Abstract
Baking soda and vinegar have been used as home remedies for generations and today we are only a mouse-click away from claims that baking soda, lemon juice, and apple cider vinegar are miracles cures for everything from cancer to COVID-19. Despite these specious claims, the therapeutic value of controlling acid-base balance is indisputable and is the basis of Food and Drug Administration-approved treatments for constipation, epilepsy, metabolic acidosis, and peptic ulcers. In this narrative review, we present evidence in support of the current and potential therapeutic value of countering local and systemic acid-base imbalances, several of which do in fact involve the administration of baking soda (sodium bicarbonate). Furthermore, we discuss the side effects of pharmaceuticals on acid-base balance as well as the influence of acid-base status on the pharmacokinetic properties of drugs. Our review considers all major organ systems as well as information relevant to several clinical specialties such as anesthesiology, infectious disease, oncology, dentistry, and surgery.
Keywords: pH, Bicarbonate, Proton, Lactate, Transporters
1. Introduction
The normal function of nearly all physiological processes in the body depends on maintenance of appropriate acid-base balance. The value of intracellular pH and interstitial pH strongly depends on the value of arterial blood pH, which ranges between 7.35 and 7.45 under normal physiological conditions. When pH deviates from its normal range, pH-dependent enzymes and membrane transport proteins may not work properly and metabolic pathways can be negatively affected. Acidemia, which is defined as arterial pH lower than 7.35, can cause a variety of disturbances including arterial vasodilation, insulin resistance, compromised immune function, and reduced neuronal excitability. Alkalemia, which is defined as arterial pH greater than7.45, can also cause many disturbances including reduced myocardial blood flow and seizures. Thus, it is imperative that the value of blood pH is tightly controlled.
Therapies for acid-base disturbances are not new. Infusion of sodium carbonate (Na2CO3) into cholera patients to compensate for loss of serum alkali in diarrhea was recorded in the 1830s [1] and the commercial production of sodium bicarbonate (NaHCO3) for use as an antacid (Brioschi®) apparently dates back to the 1880s. Since then, decades of research advances have led to a broad appreciation of the importance of acid-base balance in health and disease. This research is now coming to fruition in the form of inspired and effective medical advances. At the same time, some in the alternative medical community have seized upon anecdotes and the results of limited trials to generate ubiquitous clickbait headlines about the miraculous properties of household acids and bases such as baking soda and, in some cases, propagate conspiracy theories about suppression of this information.
In the first major section of our review (2 Acid-Base Homeostasis) we discuss how the body controls the abundance and distribution of acids and bases in order to achieve acid-base homeostasis. We describe the importance of the powerful CO2/HCO3 − buffer system, the vital functions of the lungs and kidneys in excreting excess acids and bases, the role of membrane transport proteins and carbonic anhydrases in the local redistribution of acids and bases, and the drugs that can be harnessed to control these processes. In the second major section of our review (3 Systemic Acid-Base Disturbances) we discuss the causes and consequences of generic acid-base imbalance caused by disturbances in extracellular CO2 and HCO3 − levels and how our knowledge of their etiology has informed therapeutic strategies. Our third and fourth major sections (4 Applications by Organ System and 5 Other Applications by Clinical Specialty) bring together a wealth of information from in vitro, in vivo, and clinical studies that demonstrate the current and potential utility of acid-base-balance correcting therapies. For each organ system or clinical specialization, as appropriate, we provide the fundamental physiological aspects of normal acid-base balance, the pathological consequences of systemic and local acid-base disturbances, as well as considerations of corrective therapies based on restoring (or harnessing the agents of) acid-base balance. In our fifth and final major section (6 pH-dependent Aspects of Pharmaceutical Therapy) we discuss how acid-base chemistry can influence drug pharmacokinetic properties and how this phenomenon can be advantageous for optimizing therapeutic interventions.
