Abstract
Hypertensive disorders of pregnancy (HDP) have been associated with heart failure (HF). It is unknown whether concurrent pregnancy complications (small-for-gestational age [SGA] or preterm delivery) or recurrent HDP modify HDP-associated HF risk. In this cohort study, we included Norwegian women with a first birth between 1980–2004. Follow-up occurred through 2009. Cox models examined gestational hypertension (GH) and preeclampsia in the first pregnancy as predictors of a composite of HF-related hospitalization or HF-related death, with assessment of effect modification by concurrent SGA and/or preterm delivery. Additional models were stratified by final parity (1 vs. ≥2 births) and tested associations with recurrent HDP. Among 508,422 women, 565 experienced incident HF over a median 11.8 years of follow-up. After multivariable adjustment, GH in the first birth was not significantly associated with HF (hazard ratio [HR] 1.41, 95% CI 0.84–2.35, P=0.19), whereas preeclampsia was associated with an HR of 2.00 (95% CI 1.50–2.68, P<0.001). Among women with HDP, risks were not modified by concurrent SGA and/or preterm delivery (Pinteraction = 0.42). Largest hazards of HF were observed in women whose only lifetime birth was complicated by preeclampsia and women with recurrent preeclampsia. HF risks were similar after excluding women with coronary artery disease. In summary, women with preeclampsia, especially those with one lifetime birth and those with recurrent preeclampsia, experienced increased HF risk compared to women without HDP. Further research is needed to clarify causal mechanisms.
Keywords: cardio-obstetrics, cardiovascular epidemiology, hypertension in pregnancy, heart failure, preeclampsia, women’s health
Graphical Abstract
Introduction
Hypertensive disorders of pregnancy (HDP, i.e., gestational hypertension [GH]; preeclampsia; eclampsia; and hemolysis, elevated liver enzymes, and low platelet [HELLP] syndrome) affect 5–10% of pregnancies worldwide.1,2 HDP are associated with a roughly two-fold increase in future maternal cardiovascular disease risk.3–6 Cardiovascular risks associated with HDP are best described for atherosclerotic cardiovascular disease (ASCVD, e.g., coronary artery disease [CAD] and ischemic stroke) but extend beyond ASCVD to include heart failure (HF) and cardiomyopathy.5,7–9 Although HDP are associated with peripartum cardiomyopathy,10 HDP may also be associated with long-term HF risk separate from peripartum cardiomyopathy.9
Other pregnancy complications, such as small-for-gestational age (SGA) and preterm delivery, are independently associated with increased future maternal ASCVD disease risk11–13 and modify ASCVD risk when they accompany HDP.13 In addition, ASCVD risks associated with recurrent HDP are greater than those associated with single-episode HDP.13,14 However, whether concurrent pregnancy complications (SGA and preterm delivery) or recurrent HDP also modify HF risk among women with HDP remains unclear. Understanding the contribution of concurrent pregnancy complications to the HDP-associated HF risk distribution may help with clinical risk stratification and may yield insights regarding mechanisms of HDP-associated HF.
To better characterize HF risk in women with GH and preeclampsia, we studied HF incidence in a large nationwide cohort of Norwegian women. We hypothesized (1) that HDP would be associated with long-term HF risk, (2) that concurrent SGA and/or preterm delivery would further increase risk compared with HDP alone, and (3) that recurrent HDP would be associated with greater HF risk than single-episode HDP.
Methods
Study cohort
Researchers may apply for permission to analyze data from the Cardiovascular Disease in Norway Project (CVDNOR) at www.cvdnor.no. Women with a first birth between 1980–2004 recorded in the Medical Birth Registry of Norway (MBRN) who were 16–49 years old at the first birth and had ≤5 pregnancies prior to 2010 were considered for inclusion. The MBRN is a nationwide Norwegian registry with mandatory reporting, capturing all pregnancies since 1967 lasting 16 weeks’ gestation or more.15 To ascertain incident cardiovascular diagnoses, MBRN records were linked to the CVDNOR, a database containing inpatient diagnosis codes from all hospitalizations in Norway from 1994 onward, and to the Norwegian Cause of Death Registry. The Norwegian Regional Committee for Medical and Health Research Ethics and the Partners HealthCare institutional review board approved these analyses.
Women with pre-gestational chronic hypertension registered in the MBRN or with established cardiovascular disease (defined as International Classification of Diseases [ICD]-10 codes I00-I99, ICD-9 450–454 and 456–459, and ICD-8 390–448) identified in CVDNOR prior to the first birth were excluded, as were women with extreme birthweight outliers (Z-score >4 or <−4) in the first birth, multiple gestation in the first birth, missing data on gestational duration or birth weight in the first birth, and births occurring prior to 20 weeks’ gestation. To capture each woman’s full reproductive career, women with a first birth after 2004 were excluded, and follow-up began at the last birth; we therefore also excluded women who developed HF prior to the last birth. Women who emigrated during follow-up were excluded.
Exposures
GH and preeclampsia in the first pregnancy constituted the primary exposures, with assessment of effect modification by concurrent SGA and/or preterm delivery. (Most HDP cases occur in the first pregnancy.16) In primary analyses, women without HDP in the first pregnancy served as the reference group. In analyses of recurrent HDP, women with ≥2 lifetime births and normotension in all pregnancies constituted the reference group.
