Abstract
Objectives:
Anti-inflammatory and immune-modulating effects of statins suggest that they may play a role in the risk of rheumatoid arthritis (RA). We aimed to perform a systematic review and meta-analysis of studies assessing the risk of RA in statin-users versus non-users.
Methods:
We searched Medline from inception to 01/22/2019 and Embase from 1988 to Week 03 2019 for studies that examined the association between statin use and RA without restrictions on language.
Results:
We identified 1,161 references; of them 8 studies (5 cohort studies and 3 case-control studies) were included. Four cohort studies comparing statin-users versus non-users were included in the meta-analysis. The pooled risk ratio (RR) was 1.01; 95%CI 0.93-1.10; I2=17%. Case-control studies showed highly heterogeneous results (I2=92%) and were not included in the meta-analysis. One cohort study and one case-control study assessing persistence with or intensity of treatment with statins showed lower risk of RA with higher versus lower treatment persistence or intensity of statin use (pooled RR 0.66; 95%CI 0.5-0.87; I2=83%). The certainty in the evidence was low.
Conclusion.
In this systematic review and meta-analysis, we observed no difference in risk of RA in statin users vs non-users. Risk of RA may be lower in patients with higher versus lower statin treatment persistence or intensity. Future observational studies with guards against selection bias and confounding are needed to further elucidate the impact of statin use on the risk of RA, considering potential differences by dosage, duration of use, study population and other factors.
Keywords: Rheumatoid arthritis, statins, systematic review, meta-analysis
Introduction
Statins or 3-hydroxy-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors are widely used for treatment of hyperlipidemia and prevention of cardiovascular disease (1-6). There is growing evidence on cholesterol-independent or “pleiotropic” effects of statins (7, 8). Randomized placebo-controlled trials have shown anti-inflammatory effects of statins in the general population (2) and in patients with chronic autoimmune inflammatory disease such as rheumatoid arthritis (RA) (9). Recent studies suggest that statins may also exert immune-modulating effects through their effect on signaling, gene transcription, epigenetic modifications and immune metabolism (10-12). Whether and how these immunomodulatory effects of statins impact the risk of development of autoimmune disease is unclear. Several observational studies have explored the association between statin use and onset of autoimmune conditions, including RA (13-22). A recent systematic review suggested that statin use may be associated with increased risk of systemic lupus erythematosus (SLE) and autoimmune myopathies (23). A systematic review and meta-analysis of a wide range of unintended effects of statins reported no association of statin use with “arthritis” defined as a combined category of RA and/or osteoarthritis, based on results of 4 studies (24). Effects of statins on the risk of developing RA have not been systematically reviewed. We aimed to perform a systematic review and meta-analysis of studies assessing the risk of RA in statin-users versus non-users.
Materials and Methods
The systematic review was performed according to the Cochrane Handbook for Systematic Reviews of Interventions (25) and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (26). The search strategy and subsequent literature searches were performed by an experienced medical reference librarian in collaboration with the lead author (Elena Myasoedova [EM]). The search strategy is presented in Appendix 1. There were no language restrictions. All results were downloaded into EndNote X8 (Thompson ISI Research soft, Philadelphia, Pennsylvania), a bibliographic database manager. Duplicate citations were identified and removed.
Study selection.
Two reviewers (EM and Paras Karmacharya [PK]) independently assessed the eligibility of identified studies. Only comparative studies examining the association between statin use and incidence of RA in adults were eligible for inclusion. We excluded studies in which RA was present at the time of statin use and animal studies. Disagreements among reviewers were discussed and agreement was reached by consensus.
Data extraction.
A pre-defined data collection form was used to retrieve the information from the pertinent studies. EM extracted and recorded the data in the data-collection form. Adjusted effect estimates and 95% confidence intervals (CI) were collected from each study. We used the Newcastle-Ottawa Quality Assessment Scale (27), which is intended to rate selection bias, comparability of the exposed and unexposed groups, outcome assessment, and completeness of follow-up. See Appendix 2 for the specifics of using the Newcastle-Ottawa scale for cohort and case-control studies. We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework to evaluate the certainty of evidence. Following GRADE, the certainty of evidence has an initial grade based on study design; which can be rated down based on the domains of methodological quality, indirectness, inconsistency, imprecision and publication bias (28). Certainty in evidence can be rated up in certain situations such as when the effect size is large.
Statistical Methods
Meta-analysis.
