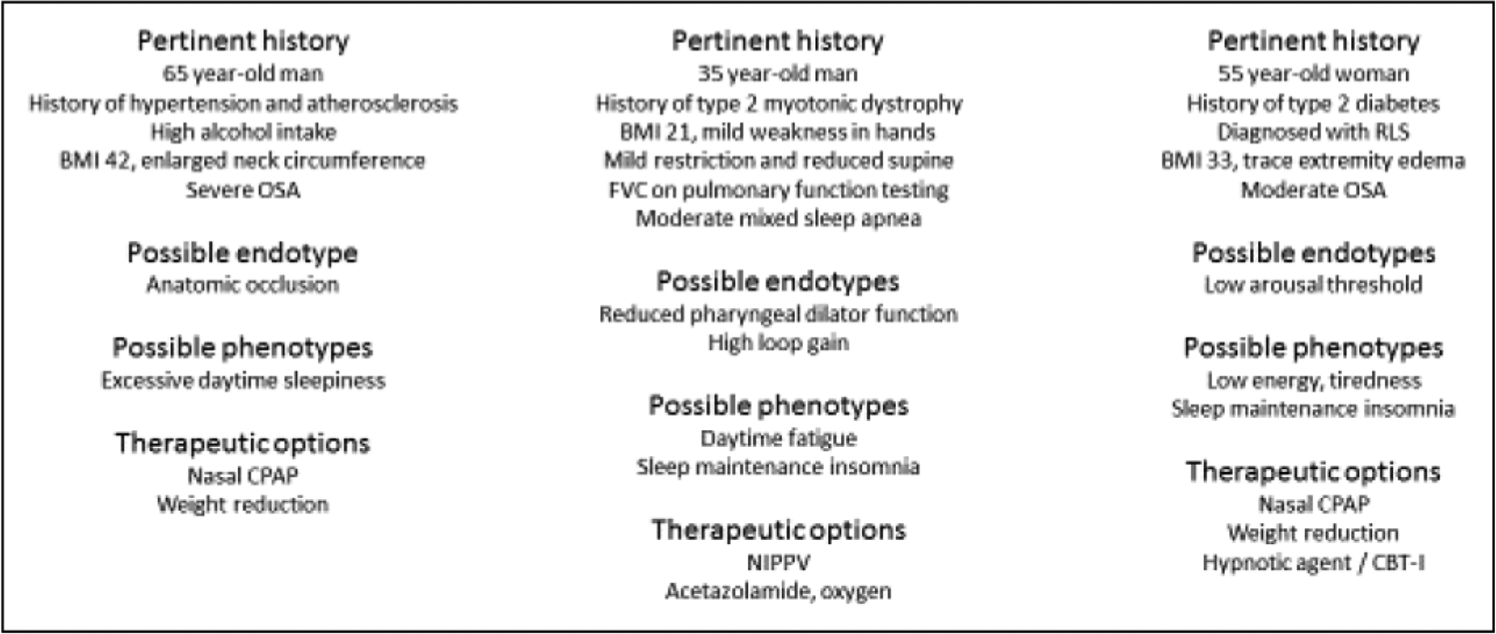
Three factitious patients to illustrate the potential variability in underlying mechanisms and clinical manifestations of obstructive sleep apnea (BMI, body mass index; CBTI, cognitive behavioral therapy for insomnia; CPAP, continuous positive airway pressure; NIPPV, noninvasive positive pressure ventilation; OSA, obstructive sleep apnea; RLS, restless legs syndrome).
Obstructive sleep apnea phenotypes
Ye et al. was one of the first teams to perform cluster analyses on a cohort of patients with OSA [7], defining three distinct groups: sleepiness with cardiovascular risk [8], disrupted sleep with insomnia, and asymptomatic patients. Further work by these authors has shown little symptomatic improvement in those ‘asymptomatic’ patients, whereas sleepy patients who adhere to PAP therapy become less somnolent. In addition, sleepy patients are apparently those patients with OSA at greatest cardiovascular risk [44,45].