The MBRN defines GH as newly elevated blood pressure after 20 weeks’ gestation with systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg, without proteinuria. The MBRN defines preeclampsia as newly elevated blood pressure, using the same blood pressure criteria, accompanied by proteinuria >0.3 g/24 h or >1+ on urine dipstick, with high positive predictive value demonstrated in validation studies.17,18 Preterm delivery was defined as delivery occurring before 37 weeks’ gestation. SGA was defined as fetal weight below the 10th percentile of the Norwegian birth weight curve.19
CAD was incorporated as an exclusion in sensitivity analyses. CAD was defined as hospitalization or death from CAD as indicated by ICD-9 codes 410–414 and ICD-10 codes I20-I25, as in previous CVDNOR analyses.13
Outcome
The study outcome was a composite of HF-related hospitalization or death with HF as an underlying, contributing, or associated cause. HF cases were identified in CVDNOR20,21 and in the Norwegian Cause of Death Registry using ICD-9 codes 425.4 and 428.0–428.9 and using ICD-10 codes I42.0, I42.8, I42.9, I50.1-I50.4, and I50.9, encompassing both HF with reduced ejection fraction and HF with preserved ejection fraction.
Statistical analysis
The population attributable risk percent (i.e., the percent of HF cases in the cohort attributable to HDP) was calculated as the difference in absolute HF incidence in the overall study cohort vs. women without HDP divided by overall HF incidence. The attributable proportion of HF risk in women with HDP was calculated as the difference in absolute HF risk in women with vs. without HDP divided by absolute risk in women with HDP.
Cox proportional hazard models tested the association of HDP with incident HF. Follow-up began at the last birth in all models. Censoring occurred at the first HF hospitalization, death, or the end of the study period (December 31, 2009), whichever occurred first. Primary models were adjusted for maternal birth year (to capture period effects), maternal age at first birth, maternal age at last birth, chronic diabetes status at the time of the last birth, and final parity. To calculate associations with overall HDP and with HDP subtypes, separate models were run for all HDP vs. no HDP (reference) and for gestational hypertension vs. preeclampsia vs. no HDP (reference). To test effect modification by concurrent SGA and/or preterm delivery, models incorporated an interaction term between SGA and/or preterm delivery and HDP status. In addition, because prior data suggested HDP-associated cardiovascular mortality risk is highest among women with only one life-time birth (“one-child mothers”),22 we also stratified models by a final parity of 1 vs. ≥2 births; in stratified models, women with multiple normotensive pregnancies served as the reference group.
To assess HF risk with respect to a woman’s entire reproductive career, we grouped women into 10 categories according to HDP status in the first birth (no HDP, GH, or preeclampsia) and HDP status in the second and all later births (all normotensive, HDP in at least one birth, or no later births), adapting the approach used by Tanz et al.11 Additional Cox models tested associations of recurrent HDP and recurrent preeclampsia with HF.
CAD is known to be associated with both HDP and HF. To test whether HDP is associated with HF independent of CAD risk, sensitivity analyses excluded women who developed CAD during the study period.
The proportional hazards assumption was tested using Schoenfeld residuals. Two-sided P-values <0.05 were considered statistically significant. Analyses were performed using Stata version 16.0 (StataCorp, LLC, College Station, TX).
Results
Study cohort
After exclusions, the final study cohort included 508,422 women (Figure 1). There were no missing data in the final cohort. Participant counts by the number of lifetime episodes of GH and preeclampsia are summarized in Table S1.
Figure 1.

Creation of the study cohort.
A total of 46,084 women (9.1%) experienced HDP in any pregnancy, of whom 8,827 women (1.7% of the overall cohort) experienced GH in the first birth and 24,326 (4.8% of the overall cohort) experienced preeclampsia in the first birth (Table 1). Women with GH and especially those with preeclampsia were more likely than women without HDP to experience SGA and/or preterm delivery in the first birth (Table 1).
Table 1.
Baseline characteristics and outcomes of study subjects by hypertensive disorder of pregnancy status in the first birth.
| Characteristic | No hypertensive disorder of pregnancy (n = 475,269) | Gestational hypertension (n = 8,827) | Preeclampsia (n = 24,326) |
|---|---|---|---|
| Maternal age at first birth, y | 25.5 (4.7) | 26.3 (4.8) | 25.7 (4.8) |
| Married/co-habitant during first pregnancy | 393,084 (82.7%) | 7,646 (86.6%) | 20,645 (84.9%) |
| Chronic diabetes diagnosis prior to first birth | 1,495 (0.3%) | 62 (0.7%) | 369 (1.5%) |
| Preterm delivery in first birth | 25,996 (5.5%) | 609 (6.9%) | 4,910 (20.2%) |
| Small-for-gestational age delivery in first birth | 57,601 (12.1%) | 1,471 (16.7%) | 5,846 (24.0%) |
| Final parity during the study period, median (IQR) | 2 (2,3) | 2 (2,3) | 2 (2,3) |
| Among participants with ≥2 pregnancies, HDP in second or subsequent pregnancy, n/N (%) | 12,931 / 395,122 (3.3%) | 1,760 / 7,155 (24.6%) | 5,252 / 19,545 (26.9%) |
| Maternal age at last birth, y | 30.3 (4.8) | 30.7 (4.7) | 30.3 (4.8) |
| Time from last birth to censoring, y | 12.4 (7.2) | 13.0 (7.6) | 11.7 (7.1) |
| Chronic diabetes diagnosed prior to last birth | 2,269 (0.5%) | 101 (1.1%) | 463 (1.9%) |
| Chronic hypertension diagnosed prior to last birth | 1,108 (0.2%) | 266 (3.0%) | 439 (1.8%) |
| Incident coronary artery disease during follow-up | 2,128 (0.5%) | 78 (0.9%) | 221 (0.9%) |
| Heart failure-related hospitalization or death during follow-up | 499 (0.1%) | 15 (0.2%) | 51 (0.2%) |
| Among women with heart failure, incident coronary artery disease during follow-up | 133 / 499 (26.7%) | 5 / 15 (33.3%) | 17 / 51 (33.3%) |
Hypertension in the first pregnancy and incident heart failure
We first assessed whether HDP in the first pregnancy associated with incident HF in our cohort. The primary endpoint of HF-related hospitalization or HF-related death occurred in 565 women (Table 1) over a median follow-up of 11.8 (interquartile range 6.4–17.8) years. The primary endpoint occurred in 499 women without HDP in the first pregnancy (0.10%, incidence rate 8.48/10,000 woman-years of follow-up [95% CI 7.77–9.24/10,000 woman-years]), 15 women with GH in the first pregnancy (0.17%, incidence rate 13.05/10,000 woman-years of follow-up [95% CI 7.96–20.44/10,000 woman-years], incidence rate difference +4.57/10,000 woman-years vs. women without HDP, P=0.06), and 51 women with preeclampsia in the first pregnancy (0.21%, incidence rate 17.90/10,000 woman-years [95% CI 13.63–23.13/10,000 woman-years], incidence rate difference +9.42/10,000 woman-years vs. women without HDP, P<0.001). Of incident HF diagnoses, 540 (95.6%) occurred >6 months after the last birth. Of 565 incident HF cases, 100 (17.7%) occurred in women with HDP, and the population risk attributable to HDP was 9.5%. Among women with HDP, the proportion of incident HF risk attributable to HDP was 53.7%.