The primary effect measures used in the studies were Odds Ratios (OR), Hazard Ratios (HR) and Relative Risks (RR). These effect measures were assumed to reasonably estimate the same association between statin use and RA occurrence given the low incidence of RA and thus were pooled together. Adjusted effect estimates were used for this analysis. If an adjusted effect estimate was not available from the study, unadjusted effect estimates were calculated using the study data available in the manuscript and used for sensitivity analyses as alternatives. Individual studies were weighted according to their log-transformed inverse variance. A random-effects model was used because of anticipated heterogeneity. The chi-square test was used to assess heterogeneity among studies; I2 statistic was also calculated. The I2 statistic estimated the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (29, 30). The possible influence of publication bias was graphically assessed using a funnel plot. All analyses were conducted using the statistical software RevMan (Version 5.3. Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Results
Figure 1 shows a flow diagram of eligible studies. The literature search identified 1,161 references which were imported for screening. Eight studies (5 cohort studies and 3 case-control studies) were included in the systematic review (13-20). All of them were English language articles. The inter-rater agreement on study selection was very good (Cohen’s Kappa coefficient 0.90) (31).
Figure 1.
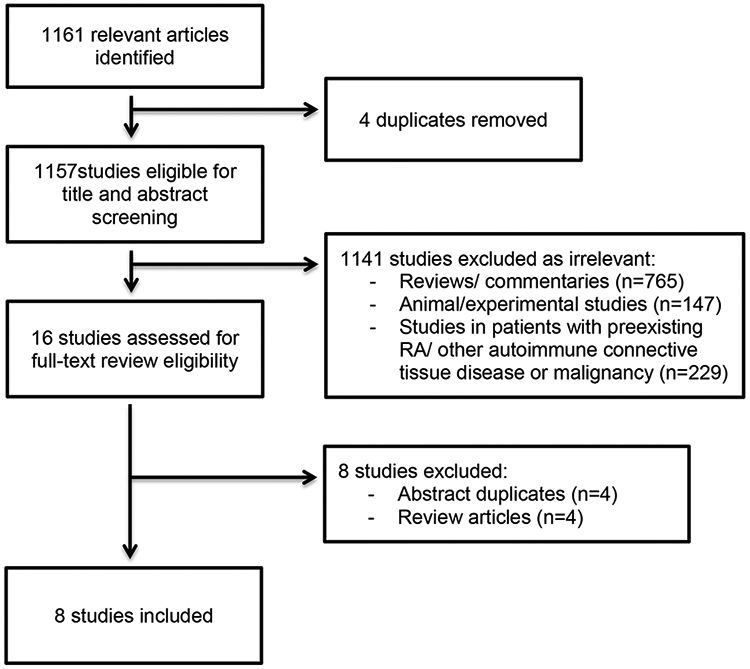
Flow diagram of eligible studies.
Table 1 summarizes the main characteristics of the studies. Most of the studies were from the UK or Europe; performed in 1990s-2000s. All studies used large data-bases for patient identification and data acquisition. All studies included adults with RA (age ≥18 years), with minimal age at inclusion specified as ≥30 years (18, 19) or ≥40 years (14, 16, 17, 20) in most studies. The majority of studies, particularly cohort studies, in this analysis were large with at least 300 and up to 5,730 RA cases.
Table 1.
Characteristics of studies
| Source, country |
Study design |
Data source for patient identification |
Years of observ ation |
Number of RA cases in comparator groups |
Age per inclusion criteria; mean age, years |
Female sex |
Length of follow- up |
Effect estimate | Newcastle -Ottawa scale |
|---|---|---|---|---|---|---|---|---|---|
| De Jong et al. (16); Netherlands | case- control | Netherlands Information Network of General Practice (LINH) database | 2001- 2006 | 508 cases of RA (81 exposed to statins) and 2369 controls (204 exposed to statins) | ≥ 40; 63.4 (cases); 62.8 (controls) | 67.5% of cases and 65.8% of controls | Not specifie d | OR 1.71; 95% CI 1.16-2.53 | S**** C** E** |
| Tascilar et al. (14); UK | nested case- control | UK Clinical Practice research datalink (CPRD) | 1997- 2009 | 1357 cases of RA (1032 exposed to statins) and 13570 controls (11,118 exposed to statins) among 528,654 new statin users | ≥ 40; 63.7 (cases); 63.8 (controls) | 60.3% of cases and controls | Mean 39.2+/− SD 30.3 months | HR 0.77; 95% CI 0.63-0.95 for highest vs low statin intensity quintile | S**** C** E** |
| Jick et al. (17); UK | nested case- control | General practice research database (GPRD) | 1992- 2001 | 313 cases of RA (41 exposed to statins) and 1252 controls (194 exposed to statins) | 40-89; mean age not specified, shown distribution by age group | 60.7% of cases and controls | Not specifie d | OR 0.59; 95% CI 0.37-0.96 | S**** C** E* |
| Smeeth et al.(20); UK | cohort, retrosp ective | The Health Improvement Network (THIN) database | 1995- 2006 | 2,532 incident RA cases overall out of 729,529 individuals; 227 cases of RA in exposed to statins, 2,305 in unexposed | 40-80; mean age not specified, shown distribution by age group | 50.2% in unexpos ed, 49.3% in exposed | Median 4.3 years | HR 0.93; 95% CI 0.73-1.18 | S** C** O* |
| Hippisley- Cox et al. (19); UK | cohort, prospe ctive | Q research database | 2002- 2008 | 5,730 RA cases among 225,922 new statin users | 30-84; 57.2 (new users); 44.4 (non- users) | 46.4% in new users, 51.1% in | Not specifie d | HR for simvastatin. Men: 0.96; 95% CI 0.84-1.09; | S*** C** O** |
| non- users | women: 1.12; 95% CI 0.96- 1.32 | ||||||||
| de Jong et al. (15); UK | matched cohort, prospe ctive | CPRD | 1995- 2009 | 837 RA cases among 511,620 current statin users | ≥ 40; 63.0 (statin users); 62.8 (non- users) | 47.9% in statin users and non-users | Mean 3 +/− SD 2.5 years | HR 1.06; 95% CI 0.93-1.22 | S**** C** O** |
