Abstract
Objectives
The aims of this study are to assess different saliva substitutes for their efficacy to lubricate the oral cavity, and to relate this oral lubrication to the ability of saliva substitutes to adsorb on and change the structure of the existing salivary conditioning film (SCF).
Materials and methods
Quartz crystal microbalance with dissipation was used to study the capability of saliva substitutes to interact with natural SCF and the ability to change the secondary SCF (S-SCF). A tongue-enamel friction system mimicking xerostomic conditions was used to assess the relief and relief period expected from these substitutes under set circumstances.
Results
Saliva Orthana spray, Biotène spray and Gum Hydral gel had an immediate effect on a SCF, increasing its structural softness. BioXtra gel, Biotène gel, Gum Hydral gel and Glandosane spray changed the S-SCF by increasing salivary protein adsorption, while others showed no sign of interaction. With respect to relief, only 2 out of the 16 saliva substitutes tested (Saliva Orthana spray and Gum Hydral gel) performed better than water. Overall, relief period correlated positively to structural softness change, whereas a positive correlation was seen between relief and mass adsorption.
Conclusions
The majority of saliva substitutes did not adsorb on the SCF, thus did not enhance lubrication. Only saliva substitutes containing carrageenan, carboxymethylcellulose, pig gastric mucin, xanthan gum and carbomer performed better in enhancing oral lubrication.
Clinical relevance
This objective assessment will help clinicians and patients make better choice of saliva substitutes. This study provides a scientific basis for future improvement in saliva substitutes.
Keywords: Ageing, Quality of life, Salivary lubrication, Xerostomia, Saliva substitutes, Friction, Biotribology
Introduction
According to the 2015 report from the Population division of the UN’s department of economics and social affairs, globally the proportion of aged as well as the overall age has never been higher and has not yet reached the peak [1]. Increasing life expectancies force countries to review and increase the retirement age, expecting the elderly to remain active, mobile and keep working for longer periods [2]. Any condition which will affect their quality of life will seriously affect their work efficiency. Xerostomia, the subjective feeling of oral dryness, is one such condition. Xerostomia is not only a symptom of increasing age [3], but it also accompanies ageing-related diseases and conditions like Sjögren’s syndrome, diabetes mellitus, side effects of several (combinations of) drugs and irradiation in the head and neck region [4, 5]. From these multiple causes, 63% of hospitalized elderly suffer from xerostomia [6].
Xerostomia is often accompanied by either decreased salivary flow or an altered composition of saliva [7, 8]. Saliva is the main substance in the mouth that provides the lubrication needed for a normal oral function like mastication, swallowing and speech, and preventing wear of mucosal tissue and dental surfaces. Therefore, the lack of saliva could have devastating effects. These effects include impeded oral functioning, a high risk of developing dental caries and oral infections and a worsened quality of life [3, 9–11].
A variety of saliva substitutes (Table 1) have been introduced to alleviate oral dryness when saliva stimulation is overall insufficient or fails to relieve xerostomia and its related complaints. Hahnel et al. and Furness et al. [12, 13] have reviewed the studies reported in literature which compare saliva substitutes. Common in both reviews is the conclusion that strong evidence is lacking for any saliva substitute to relieve dry mouth symptoms as also recognized by others [8]. This raises the question of whether the methods currently used to compare and assess saliva substitutes are valid. The methods most commonly applied include in vivo visual analogue scale, Xerostomia index, xerostomia questionnaire, dryness ranking scores [14–16] and measuring (un)stimulated whole saliva flow rate [17–20].
Table 1.
Commercially available saliva substitutes that were used in this study. The main lubricating ingredients are indicated
| Saliva substitute | Active ingredient for lubrication |
|---|---|
| Saliva Orthana (SO) spray | Porcine gastric mucin |
| BioXtra (BX) mouthwash | Hydroxyethyl cellulose (HEC), Aloe vera |
| BX gel-spray | HEC |
| BX gel | HEC, Aloe vera |
| Biotène (BT) mouthwash | HEC, Aloe vera |
| BT spray | Xanthan gum, polyethylene glycol (PEG)-hydrogenated castor oil |
| BT gel | HEC |
| Dentaid Xeros (DX) mouthwash | HEC, PEG-hydrogenated castor oil |
| DX spray | HEC |
| DX gel | HEC, Aloe vera 0.05% |
| GUM Hydral (GH) spray | PEG-hydrogenated castor oil |
| GH gel | Xanthan gum, carrageenan, PEG-hydrogenated castor oil |
| Aldiamed (ADM) spray | Carboxymethyl cellulose (CMC), Aloe vera |
| Saliva Natura (SN) spray | Plant polysaccharide |
| Glandosane (GDS) spray | CMC |
| Aequasyal (AQ) spray | Oxidized glycerol triesters |
| Entertainer’s secret (ES) spray | CMC, Aloe vera |
Although measuring salivary flow and patient questionnaires can throw some light on the efficacy of a saliva substitute to relieve xerostomia, the lubricating properties of the saliva substitutes have been completely neglected. This is probably due to the absence of a reliable method to measure the lubricating properties objectively. A recently established tongue-enamel friction system has shown a relation between salivary lubricating properties and mouth feel [21]. This system can be used to compare saliva substitutes ex vivo for their extent and duration of lubricating the patient’s oral cavity, relieving the dry mouth feeling. The aim of this study was to assess saliva substitutes on their lubricity and relate it to their ability to interact with salivary conditioning films (SCF). In order to achieve this goal, quartz crystal microbalance with dissipation (QCM-D) [22, 23] and the ex vivo tongue-enamel friction system were used.
Materials and methods
Saliva substitutes and preparation
The selection of saliva substitutes was based on the hydrating, gelling or lubricating agents present in these substitutes, aiming for coverage of commonly applied lubricating agents available in Europe (Table 1). Some brands feature an extended product line, containing sprays, gels and/or mouthwashes. Mouthwashes and sprays were used as received. Gels were diluted to 10% in demineralized water to allow for liquid flow in QCM-D experiments.
Human whole saliva collection and preparation
Human whole saliva was used as a control in this study. Both stimulated (SWS) and unstimulated (UWS) whole saliva were obtained from five healthy volunteers and collected and processed following standard protocols [24]. The whole saliva was collected in conformity with the relevant guidelines and regulations under the approval of the Medical Ethics Review Board of the University Medical Center Groningen (approval no. M17.217043, M09.069162 and UMCG IRB #2008109). Participants were asked not to eat or drink for 1 h before collection. Before collecting any saliva, the mouth was rinsed well with tap water.
