Abstract
INTRODUCTION:
Assessment of functional status is associated with risk of cognitive decline and diagnosis of dementia, and can be assessed by participants and study partners (SPs).
METHODS:
In 770 older adults enrolled in the Imaging Dementia–Evidence for Amyloid Scanning (IDEAS) study and the online Brain Health Registry (BHR), we estimated associations between online assessments and clinical variables related to Alzheimer’s disease (AD) risk.
RESULTS:
Worse online learning scores and SP-reported functional decline were associated with higher probability of AD dementia diagnosis and poor in-clinic cognitive assessment; and with higher odds of β-amyloid positivity when combined with participants’ report of less decline. SP-report of functional decline conferred predictive value independent of online cognitive assessments. Participants underreported decline compared to SPs.
DISCUSSION:
The results support the validity of online assessments, and their greater utilization in healthcare and research settings. Online SP-reported functional decline is an indicator of dementia and AD risk.
Keywords: Dementia, Mild Cognitive Impairment, Internet-based registry, Study partner, Subjective Cognitive Decline, Activities of Daily Living, Functional decline, β -Amyloid
1. Background
A major advance in the clinical Alzheimer’s disease (AD) and aging field has been the development of technology to capture information online [1], with growing evidence for construct validity [2, 3]. The use of online assessment technology has the potential to greatly facilitate identification of older adults at risk for Mild Cognitive Impairment (MCI), dementia due to AD, and other types of dementia. The use of online methods reduces the cost and time necessary for assessing clinical symptoms in individuals at risk for AD and thereby makes available to many more people access to clinical care, clinical trials and clinical research.
In older adults, decline in abilities associated with activities of daily living (ADLs), or functional decline, is an important indicator of current disease status and risk of progression. Functional decline is associated with AD pathology [4–6], and predicts future decline and a more rapid disease progression [7]. ADL preservation is important to patients and their families [8]. Estimates of individuals’ functional abilities, and the extent to which they change over time, can be determined from questions asked of the individual themselves or from someone who knows them well, often termed a study partner (SP). A number of validated instruments that measure functional decline are used in AD clinical trials and research studies [9–14]. SP-report of functional abilities are a component of diagnosis of MCI and dementia [13, 15–18], and are associated with objective measures of cognitive impairment derived from neuropsychological tests (NPTs) [18, 19] and abnormal levels of AD biomarkers, such as Positron Emission Tomography (PET) β-amyloid (Aβ) and tau [4, 5, 20]. On the other hand, self-reported measures of function are not consistently associated with the same outcomes [18], likely due to variability in insight about cognitive status in individuals with dementia [18, 21, 22], which can limit their usefulness.
Recently, an online unsupervised version of the Everyday Cognition Scale (ECog), a measure of self- and SP-reported functional change, showed evidence for construct validity in the Brain Health Registry (BHR)[3]. BHR is a public, online registry collecting longitudinal health, cognitive, and lifestyle data; and has referred 49,000 participants to 24 AD and aging studies [23]. However, the outcomes from the online version of ECog have not been verified using objective clinical data. Such validation is necessary to understand the usefulness of the online approach to facilitate AD clinical research.
In the current study, we assessed the validity of self- and SP versions of the online BHR ECog by measuring the association between BHR data and verified clinical data in a subset of BHR participants also enrolled in the Imaging Dementia-Evidence for Amyloid Scanning (IDEAS) study [24]. The IDEAS study is a multisite, longitudinal, observational study in which participants were recruited by dementia specialists from their clinical practice [24]. Participants with diagnostically uncertain MCI or dementia, who received an Aβ PET scan as part of the IDEAS study, were subsequently recruited to join BHR, with data linkage between the studies. In this sample, we first tested the hypothesis that SP-report of greater functional decline, as measured by online ECog, would be associated with higher probability of Aβ positivity and clinical diagnosis of dementia likely due to AD; as well as decreased scores on in-clinic cognitive screening instrument. The second hypothesis was that self-reported function would not show the same strength of association with objective clinical data as SP report of function. The third hypothesis was that SP-report of function would add predictive value for Aβ positivity and clinical diagnosis over and above an objective measure of cognition, an online version of the Cogstate Brief Battery (CBB) [25, 26].
2. Methods
2.1. Participants.
Participants enrolled in the IDEAS study[24] who consented to be contacted regarding participation in additional studies, were called between February 2017-February 2018. Telephone agents obtained permission to share participants’ email address with the BHR study team. BHR staff then sent recruitment emails to participants that included a link to an online registration webpage. Participants provided electronic consent to participate in the BHR [1], and to have their online BHR study data linked with IDEAS study data.
2.2. Online Brain Health Registry assessments
2.2.1. Questionnaires.
Participants completed online, self-report questionnaires within the BHR website [1]. Questionnaires were based on well-validated instruments and adapted for online collection, including a detailed medical history and demographics (age, gender, education level, race, and ethnicity). Participants were able to invite a study partner (SP) to join through the BHR Caregiver and Study Partner Portal [1, 3], a separate system within the BHR that allows a SP of a study participant to register, consent, and complete questionnaires online. SPs completed SP versions of online questionnaires regarding the participant, including ECog.
2.2.2. Everyday Cognition Scale (ECog).
Participants and SPs completed an online adaptation of the ECog within BHR, as a measure of functional change, consisting of 39 items assessing the participant’s self- or SP-reported capability to perform everyday tasks in comparison to activity levels 10 years prior, including activities that map to cognitive abilities across six domains [10]. The BHR uses an adapted version of the ECog in an online survey form, where the text of all items and response options are identical to the paper version [3].
2.2.3. Cogstate Brief Battery (CBB).
The CBB is a computerized cognitive assessment battery that has been validated in supervised [25, 26] and unsupervised [27–29] settings in a variety of patient populations. The CBB consists of four tests comprised of playing card stimuli, which measure information processing speed, visual attention, visual learning, visual memory, and working memory [3]. Participants self-administered the CBB through the BHR website, completing a practice session before each subtest. For the analyses described, we included participant scores on the CBB One Card Learning (OCL) test, with an outcome measure of accuracy (proportion of correct responses, normalized using an arcsine transformation). Lower scores reflect worse performance.
2.3. Clinical measures from the IDEAS study
2.3.1. β-Amyloid (Aβ).