In time-to-event models comparing women with vs. without any HDP in the first pregnancy and adjusted only for maternal age at the last birth, HDP in the first pregnancy was associated with 1.92-fold risk of incident HF (95% CI 1.49–2.48, P<0.001) (Table 2). In fully adjusted models, HDP remained significantly associated with HF (HR 1.83, 95% CI 1.41–2.36, P<0.001). GH was associated with an HR of 1.41 (95% CI 0.84–2.35, P=0.19) and preeclampsia with an HR of 2.00 (95% CI 1.50–2.68, P<0.001) (Figure 2). In multivariable models additionally incorporating SGA and preterm delivery as predictors in addition to HDP status, SGA delivery was independently associated with incident HF (HR 1.35, 95% CI 1.09–1.67, P=0.006) whereas preterm delivery was not (HR 1.26, 95% CI 0.93–1.70, P=0.13).
Table 2.
Hazard ratios for heart failure* by pregnancy complications in the first birth.
| Complications in the first birth | Number of women | Number of events / women-years at risk | Age-adjusted models† | Fully adjusted models‡ |
|---|---|---|---|---|
| Hazard ratio (95% confidence interval) | Hazard ratio (95% confidence interval) | |||
| By hypertensive disorder of pregnancy status only | ||||
| No HDP | 475,269 | 499 / 5,883,782 | Reference | Reference |
| HDP§ | 33,153 | 66 / 399,858 | 1.92 (1.49–2.48)** | 1.83 (1.41–2.36)** |
| Gestational hypertension | 8,827 | 15 / 114,919 | 1.38 (0.82–2.30) | 1.41 (0.84–2.35) |
| Preeclampsia | 24,326 | 51 / 284,939 | 2.17 (1.63–2.90)** | 2.00 (1.50–2.68)** |
| By hypertensive disorder of pregnancy, small-for-gestational age delivery, and preterm delivery status | ||||
| No HDP | 475,269 | 499 / 5,883,782 | Reference | Reference |
| HDP alone§ | 22,585 | 42 / 273,255 | 1.78 (1.30–2.44)** | 1.72 (1.26–2.36)# |
| HDP with preterm and/or SGA delivery§ | 10,568 | 24 / 126,603 | 2.22 (1.48–3.35)** | 2.04 (1.35–3.08)# |
| Gestational hypertension alone | 6,948 | 10 / 89,863 | 1.19 (0.63–2.22) | 1.21 (0.65–2.27) |
| Gestational hypertension with SGA and/or preterm birth | 1,879 | 5 / 25,056 | 2.02 (0.84–4.88) | 2.06 (0.85–4.98) |
| Preeclampsia alone | 15,637 | 32 / 183,393 | 2.11 (1.48–3.02)** | 1.99 (1.39–2.84)** |
| Preeclampsia with SGA and/or preterm birth | 8,689 | 19 / 101,547 | 2.28 (1.44–3.61)** | 2.04 (1.28–3.23)# |
Heart failure endpoint is a composite of heart-failure related hospitalization and heart failure-related death
Age-adjusted models are adjusted only for maternal age at the last birth
Fully adjusted models are adjusted for maternal birth year, maternal age at first birth, maternal age at last birth, chronic diabetes diagnosis prior to last birth, and final party
HDP includes both gestational hypertension and preeclampsia
P<0.05,
P<0.01,
P<0.001
Figure 2.