For QCM-D experiments, reconstituted human whole saliva was used. For this, SWS of a group of 20 donors recruited at the Department of Biomedical Engineering was pooled, dialyzed and lyophilized for storage. Reconstitution was done by dissolving freeze-dried saliva in adhesion buffer (1.5 mg ml−1) (10% 0.5 M KCl; 0.2% 0.5 M KPi; 0.1% 0.5 M CaCl2 in demineralized water) [25] and stirred for 30 min at low shear rates. KPi is a solution containing 0.5 M KH2PO4 and K2HPO4. Centrifugation of reconstituted whole saliva was performed at 10,100 g at 10 °C for 5 min.
Perturbation in structural softness of the SCF after interaction with saliva substitutes measured using QCM-D
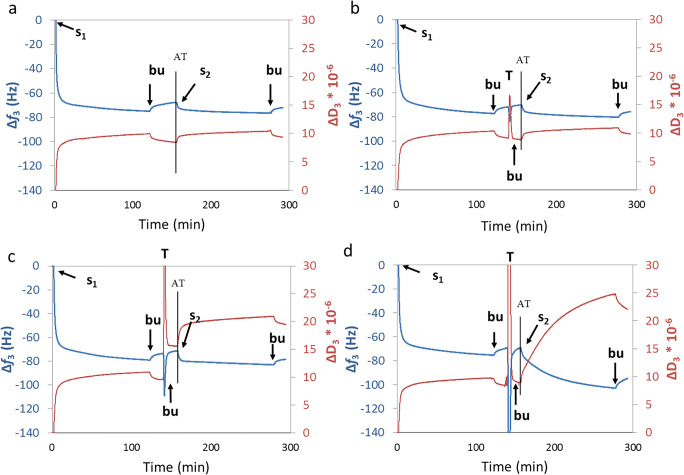
The ability of the saliva substitute to perturb the properties of an SCF was studied using QCM-D, E4-module (Q-sense, Gothenburg, Sweden). As substrates, five MHz (sensitivity constant 17 ng cm−2) AT-cut gold (Au) coated quartz crystals (Jiaxing Jingkong Electronic Co., Ltd., Jiaxing, China) were used. Before experiments, the crystals were cleaned by 10 min UV/ozone treatment, then immersed in 3:1:1 mixture of ultrapure water, NH3 and H2O2 at 75 °C for 10 min followed by another UV/ozone treatment and placed in the QCM-D flow chamber. A protocol proposed by Veeregowda et al. [22] was used for experiments where adhesion buffer was introduced in the QCM-D chamber above the resonating crystal till constant values were reached for frequency and dissipation at all the resonating frequencies, i.e. 5 to 65 MHz. The QCM-D chamber was then perfused with reconstituted whole saliva for 2 h (s1), which led to the formation of an initial SCF on the substrate. This step was followed by the perfusion of a saliva substitute (T) through the system for 2 min. This step was followed by another 2 h perfusion with reconstituted whole saliva (s2), forming a secondary SCF (S-SCF). After each perfusion step, buffer was perfused through the chamber (bu) for 15 min for rinsing (Fig. 1). The entire experiment was performed under a constant flow of 50 μl min−1 provided by a peristaltic pump at 25 °C. The frequency shift (Δf) and dissipation shift (ΔD) were continuously monitored in real-time. The structural softness of the adsorbed SCF after exposure to saliva substitutes, SCF after treatment (SCF AT), and the structural softness of the S-SCF were calculated to be able to assess the saliva substitutes on their activity. Structural softness is a measure of viscoelasticity of an SCF, and directly related to the lubricity of SCFs [26]. It is calculated by the ratio ΔD3/Δf3 for the third overtone at the end of the buffer rinsing step, as measured by the QCM-D device.
Fig. 1.
Experimental protocol used in the QCM-D to probe perturbance in SCF softness. Typical curves for frequency shift (Δf) and dissipation shift (ΔD) during QCM-D experiments. Graphs are colour-matched with the axes they correspond to. Control with intermediate buffer treatment (a), intermediate treatment with DX gel (b), with SO spray (c) and ADM spray (d). Saliva was first introduced in the QCM-D chamber (s1) to create the SCF. Adsorption of saliva proteins on the substrate led to a decreased oscillating frequency with a shift of 70 Hz. At the same time, the dissipation of energy increases (a–d), meaning that the adsorbed film becomes softer. At ‘bu’, the substrate was rinsed with adhesion buffer to wash off unbound proteins (notice the frequency rise and dissipation drop due to less mass present). At ‘T’ (in b–d), the adsorbed protein layer was treated with saliva substitutes followed by rinsing with buffer ‘bu’ which resulted in different layer properties regarding Δf and ΔD (a, control experiment; b, unchanged net dissipation and adsorption; c, increased dissipation and unchanged net adsorption; d, unchanged net dissipation and adsorption). At ‘s2’, reflow of saliva was done to create the S-SCF to study the interaction of new saliva proteins to the treated adsorbed layer (a, control experiment; b, unchanged net dissipation and adsorption; c, increased dissipation and unchanged net adsorption; d, increased dissipation and increased mass adsorption). Structural softness ΔD/Δf of the SCF and the S-SCF were calculated ‘after treatment’ (AT) and after the final rinsing step respectively
Lubricating properties of saliva substitutes
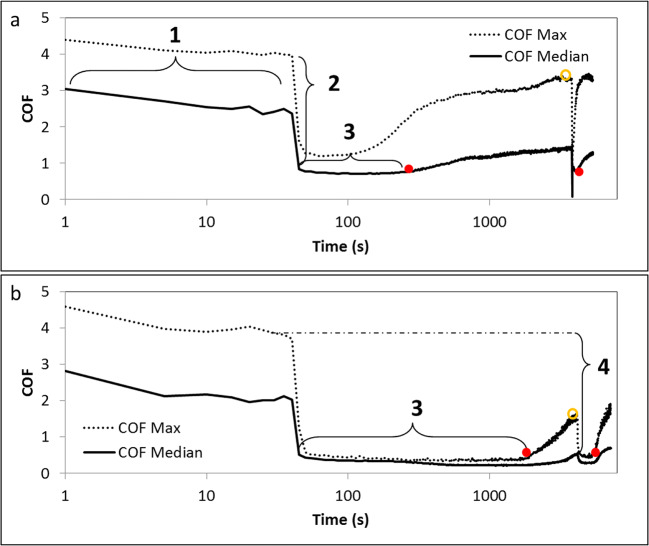
Lubricating (and rehydration) properties of saliva substitutes were studied by reciprocating sliding using a universal mechanical tester (CETR Inc., USA) and a newly developed tongue-enamel friction system to mimic dry mouth. In short, fresh porcine tongues (Kroon BV, Groningen, Netherlands) and polished bovine tooth enamel were used as sliding surfaces. With continuous monitoring of the coefficient of friction (COF), the enamel was rubbed over a flat spot on the porcine tongue over a distance of 10 mm in a reciprocating motion with a velocity of 4 mm s−1 under a constant normal load of 0.25 N. After measuring 10 cycles on dry tongue surface (stage 1 in Fig. 3a, i.e. without any lubricant), a drop of 20 μl of saliva substitute (or saliva) was added on the tongue-enamel interface, immediately causing a decrease in COF to a low value (stage 2 in Fig. 3). The ratio between COFdry and COFlubricated is termed as ‘relief’. Since every reciprocating cycle features a maximum COF and a median COF of which the latter is representing the overall plateau value, a relief calculated from maximum and median COF (Rmax and Rmedian respectively) were calculated [21]. The saliva substitute was spread all over the sliding zone to keep low COF for a certain period of time after which the saliva substitute layer dried up and the COF increased (stage 3 in Fig. 3a). The duration for which the COF remained low was called the relief period (RP).