| Chodick et al. (13); Israel | cohort, retrosp ective | Maccabi Health Services (MHS) | 1998- 2007 | 2,578 RA cases among 211,627 new statin users | ≥ 18; 57.17 years | 50.9% | Mean 4.97 years | HR for highly persistent vs non-persistent patients: 0.58; 95% CI 0.52- 0.65 | S**** C** O*** |
| Schmidt et al. (18); US | cohort, retrosp ective | San Antonio area military healthcare system, Tricare Prime/Tricare Plus | 2003- 2010 | 104 RA cases among 6,956 statin users and 122 RA cases among matched 6,956 statin non-users driven from the population of 13,640 statin users and 32,848 nonusers | 30-85; 57 in users and non-users | 41.7% in users, 56.2% in non- users | Not specifie d | Unadjusted OR for RA cases (calculated from the data in the manuscript) 0.85; 95% CI 0.65-1.11 | S**** C** O** |
Newcastle-Ottawa scale: S= selection; C=comparability; E=exposure; O=outcome
Risk of bias assessment by Newcastle-Ottawa scale for case-control and cohort studies (Table 1).
Most studies were of good quality with no evidence of selection bias, and with good comparability of cases and controls or of the exposed and unexposed groups of each cohort. Most studies except for one (20) explicitly stated their approach to verification of RA status, which was done using diagnostic codes for RA in conjunction with prescription data (14-16) and manual records review (17). All studies evaluated new/ current exposure to statins (13, 14, 16-20) and some studies additionally assessed the impact of recent (15) and/ or past (15, 17) exposure to statins on the risk of RA. Ascertainment of statin exposure was by prescription of statins, with some studies requiring only one prescription for any statin as a criterion for exposure (13, 16) and others considering duration and intensity of statin use (13-15). All but three studies (17, 19, 20) additionally assessed adherence/ persistence with statins. These differences suggest some indirectness in assessment of intervention/ exposure among the studies. None of the studies assessed response to statin treatment. In the majority of studies, there was not enough information from the manuscript regarding follow-up assessment; only one study (13) reported the number of individuals who died/ relocated. The adequacy of the length of follow-up was arguable as development of RA can take many years. We arbitrarily chose to consider a follow up duration of 3 years or more to be adequate. Verification of accuracy of data extraction and quality assessment was performed by the second reviewer (PK).
Meta-analysis.
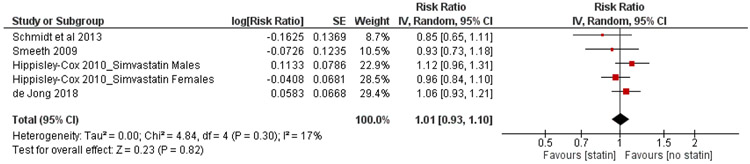
Four cohort studies (2 prospective and 2 retrospective cohorts) comparing risk of RA among statin users vs non-users were included in the meta-analysis (15, 18-20). The study by Hippisley-Cox et al. (19) reported effect estimates for males and females separately and for each type of statin without providing a combined estimate for exposed vs unexposed subjects. The authors disclosed that 70.7% of new statin users were prescribed simvastatin. Effect estimates for simvastatin as the most frequently used statin in females and males were included in the meta-analysis as separate studies as they had different control groups (i.e., female non-users and male non-uses, respectively). In the study by de Jong et al, 2018 (15) effect estimates were reported for current exposure to statins and recent exposure to statins defined as a period of time from 3 to 12 months after the end date of the most recent prescription. The effect estimate for current exposure was included in the meta-analysis. A study by Schmidt et al. (18) provided effect estimates for connective tissue disease overall (including RA), but did not report an adjusted effect estimate for patients with RA. Using the raw numbers provided in the text of the paper, we calculated the unadjusted OR for the risk of RA in statin users vs non-users and included it in the meta-analysis. Figure 2 shows the main forest plot stratified by study weight. The overall pooled effect estimate was 1.01; 95%CI 0.93-1.10; I2=17%. Although the confidence intervals were not wide, they included both potentially meaningful benefit and harm of the intervention, suggesting some imprecision in effect estimate. Thus, the message about the effect of the intervention could be different from the reported effect estimate.