Participant’s Aβ status was determined as described in [24] as part of the IDEAS study. In short, imaging specialists participating in IDEAS interpreted Aβ PET images using approved reading methodologies for each tracer (fluorine 18 (18F)–labeled florbetapir, 18F- labeled flutemetamol, and 18F-labeled florbetaben). Scans were interpreted as either “negative”, meaning retention of the Aβ tracer in cerebral white matter only, or “positive”, meaning Aβ tracer retention in cortical gray matter. Imaging specialists were board certified in diagnostic radiology or nuclear medicine and successfully completed vendor-provided training for interpreting amyloid Aβ PET scans.
2.3.2. Clinical diagnosis.
All participants were required to have completed a comprehensive diagnostic assessment, including global cognition assessed using the Mini-Mental State Examination (MMSE) or Montreal Cognitive Assessment (MoCA)[30]; laboratory testing within the past 12 months, and head computed tomography or magnetic resonance imaging within the past 24 months of enrollment in IDEAS. Participants were required to meet appropriate use criteria for Aβ PET[31, 32]. Pre-PET diagnosis of MCI or dementia (made prior to the Aβ PET scan), was determined as described in [24]. Briefly, diagnoses were made by dementia specialists overseeing the care of the participant, who were (1) board certified in neurology, psychiatry, or geriatric medicine; (2) required to devote 25% or more of patient contact time to the evaluation and care of acquired cognitive impairment; (3) recruited through professional societies, the Alzheimer’s Association, industry partners, and media outreach. Post-PET diagnosis of MCI or dementia due to AD. At a follow-up visit 90 ± 30 days after the PET can, dementia specialists completed a post-PET case report documenting changes in diagnosis. For our analyses, participants were classified as having a poet-PET diagnosis of AD dementia if the dementia specialist indicated one of the following as their diagnosis at follow-up: AD, clinically typical (memory-predominant); AD, clinically atypical (non-amnestic); AD, mixed pathology; or AD, not otherwise specified. 70 participants (n=55 MCI, n=15 dementia) with a history of stroke were excluded from the analysis.
2.4. Statistical analysis.
2.4.1. Hypotheses.
We tested the following a priori hypotheses: (1) SP-report of greater functional decline, as measured by ECog, is associated with higher probability of Aβ positivity, pre-PET diagnosis of dementia versus MCI; post-PET diagnosis of AD dementia versus MCI, and decreased MoCA performance. (2) Self-report function would not show the same strength of association with objective clinical data as the SP-report of function; (3) SP-report of function would add predictive value over objective measures of cognition.
2.4.2. Cohort comparisons.
Variables of interest were compared between various sub-groups using Mann-Whitney for continuous variables and chi-square tests of proportions for categorical variables. The comparison groups included (1) IDEAS participants enrolled in BHR with different impairment levels (MCI or dementia, Table 1); (2) the IDEAS sample included in the first aim of the IDEAS study and the BHR co-enrolled sub-sample, to evaluate the association between amyloid PET and subsequent change in clinical management, (Supplemental Table 1) [24]; (3) BHR participants enrolled in IDEAS, and BHR participants age 65 and over recruited from the general public and not IDEAS, thought to be cognitively-unimpaired (Supplemental Table 2).
Table 1:
Characteristics of participants
| Level of Impairment | ||||||
|---|---|---|---|---|---|---|
| MCI | Dementia | |||||
| Aβ + | Aβ - | Total | Aβ + | Aβ - | Total | |
| Total N | 326 | 274 | 600 | 137 | 33 | 170 |
| Demographics | ||||||
| Age | 74.3 ± 5.7 (65–90) | 74.3 ± 5.7 (65–90) | 73.8 ± 5.54 (65–90) | 74.7 ± 6.09 (65–89) | 72 ± 5.98 (65–89) | 74.2 ± 6.15 (65–89) |
| Female | 132 (40.5%) | 138 (50.4%) | 270 (45.0%) | 57 (41.6%) | 15 (45.5%) | 72 (42.4%) |
| Years of Education | 16.1 ± 2.63 (12–20) | 16.3 ± 2.48 (12–20) | 16.2 ± 2.56 (12–20) | 15.6 ± 2.98 (6–20) | 16.2 ± 2.86 (12–20) | 15.7 ± 2.96* (6–20) |
| Caucasian | 299 (91.7%) | 239 (87.2%) | 538 (89.7%) | 125 (91.2%) | 27 (81.8%) | 152 (89.4%) |
| Has an enrolled SP | 140 (42.9%) | 77 (28.1%) | 217 (36.2%) | 60 (43.8%) | 10 (30.3%) | 70 (41.2%) |
| Cognitive/Functional Measures | ||||||
| MoCA score | 24.2 ± 3.38 (7–30) | 25.2 ± 2.88 (17–30) | 24.6 ± 3.21 (7–30) | 18.7 ± 5.45 (6–29) | 21.1 ± 5.82 (5–28) | 19.2 ± 5.57* (5–29) |
| MMSE score | 27.2 ± 2.38 (16–30) | 27.8 ± 2.17 (19–30) | 27.5 ± 2.3 (16–30) | 23.5 ± 4.2 (9–30) | 25.2 ± 4.69 (10–30) | 23.8 ± 4.33* (9–30) |
| CBB OCL score | 0.87 ± 0.12 (0.59–1.16) | 0.90 ± 0.15 (0.43–1.35) | 0.89 ± 0.13 (0.43–1.35) | 0.84 ± 0.10 (0.68–1.08) | 0.85 ± 0.13 (0.61–0.97) | 0.84 ± 0.11*(0.61–1.08) |
| Self-ECog score | 1.84 ± 0.61 (1–4) | 1.85 ± 0.59 (1–3.81) | 1.85 ± 0.6 (1–4) | 1.9 ± 0.65 (1–3.85) | 2.17 ± 0.71 (1.36–3.54) | 1.95 ± 0.67 (1–3.85) |
| SP-ECog score | 2.08 ± 0.64 (1.05–4) | 1.96 ± 0.71 (1–3.95) | 2.04 ± 0.67 (1–4) | 2.51 ± 0.79 (1.05–3.92) | 2.83 ± 0.87 (1.67–3.95) | 2.56 ± 0.8* (1.05–3.95) |
Demographics and cognitive/functional profile of BHR-IDEAS participants. For continuous variables, mean ± SD (range) is shown. For categorical variables, number of participants (% of total in diagnostic group) is shown.
p<0.05 versus total MCI group using Mann-Whitney for continuous variables or chi squared test of proportions for categorical variables.