Fig. 3.
Typical output from the tongue-enamel friction system [21], shown here for two different saliva substitutes, BX gel-spray (a) and GH gel (b). Lubrication properties of saliva substitutes and their relation to the relief they provide from dry mouth. A relatively bad-performing saliva substitute with first the COF of the dry cycles (1), the relief after treatment with the saliva substitute (2) leading to a short RP (3) until the closed red dot where the slope changes clearly. After a rise of COF 25 μl of demineralized water was added causing a second drop in COF (Rmedian,r and Rmax,r) at the open orange dot leading to a secondary relief period (Rpr) at the second closed red dot (a). (b), the same for a relatively good-performing saliva substitute: the COF reaches a lower level compared with (a) and remains low for a longer RP. (4) Gives an indication of how the Rmax,r and Rmedian,r were determined (also based on the dry COF). The RPr shows to be relatively long in b
The ability of the dried saliva substitute layer to get rehydrated and to re-lubricate the oral cavity was assessed by bringing 20 μl of demineralized water at the tongue-enamel interface. A second relief, relief after rehydration (Rmax,r and Rmedian,r) and a second relief period (RPr) (stage 4 in Fig. 3b) were determined.
Statistics
The standard deviation was used for reporting the variability in the average values. For comparison between multiple saliva substitutes in the tongue-enamel friction system, a one-way analysis of variance (one-way ANOVA) was performed with a Bonferroni post-hoc test. For comparison before and after rehydration of the same sample, paired two-tailed t tests were performed. For comparison between multiple saliva substitutes in QCM-D, a two-way ANOVA was performed with a Bonferroni post-hoc test. Pearson’s correlation coefficient ‘r’ was used for correlation assessments. Statistics were done using Prism Graphpad (version 5.0).
Results
Perturbation in the structural softness of the SCF after exposure to saliva substitutes using QCM-D
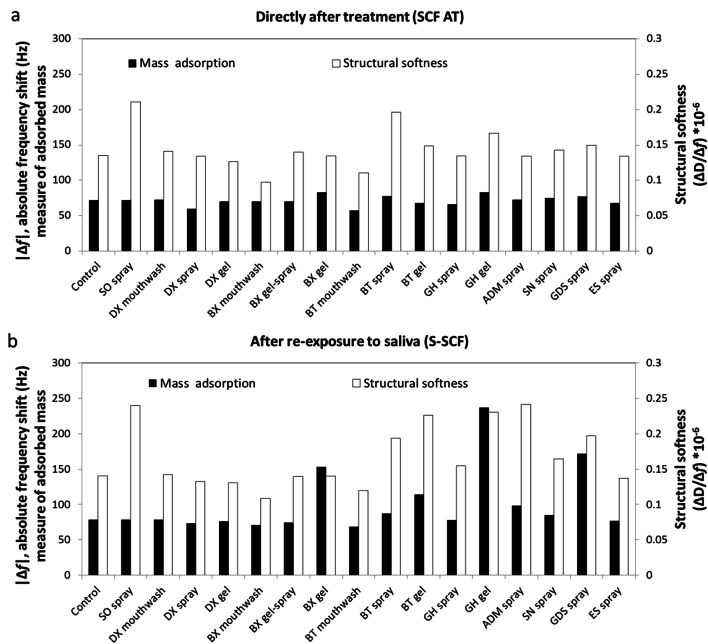
Figure 1a shows the control experiment beginning with adsorption of an initial SCF for 120 min (s1). Hereafter, loosely bound proteins were rinsed by adhesion buffer. At this point, the structural softness (ΔD3/Δf3) of the SCF was 0.14 ± 0.006 Hz−1 while the protein adsorption led to a Δf3 of 71.95 ± 1.88 Hz (Fig. 2a). In Figs. 1b–d, three different saliva substitutes were applied after the first rinsing step as being a treatment (T), showing different outputs. The structural softness of the SCF was measured after treatment (AT), i.e. after interaction of the treatment to the SCF and after rinsing off the loosely bound molecules. Figure 1b shows the Dentaid Xeros (DX) gel not interacting well with the SCF as no change in either Δf or ΔD are seen. Saliva Orthana (SO) spray interacts with the SCF by changing the softness of the layer (ΔD rises) (Fig. 1c), while ADM spray changes structural softness by changing both Δf and ΔD (Fig. 1d). Figure 2a shows that after applying saliva substitutes, the structural softness (SCF AT) increased significantly for SO spray, Biotène (BT) spray and GUM hydral (GH) gel (0.21 ± 0.006, 0.20 ± 0.009 and 0.17 ± 0.009 Hz−1 respectively). At the same time, BioXtra (BX) mouthwash and BT mouthwash led to a decreased structural softness (0.097 ± 0.009 and 0.11 ± 0.009 Hz−1 respectively) (p < 0.05). DX mouthwash, DX spray, DX gel, BX gel-spray, BX gel, GH gel, Saliva Natura (SN) spray, Glandosane (GDS) spray and entertainer’s secret (ES) spray showed a similar structural softness as the control. The Δf3, after applying and rinsing saliva substitutes, did change for some substitutes (SO spray and BX mouthwash), but not for the other substitutes tested, indicating that the change in ΔD3 was pivotal for increased structural softness changes.
Fig. 2.