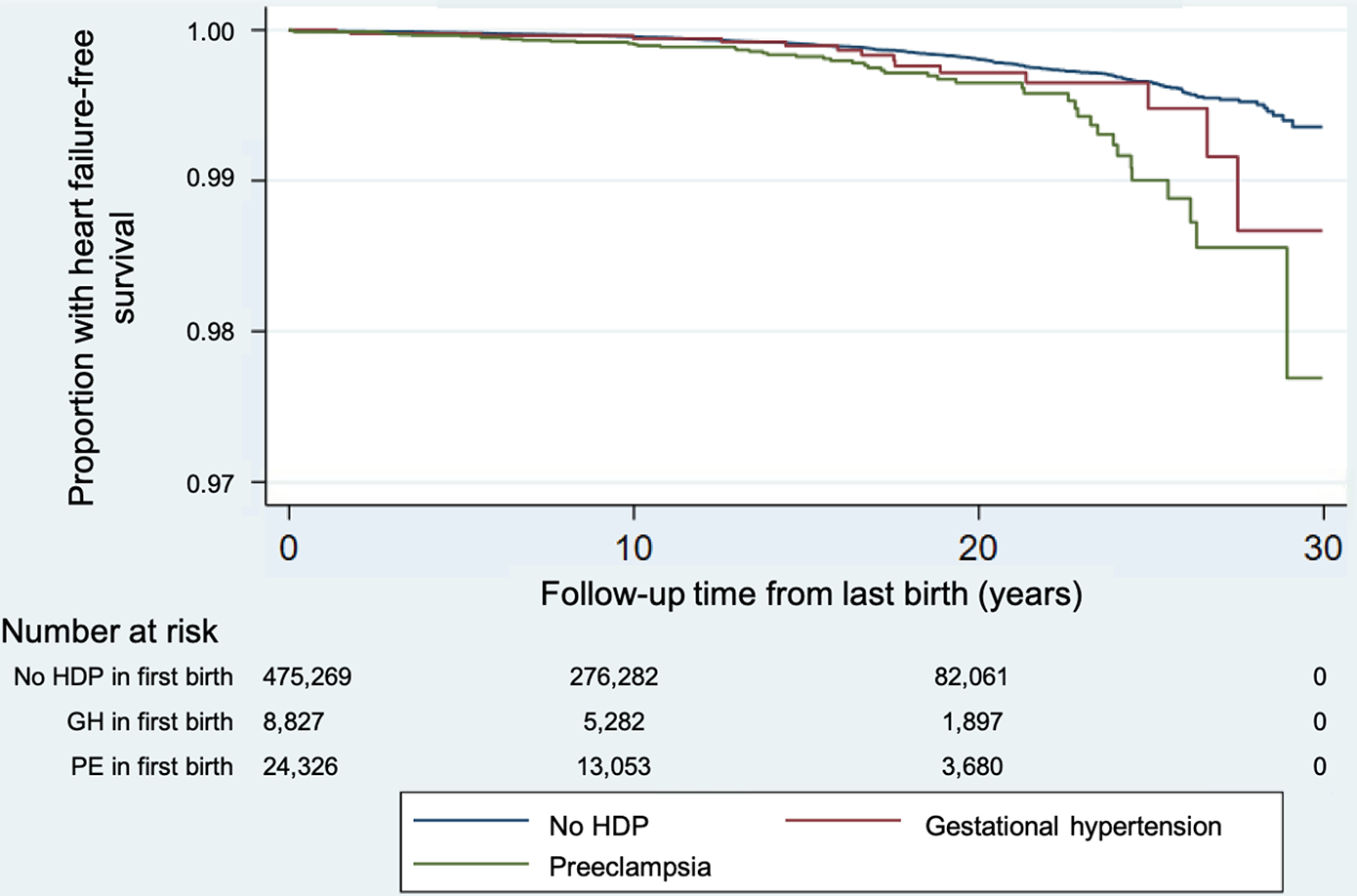
Kaplan-Meier curves for heart failure-free survival by hypertensive disorder of pregnancy status in the first birth.
HDP = hypertensive disorder of pregnancy; GH = gestational hypertension; PE = preeclampsia
Mean (SD) maternal age at the start of follow-up is 30.3 (4.8) years for women with no HDP, 30.8 (4.7) years for women with gestational hypertension, and 30.3 (4.8) years for preeclampsia in the first pregnancy
Having demonstrated an association between HDP and incident HF, we next tested whether concurrent pregnancy complications modified HF risk. HF risk after HDP in the first birth was not significantly modified by concurrent SGA and/or preterm delivery (Pinteraction = 0.42). There was no significant interaction of GH (Pinteraction = 0.81) or preeclampsia (Pinteraction = 0.80) with SGA and/or preterm delivery for incident HF in fully adjusted models (Table 2).
Coronary artery disease and incident heart failure risk
To test whether CAD explained the observed associations between HDP and HF, we conducted a sensitivity analyses that excluded women with CAD. Among 565 women with incident HF during the study period, 155 (27.4%) also developed CAD. After excluding 2,428 women from the overall cohort with incident CAD, observed hazards for incident HF were similar to the primary analysis (for GH, HR 1.32, 95% CI 0.70–2.48, P=0.39; for preeclampsia, HR 1.89, 95% CI 1.33–2.70, P<0.001).
Differential heart failure risk in women with 1 vs. multiple children
We next compared HF risks associated with HDP in women with 1 vs. ≥2 children. Compared to women with ≥2 births and normotension in all pregnancies, women with 1 lifetime birth had increased risk of HF irrespective of HDP status but especially if they experienced HDP. Among women with HDP, risk of HF was greater among women with 1 lifetime birth vs. ≥2 lifetime births (Table S2). Women with HDP in the first birth who subsequently experienced only normotensive pregnancies had attenuated HF risk compared to other subgroups of women with HDP (Table 3). Notably, elevated HF risks were observed among women with HDP in a second and/or later birth even when the first birth was normotensive (for GH in a second and/or later birth, HR 3.15, 95% CI 1.77–5.61, P<0.001; for preeclampsia in a second and/or later birth, HR 2.92, 95% CI 1.89–4.50, P<0.001).
Table 3.
Hazard ratios (95% confidence intervals) for heart failure* by hypertensive disorder of pregnancy status in the first and subsequent births.