2.4.3. Logistic and Linear Regression analyses.
Binomial logistic regression models were used to assess associations between predictors and diagnosis (pre- and post-PET), or Aβ status. Adjusted Odds Ratios (OR) and 95% confidence intervals (CI) are reported. Ordinary least squares linear regression was used to assess the association between predictors and MoCA, or between Self- and SP-report ECog. For each dependent variable (diagnosis, Aβ, MoCA), we considered separate models: (1) including either self-ECog, SP-ECog, or Cogstate OCL; (2) including both self- and SP-ECog; (3) including self- and SP-ECog and Cogstate OCL. We also considered the ratio of SP-ECog to Self-ECog (ECog ratio) as a predictor; this variable measures the discrepancy between SP- and self-report. We also considered interactions between ECog and Aβ (for models with diagnosis and MoCA as the dependent variable) or ECog and pre-PET diagnosis (for models with Aβ and MoCA as the dependent variable). All models included age, education, and gender as covariates. All analyses were completed using SAS Version 9.4.
3. Results
3.1. Cohort description and feasibility of online approach
3.1.1. Demographic and cognitive profile of participants.
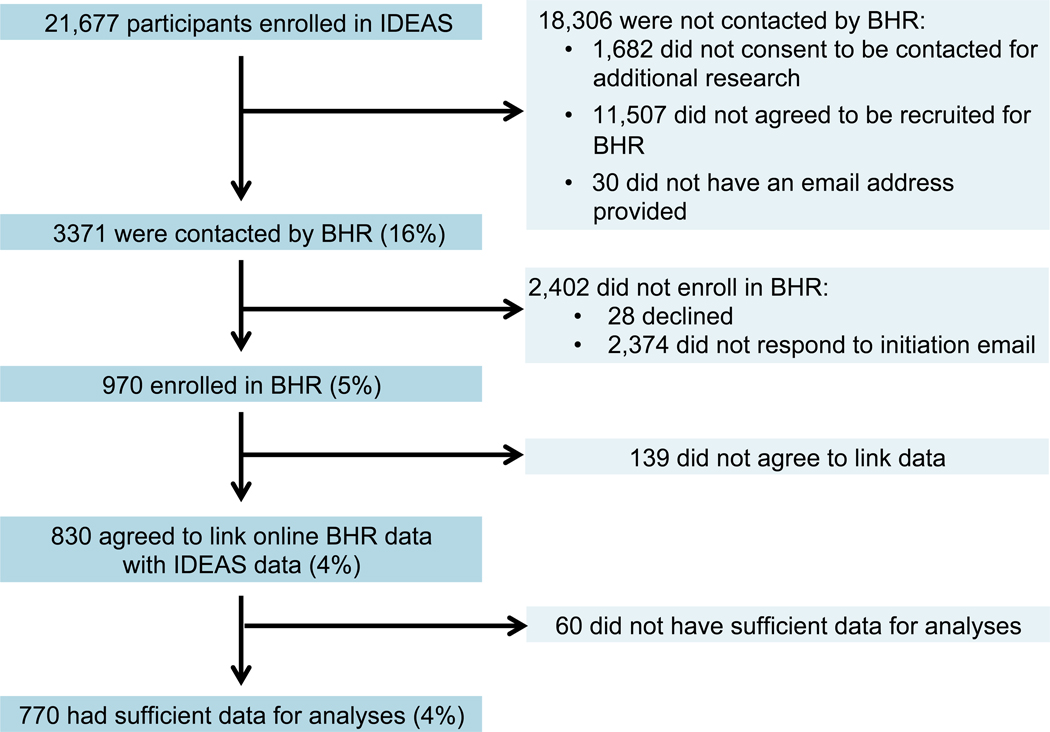
Over 21,600 participants enrolled in the IDEAS study. Of this group, 14,912 (69%) agreed to be contacted for future research studies, 3371 (16%) were contacted by email by BHR, 970 (5%) enrolled in the BHR, 830 (4%) agreed to data linkage between IDEAS clinical data and BHR, and 770 (4%) provided sufficient data for analyses (Figure 1 and Table 1). In this group (BHR-IDEAS), 77.9% had a pre-PET diagnosis of MCI and 22.1% had a pre-PET diagnosis of dementia. Fifty-four percent of participants with MCI and 80.1% of those with dementia were Aβ+ (Table 1).
Figure 1:
Study Flow for enrollment of IDEAS participants into BHR.
3.1.2. Comparison between BHR-IDEAS and IDEAS cohort.
Compared to the entire IDEAS cohort included in Aim 1 of the main IDEAS study (Supplemental Table 1), the BHR-IDEAS cohort had lower proportions of diagnosed dementia cases, females, African Americans, and those with education levels of high school or less. BHR-IDEAS participants also had higher MMSE and MoCA scores compared to the entire IDEAS cohort, indicating lower levels of impairment. A higher proportion of BHR-IDEAS participants with dementia were Aβ+ compared to the entire IDEAS cohort.
3.1.3. Online task completion.
BHR-IDEAS participants completed most BHR questionnaires and CBB at rates lower than cognitively-unimpaired older adults enrolled in BHR from the general public, who were not part of the IDEAS study (Supplemental Table 2).