Our review highlights an emerging and dynamic field of research that is in the process of translating numerous basic scientific findings into clinical therapies. These findings appear to touch on nearly all aspects of health. We note a wide array of therapeutic paradigms developed around the control of acid-base balance including numerous reports of the successful application of NaHCO3, the so-called ‘enemy of the pharmaceutical industry,’ to the amelioration of disease signs in animal models and in limited clinical trials. Although studies of the role of acid-base balance in health and disease have resulted in the generation of several FDA-approved pharmaceuticals such as contraceptive gels and gastric-acid suppressors, systematic reviews of random trials of the clinical effectiveness of NaHCO3 itself tend to be circumspect in their conclusions.
A note to the reader: We, the authors, are basic scientists and do not intend this review to serve as a diagnostic or therapeutic guide. In many cases, a lack of consistency among study methods and subject demographics makes it difficult to draw firm conclusions regarding outcomes. For these reasons it is also impossible to extrapolate findings of therapeutic effectiveness in animal models and limited trials into an assessment of general clinical utility. But, in as much as we are reporting potential, we have not discounted any positive outcomes. Finally, we note that the scope of this narrative review is extremely broad and the literature is extensive. For this reason, we have often cited reviews instead of primary literature in order to simplify the document and provide a cue to further reading. We apologize in advance to any authors whose work we have omitted.
2. Acid-Base Homeostasis
2.1. The CO2/HCO3− Buffer System
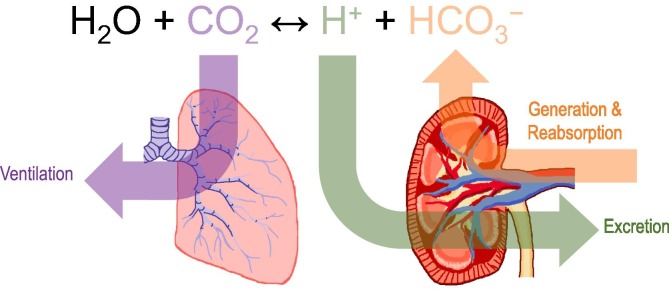
The maintenance of blood pH in the face of a ~40–70 mEq H+/day acid-load imposed by diet and metabolism (net endogenous acid production: NEAP [2], [3]), requires robust homeostatic mechanisms. Regulation of blood pH and, by extension, the entire extracellular fluid compartment depends on the interplay between (i) the urinary system, which controls the blood bicarbonate concentration ([HCO3 −]), and (ii) the neuro-respiratory systems, which control the partial pressure of CO2 (pCO2, see Fig. 1 ). The kidneys perform the tasks of generating HCO3 −, depositing it into circulation, and recycling HCO3 − from filtered plasma back into circulation (see Fig. 2 ). The lungs exhale CO2, with respiratory drive being controlled by chemosensitive neural circuitry [4]. A third mechanism of defense, which tends to minimize pH changes, is provided by the multitude of buffer systems present in the extracellular fluid compartment. Among these buffers, the most powerful is the CO2/HCO3 − buffer system, the efficacy of which is conferred by the body behaving as an open system, with respect to CO2, from which CO2 can escape [5]. A feature of the CO2/HCO3 − buffer system is that the first of the two-step reactions that describe the interconversion between CO2 and HCO3 − (CO2 + H2O H2CO3 HCO3 −+ H+) is very slow unless catalyzed by a carbonic anhydrase (CA) enzyme. Thus, efficient buffering requires the presence of a CA.
Fig. 1.
Whole-body acid base homeostasis. Because the body behaves as an open CO2/HCO3− buffer system, maintenance of blood/extracellular pH within a narrow physiological range depends on the dual and independent action of the kidneys which, by adjusting urine acid secretion, control blood [HCO3−], and of the lungs which, by adjusting ventilation, control blood pCO2.