| HDP status in first birth | HDP status in second and/or subsequent births | Number of women | Events / women-years at risk | Age-adjusted models† | Fully adjusted models‡ |
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | ||||
| No HDP | No HDP | 382,191 | 333 / 4,474,400 | Reference | Reference |
| No HDP | No later births | 80,147 | 132 / 1,273,070 | 1.12 (0.91–1.38) | 1.64 (1.26–2.12)** |
| No HDP | Gestational hypertension but no preeclampsia in later births | 4,405 | 12 / 45,790 | 3.39 (1.91–6.03)** | 3.15 (1.77–5.61)** |
| No HDP | Preeclampsia in later births | 8,526 | 22 / 90,522 | 3.33 (2.16–5.14)** | 2.92 (1.89–4.50)** |
| Gestational hypertension | No HDP | 5,395 | 7 / 66,242 | 1.32 (0.62–2.78) | 1.34 (0.64–2.84) |
| Gestational hypertension | No later births | 1,672 | 5 / 27,200 | 1.68 (0.69–4.08) | 2.58 (1.05–6.34)|| |
| Gestational hypertension | HDP in second and/or later births | 1,760 | 3 / 21,477 | 1.70 (0.55–5.31) | 1.66 (0.53–5.18) |
| Preeclampsia | No HDP | 14,293 | 17 / 156,015 | 1.55 (0.95–2.53) | 1.46 (0.90–2.39) |
| Preeclampsia | No later births | 4,781 | 18 / 71,854 | 2.75 (1.70–4.43)** | 3.63 (2.19–6.01)** |
| Preeclampsia | HDP in second and/or later births | 5,252 | 16 / 57,070 | 3.83 (2.32–6.32)** | 3.47 (2.10–5.75)** |
Heart failure endpoint is a composite of heart-failure related hospitalization and heart failure-related death
Age-adjusted models are adjusted only for maternal age at the last birth
Fully adjusted models are adjusted for maternal birth year, maternal age at first birth, maternal age at last birth, and chronic diabetes diagnosis prior to last birth
P<0.05,
P<0.01,
P<0.001
Heart failure risk in women with recurrent hypertension in pregnancy
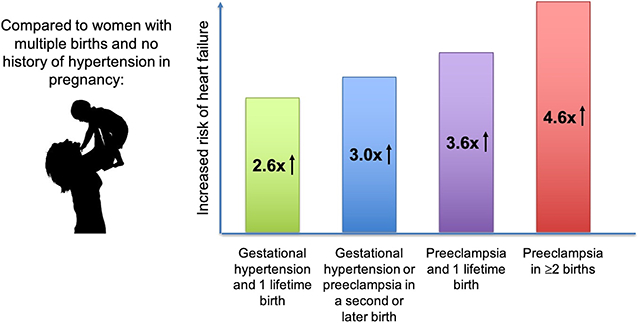
Finally, we compared HF risks among women with recurrent HDP, women with one lifetime birth, and multiparous women with normotension in all pregnancies. Women with preeclampsia in the first birth and HDP in a subsequent birth had similarly elevated HF risk compared to women with 1 lifetime birth complicated by preeclampsia (Table 3). HF risk associated with recurrent HDP was driven predominantly by recurrent preeclampsia (Figure 3, Table S3), with a progressive increase in HF incidence rate was observed with increasing number of preeclampsia episodes (Ptrend <0.001). Compared to women without preeclampsia (irrespective of GH status), single-episode preeclampsia was associated with an HR of 1.93 (95% CI 1.47–2.55, P<0.001), and recurrent preeclampsia was associated with an HR of 4.36 (95% CI 2.71–7.02, P<0.001) for HF (Table S4). After excluding 2,428 women with incident CAD during the study period, these associations with HF were only mildly attenuated (for single-episode preeclampsia, HR 1.55, 95% CI 1.08–2.22, P=0.02; for recurrent preeclampsia, HR 3.82, 95% CI 2.09–6.99, P<0.001).
Figure 3.
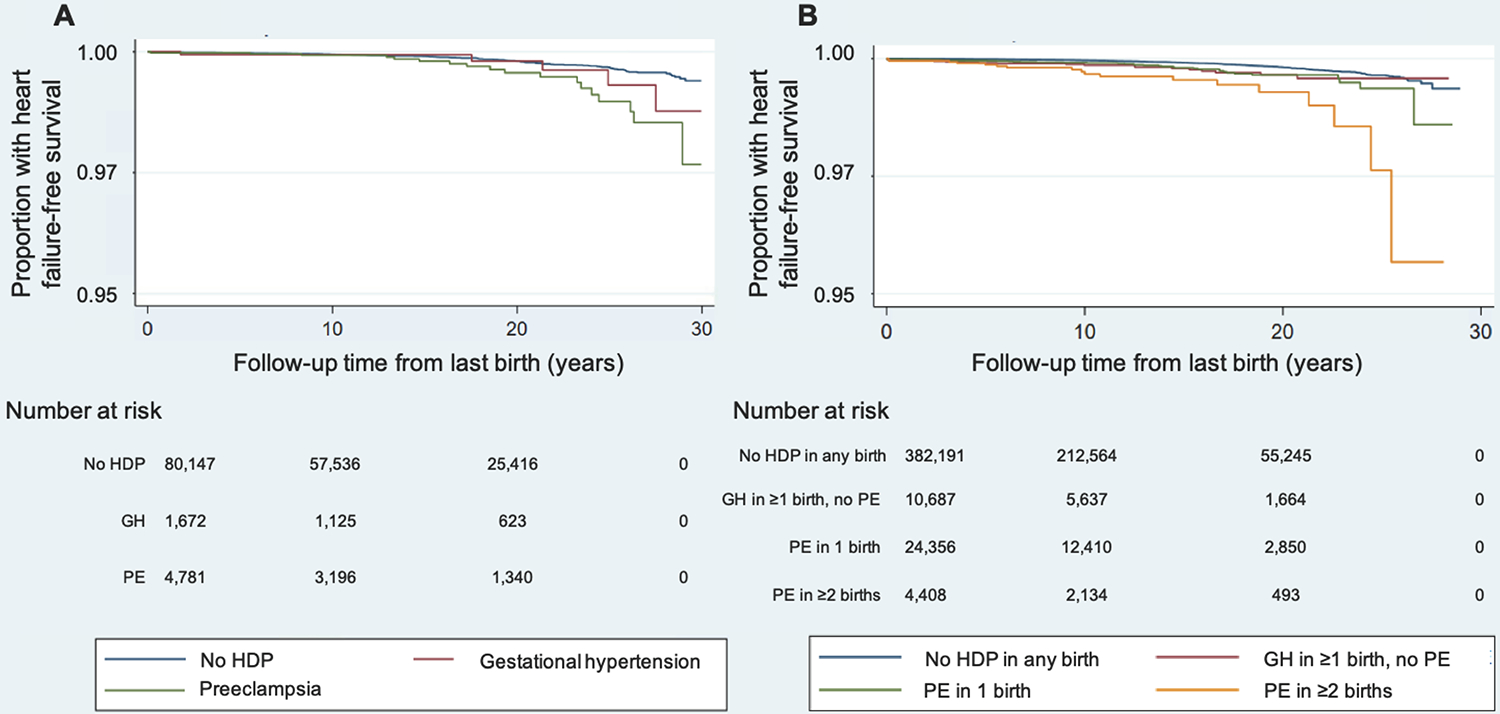
Kaplan-Meier curves for heart failure-free survival (A) by hypertensive disorder of pregnancy status among one-child mothers and (B) by recurrent hypertensive disorder of pregnancy status among women with ≥2 lifetime births.