Figure 2.
Forest-plot random effect model meta-analysis of the association between statin use and risk of rheumatoid arthritis
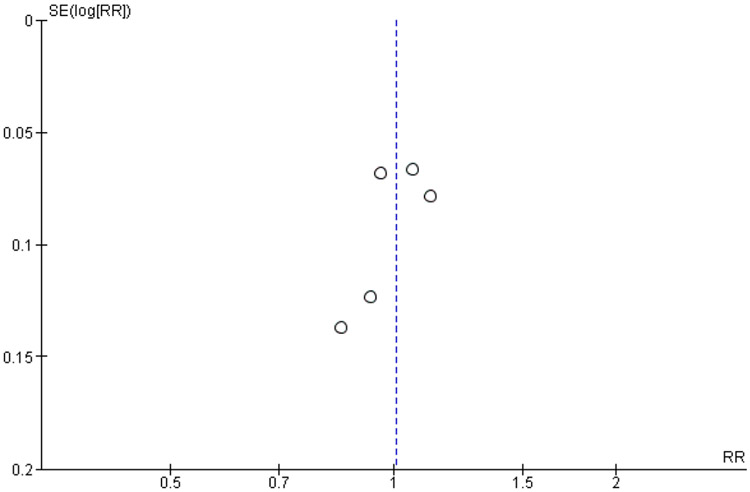
The funnel plot of the association between the estimated effect size and its standard error in the included studies shows a fairly symmetrical distribution (Figure 3). The Egger Regression Asymmetry test was not performed as the power of this method to detect asymmetry in the distribution of the estimates may be low with the small number of studies included in this analysis.
Figure 3.
Funnel plot of the association between the estimated effect size and its standard error
Sensitivity analysis.
We calculated effect estimates for current and recent statin users vs non-users for the study by de Jong et al. (15). Including these estimates in the meta-analysis did not change the results (RR 1.08; 95%CI 0.83-1.4).
Studies by Tascilar et al. (14) and Chodick et al. (13) were not included in the meta-analysis as these studies assessed association between persistence with or intensity of treatment with statins and the risk of RA, and none of these studies had a statin non-user group. When these studies were analyzed separately, the pooled effect estimate was 0.66; 95%CI 0.5-0.87; I2=83%.
Two case-control studies evaluating relationship between statin use and the risk of RA showed drastically different, inconsistent results. Study by Jick et al. (17) showed protective effect (OR 0.59; 95% CI 0.37-0.96) and study by de Jong et al., 2012 (16) showed harmful effect of statins on the risk of RA (OR 1.71; 95% CI 1.16-2.53), yielding the pooled RR 1.01; 95%CI 0.36-2.87, I2=92%. Given extreme heterogeneity of the results, limited information on individual’s statin exposure status over time, and relatively small number of patients exposed to statins these studies were not included in the meta-analysis.
Certainty in the evidence
Most of the included studies had low risk of bias based on the Newcastle-Ottawa scale. Heterogeneity in the analysis of cohort studies was small. The number of events was sufficiently large but with confidence intervals that include appreciable benefit and harm. There was no large magnitude of effect. Two studies showed dose-response associations between persistence with statins and risk of RA (13), and quintiles of duration-weighted average statin intensity and risk of RA (14). Study by Chodik et al. (13) estimated that the risk of RA was lower in patients who were highly persistent with statins (i.e. proportion of follow-up days covered [PDC] with statins ≥80%) compared to non-persistent patients (i.e. PDC < 20%): HR 0.58, 95%CI 0.52-0.65, adjusting for sociodemographics, comorbidities, LDL levels and efficacy of initial statin therapy. Increase in PDC by 10% was linked to 5.3% lower risk of RA. Extending these findings, a study by Tascilar et al. (14) showed that in a population of patients with persistence to statins (i.e., median PDC >80%) the incidence of RA was lower in the highest statin intensity quintile versus lowest quintile: HR 0.77, 95% CI 0.63–0.95, adjusting for smoking status, total cholesterol level, obesity, history of cardiovascular disease, coexistent autoimmune disease, hypothyroidism, and persistence with treatment., with higher as opposed to lower treatment persistence and intensity being associated with decreased risk of RA. However, as described in the sections above, the design of these two studies did not fully match the pre-specified question of assessing the risk of RA in statin users vs nonusers, and these studies were not included in the meta-analysis. Based on these domains of the GRADE framework, the certainty in the evidence was considered low.