OSA phenotypes have been principally identified through different methodologies of cluster analysis such as latent class analysis, hierarchical ascending clustering, or K-means clustering. The goal of these efforts has been to identify clearly distinct subgroups of OSA with minimal differences between two individuals within a same phenotype and clear differences between two individuals having distinct phenotypes. Across previous studies, anywhere from three to seven clusters have been identified in such analyses, the number depending on the clustering method employed, and the different selections and combinations of variables included in the clustering analyses. Some studies have been based on the anatomic and/or craniofacial characteristics of OSA patients, others on symptoms, polysomnographic indices, or comorbidities. A combination of comorbidities and symptoms has been mostly used. Few studies have included extensive combinations of lifestyle, clinical, polysomnographic, and comorbidity variables. The majority of studies have been based on local or national databases whereas geographical factors, lifestyle, behaviors and genetic background might also impact OSA phenotypes [46].
The importance of these phenotypic clusters has drawn increasing attention in part because of the failure of some large multicenter trials to improve cardiovascular risk of OSA [47]. Given that only a subset of patients appear to be at OSA-related cardiovascular risk [8], the finding that a large group of patients with OSA does not derive cardiovascular benefit from OSA treatment is perhaps not surprising [48]. Major efforts are underway to identify those patients who are likely to be at high cardiovascular risk so that such patients can be identified prospectively in designing interventional trials.
Several approaches may be viable to identify patients with OSA who may be at high cardiovascular risk:
Certain endotypes may be important in predicting cardiovascular risk. For example, short respiratory events, perhaps associated with high loop gain and low arousal threshold, have been associated with cardiovascular risk [49]. Short respiratory events compared to longer events are typically those with minimal desaturation. In contrast, hypoxic burden, based on the area under the hypoxia curve, is predictive of cardiovascular risk. Marked hypoxemia can be a marker of high arousal threshold [15,50▀]. Thus, the data are not consistent regarding the epidemiological findings, emphasizing the need for further mechanistic research.
Cardiovascular biomarkers, or panels of biomarkers, may be useful in predicting cardiovascular risk [51]. hsCRP is a robust marker of cardiovascular risk but has been inconsistent in its association with OSA versus obesity. A panel of biomarkers could assess sympathoexcitation (e.g. muscle sympathetic nerve activity, heart rate variability), oxidative stress (e.g. lipid peroxidation), and inflammatory path ways (interleukin-6, hsCRP), and may be useful to optimize the prediction of OSA-associated cardiovascular risk [51–53].
Novel biomarkers such as microRNAs, the air way or gut microbiome, metabolomics, exosomes, and other techniques are now being assessed in the context of OSA-associated cardiovascular risk. Trimethylamine N-oxide (TMAO) is a cardiovascular biomarker which has been shown to be released by gut bacteria; antibiotics have been shown to suppress TMAO in humans. Recurrent hypoxia from OSA may lead to changes in gut bacteria which could release metabolites affecting cardiovascular risk. In theory, drug targets could block the impact of the metabolites thereby diminishing cardiovascular risk. Studies in rodents have shown proof of concept although human interventional data with hard outcomes are lacking [54–56].
The use of exosomes is being increasingly examined whereby extracellular vesicles acquired from humans can be examined in vitro to assess their impact on endothelial cell function and barrier permeability. Bhattacharjee et al. [57] have shown endothelial dysfunction induced by exosomes from humans with obesity hypoventilation syndrome; these abnormalities largely resolved after the patients were treated with PAP. Such approaches could be used in clinical trials since patients who had high cardiovascular risk could ostensibly be defined based on in vitro analyses of the participant’s exosomes.
A subset of patients with OSA do not respond well to PAP. Some individuals have treatment emergent central sleep apnea, which in some cases can persist in the long term (treatment persistent central sleep apnea) [58]. The natural history of these patients is unclear, but long-term adherence and cardiovascular protection may well be compromised. Individuals with high loop gain are those thought to be at risk of reduced PAP efficacy, and thus their disease may be particularly amenable to interventions targeting this endotype (e.g. acetazolamide or oxygen).