The structural softness as a measure of lubricity of the SCF film due to saliva substitute exposure using QCM-D. The mass of the adsorbed proteins on the quartz crystal measured in absolute frequency shift (|Δf3|) and differences in the structural softness (ΔD3/Δf3) of the SCF ‘after treatment’ (AT) with saliva substitutes (a) and after re-exposure of the SCF treated with saliva substitutes to saliva again (s-SCF: secondary SCF; b). The white bars represent the structural softness of the salivary conditioning film and the black bars represent the absolute frequency shift. Saliva substitutes tested: Buffer (control), Saliva Orthana (SO), Dentaid Xeros (DX), BioXtra (BX), Biotène (BT), Gum Hydral (GH), Aldiamed (ADM), Saliva Natura (SN), Glandosane (GDS), Entertainers secret (ES). All saliva substitutes except BX gel showed an increase in structural softness
QCM-D experiments featured a second cycle of 120-min exposure of the SCF to saliva (s2) followed by a second rinsing step (Fig. 1). For the control, ΔD3/Δf3 of S-SCF was 0.14 ± 0.004 Hz−1 (Fig. 2b), which equals ΔD3/Δf3 of the SCF (Fig. 2a). Comparing ΔD3/Δf3 of SCF AT and S-SCF, a slight increase was found for SO spray, 0.24 ± 0.008 Hz−1 at the S-SCF compared with SCF AT while Δf3 increased to 78.3 Hz. Meanwhile, a substantial increase of ΔD3/Δf3 between the SCF AT and S-SCF occurred for BT gel, GH gel, ADM spray and GDS spray treatments. For BT spray, ΔD3/Δf3 of S-SCF was not substantially higher than that of the SCF AT, although the Δf3 increased by 10 Hz, i.e. from 77.8 to 87.0 Hz. The structural softness of DX mouthwash, DX spray, DX gel, BX gel-spray and ES spray show similar values for S-SCF as compared with the control. ΔD3/Δf3 of BX mouthwash and BT mouthwash did not change between the SCF AT and S-SCF, but ΔD3/Δf3 was lower compared with the control (0.11 and 0.12 Hz−1 respectively).
Lubrication and dry mouth relief provided by saliva substitutes
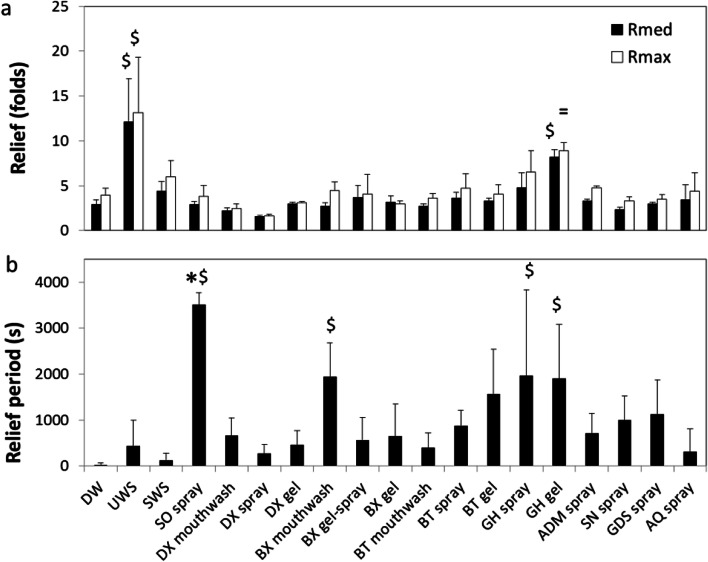
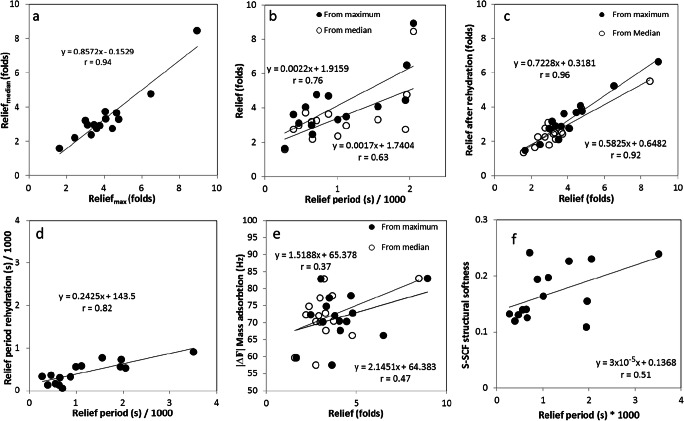
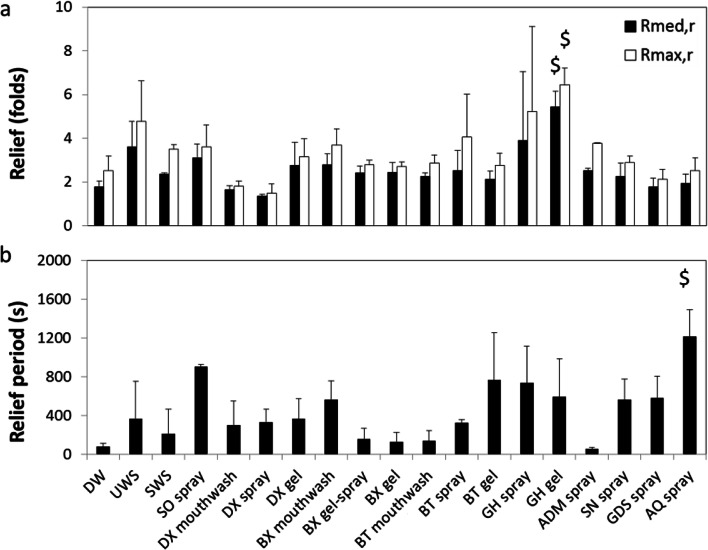
Figure 3 shows examples of typical output from the tongue-enamel friction measurements on the universal mechanical tester for both a relatively bad performing (BX gel-spray) and a relatively good performing (GH gel) saliva substitute. Figure 3a shows that the COF drops to just below 1, whereas in Fig. 3b the COF drops to below 0.5 for the median COF. The latter one led to a higher Relief. The RP in Fig. 3a is about 200 s, whereas the RP in Fig. 3b is around 1900 s, which depicts a clear difference in RP provided by the two saliva substitutes. After rehydration (open dot) a secondary relief and relief period (until next closed dot) have been visualized (Rmedian,r, Rmax,r and RPr). For these two, and all other tested saliva substitutes, both the Rmax and Rmedian are displayed in Fig. 4a. Demineralized water (DW) showed a Rmax and Rmedian of 3.9 ± 0.7 and 2.9 ± 0.5, respectively. UWS showed significantly (p < 0.01) higher relief of 12.1 ± 4.8 and 13.1 ± 6.2, which is about 3.3–4.2 times higher than DW. All the other tested natural saliva and saliva substitutes did not show any significant difference in relief as compared with water. For SWS, the Rmax and Rmedian were 6.0 ± 1.8 and 4.3 ± 1.1, respectively. For the saliva substitutes, DX spray revealed the lowest relief of 1.65 ± 0.2 and 1.6 ± 0.2 for Rmax and Rmedian, whereas GH gel displayed the highest relief with Rmax and Rmedian of 9 ± 1 and 8.5 ± 0.8 respectively. GH gel was the only saliva substitute that did not perform significantly (p < 0.05) worse than UWS in both parameters. The other saliva substitutes showed Rmax ranging between 2.5 ± 0.6 (DX mouthwash) to 6.5 ± 2.5 (GH spray) and for Rmedian between 2.2 ± 0.4 (DX mouthwash) and 4.7 ± 1.7 (GH mouthwash). Relief determined using median COF per cycle correlated very well with the relief determined using the maximum COF per cycle (r = 0.94 in Fig. 6a).