Discussion
Growing evidence of immune-modulating effects of statins suggests that this class of medications may affect the risk of development of autoimmune disease. Observational studies evaluating the association between statin use and RA onset have shown conflicting results suggesting protective effect (13, 14, 17), harmful effect (15, 16) or no effect (19, 20). This systematic review and meta-analysis provides qualitative and quantitative summary of the association between statin use and risk of RA from existing observational studies. In this systematic review and meta-analysis, we observed no difference in risk of RA in statin users vs non-users. The overall quality of the evidence supporting these associations was low by GRADE framework. Cohort studies included in the meta-analysis showed low heterogeneity of the results with lack of a statistically significant effect of statin exposure on RA onset. Case-control studies were not included in the meta-analysis primarily due to extreme heterogeneity of the results (I2=92%). When analyzed separately, the pooled RR for these studies averaged into lack of effect but with wide 95%CI extending from potential 64% reduction to 287% increased risk of RA. Pooling the results of the studies assessing persistence with or intensity of treatment with statins (13, 14), we found 34% lower risk of RA in those who have higher as opposed to lower treatment persistence or intensity of statin use. However, there was a high degree of heterogeneity in this analysis (I2=83%).
Biological plausibility.
The presence of either protective of harmful effect of statin use on the risk of RA is biologically plausible. Prior clinical and experimental studies suggested anti-inflammatory and immune-modulating effects of statins. Statins have been found to beneficially impact the T helper cells (Th1)/Th2 balance in patients with acute coronary syndrome, heart failure and RA (32-34) and decrease CD40 expression and CD40-related activation of vascular cells (35). Atorvastatin has been shown to ameliorate experimental autoimmune neuritis by decreased Th1/Th17 cytokines and up-regulated T regulatory cells (36). Literature on anti-inflammatory effects of statins is growing, and two recent meta-analyses demonstrated beneficial effects of statins on RA disease activity (37, 38). Statins have been shown to reduce the rates of heart and kidney transplant rejection (39-41).
In contrast, there have been studies suggesting that statins may precipitate autoimmunity and predispose to development of SLE and lupus-like syndromes (23). Some of the included studies showed an adverse impact of statins on the risk of RA, particularly in the first year of statin use (15, 16). Taken together with the results from the studies reporting lower risk of RA in those who have higher as opposed to lower treatment persistence or intensity of statin use (13, 14), it may be suggested that there is a real variation in the treatment effect depending on patient characteristics or intervention.
Clinical implications.
Given the overall lack of association between statin use and risk of RA from our main analysis, there is not enough evidence to suggest that any additional considerations should be undertaken in statin users at risk for RA. The results of this study do not support the need for change to the current clinical practice at this point. However, our study outlines directions for future research. While randomized controlled trials on the subject of this study may not be feasible, large prospective studies with long follow-up and adequate uniform assessment of exposure and outcome can help identify factors that may modify the impact of treatment and direct the evidence on the association between statin use and risk of RA.
This systematic review has important strengths. This is the first systematic evaluation providing qualitative and quantitative assessment of the association between statins and the risk of RA. The study takes advantage of a comprehensive, up-to-date literature search and formal assessment of the methodological quality of pertinent studies. There was no apparent publication bias based on the graphical assessment with a funnel plot.
There are several potential limitations to this study. While only few studies were included in the meta-analysis, heterogeneity of these studies was low (I2=17%), suggesting that estimates from these studies can be reliably combined in the meta-analysis. Extreme heterogeneity that was found for the two eligible case-control studies (I2=92%) was felt to be unacceptable for inclusion in the meta-analysis. The reasons for potential heterogeneity may include differences in patient level parameters (i.e., demographics, comorbidities), intervention factors (i.e., dose, timing or duration of treatment), comparator factors (i.e., control group treatment or the co-interventions), study design (i.e., duration of follow-up or the reliability of exposure and outcome measures). The overall quality of evidence supporting the reported associations was low, which is not unexpected in the meta-analysis of observational studies and is inherent to the limitations of individual studies included in this analysis. Quantifying the length of statin exposure was not possible due to lacking information on exposure and follow-up from the original studies. This should be taken into consideration, and caution should be used while interpreting the result of this analysis. With only few studies included in the meta-analysis, the results of the funnel plot should be viewed with caution.
In summary, in this rigorous systematic review and meta-analysis, the overall effect estimate was consistent with similar risk of RA in statin users and non-users. However, the confidence interval included both potentially meaningful benefit and harm of the intervention, suggesting that the message about the effect of the intervention could be different from the reported effect estimate if more studies become available. Risk of RA may be lower in patients who have higher treatment persistence or intensity of statin use, suggesting potential variation in treatment effect. Inherent to the limitations of the included studies, the certainty in the evidence was low. Future observational studies with guards against selection bias and confounding are needed to further elucidate the impact of statin use on the risk of RA, considering potential differences by dosage, duration of use, study population and other factors.
Highlights:
Current evidence suggests similar risk of rheumatoid arthritis (RA) statin users vs non-users.
Risk of RA may be decreased with higher statin treatment persistence or intensity.
Studies evaluating cumulative statin exposure on the risk of RA are warranted.
Acknowledgements:
We would like to thank our librarian, Patricia Erwin for help with the extensive search strategy; Drs. Victor M. Montori and Colin P. West for their valuable advice on the systematic review and meta-analysis process.
Financial support: This publication was made possible by CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH).