HDP = hypertensive disorder of pregnancy; GH = gestational hypertension; PE = preeclampsia
Mean (SD) maternal age at the start of follow-up is 27.6 (5.8) years for one-child mothers with no HDP, 29.0 (5.9) years for one-child mothers with GH, 28.1 (5.9) years for one-child mothers with PE, 30.8 (4.4) years in women with ≥2 births and no HDP, 31.5 (4.4) years in women with GH in ≥1 birth and no PE, 31.1 (4.4) years in women with ≥2 births and 1 PE episode, and 31.5 (4.4) years in women with PE in ≥2 births
Discussion
In a large, nationwide study of Norwegian women followed for a median 12 years after the last birth, we observed a two-fold risk of incident HF in women with a history of preeclampsia. Contrary to our hypothesis, concurrent SGA and/or preterm delivery did not appear to modify HF risks among women with HDP. However, women with preeclampsia in their only lifetime birth, as well as women with more than 1 birth who experienced recurrent preeclampsia, demonstrated further increases in HF risk. Although HDP is associated with incident CAD and CAD is in turn associated with HF, HF risks associated with HDP in our cohort appeared largely independent of CAD. In this relatively young cohort of women, absolute risks of HF were expectedly low, but a disproportionate number of incident HF cases (17.7%) occurred in the 9.1% of women with prior HDP.
The results of the present study align with prior evidence that preeclampsia-associated risk of cardiovascular mortality is highest among women who bear only 1 child22 and extend this finding to HF. A similar pattern of risk concentration among one-child mothers has also been shown with respect to ASCVD risk after preterm delivery.11 Although greater parity, especially extreme parity, has been associated with elevated cardiovascular disease risk,23 studies have suggested a J-shaped relationship between parity and cardiovascular risk.24–26 The causes of HDP-associated risk concentration among women whose only birth is hypertensive are currently unknown but may reflect avoidance of additional pregnancies and/or impaired fertility related to accelerated development of comorbidities in women with more severe HDP, as well as unmeasured socioeconomic factors; further research to clarify mechanisms is needed.
Among women with ≥2 lifetime births, we observed greater HF risk among women with HDP in a second and/or later birth compared to women who experienced HDP only in a first birth and normotension in subsequent pregnancies. HDP occurring in a second or subsequent birth—a relatively rare occurrence, observed in only 4.7% of women in our cohort with ≥2 lifetime births—may serve as an especially important cardiovascular risk signal and warrants assessment across other cardiovascular conditions and in other cohorts.
Beyond strengthening evidence of an association between HDP and HF, this study’s findings may have other implications for understanding long-term cardiovascular risk after HDP. First, among women with preeclampsia, we did not observe evidence of effect modification for HF risk by concurrent SGA and/or preterm delivery, despite the fact that SGA was an independent predictor of HF. Similarly, a prior analysis observed similar hazards for HF in women with term and preterm preeclampsia.9 By contrast, further elevation in ASCVD risk among women with HDP and SGA and/or preterm delivery has now been well described.4,6,13,27 This discrepancy suggests that the mechanisms underlying ASCVD and HF after HDP may be different, at least among women in early midlife. Additionally, our results replicate the important finding from Behrens et al.9 that a significant portion of HF following HDP is non-ischemic, even though HDP is also associated with development of CAD. In a previous study of women followed prospectively from midlife (mean age 57 years), those with a history of HDP had persistently elevated risk of incident HF, and HDP-associated HF risk appeared additive to CAD-associated HF risk.7 It is likely that the relative contribution of ischemic heart disease to HF risk increases in women with HDP with increasing age as ASCVD risk increases with age in the population overall. In our younger cohort, however, only one-quarter of women with incident HF also had CAD, and exclusion of women with CAD did not meaningfully change the observed risks of HF. Further, most HF after HDP (>95%) occurred >6 months after the first birth, reinforcing that the vast majority of HF in this population is not peripartum cardiomyopathy.10
Second, our results support and extend the limited prior literature on cardiovascular disease risk after recurrent HDP27,28 by demonstrating greater HF risk following recurrent preeclampsia compared with single-episode preeclampsia. Prior work suggested further increase in HF risk after recurrent vs. single-episode preeclampsia,28 but this analysis only tested risk differences among women with multiple births and did not test the influence of comorbid CAD. In the present analysis, we observed these associations even among women without CAD, despite the dose-dependent relationship shown previously between recurrent preeclampsia and CAD.6,13,14 Taken together with prior literature, our findings identify women with recurrent preeclampsia and women with a single lifetime birth complicated by preeclampsia as highest-risk for both ASCVD and non-ASCVD cardiovascular disease within the spectrum of HDP-associated risk. Further investigation is needed to disentangle whether HF risk associated with recurrent preeclampsia reflects stronger shared upstream predisposition to both conditions such as a shared genetic risk,29 enduring and additive cardiovascular damage imparted by each preeclampsia episode, or both.
GH in the first pregnancy was not significantly associated with incident HF in this analysis, in contrast to findings reported by Behrens et al., who observed similar HF risks associated with GH and moderate preeclampsia.9 Although they did not meet the threshold for statistical significance in primary models, the hazards associated with GH plus SGA and/or preterm delivery, as well as the hazards associated with GH among one-child mothers, were similar to those associated with preeclampsia in the overall cohort. Given low absolute HF case counts (N=15) among women with GH in the first birth, low power may have hindered our ability to detect significant associations between GH and HF.