3.2. Associations between Aβ and BHR assessments.
3.2.1. Associations between Aβ and online CBB.
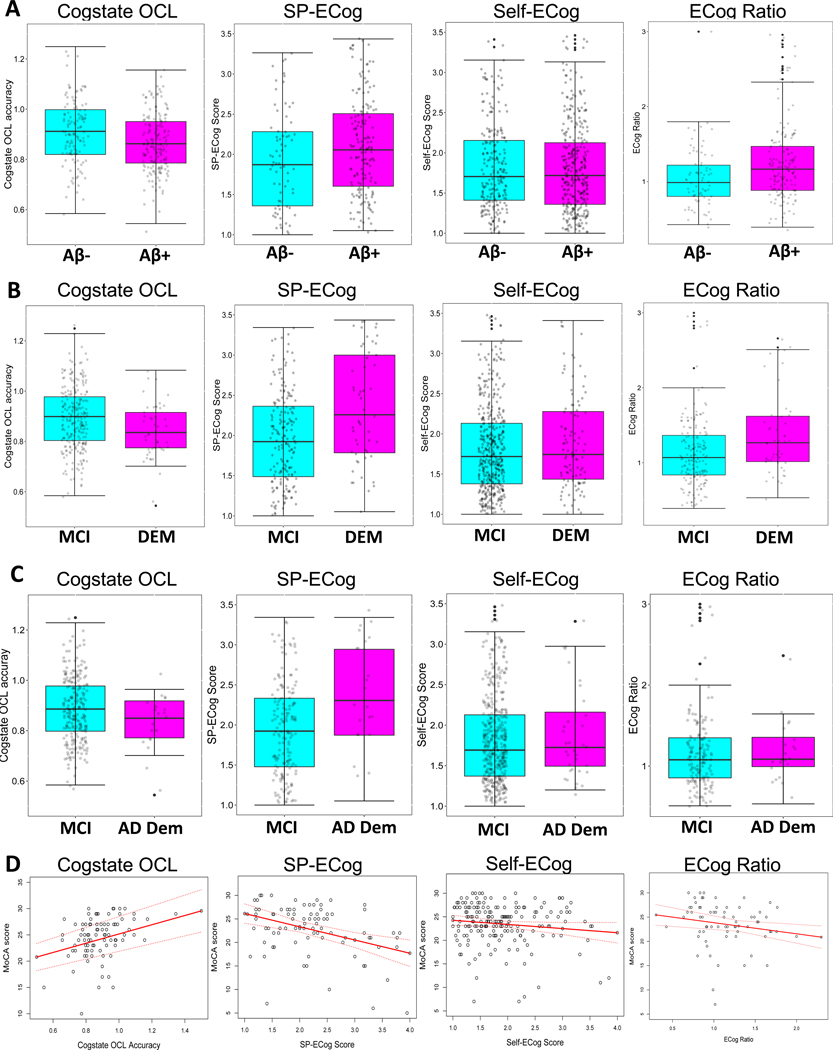
Increasing accuracy on the Cogstate OCL test was associated with lower probability of Aβ positivity (Table 2A and Figure 2A). There was no effect of pre-PET diagnosis on the association (OCL by diagnosis interaction, OR: 1.33, CI: 0.02–1.66, p= 0.95).
Table 2:
Associations betweenAβ or clinical diagnosis and BHR online assessments.
| A. Associations with Aβ+ | |||
|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | p-value | |
| Individual Models | |||
| CBB OCL | 0.13 | 0.02, 0.85 | 0.03 |
| Self-ECog | 0.97 | 0.73, 1.29 | 0.85 |
| SP-ECog | 1.36 | 0.93, 2.00 | 0.11 |
| ECog Ratio | 2.74 | 1.37–5.48 | 0.005 |
| Combined model | |||
| CBB OCL | 0.087 | 0.004, 1.95 | 0.12 |
| Self-ECog | 0.526 | 0.25, 1.13 | 0.10 |
| SP-ECog | 2.084 | 0.98, 4.41 | 0.05 |
| B. Associations with pre-PET diagnosis (MCI versus dementia) | |||
| Odds Ratio | 95% Confidence Interval | p-value | |
| Individual Models | |||
| CBB OCL | 0.07 | 0.01, 0.94 | 0.04 |
| Self-ECog | 1.25 | 0.89, 1.75 | 0.20 |
| SP-ECog | 2.60 | 1.72, 3.93 | <0.001 |
| ECog Ratio | 2.99 | 1.66, 5.39 | <0.001 |
| Combined model | |||
| CBB OCL | 0.36 | 0.01, 23.78 | 0.63 |
| Self-ECog | 0.61 | 0.23, 1.62 | 0.32 |
| SP-ECog | 4.36 | 1.63, 11.68 | 0.003 |
| C. Associations with post-PET diagnosis (MCI versus AD dementia) | |||
| Odds Ratio | 95% Confidence Interval | p-value | |
| Individual models | |||
| CBB OCL | 0.02 | 0, 1.29 | 0.06 |
| Self-ECog | 1.33 | 0.79, 2.25 | 0.29 |
| SP-ECog | 2.40 | 1.33, 4.32 | 0.003 |
| ECog Ratio | 1.38 | 0.55, 3.47 | 0.51 |
| Combined model | |||
| CBB OCL | 0.06 | 0, 15.41 | 0.30 |
| Self-ECog | 0.71 | 0.2, 2.53 | 0.59 |
| SP-ECog | 4.95 | 1.24, 19.74 | 0.01 |
Results of logistic regression models with (A) Aβ; (B) pre-PET diagnosis (dementia or MCI); or (C) post-PET diagnosis (MCI or AD dementia) as the dependent variable. Results are shown for a series of models including either CBB OCL, Self-ECog, SP-ECog, or ECog ratio (Individual Models) as predictors; or a single multivariable model including both CBB OCL and ECog (Combined Model). All analyses included participant age, education level, and gender as covariates. P-values < 0.05 are indicated by bold italics.
Figure 2: Online variable associated with Aβ and clinical diagnosis.
Box and whisker plots showing (A) CBB OCL scores, SP-ECog scores, self-ECog scores, and ECog ratio in A- (cyan) and A+ (magenta) participants; or (B) the same online variables in participants diagnosed with MCI (cyan) or dementia (DEM, magenta), or (C) diagnosed with MCI (cyan) or dementia due to AD (AD Dem, magenta). Top and bottom limits of the boxes represent the 25th and 75th percentile, box centerlines represent the median value, and whiskers extend to the most extreme data point which is no more than 1.5 times the length of the box away from the box. (D) Scatter plots showing the association between MoCA scores and CBB OCL scores, SP-ECog scores, self-ECog scores, or ECog ratio. Linear regression analysis (red solid line) shows significant associations between MoCA and CBB OCL, SP-ECog, and ECog ratio. MoCA scores of individual participants are indicated by black dots. Dotted red lines represent 95% confidence intervals.