This work was partially funded by a grant from the National Institutes of Health, NIAMS (R01 AR46849). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Elena Myasoedova is supported by the Gerstner Family Career Development Award through the Center for Individualized Medicine (CIM), Mayo Clinic.
Dr. Ali Duarte-Garcia is supported by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic.
Appendix 1. Search strategy*
Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily <1946 to January 22, 2019>
Search history sorted by search number ascending
| # | Searches | Results | Type |
|---|---|---|---|
| 1 | exp Hydroxymethylglutaryl-CoA Reductase Inhibitors/ | 37420 | Advanced |
| 2 | hmg coa.mp. | 8300 | Advanced |
| 3 | 1 or 2 or statin* 1.mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 58882 | Advanced |
| 4 | rheumatoid arthritis.mp. or exp Arthritis, Rheumatoid/ | 139497 | Advanced |
| 5 | 3 and 4 | 346 | Advanced |
| 6 | exp Dyslipidemias/dt, pc [Drug Therapy, Prevention & Control] | 24323 | Advanced |
| 7 | 6 and 4 | 66 | Advanced |
| 8 | 5 or 7 | 381 | Advanced |
| 9 | (hydroxymethylglutaryl coenzyme a reductase inhibitor* or atorvastatin or cerivastatin or compactin or fluindostatin or lovastatin or mevinolin or pitavastatin or pravastatin or rosuvastatin or simvastatin).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 27154 | Advanced |
| 10 | 4 and 9 | 112 | Advanced |
| 11 | 8 or 10 | 394 |
Central - same strategy as above = 95
Embase <1988 to 2019 Week 03>
Search history sorted by search number ascending
| # | Searches | Results | Type |
|---|---|---|---|
| 1 | exp hydroxymethylglutaryl coenzyme A reductase inhibitor/ | 134783 | Advanced |
| 2 | exp rheumatoid arthritis/ | 160731 | Advanced |
| 3 | 1 and 2 | 1862 | Advanced |
| 4 | clinical study/ or exp case control study/ or exp case study/ or exp clinical trial/ or exp longitudinal study/ or exp major clinical study/ or exp prospective study/ or exp retrospective study/ | 4485701 | Advanced |
| 5 | methodology/ or exp "clinical trial (topic)"/ or exp cohort analysis/ or exp correlational study/ or exp cross-sectional study/ or exp double blind procedure/ or exp evidence based practice/ | 2986990 | Advanced |
| 6 | 3 and (4 or 5) | 911 | Advanced |
| 7 | exp rheumatoid arthritis/ep and 3 | 25 | Advanced |
| 8 | 6 or 7 | 921 |
Footnote:
Description of search strategy: The initial strategy was developed in Ovid MEDLINE (1946-January 22, 2019), using MeSH (Medical Subject Headings) controlled vocabulary and text words for those articles not yet indexed, and then modified the strategy for Ovid EMBASE (1988 through week 03, January 2019). Primary terms were: (Explode Hydroxymethylglutaryl-CoA Reductase Inhibitors/) OR (hmg coa.mp) OR (statin.mp [multiple posting]) OR (hydroxymethylglutaryl coenzyme a reductase inhibitor or atorvastatin or cerivastatin or compactin or fluindostatin or lovastatin or mevinolin or pitavastatin or pravastatin or rosuvastatin or simvastatin OR (explode Dyslipidemias [Drug Therapy, Prevention and Control]) AND (rheumatoid arthritis.mp or explode Arthritis, Rheumatoid/). Explode allows including all of the specific terms without having to use all of the variable terms and synonyms. Multiple posting allows for searching in title, abstract, name of substance word, subject heading word, floating sub-heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, and synonyms. The same process was used with Ovid EMBASE with changes to accommodate more granular subject headings in EMBASE. Search terms were as follows: (explode hydroxymethylglutaryl coenzyme A reductase inhibitor/) AND (explode rheumatoid arthritis/) AND (clinical study/ or explode case control study/ or explode case study/ or explode clinical trial/ or explode longitudinal study/ or explode major clinical study/ or explode prospective study/ or explode retrospective study/) OR (methodology/ or explode "clinical trial (topic)"/ or explode cohort analysis/ or explode correlational study/ or explode cross-sectional study/ or explode double blind procedure/ or explode evidence based practice/).
Appendix 2. Newcastle-Ottawa scale for observational studies
NEWCASTLE - OTTAWA QUALITY ASSESSMENT SCALE FOR CASE CONTROL STUDIES
Note: A study can be awarded a maximum of one star for each numbered item within the Selection and Exposure categories. A maximum of two stars can be given for Comparability.
Selection
- Is the case definition adequate?
- yes, with independent validation ✵
- yes, eg record linkage or based on self reports
- no description
- Representativeness of the cases
- consecutive or obviously representative series of cases ✵
- potential for selection biases or not stated
- Selection of Controls
- community controls ✵
- hospital controls
- no description
- Definition of Controls
- no history of disease (endpoint) ✵
- no description of source
Comparability
- Comparability of cases and controls on the basis of the design or analysis
- study controls for _______________ (Select the most important factor.) ✵
- study controls for any additional factor ✵ (This criteria could be modified to indicate specific control for a second important factor.)