Our findings should be interpreted in the context of study design. First, although the CVDNOR Project captures nationwide Norwegian data, diagnoses are ascertained based on inpatient codes and the national death registry; thus, HF cases that were evaluated and managed exclusively in the outpatient setting (i.e., milder HF presentations) may not be captured. Prior work suggests that administrative codes yield high specificity but limited sensitivity for HF.30 Second, due to low absolute risks and low overall HF case counts in our relatively young cohort, we were unable to test effect modification by preterm delivery and SGA delivery separately due to insufficient power and therefore assessed SGA and/or preterm delivery as a lumped effect-modifier. Third, we were unable to ascertain development of cardiometabolic risk factors in the interval between the last birth and development of HF. Fourth, given lack of racial/ethnic diversity, whether findings in this white northern European cohort extend to non-white populations requires further study. However, the study also has several strengths, most notably a very large, nationwide cohort with detailed pregnancy phenotyping and complete, long-term follow-up.
Supplementary Material
Novelty and Significance.
What Is New?
Compared to women without hypertensive disorders of pregnancy (HDP), women with preeclampsia had two-fold risk of future heart failure. Risk of heart failure was not modified by concurrent small-for-gestational age and/or preterm birth.
Women with a single lifetime birth complicated by preeclampsia and women with recurrent preeclampsia demonstrated further elevation in heart failure risk.
Heart failure risks were largely independent of HDP’s association with coronary artery disease.
What Is Relevant?
The hypertensive disorders of pregnancy (e.g., gestational hypertension, preeclampsia) are strongly associated with development of chronic hypertension and diverse cardiovascular disease.
Summary
Women with preeclampsia experienced two-fold risk of HF, without evidence of effect modification by concurrent SGA and/or preterm delivery. Risks increased further in women whose only lifetime birth was complicated by preeclampsia and in women with recurrent preeclampsia.
Perspectives.
Women with preeclampsia experienced two-fold risk of HF, without evidence of effect modification by concurrent SGA and/or preterm delivery. Risks increased further in women with preeclampsia and only one lifetime birth and in women with recurrent preeclampsia. These risks were largely independent of HDP’s association with CAD. Further research is needed to elucidate underlying mechanisms and define optimal cardiovascular disease prevention and treatment in affected women.
Acknowledgements:
The authors thank Tomislav Dimoski at The Norwegian Institute of Public Health, Norway, for his contribution by developing the software necessary for obtaining data from Norwegian hospitals, conducting the data collection, and quality assurance of data in this project.
Funding: This work was supported by a grant from the Massachusetts General Hospital Corrigan Women’s Heart Health Program. The authors are partially supported by the U.S. National Heart, Lung, and Blood Institute (T32HL094301-07 to M.C.H.; and R01HL142711, R01HL148565, and R01HL148050 to P.N.), Fondation Leducq (TNE-18CVD04 to P.N.), the Massachusetts General Hospital (Hassenfeld Award to P.N.), and the European Research Council (ERC Advanced Grant 833076 to K.K.).
Footnotes
Conflict of interest: P.N. reports grant support from Amgen, Apple, and Boston Scientific, as well as consulting income from Apple, all unrelated to this work. All remaining authors have no conflict of interest.
Publisher's Disclaimer: Disclaimer: Data from the Norwegian Cause of Death Registry have been used in this publication. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by this registry should be inferred.
References
- 1.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–83. [DOI] [PubMed] [Google Scholar]
- 2.LifeCycle Project-Maternal Obesity and Childhood Outcomes Study Group, Voerman E, Santos S, Inskip H, Amiano P, Barros H, Charles MA, Chatzi L, Chrousos GP, Corpeleijn E, Crozier S, Doyon M, Eggesbø M, Fantini MP, Farchi S, Forastiere F, Georgiu V, Gori D, Hanke W, Hertz-Picciotto I, Heude B, Hivert MF, Hryhorczuk D, Iñiguez C, Karvonen AM, Küpers LK, Lagström H, Lawlor DA, Lehmann I, Magnus P, Majewska R, Mäkelä J, Manios Y, Mommers M, Morgen CS, Moschonis G, Nohr EA, Nybo Andersen AM, Oken E, Pac A, Papadopoulou E, Pekkanen J, Pizzi C, Polanska K, Porta D, Richiardi L, Rifas-Shiman SL, Roeleveld N, Ronfani L, Santos AC, Standl M, Stigum H, Stoltenberg C, Thiering E, Thijs C, Torrent M, Trnovec T, van Gelder MMHJ, van Rossem L, von Berg A, Vrijheid M, Wijga A, Zvinchuk O, Sørensen TIA, Godfrey K, Jaddoe VWV, Gaillard R. Association of Gestational Weight Gain With Adverse Maternal and Infant Outcomes. JAMA. 2019;321(17):1702–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA, Mamas MA. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2). [DOI] [PubMed] [Google Scholar]
- 4.Leon LJ, McCarthy FP, Direk K, Gonzalez-Izquierdo A, Prieto-Merino D, Casas JP, Chappell L. Preeclampsia and Cardiovascular Disease in a Large UK Pregnancy Cohort of Linked Electronic Health Records: A CALIBER Study. Circulation. 2019;140(13):1050–60. [DOI] [PubMed] [Google Scholar]