3.2.2. Associations between Aβ and functional status.
In individual logistic regression models including either SP-ECog or self-ECog, neither was associated with Aβ (Table 2A). In a multivariable model including both SP-ECog and self-ECog, higher SP-ECog scores (indicating report of greater amounts of functional decline) were associated with higher probability of being Aβ+ (OR: 1.67, CI: 1.07–2.61, p=0.03). Conversely, higher (worse) self-ECog scores were significantly associated with higher probability of being Aβ- (OR: 0.58, CI: 0.36–0.93, p=0.024). To further explore this inverse relationship, we determined the ratio of SP-ECog to self-ECog, and considered ECog ratio as a predictor in subsequent analyses. Higher ECog ratios, meaning a larger discrepancy between SP and self-report ECog, with SPs reporting more decline than participants themselves; were associated with higher probability of being Aβ+ (Table 2A). The associations did not depend on pre-PET diagnosis (self-ECog by diagnosis interaction OR: 0.56, CI: 0.26–1.21, p=0.14; SP-ECog by diagnosis interaction OR: 0.47, CI: 0.16–1.39, p=0.17; ECog ratio by diagnosis interaction OR: 1.87, CI: 0.22–15.77, p=0.57).
3.2.3. Online composites to predict Aβ.
In a model including ECog and Cogstate, worse SP-ECog scores were associated with greater probability of being Aβ. There was no association between Cogstate OCL or Self-ECog and Aβ in this model (Table 2A).
3.3. Associations between clinical diagnosis and BHR assessments.
3.3.1. Association between pre-PET diagnosis and online CBB.
Lower accuracy on the Cogstate OCL test was associated with higher probability of being diagnosed with dementia versus MCI (Table 2B and Figure 2B). The associations did not differ between Aβ+ and Aβ- participants (CBB OCL by Aβ interaction, OR: 0.72, CI: 0.01–39.72, p = 0.92).
3.3.2. Association between pre-PET diagnosis and functional status.
Higher (worse) SP-ECog scores, and higher SP to self ECog ratio, were associated with higher probability of dementia versus MCI diagnosis (Table 2B and Figure 2B). The associations did not depend on participant Aβ status (SP-ECog by Aβ interaction, OR: 0.58, CI: 0.19–1.74, p=0.33; ECog ratio by Aβ interaction, OR: 1.40, CI: 0.23–8.69, p = 0.72).
3.3.3. Online composites to predict participant pre-PET diagnosis.
In a model including ECog and Cogstate, worse SP-ECog scores were associated with higher probability of dementia. Neither Cogstate OCL nor self-ECog were associated with pre-PET diagnosis in this model (Table 2B).
3.3.4. Association between post-PET diagnosis and online variables.
Worse SP-ECog scores were associated with greater probability of having a diagnosis of dementia due to AD versus MCI (Table 2C and Figure 2C). No other online variables were associated with post-PET diagnosis.
3.4. Associations between MoCA performance and BHR assessments.
Lower accuracy on Cogstate OCL was associated with lower (worse) MoCA scores. (Table 3 and Figure 2D). Worse SP-ECog scores, but not self-ECog scores, were associated with lower MoCA scores (Table 3). In a model including ECog and OCL, no predictors were associated with MoCA scores, although there was a trend-level association between SP-ECog and MoCA (Table 3). None of the associations between MoCA and online variables depended on participant diagnosis or Aβ status (p-values >0.10 for all diagnosis and Aβ interaction terms).
Table 3:
Associations between MoCA and online variables
| β | 95% Confidence Interval | p-value | R2 | |
|---|---|---|---|---|
| Individual models | ||||
| CBB OCL | 8.53 | 2.24, 14.83 | 0.009 | 0.11 |
| Self-ECog | −0.88 | −1.87, 0.11 | 0.08 | 0.03 |
| SP-ECog | −2.81 | −4.28, −1.34 | <0.001 | 0.16 |
| ECog ratio | −2.29 | −4.25, −0.37 | 0.02 | 0.08 |
| Combined Model (online composite) | 0.15 | |||
| CBB OCL | 7.11 | −4.02, 18.23 | 0.20 | |
| Self-ECog | 1.103 | −2.246, 4.45 | 0.51 | |
| SP-ECog | −3.00 | −6.29, 0.29 | 0.07 |
Results of linear regression models with MoCA score as the dependent variable. Results are shown for a series of models including either CBB OCL, Self-ECog, SP-ECog, or ECog ratio (Individual Models) as predictors; or a single multivariable model including both CBB OCL and ECog (Combined Model). All analyses included participant age, education level, and gender as covariates. P-values < 0.05 are indicated by bold italics.
3.5. Associations between self- and SP-report functional status.
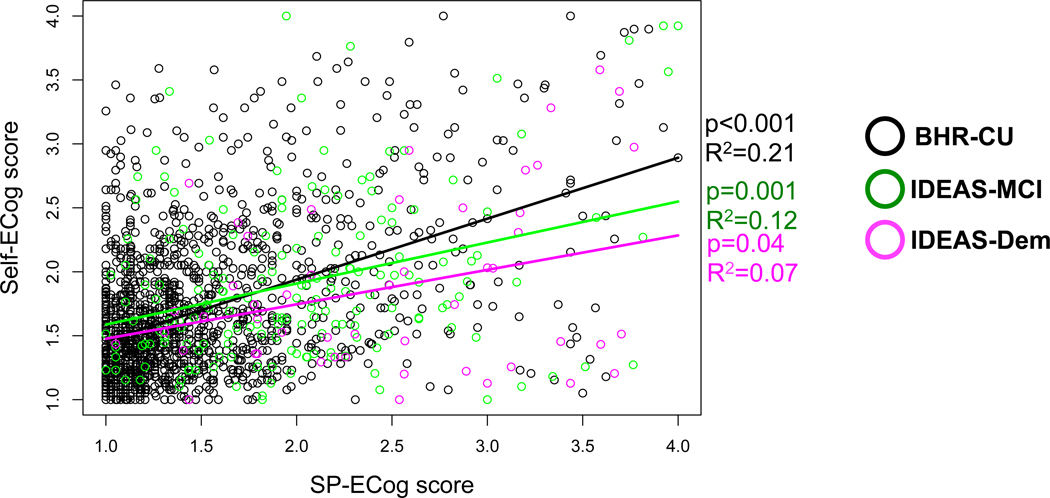
In order to further explore the relationship between SP- and self-report ECog, we estimated associations between self- and SP-report ECog in three groups: (1) BHR-IDEAS with MCI; (2) BHR-IDEAS with dementia; (3) a cohort of older adults with no evidence of cognitive impairments who were enrolled in BHR from the general public and did not participate in IDEAS (BHR-CU, see Supplemental Table 1). There was an association between higher SP-ECog and higher self-ECog scores in the BHR-CU and IDEAS-MCI groups, but not in the IDEAS dementia group (Figure 3). Compared to BHR-CU, IDEAS-MCI and IDEAS dementia participants underreported subjective decline compared to their SPs (Figure 3).