Exposure
- Ascertainment of exposure
- secure record (eg surgical records) ✵
- structured interview where blind to case/control status ✵
- interview not blinded to case/control status
- written self report or medical record only
- no description
- Same method of ascertainment for cases and controls
- yes ✵
- no
- Non-Response rate
- same rate for both groups ✵
- non respondents described
- rate different and no designation
NEWCASTLE - OTTAWA QUALITY ASSESSMENT SCALE FOR COHORT STUDIES
Note: A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability
Selection
- Representativeness of the exposed cohort
- truly representative of the average _______________ (describe) in the community ✵
- somewhat representative of the average _______________ in the community ✵
- selected group of users eg nurses, volunteers
- no description of the derivation of the cohort
- Selection of the non exposed cohort
- drawn from the same community as the exposed cohort ✵
- drawn from a different source
- no description of the derivation of the non exposed cohort
- Ascertainment of exposure
- secure record (eg surgical records) ✵
- structured interview ✵
- written self report
- no description
- Demonstration that outcome of interest was not present at start of study
- yes ✵
- no
Comparability
- Comparability of cohorts on the basis of the design or analysis
- study controls for _____________ (select the most important factor) ✵
- study controls for any additional factor ✵ (This criteria could be modified to indicate specific control for a second important factor.)
Outcome
- Assessment of outcome
- independent blind assessment ✵
- record linkage ✵
- self report
- no description
- Was follow-up long enough for outcomes to occur
- yes (select an adequate follow up period for outcome of interest) ✵
- no
- Adequacy of follow up of cohorts
- complete follow up - all subjects accounted for ✵
- subjects lost to follow up unlikely to introduce bias - small number lost - > ____% (select an adequate %) follow up, or description provided of those lost) ✵
- follow up rate < ____% (select an adequate %) and no description of those lost
- no statement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- 1.Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, et al. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol. 2009;54(4):293–302. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. [DOI] [PubMed] [Google Scholar]
- 3.Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–58. [DOI] [PubMed] [Google Scholar]
- 4.Long-Term Intervention with Pravastatin in Ischaemic Disease Study G. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349–57. [DOI] [PubMed] [Google Scholar]
- 5.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615–22. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301–7. [DOI] [PubMed] [Google Scholar]
- 7.Abeles AM, Pillinger MH. Statins as antiinflammatory and immunomodulatory agents: a future in rheumatologic therapy? Arthritis Rheum. 2006;54(2):393–407. [DOI] [PubMed] [Google Scholar]
- 8.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I, et al. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet. 2004;363(9426):2015–21. [DOI] [PubMed] [Google Scholar]
- 10.Hechinger AK, Maas K, Durr C, Leonhardt F, Prinz G, Marks R, et al. Inhibition of protein geranylgeranylation and farnesylation protects against graft-versus-host disease via effects on CD4 effector T cells. Haematologica. 2013;98(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Husein BA, Dawah B, Bani-Hani S, Al Bashir SM, Al-Sawalmeh KM, Ayoub NM. Immunomodulatory effect of statins on Regulatory T Lymphocytes in human colorectal cancer is determined by the stage of disease. Oncotarget. 2018;9(87):35752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goncalves RSG, Dantas AT, Pereira MC, de Almeida AR, Rego M, da Rocha Pitta I, et al. Statins Inhibit Cytokines in a Dose-Dependent Response in Patients with Systemic Sclerosis. Inflammation. 2018. [DOI] [PubMed] [Google Scholar]
- 13.Chodick G, Amital H, Shalem Y, Kokia E, Heymann AD, Porath A, et al. Persistence with statins and onset of rheumatoid arthritis: a population-based cohort study. PLoS Med. 2010;7(9):e1000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tascilar K, Dell'Aniello S, Hudson M, Suissa S. Statins and Risk of Rheumatoid Arthritis: A Nested Case-Control Study. Arthritis rheumatol. 2016;68(11):2603–11. [DOI] [PubMed] [Google Scholar]
- 15.de Jong HJI, Cohen Tervaert JW, Lalmohamed A, de Vries F, Vandebriel RJ, van Loveren H, et al. Pattern of risks of rheumatoid arthritis among patients using statins: A cohort study with the clinical practice research datalink. PLoS ONE. 2018;13(2):e0193297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jong HJ, Klungel OH, van Dijk L, Vandebriel RJ, Leufkens HG, van der Laan JW, et al. Use of statins is associated with an increased risk of rheumatoid arthritis. Ann Rheum Dis. 2012;71(5):648–54. [DOI] [PubMed] [Google Scholar]