Fig. 4.
Dry mouth relief provided by saliva substitutes. Relief (a) and relief period (b) as explained in Fig. 3 obtained for different natural saliva and saliva substitutes on the tongue-enamel friction system. Relief was obtained based on both the maximum (Rmax) and median (Rmedian) COF. Error bars represent standard deviations over triplicate measurements. Substances tested: demineralized water (DW), unstimulated and stimulated human whole saliva (UWS and SWS respectively), Saliva Orthana (SO), Dentaid Xeros (DX), BioXtra (BX), Biotène (BT), Gum Hydral (GH), Aldiamed (ADM), Saliva Natura (SN), Glandosane (GDS), Aequasyal (AQ). In (a), all tested agents were significantly different (p < 0.05) to UWS in both Rmax and Rmed except GH gel, depicted by =. $ depicts significant differences compared with DW (p < 0.05). * Shows significant difference compared with UWS in Relief Period. Data for DW, SWS, and UWS was taken from [21]
Fig. 6.
Lubrication depends on the perturbation to the structural softness of the salivary conditioning films by saliva substitutes. Correlation between different lubricating properties of saliva substitutes and with the perturbation caused to the SCF; Reliefmax vs. Reliefmedian (a), relief period vs. relief (b), relief before and after rehydration (c), relief period before and after rehydration (d), relief vs. protein adsorption after treatment with saliva substitutes (e) and relief period vs. structural softness of the S-SCF after second perfusion with saliva (f). For every graph, the Pearson’s correlation coefficient ‘r’ has been visualized
Human whole saliva showed a relief period of 439 ± 561 s and 125 ± 155 s for UWS and SWS respectively, which was not significantly different from water (28 ± 44 s). SO spray showed a relief period from 3507 ± 259 s which was significantly higher than all other lubricants (p < 0.05) except four other saliva substitutes which showed a high mean in relief period accompanied by a high standard deviation (GH gel and spray, BT gel and BX mouthwash) (Fig. 4b). Despite the high mean values of relief period, these saliva substitutes showed no significant differences from water. Regardless of the high standard deviation in the relief period, Fig. 6b illustrates a fairly strong correlation between relief and relief period, (r = 0.63 and 0.76 for median and maximum respectively), although beyond the relief period of 2000 s, we only observed an increase in relief.
Dry mouth relief provided by saliva substitutes upon rehydration with water
Figure 5a shows the relief after rehydration (Rmed,r and Rmax,r) of the once dried-up layer of saliva substitutes. This was done to study the possibility of the reuse of the adsorbed layer of the saliva substitute in the patient’s mouth simply with the help of water. The figure shows that the relief after rehydration is highly comparable for most saliva substitutes. DW had Rmed,r of 1.79 ± 0.265 which was significantly lower than the first time (p < 0.05). SWS, the BT product family, GH gel and GDS had a significantly worse Rmed,r compared with Rmed. The remainder of the saliva substitutes did not show any differences between Rmed and Rmed,r. Overall GH gel is the only saliva substitute that performed significantly better than water in both Rmed,r and Rmax,r. Altogether, there is a strong correlation between the initial relief and the relief after rehydration (r = 0.96 and r = 0.92 for Rmax and Rmed respectively) (Fig. 6c). In relief period (Fig. 5b) after rehydration (RPr), AQ spray revealed a significant longer relief period than demineralized water (but not to UWS). No significant differences were found in relief period duration between initial RP and RPr except for SO spray, which performed significantly worse than the first time. Altogether, the overall RP and RPr correlate well (r = 0.82) (Fig. 6d). RPr is only about one-fourth of the initial relief period.
Fig. 5.
Dry mouth relief provided by saliva substitutes upon rehydration with water. Relief (a) and relief period (b) after rehydration obtained for different natural saliva and saliva substitutes on the tongue-enamel friction system. Relief after rehydration was obtained based on both the maximum (Rmax,r) and median (Rmedian,r) COF. Error bars represent standard deviations over triplicate measurements. Substances tested: demineralized water (DW), unstimulated and stimulated human whole saliva (UWS and SWS respectively), Saliva Orthana (SO), Dentaid Xeros (DX), BioXtra (BX), Biotène (BT), Gum Hydral (GH), Aldiamed (ADM), Saliva Natura (SN), Glandosane (GDS), Aequasyal (AQ). $ shows significant differences (p < 0.05) to DW
Correlation between structural and lubrication parameters
Besides the correlation of tongue-enamel parameters, the parameters between the methods have been analysed. Figure 6e shows a fair correlation with r = 0.47 and 0.37 for Rmedian and Rmax, respectively and adsorbed protein mass (Δf3) on the SCF, while the relief period correlates better with the structural softness (Fig. 6f) of the SCF (r = 0.51).
Discussion
In this study, we have assessed the ability of various saliva substitutes to lubricate, as a means to provide relief to xerostomia patients, in relation to their capacity to interact with the existing SCF by changing its structural softness.
The lubricating properties were measured using a tongue-enamel friction system [21], where the relief and relief period were calculated based on median and maximum COF per reciprocating cycle. Since relief determined using median COF per cycle correlated very well with the relief determined using the maximum COF per cycle, either of them may be used for further comparison of the lubricating properties of saliva substitutes and natural saliva. The fairly strong correlation between relief and relief period (Fig. 6b) exists up to about 2000 s of relief period and this might be explained by the nature of the two parameters. Both relief and relief period provided by human whole saliva and saliva substitutes are dependent on their chemical composition and presence of specialized lubricating and water holding molecules.
Mechanical stimulation of the salivary flow increases the parotid contribution to the total saliva [27]. The result is that UWS contains a higher contribution from the thick, mucin-containing, submandibular and sublingual saliva than SWS. The thicker composition of UWS provides three times higher relief as compared with SWS (Fig. 4 and [21, 28]). All of the saliva substitutes but GH gel showed the relief which was significantly lower than UWS but similar to SWS and water (Fig. 4a). This indicates that with respect to relief, saliva substitutes do not perform any better than water and some even worse. It suggests that most saliva substitutes lack good lubricating properties. This corroborates the concluding statement from Furness et al. [12], i.e. “There is no strong evidence from this review that any topical therapy is effective in relieving the symptoms of dry mouth.”