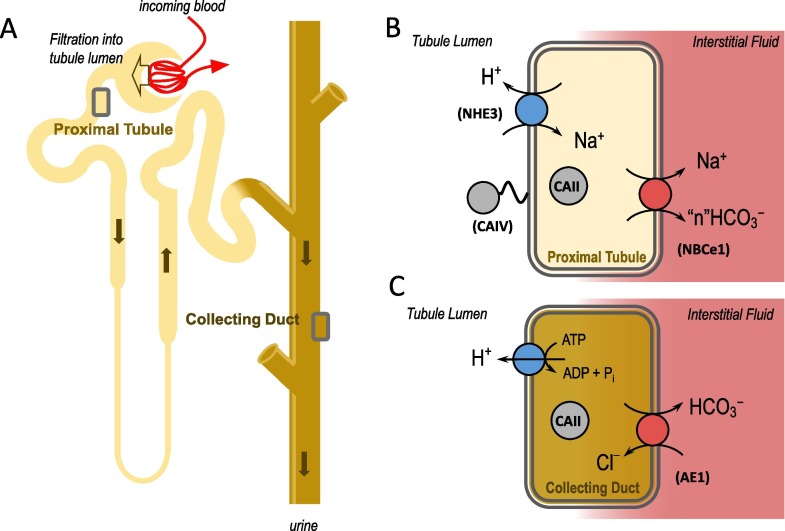
Fig. 2.
Simplified schematization of H+ and HCO3− handling by the kidneys. Plasma is filtered from the renal blood supply at the glomerulus of each nephron (panel A). Acids are secreted into the filtered fluid by epithelial cells in the proximal tubule (panel B) and the collecting duct (panel C), generating new HCO3− that is absorbed into circulation to replace that titrated by acids in circulation. In the tubule lumen, if secreted H+ is titrated by a non-HCO3− buffer such as NH3 or phosphate, it can be excreted. If a secreted H+ reacts with a filtered HCO3− in the proximal tubule lumen (catalyzed by CAIV), the CO2 enters the proximal tubule cell and is converted back into a H+ and HCO3− (by CAII). In that case no H+ is excreted and the HCO3− is considered to have been reabsorbed. Virtually no filtered HCO3− is excreted in the urine and the amount of H+ excreted varies with the body’s acid load. NHE3: Na+/H+ exchanger 3; CAIV: carbonic anhydrase IV; CAII: carbonic anhydrase II; NBCe1: electrogenic Na+/HCO3− cotransporter. Note that, because the stoichiometry of NBCe1 in the renal proximal tubule is not yet established [43], we have indicated with "n" the number of HCO3− ions carried by NBCe1; AE1: Cl–/HCO3– exchanger 1.
The Henderson-Hasselbalch equation [6] describes how [HCO3 −] and pCO2 determine pH:
| (1) |
Here, , with being the equilibrium constant of the CO2/HCO3 − buffer system and the solubility coefficient of CO2. At 37 °C, it can be assumed that is 6.1 and is 0.03. Eq. (1) shows that pH depends on the [HCO3 −]:pCO2 ratio.
According to the National Institutes of Health, the reference range for [HCO3 −] is 22–26 mEq/L and the reference range for pCO2 is 35–45 mmHg [7]. However, it is important to recognize that there are differences among individuals that could impact diagnosis, susceptibility to disease, and responsiveness to treatment. One study, for example, suggests that the reference range for [HCO3 −] varies with sex and ethnicity, reporting (i) higher upper-range and lower-range limits in males, (ii) higher lower-range limit in individuals who identified as Asian compared to those who identified as white and (iii) higher upper-range limit in those who identified as black compared to those who identified as white [8]. Data that demonstrate the practical consequence of these specific observations are currently lacking, but there are clear demonstrations of acid-base-related health disparities among minorities [9], [10], [11], [12] and studies in animal models report physiological differences in acid-base handling in males compared to females [13]. Although there are reports of age-related changes in serum [HCO3 −], there is a surprising lack of consensus regarding whether the correlation is positive [2] or negative [14], probably due to subgroup effects.
The extent to which extracellular pH (pHe) influences intracellular pH (pHi) varies depending on the complement of acid-extruding or acid-loading membrane transport proteins that a cell expresses [15]. Typically, cells express an array of these proteins as defense against acidosis. The protective role of these proteins is important for cell survival [16], [17], proliferation [18], and migration [19] in a variety of cell types. The actions of these proteins can make cell sensitive to changes in pHe by allowing permeation of acids and bases or help them defend against changes in pHe by eliminating or dissipating acids and bases to maintain pHi. An overview of these proteins is provided in the following section.