Adaptive trial designs are being used such that the outcomes and interventions in studies can be changed on an ongoing basis depending on response to therapies. Such studies have been used in the oncology world but are increasingly being applied to trials in sepsis, asthma, and other respiratory diseases. One example would be to enroll patients and, prior to randomization, determine for which outcomes a particular patient is at risk. In theory some patients could be enrolled to assess Alzheimer’s risk (perhaps based on impaired memory or ApoE status), whereas others could be enrolled examining cardiovascular outcomes (perhaps based on endothelial dysfunction or other biomarkers), and yet others may be likely to remain asymptomatic and thus treatment benefits would be difficult to demonstrate [52,53,59▀ ▀,60,61].
CONCLUSION
Major progress has been made in the OSA field via recognition of the heterogeneity of this disease, both in terms of its pathogenesis (endotypes) and its expression (phenotypes). Only through further mechanistic and multidisciplinary clinical translational and basic research are new therapeutic targets and approaches likely to emerge. Further research is also needed for recognizing sex-based phenotypes and the impact of these subtypes on treatment prescription. The majority of the relevant studies have been cross-sectional and the impact on treatment adherence or prognosis of these clusters remains to be studied ideally by combining existing databases in large international consortia.
KEY POINTS.
OSA is a heterogeneous disease with multiple underlying mechanisms.
The mechanism underlying OSA might affect clinical manifestations.
The clinical presentation of OSA is highly variable with some patients asymptomatic and others at major cardiovascular risk.
Further research regarding OSA endotypes and phenotypes is imperative.
Financial support and sponsorship
A.M. is funded by NHLBI and NIA. O.M. is funded by K08. R.L.O. is funded by NHLBI RO1.
Footnotes
Conflicts of interest A.M. reports income from Merck and Livanova related to medical education. ResMed provided a philanthropic donation for UC San Diego.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▀ of special interest
▀ ▀ of outstanding interest
- 1.▀ ▀.Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 2019; 7:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]; The paper estimated the global prevalence of sleep apnea based on published studies and available demographic variables. Using publicly available data the authors estimated global prevalence of OSA up to 1 billion people.
- 2.Phillipson EA. Control of breathing during sleep. Am Rev Respir Dis 1978; 118:909–939. [DOI] [PubMed] [Google Scholar]
- 3.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 2010; 90:47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet 1999; 353:2100–2105. [DOI] [PubMed] [Google Scholar]
- 5.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med 2005; 142:187–197. [DOI] [PubMed] [Google Scholar]
- 6.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014; 383:736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye L, Pien GW, Ratcliffe SJ, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J 2014; 44:1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazzotti DR, Keenan BT, Lim DC, et al. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med 2019; 200:493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014; 2:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.▀ ▀.Zinchuk A, Yaggi HK. Phenotypic subtypes of OSA: a challenge and opportunity for precision medicine. Chest 2020; 157:403–420. [DOI] [PMC free article] [PubMed] [Google Scholar]; A summary of the existing literature emphasizing the heterogeneity of sleep apnea.
- 11.Zinchuk AV, Gentry MJ, Concato J, Yaggi HK. Phenotypes in obstructive sleep apnea: a definition, examples and evolution of approaches. Sleep Med Rev 2017; 35:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pepin JL, Bailly S, Tamisier R. Incorporating polysomnography into obstructive sleep apnoea phenotyping: moving towards personalised medicine for OSA. Thorax 2018; 73:409–411. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Garcia MA, Campos-Rodriguez F, Barbe F, et al. Precision medicine in obstructive sleep apnoea. Lancet Respir Med 2019; 7:456–464. [DOI] [PubMed] [Google Scholar]
- 14.Eckert DJ, White DP, Jordan AS, et al. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med 2013; 188:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards BA, Eckert DJ, McSharry DG, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2014; 190:1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Younes M Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med 2004; 169:623–633. [DOI] [PubMed] [Google Scholar]
- 17.Younes M, Ostrowski M, Thompson W, et al. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2001; 163:1181–1190. [DOI] [PubMed] [Google Scholar]
- 18.Redolfi S, Arnulf I, Pottier M, et al. Attenuation of obstructive sleep apnea by compression stockings in subjects with venous insufficiency. Am J Respir Crit Care Med 2011; 184:1062–1066. [DOI] [PubMed] [Google Scholar]
- 19.Isono S, Morrison DL, Launois SH, et al. Static mechanics of the velopharynx of patients with obstructive sleep apnea. J Appl Physiol 1993; 75:148–154. [DOI] [PubMed] [Google Scholar]
- 20.▀.Wang SH, Keenan BT, Wiemken A, et al. Effect of weight loss on upper airway & anatomy and the apnea-hypopnea index. The importance of tongue fat. Am J Respir Crit Care Med 2020; 201:718–727. [DOI] [PMC free article] [PubMed] [Google Scholar]; An imaging study defining the potential important of tongue fat in OSA pathogenesis.