Figure 3: Association between self- and SP-report ECog, stratified by diagnosis.
Scatter plots showing association between self- and SP-report ECog scores for cognitively-unimpaired older adults enrolled in BHR who did not participate in IDEAS (BHR-CU, black), and IDEAS participants diagnosed with MCI (green) or dementia (magenta). Linear regression analysis (solid black, green, magenta lines) adjusted for age, gender, and education.
4. Discussion
The major findings of this study were that in a large cohort of older adults with diagnosed cognitive impairments (1) Enrollment into an online registry is feasible. The online approach has a selection bias for younger and less cognitively-impaired individuals, with higher educational attainment; (2) Online SP-report of greater functional decline is associated with higher probability of AD dementia diagnosis, and worse cognitive screening test (MoCA) scores ; (3) Online SP-report of greater functional decline is associated with Aβ positivity, impairment level, and dementia diagnosis when accounting for self-reported functional decline and an objective measure of cognition, online Cogstate visual learning test; (4) Participants underreported functional changes compared to their SPs, which may be due to lack of insight in participants with cognitive impairment. Greater discrepancy between SP-report and self-report of functional decline is associated with greater probability of Aβ positivity and dementia diagnosis. Taken together, the results support the validity of measures collected unsupervised and online in a cohort of cognitively-impaired older adults, and highlight the importance of online SP-reported information for dementia clinical research.
Compared to the entire IDEAS cohort included in Aim 1 of the IDEAS study, the subsample (4%) who enrolled in BHR were younger, more highly educated, and had less cognitive impairment. (Supplemental Table 1). These differences highlight the selection bias of the online cohort for those with sufficient cognitive and functional abilities and computer access and literacy to navigate through the BHR website to complete online tasks. The BHR-IDEAS cohort also underrepresented African Americans compared to the larger IDEAS cohort, highlighting the need for additional strategies to recruit traditionally-underserved groups. Nevertheless, 770 cognitively impaired participants were able to successfully enroll in BHR and complete online questionnaires and cognitive tests.
Lower accuracy on CBB OC was associated with increased likelihood of being Aβ+. For SP-ECog, the relationship reached statistical significance only when self-ECog was included in the model. These findings agree with previous findings using in-clinic measures of objective cognition and subjective decline [4, 20, 33, 34]. We observed an inverse relationship between self-ECog and Aβ status, in which participant report of less functional decline was associated with greater likelihood of being Aβ+. Furthermore, the ratio of SP to self-report ECog was significantly associated with Aβ. Specifically, a greater ECog ratio (greater discrepancy between the SP and self-report, in which the SP reports more decline than the participant) was associated with Aβ positivity. Our findings suggest that participant lack of reported functional changes, together with SP-report of increased functional decline, may help identify participants with high levels of brain Aβ. Loss of insight about one’s own cognitive and functional status are likely to contribute to the participants’ under-reporting of functional decline that we observed [18, 21, 22]. The results contrast with [35], in which mutual (self and SP) cognitive complaint was associated with Aβ in MCI participants. Thus, different subjective constructs (complaint, concerns, decline in ADLs) may be differentially predictive of AD-related risk outcomes. Cohort differences between IDEAS and more selected academic samples may also contribute to observed differences. Our novel results support the validity of an online, unsupervised measure of SP-report subjective decline for identifying those at risk for MCI and dementia due to AD.
Worse SP-ECog was associated with higher probability of both a pre-PET diagnosis of dementia, and as post-PET diagnosis of AD dementia. Higher ECog ratio and lower accuracy on CBB OCL were also associated with higher probability of pre-PET dementia diagnosis. Our results are not surprising, considering that objective evidence of cognitive impairment, as well as changes reported by an SP, are diagnostic criteria for MCI and AD dementia [36–38]. The novelty of our result is the use of unsupervised, online measures of objective cognition and subjective functional decline to identify those with MCI, dementia, and AD. Furthermore, we found that in a multivariable model that included both ECog and CBB OCL, only worse SP-ECog scores remained associated with higher likelihood of AD dementia diagnosis. This supports the idea, recently confirmed using in clinic data [39] that SP-reported functional decline confers value in identifying those at risk for MCI and dementia, over and above the contribution of objective cognitive measures.
Providing further evidence for the construct validity of our online approach, worse SP-ECog and CBB OCL scores were associated with worse MoCA scores. The associations between BHR variables and MoCA scores that we observed were relatively modest (R2=0.15 in the composite model). In future studies it will be important to investigate the relationship between online variables and more sensitive, in-clinic measures of cognition, such as neuropsychological test results. Nevertheless, our present findings with the MoCA demonstrate that the online measures are useful for identifying those with in-clinic, well-validated objective evidence of cognitive impairment, and further supports the use of online measures to identify those with cognitive impairments, including MCI and dementia.
Limitations of this study that may affect generalizability to the older adults population include the selection biases described above, the lack of sociodemographic diversity of the sample, and IDEAS study inclusion criteria requiring cognitive impairment due to unknown etiology. On the other hand, the IDEAS study is unique in that participants are recruited primarily from non-academic memory clinics, and data therefore results maybe more generalizable than measures in more selected, academic cohorts. Reliability of Aβ PET visual reads may be affected by community reads of Aβ+ by local imaging specialists, as opposed to standardized, central reads; and variability in diagnostic methodology used by local dementia specialists could influence diagnostic accuracy . We limited our analysis of subjective assessment to ECog, which measures changes in complex, instrumental ADLs. In future studies it will be important to include additional subjective measures, such as basic ADLs, cognitive complaints, memory concerns, and behavioral and neuropsychiatric symptoms. These additional measures, which are being collected as part of the BHR study, may confer additional predictive power [40–45].