2.2. Agents of Acid-Base Balance and their Blockers
In this section, we provide a brief description of the nature, molecular action, and clinically important blockers of each of the major classes of acid-base transporters (ABTs) or carbonic anhydrases that are pertinent to the current review. Examples from each of the classes of protein discussed below in 2.2.1, 2.2.2, 2.2.3, 2.2.4, 2.2.5, 2.2.6, 2.2.7, 2.2.8 are illustrated in Fig. 3 . Here, we also establish the nomenclature and abbreviations for the proteins that are cited in this manuscript. Because many compounds that are useful to block ABTs in model systems (e.g., the anion transport blocker DIDS) are not sufficiently specific for targeted therapeutic use, we will confine our consideration to highlighting only those few compounds that have either been approved by the Food and Drug Administration (FDA) for specific uses, drugs currently in clinical trial, or drugs that failed clinical trials that may be repurposed in other settings. Additional experimental compounds are discussed in 3, 4, 5. The distribution, roles in physiology and pathophysiology, and therapeutic relevance of specific proteins are discussed later in 3, 4, 5.
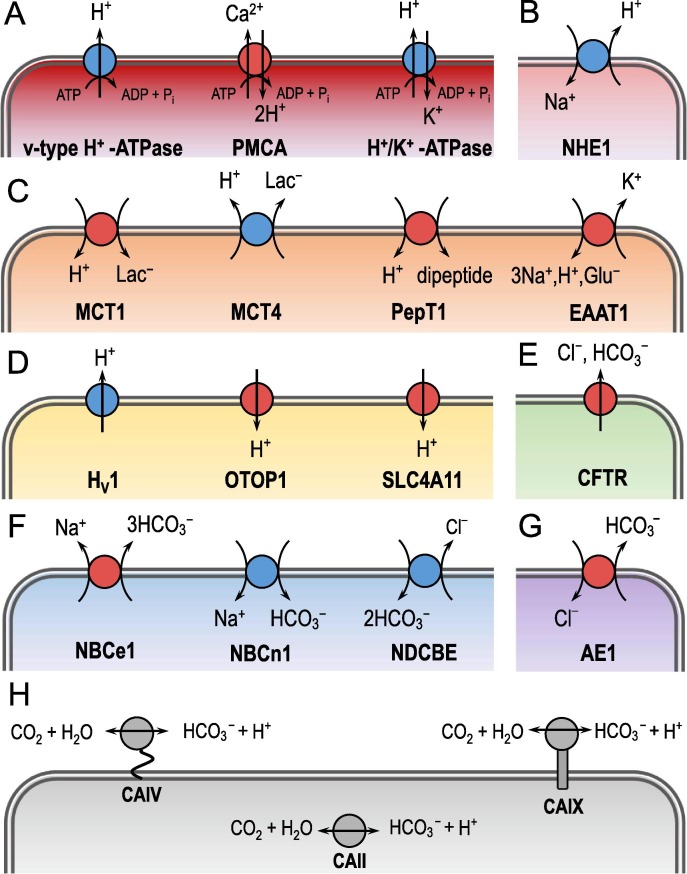
Fig. 3.
Examples of various classes of acid-base handling proteins. Panel A shows the “v-type” H+-ATPase, the plasma membrane Ca2+-ATPase (PMCA) and the H+/K+-ATPase. All three transporters are H+ pumps. Panel B shows the Na+/H+ exchanger NHE1. Panel C shows a selection of H+-coupled solute transporters. Specifically, it shows the H+-coupled lactate transporters MCT1 and MCT4, the H+-coupled oligopeptide transporter PeptT1 and the excitatory amino acid transporter EAAT1. Panel D shows examples of H+ channels such as the voltage-gated H+-channel HV1, otopetrin 1 (OTOP1) and the voltage-independent H+ conductor SLC4A11. Panel E shows the cystic fibrosis transmembrane regulator CFTR as an example of a HCO3−-permeable anion channel. Panel F shows members of the Na+-coupled HCO3−-transporter family, including the electrogenic Na+/3HCO3− cotransporter NBCe1, the electroneutral Na+–HCO3− cotransporter NBCn1 and the electroneutral Na+-driven Cl−/HCO3− exchanger NDCBE. Note that NBCe1 normally operates as an acid extruder with a stoichiometry of 1 Na+: 2HCO3−. An exception is the renal proximal tubule where NBCe1 operates as acid loader with a presumed 1 Na+: 3HCO3− stoichimetry [43]. Panel G shows the Cl−/HCO3− exchanger AE1. Panel H shows members of the carbonic anhydrase family (CAs) like the glycosylphosphatidylinositol (GPI)-anchored extracellular isoform CAIV, the cytosolic isoform CAII and the transmembrane isoform CAIX.