- 21.Sforza E, Bacon W, Weiss T, et al. Upper airway collapsibility and cephalometric variables in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2000; 161:347–352. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz AR, Smith PL, Wise RA, et al. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol 1988; 64:535–542. [DOI] [PubMed] [Google Scholar]
- 23.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal EMG in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanisms). J Clin Invest 1992; 89:1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathew OP. Upper airway negative pressure effects on respiratory activity of upper airway muscles. J Appl Physiol 1984; 56:500. [DOI] [PubMed] [Google Scholar]
- 25.Malhotra A, Pillar G, Fogel R, et al. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med 2000; 162:1058–1062. [DOI] [PubMed] [Google Scholar]
- 26.Jordan AS, Wellman A, Heinzer RC, et al. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax 2007; 62:861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordan AS, White DP, Lo YL, et al. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep 2009; 32:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer P, Dematteis M, Pepin JL, et al. Peripheral neuropathy in sleep apnea. A tissue marker of the severity of nocturnal desaturation. Am J Respir Crit Care Med 1999; 159:213–219. [DOI] [PubMed] [Google Scholar]
- 29.Stanchina ML, Malhotra A, Fogel RB, et al. Genioglossus muscle responsiveness to chemical and mechanical stimuli during nonrapid eye movement sleep. Am J Respir Crit Care Med 2002; 165:945–949. [DOI] [PubMed] [Google Scholar]
- 30.Sands SA, Eckert DJ, Jordan AS, et al. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med 2014; 190: 930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khoo M Determinants of ventilatory instability and variability. Respir Physiol 2000; 122:167–182. [DOI] [PubMed] [Google Scholar]
- 32.Malhotra A, Jordan AS. Did fat boy Joe need hormone replacement? Sleep 2006; 29:16–18. [PubMed] [Google Scholar]
- 33.Javaheri S A mechanism of central sleep apnea in patients with heart failure. N Engl J Med 1999; 23:985–987. [DOI] [PubMed] [Google Scholar]
- 34.Salloum A, Rowley JA, Mateika JH, et al. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med 2010; 181:189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orr JE, Heinrich EC, Djokic M, et al. Adaptive servoventilation as treatment for central sleep apnea due to high-altitude periodic breathing in nonacclimatized healthy individuals. High Alt Med Biol 2018; 19:178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med 2004; 170:1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Ye J, Han D, et al. The effect of upper airway surgery on loop gain in obstructive sleep apnea. J Clin Sleep Med 2019; 15:907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sands SA, Edwards BA, Terrill PI, et al. Identifying obstructive sleep apnoea patients responsive to supplemental oxygen therapy. Eur Respir J 2018; 52:1800674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. [Review] [83 refs]. Sleep 1997; 20:654–675. [DOI] [PubMed] [Google Scholar]
- 40.Smales ET, Edwards BA, Deyoung PN, et al. Trazodone effects on obstructive sleep apnea and non-REM arousal threshold. Ann Am Thorac Soc 2015; 12:758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orr JE, Smales C, Alexander TH, et al. Treatment of OSA with CPAP is associated with improvement in PTSD symptoms among veterans. J Clin Sleep Med 2017; 13:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zinchuk A, Edwards BA, Jeon S, et al. Prevalence, associated clinical features, and impact on continuous positive airway pressure use of a low respiratory arousal threshold among male united states veterans with obstructive sleep apnea. J Clin Sleep Med 2018; 14:809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orr JE, Sands SA, Edwards BA, et al. Measuring loop gain via home sleep testing in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2018; 197:1353–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vavougios GD, George DG, Pastaka C, et al. Phenotypes of comorbidity in OSAS patients: combining categorical principal component analysis with cluster analysis. J Sleep Res 2016; 25:31–38. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Keenan BT, Lim DC, et al. Symptom-based subgroups of Koreans with obstructive sleep apnea. J Clin Sleep Med 2018; 14:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailly S, Destors M, Grillet Y, et al. , scientific council and investigators of the French national sleep apnea registry (OSFP). Obstructive sleep apnea: a cluster analysis at time of diagnosis. PLoS One 2016; 11: e0157318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McEvoy RD, Antic NA, Heeley E, et al. , Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375:919–931. [DOI] [PubMed] [Google Scholar]
- 48.Zheng D, Xu Y, You S, et al. Effects of continuous positive airway pressure on depression and anxiety symptoms in patients with obstructive sleep apnoea: results from the sleep apnoea cardiovascular endpoint randomised trial and meta-analysis. EClinicalMedicine 2019; 11:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butler MP, Emch JT, Rueschman M, et al. Apnea-hypopnea event duration predicts mortality in men and women in the sleep heart health study. Am J Respir Crit Care Med 2019; 199:903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.▀.Azarbarzin A, Sands SA, Stone KL, et al. The hypoxic burden of sleep apnoea & predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J 2019; 40:1149–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]; An analysis of the hypoxic burden (area under the hypoxia curve) as a predictor of cardiovascular disease related mortality.
- 51.Montesi SB, Bajwa EK, Malhotra A. Biomarkers of sleep apnea. Chest 2012; 142:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren R, Covassin N, Zhang Y, et al. Interaction between slow wave sleep and obstructive sleep apnea in prevalent hypertension. Hypertension 2020; 75:516–523. [DOI] [PubMed] [Google Scholar]
- 53.Venkataraman S, Vungarala S, Covassin N, Somers VK. Sleep apnea, hypertension and the sympathetic nervous system in the adult population. J Clin Med 2020; 9:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011; 472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013; 368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xue J, Zhou D, Poulsen O, et al. Intermittent hypoxia and hypercapnia accelerate atherosclerosis, partially via trimethylamine-oxide. Am J Respir Cell Mol Biol 2017; 57:581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhattacharjee R, Khalyfa A, Khalyfa AA, et al. Exosomal cargo properties, endothelial function and treatment of obesity hypoventilation syndrome: a proof of concept study. J Clin Sleep Med 2018; 14:797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stanchina M, Robinson K, Corrao W, et al. Clinical use of loop gain measures to determine continuous positive airway pressure efficacy in patients with complex sleep apnea. A pilot study. Ann Am Thorac Soc 2015; 12:1351–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.▀ ▀.Djonlagic I, Guo M, Igue M, et al. REM-related obstructive sleep apnea: when && does it matter? Effect on motor memory consolidation versus emotional health. J Clin Sleep Med 2020; 16:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]; A recent study defining the impact of OSA during REM sleep on various outcome measures.
- 60.Treptow E, Pepin JL, Bailly S, et al. Reduction in sympathetic tone in patients with obstructive sleep apnoea: is fixed CPAP more effective than APAP? A randomised, parallel trial protocol. BMJ Open 2019; 9:e024253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joyeux-Faure M, Baguet JP, Barone-Rochette G, et al. Continuous positive airway pressure reduces night-time blood pressure and heart rate in patients with obstructive sleep apnea and resistant hypertension: the RHOOSAS randomized controlled trial. Front Neurol 2018; 9:318. [DOI] [PMC free article] [PubMed] [Google Scholar]