In conclusion, online SP-report of greater functional decline is significantly associated with clinically-verified participant diagnosis, and cognitive screening instrument scores in a cohort of cognitively-impaired older adults enrolled in the Brain Health Registry. Online SP-report functional decline confers independent predictive power compared to cognitive test scores alone in predicting diagnosis and brain Aβ levels. In addition, SP-report of more decline, combined with participant report of less decline, significantly predicts AD risk. These findings have important implications for using online SP-reported measures to identify those at risk for cognitive decline, MCI, dementia, and AD in clinical research and healthcare settings.
Supplementary Material
5. Acknowledgments and Funding Sources.
This work was funded by the National Institutes of Health [K01AG055692], Alzheimer’s Association [BHR-16–459161], Larry L. Hillblom Foundation [2015-A-011-NET], and the California Department of Public Health [16–10323]. We would like to acknowledge the contributions of all Brain Health Registry staff members and all Brain Health Registry participants and study partners. We would like to acknowledge the individuals who contributed to the IDEAS study, including faculty and staff who contributed to study planning, operations, and participation in study committees; and all dementia specialists, imaging specialists, and patients who participated in the study. The IDEAS study was funded by the Alzheimer’s Association, the American College of Radiology, Avid Radiopharmaceuticals Inc (a wholly owned subsidiary of Eli Lilly and Company), General Electric Healthcare, and Life Molecular Imaging (formerly Piramal Imaging).
Footnotes
Author Declarations of Interest Statement:
RLN, MRC, CJ, JN, DT, DF, MA, MCC, KNF, JH, and LH have no interests to declare. RSM has received grant funding from the National Institute of Mental Health and has received research support from Johnson&Johnson. PM is an employee of Cogstate, Ltd. GDR is Study Chair for the IDEAS study; Additional research support from NIH, Alzheimer’s Association, Tau Consortium, Avid Radiopharmaceuticals, Eli Lilly. Consultant for Axon Neurosciences, GE Healthcare, Eisai, Merck. Associate Editor for JAMA Neurology. MWW receives support for his work from the following funding sources: NIH: 5U19AG024904–14; 1R01AG053798–01A1; R01 MH098062; U24 AG057437–01; 1U2CA060426–01; 1R01AG058676–01A1; and 1RF1AG059009–01, DOD: W81XWH-15–2-0070; 0W81XWH-12–2-0012; W81XWH-14–1-0462; W81XWH-13–1-0259, PCORI: PPRN-1501–26817, California Dept. of Public Health: 16–10054, U. Michigan: 18-PAF01312, Siemens: 444951–54249, Biogen: 174552, Hillblom Foundation: 2015-A-011-NET, Alzheimer’s Association: BHR-16–459161 ; The State of California: 18–109929. He also receives support from Johnson&Johnson, Kevin and Connie Shanahan, GE, VUmc, Australian Catholic University (HBI-BHR), The Stroke Foundation, and the Veterans Administration. He has served on Advisory Boards for Eli Lilly, Cerecin/Accera, Roche, Alzheon, Inc., Merck Sharp & Dohme Corp., Nestle/Nestec, PCORI/PPRN, Dolby Family Ventures, National Institute on Aging (NIA), Brain Health Registry and ADNI. He serves on the Editorial Boards for Alzheimer’s & Dementia, TMRI and MRI. He has provided consulting and/or acted as a speaker/lecturer to Cerecin/Accera, Inc., Alzheimer’s Drug Discovery Foundation (ADDF), Merck, BioClinica, Eli Lilly, Indiana University, Howard University, Nestle/Nestec, Roche, Genentech, NIH, Lynch Group GLC, Health & Wellness Partners, Bionest Partners, American Academy of Neurology (AAN), NYU, Japanese Government Alliance, National Center for Geriatrics and Gerontology (Japan), US Against Alzheimer’s, Society for Nuclear Medicine and Molecular Imaging (SNMMI), The Buck Institute for Research on Aging, and FUJIFILM-Toyama Chemical (Japan). He holds stock options with Alzheon, Inc., Alzeca, and Anven. The following entities have provided funding for academic travel: Kenes, Intl., Merck, ADCS, ATRI, Eli Lilly, The Alzheimer’s Association, Merck, Tokyo University, Kyoto University, AAN, AC Immune, CHU Toulouse, St. George Hospital University, Indiana U., U. Melbourne, Australian Catholic University, Japanese Government Alliance, National Center for Geriatrics and Gerontology (Japan), US Against Alzheimer’s, NYU, USC, and SNMMI.
8. References Cited
- 1.Weiner MW, et al. , The Brain Health Registry: An internet-based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimer’s & Dementia, 2018. 14(8): p. 1063–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackin RS, et al. , Unsupervised online neuropsychological test performance for individuals with mild cognitive impairment and dementia: Results from the Brain Health Registry. Alzheimers Dement (Amst), 2018. 10: p. 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nosheny RL, et al. , Online study partner-reported cognitive decline in the Brain Health Registry. Alzheimers Dement (N Y), 2018. 4: p. 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okonkwo OC, et al. , Cerebrospinal fluid abnormalities and rate of decline in everyday function across the dementia spectrum: normal aging, mild cognitive impairment, and Alzheimer disease. Arch Neurol, 2010. 67(6): p. 688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall GA, et al. , Regional cortical thinning and cerebrospinal biomarkers predict worsening daily functioning across the Alzheimer’s disease spectrum. J Alzheimers Dis, 2014. 41(3): p. 719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lilamand M, et al. , Brain Amyloid Deposition Is Associated With Lower Instrumental Activities of Daily Living Abilities in Older Adults. Results From the MAPT Study. J Gerontol A Biol Sci Med Sci, 2016. 71(3): p. 391–7. [DOI] [PubMed] [Google Scholar]
- 7.Farias ST, et al. , Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol, 2009. 66(9): p. 1151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dauphinot V, et al. , Risk factors of caregiver burden among patients with Alzheimer’s disease or related disorders: a cross-sectional study. J Alzheimers Dis, 2015. 44(3): p. 907–16. [DOI] [PubMed] [Google Scholar]
- 9.Morris JC, The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology, 1993. 43(11): p. 2412–4. [DOI] [PubMed] [Google Scholar]
- 10.Farias ST, et al. , The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology, 2008. 22(4): p. 531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeffer RI, et al. , Measurement of functional activities in older adults in the community. J Gerontol, 1982. 37(3): p. 323–9. [DOI] [PubMed] [Google Scholar]
- 12.Jorm AF, et al. , Performance of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) as a screening test for dementia. Psychol Med, 1991. 21(3): p. 785–90. [DOI] [PubMed] [Google Scholar]