Relief period is highly dependent on ambient air humidity and temperature. Ambient air humidity and temperature were hard to control in our setup, which might have influenced the drying rate of the saliva and saliva substitutes ex vivo. One way in which human saliva lubricates the oral cavity is via salivary mucin MUC5B, which adsorbs on the mucosa in both healthy and dry mouth patients [29]. In a highly humid oral cavity, mucins enable lubrication by trapping water molecules [29, 30]. In a less humid environment, i.e. in air like in our experiments, much less water can be retained by the mucins leading to easier drying of the mucin layer. This probably caused high standard deviations in some measurements, resulting in insignificant differences. A larger sample size could have been more conclusive regarding the relief period; however, our results provide an overview of the efficacy of saliva substitutes in general. With respect to relief period, one saliva substitute relieved the dry mouth for a much longer period of time than UWS, i.e. SO spray. Some others (BX mouthwash, GH spray, and GH gel) provided relief significantly longer than DW, while the remainder performed no better than DW, i.e. < 300 s (Fig. 4b). A short relief period provided by UWS and SWS might be explained by the differences in humidity between the ex vivo system used and the oral environment. In the ex vivo setup, the mucin layer is more easily dried out compared with the oral cavity. However, when the dried salivary layer was rehydrated, the functionality of the UWS was restored for a longer period of time (Fig. 5b). This suggests that even after drying the mucins are still able to reabsorb and retain water upon rehydration.
With respect to RPr, the outstanding results of AQ spray could be due to the oxidized glycerol triesters. It is likely that these lipid molecules will form a slippery emulsion of oil and water. SO spray had good RPr here as well (~ 900 s), which shows the capacity of the porcine gastric mucin to reabsorb and bind water molecules for a longer period of time.
Notable is that the saliva substitutes which show better performance in the tongue-enamel system (Fig. 4) also show changes in the frequency shift and structural softness of SCF determined by the QCM-D (Figs. 2, 6e, f). This indicates that these saliva substitutes contain molecules that actively adsorb on the SCF and by doing so increase the Relief. We presume that mucin (from SO spray), carboxymethyl cellulose (CMC) (from ADM and GDS sprays), carrageenan and xanthan (from GH gel) and carbomer (from BX gel and BT gel) adsorb on the SCF and help increase the Relief. The relief period better correlates with the SCF softness (Fig. 6f). Saliva substitutes, which upon adsorption increase the SCF structural softness, increase the relief period. An increase in structural softness of the salivary conditioning films has been shown to cause a decrease in the COF of SCF in vitro [22]. These results suggest that if a saliva substitute is able to interact with the SCF, it will provide better lubricating properties.
The makers of most of the saliva substitutes only mention the molecules they have used in the formulation but do not mention the amounts (% w/v) used, which makes it difficult to relate the changes caused by them to the SCF and its lubricity. Also, synergistic effects between different constituents cannot be ruled out. Only one out of eight saliva substitutes containing hydroxyethyl cellulose (HEC) (BT gel) increased the relief period and increased the structural softness of the S-SCF. A major difference in BT gel composition and the other saliva substitutes containing HEC is that it also contains the polymer carbomer (poly(acrylic acid)), which has been shown to have mucoadhesive properties [31, 32]. However, the other saliva substitute containing only carbomer (BX gel) only increased the mass adsorption but did not increase structural softness after the treatment. This strongly indicates that the synergistic effect of HEC and carbomer, i.e. HEC containing saliva substitutes, seems only effective when carbomer is added to the substitute. HEC on its own is highly soluble in water [33] and increases viscosity.
Saliva substitutes containing CMC (ADM spray, GDS spray) changed the structural softness of the SCF, while not containing a lubrication-inducing polymer like carbomer. CMC is mucoadhesive [34] and our QCM-D results suggest that there is a strong interaction between CMC and the SCF. GDS spray caused a frequency shift of − 172 Hz, which was the highest seen after GH gel, while the S-SCF with ADM shows the highest structural softness.
Carrageenan is able to form a polymer/mucus gel [35], which could explain the differences between GH gel and GH spray. The interaction of GH gel on the structural softness of the SCF reveals a high value (~ 0.23) and very high mass adsorption (3 times control), whereas GH spray was highly comparable with the control. GH gel contains only four extra ingredients as compared with GH spray, i.e. two dyes (tartrazine and brilliant blue FCF), xanthan gum and carrageenan powder. For GH gel, it was proposed that carrageenan was the main ingredient resolving muco-adhesiveness and lubricating properties; however, xanthan gum is also likely to interact with an SCF. From in vivo intraoral bio-adhesion tests, xanthan remained for 2.5 h on four different sites in the oral cavity [36]. Furthermore, xanthan gum is generally used as a thickening agent in food and the cosmetic industry, revealing high viscosity at low concentrations [37] and has antifungal properties [38]. Other common ingredients such as glycerine, xylitol, sorbitol, starch hydrolysates and propylene or butylene glycols seem not to bear any major lubricating or SCF adapting properties. Polyethylene glycol (PEG)-hydrogenated castor oils are more frequently used as solubilizers, and to our knowledge, do not have lubricating properties [39]. Some saliva substitutes only increase viscosity instead of lubrication, while those terms are not exchangeable [13, 40].
Upon reflow of saliva on the saliva substitute-exposed SCF, we observe that salivary proteins adsorb and form an S-SCF with a different structural softness (Fig. 2b) compared with before (Fig. 2a). This implies that the components of saliva substitutes (SO spray, BT gel, GH gel, ADM spray, SN spray and GDS spray), which had adsorbed to the SCF, change the way the SCF interacts with the salivary components and thus modify the structural softness of S-SCF as compared with control. Components like mucin, CMC, xanthan gum, carrageenan, carbomer or their combinations help change the S-SCF structural softness. This increase in softness would imply that they would further enhance the ability of the S-SCF to retain water and thus increase the relief period (Fig. 6f), although this remains to be experimentally confirmed.
Conclusions
Saliva substitutes which caused mass adsorption to SCF increased the relief, whereas the ones which increased the structural softness tend to increase the relief period. Overall, only those saliva substitutes which perturbed the existing SCF were able to enhance the lubricating and water holding capacity of the SCF and hence provide relief against dry mouth. So altogether, the presence of constituents like carrageenan, CMC, xanthan gum, carbomer and porcine gastric mucin in saliva substitute formulations were found important for their performance. Thus, there is a great need to rethink the strategy for new saliva substitute formulations. They need to contain ingredients that specifically adsorb to the existing salivary conditioning films in the patient’s oral cavity and drastically enhance the layer softness.
Acknowledgements
We are thankful to all healthy volunteers from the department of Biomedical Engineering, University Medical Center Groningen (UMCG) and the first-year medical students from the learning community molecular medicine who donated the stimulated and unstimulated saliva. We are thankful to Dr. Brandon Peterson for his final review of the manuscript as a lifelong speaker of American English. We are also thankful to the graduate school of medical sciences (GSMS) of the UMCG for the providing scholarship to Drs. Jeroen Vinke. The universal mechanical tester was purchased using the grant no. ZonMW91112026 from the Netherlands Organization for Scientific Research.