- 17.Jick SS, Choi H, Li L, McInnes IB, Sattar N. Hyperlipidaemia, statin use and the risk of developing rheumatoid arthritis. Ann Rheum Dis. 2009;68(4): 546–51. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt T, Battafarano DF, Mortensen EM, Frei CR, Mansi I. Frequency of development of connective tissue disease in statin-users versus nonusers. Am J Cardiol. 2013;112(6):883–8. [DOI] [PubMed] [Google Scholar]
- 19.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. Bmj. 2010;340:c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol. 2009;67(1):99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jong HJ, Saldi SR, Klungel OH, Vandebriel RJ, Souverein PC, Meyboom RH, et al. Statin-associated polymyalgia rheumatica. An analysis using WHO global individual case safety database: a case/non-case approach. PLoS ONE. 2012;7(7):e41289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Jong HJ, Tervaert JW, Saldi SR, Vandebriel RJ, Souverein PC, Meyboom RH, et al. Association between statin use and lupus-like syndrome using spontaneous reports. Semin Arthritis Rheum. 2011;41(3):373–81. [DOI] [PubMed] [Google Scholar]
- 23.Noel B Lupus erythematosus and other autoimmune diseases related to statin therapy: a systematic review. J Eur Acad Dermatol Venereol. 2007;21(1): 17–24. [DOI] [PubMed] [Google Scholar]
- 24.Macedo AF, Taylor FC, Casas JP, Adler A, Prieto-Merino D, Ebrahim S. Unintended effects of statins from observational studies in the general population: systematic review and meta-analysis. BMC Med. 2014;12:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JPT GS. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. 2011.
- 26.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj. 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 27.Wells G SB, O’Connell J, Robertson J, Peterson V, Welch V, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. (Available at http://www.ohri.ca/programs/clinical_epidemiology/oxfordasp. Accessed March 1, 2019).
- 28.Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. [DOI] [PubMed] [Google Scholar]
- 29.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. J Clin Epidemiol. 2011;64(12): 1294–302. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276–82. [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng X, Ding Y, Xia C, Tang T, Yu X, Xie J, et al. Atorvastatin modulates Th1/Th2 response in patients with chronic heart failure. J Card Fail. 2009;15(2):158–62. [DOI] [PubMed] [Google Scholar]
- 33.Kanda H, Yokota K, Kohno C, Sawada T, Sato K, Yamaguchi M, et al. Effects of low-dosage simvastatin on rheumatoid arthritis through reduction of Th1/Th2 and CD4/CD8 ratios. Mod Rheumatol. 2007;17(5):364–8. [DOI] [PubMed] [Google Scholar]
- 34.Shimada K, Miyauchi K, Daida H. Early intervention with atorvastatin modulates TH1/TH2 imbalance in patients with acute coronary syndrome: from bedside to bench. Circulation. 2004;109(18):e213–4; author reply e-4. [DOI] [PubMed] [Google Scholar]
- 35.Mulhaupt F, Matter CM, Kwak BR, Pelli G, Veillard NR, Burger F, et al. Statins (HMG-CoA reductase inhibitors) reduce CD40 expression in human vascular cells. Cardiovasc Res. 2003;59(3):755–66. [DOI] [PubMed] [Google Scholar]
- 36.Li XL, Dou YC, Liu Y, Shi CW, Cao LL, Zhang XQ, et al. Atorvastatin ameliorates experimental autoimmune neuritis by decreased Th1/Th17 cytokines and up-regulated T regulatory cells. Cell Immunol. 2011;271(2):455–61. [DOI] [PubMed] [Google Scholar]
- 37.Lv S, Liu Y, Zou Z, Li F, Zhao S, Shi R, et al. The impact of statins therapy on disease activity and inflammatory factor in patients with rheumatoid arthritis: a meta-analysis. Clin Exp Rheumatol. 2015;33(1):69–76. [PubMed] [Google Scholar]
- 38.Li GM, Zhao J, Li B, Zhang XF, Ma JX, Ma XL, et al. The anti-inflammatory effects of statins on patients with rheumatoid arthritis: A systemic review and meta-analysis of 15 randomized controlled trials. Autoimmun Rev. 2018;17(3):215–25. [DOI] [PubMed] [Google Scholar]
- 39.Rostami Z, Moteshaker Arani M, Salesi M, Safiabadi M, Einollahi B. Effect of Statins on Patients and Graft Survival in Kidney Transplant Recipients: a Survival Meta-analysis. Iran J Kidney Dis. 2017;11(5):329–38. [PubMed] [Google Scholar]
- 40.Wenke K, Meiser B, Thiery J, Nagel D, von Scheidt W, Steinbeck G, et al. Simvastatin reduces graft vessel disease and mortality after heart transplantation: a four-year randomized trial. Circulation. 1997;96(5):1398–402. [DOI] [PubMed] [Google Scholar]
- 41.Kobashigawa JA, Katznelson S, Laks H, Johnson JA, Yeatman L, Wang XM, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333(10):621–7. [DOI] [PubMed] [Google Scholar]