2.2.1. H+ pumps
The heterodimeric ‘gastric’ H+/K+-ATPase (ATP4A and ATP4B encode its two subunits [20]) and the multi-subunit “v-type” H+-ATPase expressed in the kidney (ATP6V0A4 and ATP6V1B1 encode two of its subunits [21]) both use the energy of ATP hydrolysis to extrude H+. On the other hand, the plasma membrane Ca2+-ATPase (PMCA, ATP2B1), which is expressed in a variety of excitable cells, uses ATP to extrude Ca2+ and mediates an acid-loading Ca2+/H+ exchange activity [22]. The most important class of pharmaceutical associated with this group are the proton pump inhibitors (PPIs). Benzimidazole derivatives such as the FDA-approved esomeprazole (NEXIUM®), when taken orally, target the H+/K+-ATPase as therapy for pathologies such as peptic ulceration caused by stomach acidity [23] (see Section 4.7).
2.2.2. Na+/H+ exchangers
Five of the -9 members of the Na+/H+ exchanger family (NHEs, SLC9A1-9 [24]) are referenced in our review. Of these, four NHEs are commonly expressed in the plasma membrane (NHE1, NHE2, NHE3, and NHE8) where they harness the inwardly directed Na+ gradient (established by the Na+/K+-ATPase) to mediate acid-extrusion. NHE6, on the other hand, is expressed in the endosomal membrane. The endosome interior is acidified by a “v-type” H+-ATPase, while NHE6 action extrudes H+ to prevent compartmental over-acidification [25]. Pyrazinoylguanidine-derivatives such as the FDA-approved amiloride exhibit imperfect NHE specificity and their therapeutic usefulness as antihypertensive diuretics results mainly from block of the epithelial Na+ channel ENaC in the renal collecting duct [26]. The benzoylguanidine-derivative inhibitor cariporide exhibits a greater specificity for NHE1 and entered Phase III clinical trials as a cardioprotective agent. However, despite some positive retrospective findings, the drug failed due to increased mortality caused by thromboembolic stroke, a possible effect of NHE1 inhibition in platelets [27], [28] (see Section 4.4). Tenapanor (IBSRELA®) is an NHE3 inhibitor approved by the FDA to treat irritable bowel syndrome with constipation [29] (see Section 4.8).
2.2.3. H+-coupled solute transporters
Four of the 14 members of the monocarboxylate transporter family (MCTs, SLC16A1-14 [30]) are H+-coupled lactate transporters (MCT1-4). MCT1 and MCT4, being expressed in tumors, are of main therapeutic interest. The directionality of their transport process is determined by kinetic, thermodynamic, and situational considerations; for example, the widely expressed isoform MCT1 typically mediates H+/Lac− import, while MCT4, which is abundantly expressed in glycolytic (i.e., lactate-producing) cells such as cancer cells and astrocytes, mediates H+/Lac− export [31]. Members of several other solute carrier families also cotransport H+ with their substrates such as the H+-coupled oligopeptide transporters of the SLC15 family (e.g., PeptT1 [32]) or the H+-coupled neurotransmitter transporters of the SLC1 family (e.g., the excitatory amino acid transporter EAAT1 [33]). The MCT1 inhibitor AZD3965 [34] is currently in Phase I clinical trial for its effectiveness in treating cancer (ClinicalTrials.gov Identifier: NCT01791595).