Abbreviations
- ADM
Aldiamed
- AT
After treatment
- AQ
Aequasyal
- BT
Biotène
- BX
BioXtra
- CMC
Carboxymethyl cellulose
- COF
Coefficient of friction
- DW
Demineralized water
- DX
Dentaid Xeros
- E4
A specific type of module for QCM-D
- ES
Entertainer’s secret
- GDS
Glandosane
- GH
GUM hydral
- HEC
Hydroxyethyl cellulose
- PEG
Polyethylene glycol
- QCM-D
Quartz crystal microbalance with disspation
- Rmedianr
Relief calculated from median COF
- Rmedian,r
Rmedian after rehydration
- Rmax
Relief calculated from maximum COF
- Rmax,r
Rmax after rehydration
- RP
Relief period
- RPr
RP after rehydration
- S-SCF
Secondary salivary conditioning film
- SCF
Salivary conditioning film
- SCF AT
Salivary conditioning film after treatment
- SN
Saliva Natura
- SO
Saliva Orthana
- SWS
Stimulated whole saliva
- T
Treatment
- UWS
Unstimulated whole saliva
Funding information
We are thankful to the graduate school of medical sciences (GSMS) of the University Medical Center Groningen for the 3-year scholarship provided to J. Vinke. The universal mechanical tester was purchased using the grant no. 91112026 from the Netherlands Organization for Health Research and Development (ZonMW).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Saliva was obtained in conformity with relevant guidelines and regulations under the approval of the Medical Ethics Review Board of the University Medical Center Groningen (approval no. M17.217043, M09.069162 and UMCG IRB #2008109).
Informed consent
Informed consent was obtained from all the donors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/15/2020
After publication of this paper, the authors observed that that figure 6 appears before figure 5.
References
- 1.United Nations, Department of Economic and Social Affairs, Population Division (2015) World population ageing. ST/ESA/SER.A/390
- 2.Wiatrowski WJ (2001) Changing retirement age: ups and downs. Mon Labor Rev 124:3–12
- 3.Hopcraft M, Tan C. Xerostomia: an update for clinicians. Aust Dent J. 2010;55:238–244. doi: 10.1111/j.1834-7819.2010.01229.x. [DOI] [PubMed] [Google Scholar]
- 4.Von Bültzingslöwen I, Sollecito TP, Fox PC, Daniels T, Jonsson R, Lockhart PB, et al. Salivary dysfunction associated with systemic diseases: systematic review and clinical management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(Suppl):S57.e1–S57.15. doi: 10.1016/j.tripleo.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Wolff A, Joshi RK, Ekström J, Aframian D, Lynge Pedersen AM, Proctor G, et al. A guide to medications inducing salivary gland dysfunction, xerostomia, and subjective sialorrhea: a systematic review sponsored by the world workshop on oral medicine VI. Drugs R&D. 2017;17:1–28. doi: 10.1007/s40268-016-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pajukoski H, Meurman JH, Halonen P, Sulkava R. Prevalence of subjective dry mouth and burning mouth in hospitalized elderly patients and outpatients in relation to saliva, medication, and systemic diseases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:641–649. doi: 10.1067/moe.2001.118478. [DOI] [PubMed] [Google Scholar]
- 7.Tabak LA. In defense of the oral cavity: structure, biosynthesis, and function of salivary mucins. Annu Rev Physiol. 1995;57:547–564. doi: 10.1146/annurev.ph.57.030195.002555. [DOI] [PubMed] [Google Scholar]
- 8.Villa A, Wolff A, Aframian D, Vissink A, Ekström J, Proctor G, McGowan R, Narayana N, Aliko A, Sia YW, Joshi RK, Jensen SB, Kerr AR, Dawes C, Pedersen AM. World workshop on Oral medicine VI: a systematic review of medication-induced salivary gland dysfunction: prevalence, diagnosis, and treatment. Clin Oral Investig. 2015;19:1563–1580. doi: 10.1007/s00784-015-1488-2. [DOI] [PubMed] [Google Scholar]
- 9.Napeñas JJ, Brennan MT, Fox PC. Diagnosis and treatment of xerostomia (dry mouth) Odontology. 2009;97:76–83. doi: 10.1007/s10266-008-0099-7. [DOI] [PubMed] [Google Scholar]
- 10.Delli K, Spijkervet FKL, Kroese FGM, Bootsma H, Vissink A (2014) Xerostomia. Monogr Oral Sci 24:109–125 [DOI] [PubMed]
- 11.Dawes C, Lynge Pedersen AM, Villa A, Ekström J, Proctor GB, Vissink A, et al. The functions of human saliva: a review sponsored by the world workshop on oral medicine VI. Arch Oral Biol. 2015;60:863–874. doi: 10.1016/j.archoralbio.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Furness S, Worthington HV, Bryan G, Birchenough S, McMillan R. Interventions for the management of dry mouth: topical therapies. Cochrane Database Syst Rev. 2011;8:CD009603. doi: 10.1002/14651858.CD008934.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Hahnel S, Behr M, Handel G, Bürgers R. Saliva substitutes for the treatment of radiation-induced xerostomia-a review. Support Care Cancer. 2009;17:1331–1343. doi: 10.1007/s00520-009-0671-x. [DOI] [PubMed] [Google Scholar]
- 14.Davies AN. A comparison of artificial saliva and chewing gum in the management of xerostomia in patients with advanced cancer. Palliat Med. 2000;14:197–203. doi: 10.1191/026921600672294077. [DOI] [PubMed] [Google Scholar]
- 15.Shahdad SA, Taylor C, Barclay SC, Steen IN, Preshaw PM. A double-blind, crossover study of Biotène Oralbalance and BioXtra systems as salivary substitutes in patients with post-radiotherapy xerostomia. Eur J Cancer Care (Engl) 2005;14:319–326. doi: 10.1111/j.1365-2354.2005.00587.x. [DOI] [PubMed] [Google Scholar]
- 16.Mouly S, Salom M, Tillet Y, Coudert A-C, Oberli F, Preshaw PM, Desjonquères S, Bergmann JF. Management of xerostomia in older patients: a randomised controlled trial evaluating the efficacy of a new oral lubricant solution. Drugs Aging. 2007;24:957–965. doi: 10.2165/00002512-200724110-00007. [DOI] [PubMed] [Google Scholar]