- 5.Männistö T, Mendola P, Vääräsmäki M, Järvelin MR, Hartikainen AL, Pouta A, Suvanto E. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127(6):681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riise HKR, Sulo G, Tell GS, Igland J, Nygård O, Iversen AC, Daltveit AK. Association Between Gestational Hypertension and Risk of Cardiovascular Disease Among 617 589 Norwegian Women. J Am Heart Assoc. 2018;7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honigberg MC, Zekavat SM, Aragam K, Klarin D, Bhatt DL, Scott NS, Peloso GM, Natarajan P. Long-Term Cardiovascular Risk in Women With Hypertension During Pregnancy. J Am Coll Cardiol. 2019;74(22):2743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haug EB, Horn J, Markovitz AR, Fraser A, Klykken B, Dalen H, Vatten LJ, Romundstad PR, Rich-Edwards JW, Asvold BO. Association of Conventional Cardiovascular Risk Factors With Cardiovascular Disease After Hypertensive Disorders of Pregnancy: Analysis of the Nord-Trøndelag Health Study. JAMA Cardiol. 2019;4(7):628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behrens I, Basit S, Lykke JA, Ranthe MF, Wohlfahrt J, Bundgaard H, Melbye M, Boyd HA. Association Between Hypertensive Disorders of Pregnancy and Later Risk of Cardiomyopathy. JAMA. 2016;315(10):1026–33. [DOI] [PubMed] [Google Scholar]
- 10.Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ. 2019;364:k5287. [DOI] [PubMed] [Google Scholar]
- 11.Tanz LJ, Stuart JJ, Williams PL, Rimm EB, Missmer SA, Rexrode KM, Rich-Edwards JW. Preterm Delivery and Maternal Cardiovascular Disease in Young and Middle-Aged Adult Women. Circulation. 2017;135(6):578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, Smith GN, Gore GC, Ray JG, Nerenberg K, Platt RW. Cardiovascular Disease-Related Morbidity and Mortality in Women With a History of Pregnancy Complications. Circulation. 2019;139(8):1069–79. [DOI] [PubMed] [Google Scholar]
- 13.Riise HK, Sulo G, Tell GS, Igland J, Nygård O, Vollset SE, Iversen AC, Austgulen R, Daltveit AK. Incident Coronary Heart Disease After Preeclampsia: Role of Reduced Fetal Growth, Preterm Delivery, and Parity. J Am Heart Assoc. 2017;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brouwers L, van der Meiden-van Roest AJ, Savelkoul C, Vogelvang TE, Lely AT, Franx A, van Rijn BB. Recurrence of pre-eclampsia and the risk of future hypertension and cardiovascular disease: a systematic review and meta-analysis. BJOG. 2018;125(13):1642–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000;79(6):435–9. [PubMed] [Google Scholar]
- 16.Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. [DOI] [PubMed] [Google Scholar]
- 17.Thomsen LC, Klungsøyr K, Roten LT, Tappert C, Araya E, Baerheim G, Tollaksen K, Fenstad MH, Macsali F, Austgulen R, Bjørge L. Validity of the diagnosis of pre-eclampsia in the Medical Birth Registry of Norway. Acta Obstet Gynecol Scand. 2013;92(8):943–50. [DOI] [PubMed] [Google Scholar]
- 18.Klungsøyr K, Harmon QE, Skard LB, Simonsen I, Austvoll ET, Alsaker ER, Starling A, Trogstad L, Magnus P, Engel SM. Validity of pre-eclampsia registration in the medical birth registry of norway for women participating in the Norwegian mother and child cohort study, 1999–2010. Paediatr Perinat Epidemiol. 2014;28(5):362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand. 2000;79(6):440–9. [PubMed] [Google Scholar]
- 20.Sulo G, Igland J, Vollset SE, Nygard O, Oyen N, Tell GS. Cardiovascular disease and diabetes mellitus in Norway during 1994–2009: CVDNOR – a nationwide research project. Norsk Epidemiologi. 2013;23:101–7. [Google Scholar]
- 21.Sulo G, Igland J, Nygård O, Vollset SE, Ebbing M, Tell GS. Favourable trends in incidence of AMI in Norway during 2001–2009 do not include younger adults: a CVDNOR project. Eur J Prev Cardiol. 2014;21(11):1358–64. [DOI] [PubMed] [Google Scholar]
- 22.Skjaerven R, Wilcox AJ, Klungsøyr K, Irgens LM, Vikse BE, Vatten LJ, Lie RT. Cardiovascular mortality after pre-eclampsia in one child mothers: prospective, population based cohort study. BMJ. 2012;345:e7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters SA, Woodward M. Women’s reproductive factors and incident cardiovascular disease in the UK Biobank. Heart. 2018;104(13):1069–75. [DOI] [PubMed] [Google Scholar]
- 24.Lv H, Wu H, Yin J, Qian J, Ge J. Parity and Cardiovascular Disease Mortality: a Dose-Response Meta-Analysis of Cohort Studies. Sci Rep. 2015;5:13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Ruan W, Lu Z, Wang D. Parity and risk of maternal cardiovascular disease: A dose-response meta-analysis of cohort studies. Eur J Prev Cardiol. 2019;26(6):592–602. [DOI] [PubMed] [Google Scholar]
- 26.Halland F, Morken NH, DeRoo LA, Klungsøyr K, Wilcox AJ, Skjærven R. Association of Women’s Reproductive History With Long-term Mortality and Effect of Socioeconomic Factors. Obstet Gynecol. 2015;126(6):1181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53(6):944–51. [DOI] [PubMed] [Google Scholar]
- 28.Auger N, Fraser WD, Schnitzer M, Leduc L, Healy-Profitós J, Paradis G. Recurrent pre-eclampsia and subsequent cardiovascular risk. Heart. 2017;103(3):235–43. [DOI] [PubMed] [Google Scholar]
- 29.Gammill HS, Chettier R, Brewer A, Roberts JM, Shree R, Tsigas E, Ward K. Cardiomyopathy and Preeclampsia. Circulation. 2018;138(21):2359–66. [DOI] [PubMed] [Google Scholar]
- 30.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of heart failure diagnoses in administrative databases: a systematic review and meta-analysis. PLoS One. 2014;9(8):e104519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