2.2.4. H+ channels
There are several membrane proteins that conduct H+ but share no obvious commonality in protein sequence. One group act as H+-selective channels and include the voltage-gated H+-channel HV1 (HVCN1 [35]) and the voltage-independent H+ conductors SLC4A11 [36] and otopetrin 1 (OTOP1 [37]). All permit the movement of H+ down their transmembrane electrochemical gradient, but each differ in their regulation of gating. HV1 only opens when the gradient favors H+ efflux, SLC4A11 favors H+ influx (particularly at elevated pHi [38], perhaps to defend pHi), and OTOP1 also favors H+ influx (particularly at low pHe, perhaps consistent with its sensory role). In acidotic conditions, the transient receptor potential cation channel subfamily V member 1 (TRPV1) can also mediate a significant, but non-canonical, acid-loading H+ conductance [39]. We are unaware of any FDA-approvals for inhibition of this class of proteins, with the exception of TRPV1 agonists whose influence on H+ conductance is untested.
2.2.5. HCO3−-permeable anion channels
Although there is no description of a HCO3 −-specific ion channel, several anion channels have significant HCO3 − permeability. The electrochemical gradient for HCO3 − typically favors HCO3 −-efflux. These channels include the cystic fibrosis transmembrane regulator (CFTR [40]), the Ca2+-activated Cl−–channel anoctamin 1 (ANO1 [41]), as well GABA- and glycine-activated Cl−- channels (GABR and GLR families [42]). Drugs such as ivacaftor (KALYDECO®, increases CFTR channel open probability), or cocktails that include ivacaftor and one or more of the CFTR-folding-chaperone drugs elexacaftor/lumacaftor/tezacaftor (e.g., ORKAMBI®, SYMDEKO®, TRIKAFTA®) are indicated for the treatment of cystic fibrosis (CF) by the restoration of certain defective CFTR channels. Both ivacaftor and tezacaftor rescue the HCO3 − permeability of the Δ508-CFTR mutant. In fact, the rescued mutant has a greater HCO3 −:Cl− permeability ratio than wild-type CFTR, which may be therapeutically valuable. The importance of CFTR-mediated HCO3 − secretion is discussed in Section 4.3 The Respiratory System, Section 4.8 The Lower Digestive System, and Section 4.9 The Urinary System).
2.2.6. Na+-coupled HCO3−-transporters
Five of the 10 members of the SLC4 family of proteins mediate some form of Na+-coupled HCO3 −-transport [43]. NBCe1 and NBCe2 (SLC4A4 and SLC4A5) are electrogenic Na+/2HCO3 − cotransporters that may either act as acid-extruders or acid-loaders, depending on the electrochemical gradient. For example, NBCe1 mediates HCO3 − efflux in renal proximal tubule epithelia, but HCO3 − influx in pancreatic duct epithelia. The remaining three are all acid extruders. NBCn1 (SLC4A7) is an electroneutral Na+–HCO3 − cotransporter, while NDCBE (SLC4A8) is an electroneutral Na+-driven Cl−/HCO3 − exchanger. NBCn2/NCBE (SLC4A10) has been described as being capable of both actions. We are unaware of any FDA-approvals for inhibition of this class of proteins, nor of any drug that is specific for this class of protein. However, -we note that the non-steroidal anti-inflammatory drug tenidap, which failed clinical trials for the treatment of rheumatoid arthritis due to renal and hepatic toxicity, is an effective blocker of NBCe1 and NBCe2 [44], [45].
2.2.7. Cl−/HCO3−-exchangers
Three of the 10 members of the SLC4 family of proteins (AE1, AE2, and AE3, SLC4A1-3) mediate electroneutral Cl−/HCO3 − exchange [43] as do seven of the 11 members of the SLC26 family of anion exchange proteins [46]. Three of the latter have specific importance to the present review. These are the ‘downregulated in adenoma’ protein DRA (SLC26A3), pendrin (SLC26A4), and the ‘pancreatic anion transporter’ protein PAT1 (SLC26A6). In all cases, prevailing physiological conditions favor HCO3 −-efflux. Although there are exceptions, as a general rule SLC4s protect cells from alkalosis by extruding HCO3 − across the basolateral membrane of epithelial cells into the interstitial fluid, whilst SLC26s mediate HCO3 − secretion across the apical membrane. We are unaware of any FDA-approvals for modulation of this class of proteins, although tenidap (mentioned in the previous section) is a blocker of DRA [47].