- 17.Sweeney MP, Bagg J, Baxter WP, Aitchison TC. Clinical trial of a mucin-containing oral spray for treatment of xerostomia in hospice patients. Palliat Med. 1997;11:225–232. doi: 10.1177/026921639701100307. [DOI] [PubMed] [Google Scholar]
- 18.Mouly S, Orler J-B, Tillet Y, Coudert A-C, Oberly F, Preshaw PM, et al. Efficacy of a new oral lubricant solution in the management of psychotropic drug-induced xerostomia: a randomized controlled trial. J Clin Psychopharmacol. 2007;27:437–443. doi: 10.1097/jcp.0b013e31814db434. [DOI] [PubMed] [Google Scholar]
- 19.Ship JA, McCutcheon JA, Spivakovsky S, Kerr AR. Safety and effectiveness of topical dry mouth products containing olive oil, betaine, and xylitol in reducing xerostomia for polypharmacy-induced dry mouth. J Oral Rehabil. 2007;34:724–732. doi: 10.1111/j.1365-2842.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- 20.Femiano F, Rullo R, Di Spirito F, Lanza A, Festa VM, Cirillo N. A comparison of salivary substitutes versus a natural sialogogue (citric acid) in patients complaining of dry mouth as an adverse drug reaction: a clinical, randomized controlled study. Oral Surgery, Oral Med Oral Pathol Oral Radiol Endodontology. 2011;112:e15–e20. doi: 10.1016/j.tripleo.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 21.Vinke J, Kaper HJ, Vissink A, Sharma PK. An ex vivo salivary lubrication system to mimic xerostomic conditions and to predict the lubricating properties of xerostomia relieving agents. Sci Rep. 2018;8:9087. doi: 10.1038/s41598-018-27380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veeregowda DH, Kolbe A, Van der Mei HC, Busscher HJ, Herrmann A, Sharma PK (2013) Recombinant supercharged polypeptides restore and improve biolubrication. Adv Mater 25:3426–3431 [DOI] [PubMed]
- 23.Ash A, Wilde P, Bradshaw DJ, King SP, Pratten JR. Structural modifications of the salivary conditioning film upon exposure to sodium bicarbonate: implications for lubrication and mouthfeel. Soft Matter. 2016;12:2794–2801. doi: 10.1039/C5SM01936B. [DOI] [PubMed] [Google Scholar]
- 24.Vissink A, Wolff A, Veerman ECI (2008) Saliva collectors. In: Wong DT (ed) Salivary diagnostics. Wiley-Blackwell, Ames, pp 37–59
- 25.Verkaik MJ, Busscher HJ, Jager D, Slomp AM, Abbas F, Van der Mei HC. Efficacy of natural antimicrobials in toothpaste formulations against oral biofilms in vitro. J Dent. 2011;39:218–224. doi: 10.1016/j.jdent.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Veeregowda DH, Busscher HJ, Vissink A, Jager D-J, Sharma PK, Van der Mei HC. Role of structure and glycosylation of adsorbed protein films in biolubrication. PLoS One. 2012;7:e42600. doi: 10.1371/journal.pone.0042600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Almeida PDV, Grégio AMT, Machado MÂN, De Lima AAS, Azevedo LR. Saliva composition and functions: a comprehensive review. J Contemp Dent Pract. 2008;9:72–80. doi: 10.5005/jcdp-9-3-72. [DOI] [PubMed] [Google Scholar]
- 28.Prinz JF, De Wijk RA, Huntjens L. Load dependency of the coefficient of friction of oral mucosa. Food Hydrocoll. 2007;21:402–408. doi: 10.1016/j.foodhyd.2006.05.005. [DOI] [Google Scholar]
- 29.Chaudhury NMA, Shirlaw P, Pramanik R, Carpenter GH, Proctor GB. Changes in saliva rheological properties and mucin glycosylation in dry mouth. J Dent Res. 2015;94:1660–1667. doi: 10.1177/0022034515609070. [DOI] [PubMed] [Google Scholar]
- 30.Castro I, Sepúlveda D, Cortés J, Quest AFG, Barrera MJ, Bahamondes V, et al. Oral dryness in Sjögren’s syndrome patients. Not just a question of water. Autoimmun Rev. 2013;12:567–574. doi: 10.1016/j.autrev.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Khutoryanskiy VV. Advances in Mucoadhesion and Mucoadhesive polymers. Macromol Biosci. 2011;11:748–764. doi: 10.1002/mabi.201000388. [DOI] [PubMed] [Google Scholar]
- 32.Kerr AR, Corby PM, Shah SS, Epler M, Fish GS, Norman RG. Use of a mucoadhesive disk for relief of dry mouth. J Am Dent Assoc. 2010;141:1250–1256. doi: 10.14219/jada.archive.2010.0053. [DOI] [PubMed] [Google Scholar]
- 33.Aqualon Natrosol. Ind Eng Chem. 1945;37:526–533. doi: 10.1021/ie50426a010. [DOI] [Google Scholar]
- 34.Madsen F, Eberth K, Smart JD. A rheological assessment of the nature of interactions between mucoadhesive polymers and a homogenised mucus gel. Biomaterials. 1998;19:1083–1092. doi: 10.1016/S0142-9612(98)00037-4. [DOI] [PubMed] [Google Scholar]
- 35.Madsen F, Eberth K, Smart JD. A rheological evaluation of various mucus gels for use in in-vitro mucoadhesion testing. Pharm Sci. 1996;1:563–566. [Google Scholar]
- 36.Needleman IG, Martin GP, Smales FC. Characterisation of bioadhesives for periodontal and oral mucosal drug delivery. J Clin Periodontol. 1998;25:74–82. doi: 10.1111/j.1600-051X.1998.tb02366.x. [DOI] [PubMed] [Google Scholar]
- 37.De Vicente J, Stokes JRR, Spikes HAA. Lubrication properties of non-adsorbing polymer solutions in soft elastohydrodynamic (EHD) contacts. Tribol Int. 2005;38:515–526. doi: 10.1016/j.triboint.2004.11.001. [DOI] [Google Scholar]
- 38.Ruissen ALA, Van der Reijden WA, Van’ t Hof W, Veerman ECI, Van Nieuw Amerongen A. Evaluation of the use of xanthan as vehicle for cationic antifungal peptides. J Control Release. 1999;60:49–56. doi: 10.1016/S0168-3659(99)00052-8. [DOI] [PubMed] [Google Scholar]
- 39.Jang H-J, Shin CY, Kim K. Safety evaluation of polyethylene glycol (PEG) compounds for cosmetic use. Toxicol Res. 2015;31:105–136. doi: 10.5487/TR.2015.31.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hatton MN, Levine MJ, Margarone JE, Aguirre A. Lubrication and viscosity features of human saliva and commercially available saliva substitutes. J Oral Maxillofac Surg. 1987;45:496–499. doi: 10.1016/S0278-2391(87)80009-5. [DOI] [PubMed] [Google Scholar]