- 13.Amariglio RE, et al. , Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: the Alzheimer’s Disease Cooperative Study Cognitive Function Instrument. JAMA Neurol, 2015. 72(4): p. 446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galvin JE, et al. , The AD8: a brief informant interview to detect dementia. Neurology, 2005. 65(4): p. 559–64. [DOI] [PubMed] [Google Scholar]
- 15.Marshall GA, et al. , Everyday cognition scale items that best discriminate between and predict progression from clinically normal to mild cognitive impairment. Curr Alzheimer Res, 2014. 11(9): p. 853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donovan NJ, et al. , Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. Am J Geriatr Psychiatry, 2014. 22(12): p. 1642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomaszewski Farias S, et al. , The measurement of everyday cognition: development and validation of a short form of the Everyday Cognition scales. Alzheimers Dement, 2011. 7(6): p. 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rueda AD, et al. , Self-rated and informant-rated everyday function in comparison to objective markers of Alzheimer’s disease. Alzheimers Dement, 2015. 11(9): p. 1080–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong Y, et al. , The informant AD8 is superior to participant AD8 in detecting cognitive impairment in a memory clinic setting. J Alzheimers Dis, 2013. 35(1): p. 159–68. [DOI] [PubMed] [Google Scholar]
- 20.Halawa OA, et al. , Inferior and medial temporal tau and cortical amyloid are associated with daily functional impairment in Alzheimer’s disease. Alzheimers Res Ther, 2019. 11(1): p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farias ST, Mungas D, and Jagust W, Degree of discrepancy between self and other-reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. Int J Geriatr Psychiatry, 2005. 20(9): p. 827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherling CS, et al. , Decreased Self-Appraisal Accuracy on Cognitive Tests of Executive Functioning Is a Predictor of Decline in Mild Cognitive Impairment. Front Aging Neurosci, 2016. 8: p. 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiner MW, et al. , The Brain Health Registry: An internet-based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimers Dement, 2018. 14(8): p. 1063–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabinovici GD, et al. , Association of Amyloid Positron Emission Tomography With Subsequent Change in Clinical Management Among Medicare Beneficiaries With Mild Cognitive Impairment or Dementia. JAMA, 2019. 321(13): p. 1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maruff P, et al. , Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol, 2009. 24(2): p. 165–78. [DOI] [PubMed] [Google Scholar]
- 26.Maruff P, et al. , Clinical utility of the cogstate brief battery in identifying cognitive impairment in mild cognitive impairment and Alzheimer’s disease. BMC Psychol, 2013. 1(1): p. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuiper JS, et al. , Comparison of cognitive functioning as measured by the Ruff Figural Fluency Test and the CogState computerized battery within the LifeLines Cohort Study. BMC Psychol, 2017. 5(1): p. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cromer JA, et al. , Comparison of Cognitive Performance on the Cogstate Brief Battery When Taken In-Clinic, In-Group, and Unsupervised. Clin Neuropsychol, 2015. 29(4): p. 542–58. [DOI] [PubMed] [Google Scholar]
- 29.Sumner JA, et al. , Posttraumatic stress disorder symptoms and cognitive function in a large cohort of middle-aged women. Depress Anxiety, 2017. 34(4): p. 356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasreddine ZS, et al. , The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc, 2005. 53(4): p. 695–9. [DOI] [PubMed] [Google Scholar]
- 31.Johnson KA, et al. , Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement, 2013. 9(1): p. e-1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson KA, et al. , Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. J Nucl Med, 2013. 54(7): p. 1011–3. [DOI] [PubMed] [Google Scholar]
- 33.Lim YY, et al. , Performance on the Cogstate Brief Battery Is Related to Amyloid Levels and Hippocampal Volume in Very Mild Dementia. J Mol Neurosci, 2016. 60(3): p. 362–370. [DOI] [PubMed] [Google Scholar]
- 34.Lim YY, et al. , Relationships between performance on the Cogstate Brief Battery, neurodegeneration, and Abeta accumulation in cognitively normal older adults and adults with MCI. Arch Clin Neuropsychol, 2015. 30(1): p. 49–58. [DOI] [PubMed] [Google Scholar]
- 35.Gifford KA, et al. , A Mutual Self- and Informant-Report of Cognitive Complaint Correlates with Neuropathological Outcomes in Mild Cognitive Impairment. PLoS One, 2015. 10(11): p. e0141831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersen RC, et al. , Mild cognitive impairment: clinical characterization and outcome. Arch Neurol, 1999. 56(3): p. 303–8. [DOI] [PubMed] [Google Scholar]
- 37.McKhann GM, et al. , The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement, 2011. 7(3): p. 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albert MS, et al. , The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement, 2011. 7(3): p. 270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nosheny RL, et al. , Study partner-reported decline identifies cognitive decline and dementia risk. Ann Clin Transl Neurol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Harten AC, et al. , Subjective cognitive decline and risk of MCI: The Mayo Clinic Study of Aging. Neurology, 2018. 91(4): p. e300–e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masters MC, Morris JC, and Roe CM, “Noncognitive” symptoms of early Alzheimer disease: a longitudinal analysis. Neurology, 2015. 84(6): p. 617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Creese B, et al. , Mild Behavioral Impairment as a Marker of Cognitive Decline in Cognitively Normal Older Adults. Am J Geriatr Psychiatry, 2019. 27(8): p. 823–834. [DOI] [PubMed] [Google Scholar]
- 43.Yoon JS, et al. , Depressive Symptoms as a Predictor of Memory Complaints in the PRISM Sample. J Gerontol B Psychol Sci Soc Sci, 2017. 00(00): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yates JA, et al. , Subjective Memory Complaints are Involved in the Relationship between Mood and Mild Cognitive Impairment. J Alzheimers Dis, 2015. 48 Suppl 1: p. S115–23. [DOI] [PubMed] [Google Scholar]
- 45.Jimenez-Huete A, et al. , Subjective Evaluation of Mood and Cognitive Functions in a General Neurology Clinic: Patients versus Informants. J Clin Neurol, 2017. 13(3): p. 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.