2.2.8. Carbonic anhydrases
The 14 mammalian CAs (CA1-CA14) include cytosolic, mitochondrial, transmembrane, membrane-bound, and secreted isoforms [48]. All catalyze the interconversion of CO2 and H2O with HCO3 − and H+, the direction depending on prevailing gradients, enhancing buffering of H+ and promoting the facilitated diffusion of CO2 across the plasma membrane [49]. The five CAs of greatest relevance to this review are the ubiquitous cytosolic isoform CAII, the skeletal muscle cytosolic isoform CAIII, the glycosylphosphatidylinositol (GPI)-anchored extracellular isoform CAIV, and the two transmembrane isoforms CAIX and CAXII, the CA-activities of which are also extracellular. Acetazolamide (ACZ, DIAMOX®) is one of a number of FDA-approved CA inhibitors that are indicated for treatment of epilepsy and glaucoma (see 4.1, 4.2) as well as for induction of diuresis as a therapy for congestive heart failure or drug-induced edema [50] (see Section 4.9).
3. Systemic Acid-Base Disturbances
3.1. The Four Classic/Simple Acid-Base Disturbances
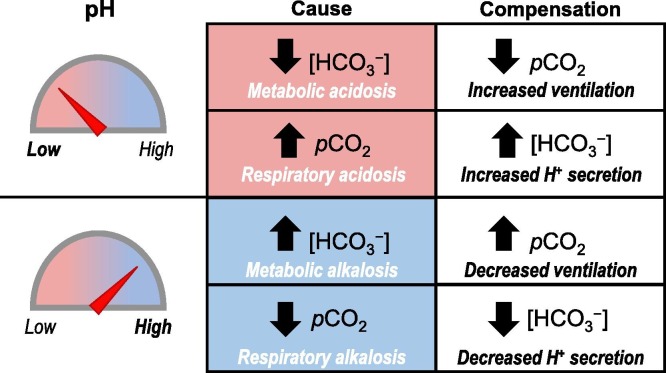
Eq. (1) and Fig. 4 show that, provided that pCO2 remains constant, (i) a fall in extracellular [HCO3 −] causes blood pH to decrease whereas (ii) a rise in extracellular [HCO3 −] causes blood pH to increase. These two cases describe states of metabolic acidosis (MAc) and metabolic alkalosis (MAlk), respectively. Eq. (1) also indicates that pH can return towards its normal physiological value by a decrease in extracellular pCO2 (in case ‘i’) or an increase in extracellular pCO2 (in case ‘ii’). This compensatory normalization of the [HCO3 −]:pCO2 ratio to restore pH, describes the physiological response of the neuro-respiratory system to MAc and MAlk. Moreover, Eq. (1) and Fig. 4 show that, provided that [HCO3 −] remains constant, (iii) a rise in extracellular pCO2 causes blood pH to decrease whereas (iv) a decrease in extracellular pCO2 causes blood pH to rise. These two cases describe states of respiratory acidosis (RAc) and respiratory alkalosis (RAlk), respectively. Again, Eq. (1) indicates that pH can return towards its normal value by an increase (case ‘iii’) or decrease (case ‘iv’) in extracellular [HCO3 −], describing the compensatory physiological response of the urinary system to RAc and RAlk. MAc, MAlk, RAc, and RAlk are usually referred to as the four classic/simple acid-base disturbances.
Fig. 4.
The four classic systemic acid-base disturbances. Alterations in [HCO3−] and pCO2 can both cause derangements of pH. Metabolic pH disturbances can be compensated by altering the ventilation rate to normalize the [HCO3−]:pCO2 ratio. Respiratory pH disturbances can be compensated by altering the renal acid excretion rate.
Respiratory compensations usually occur quite rapidly, within an hour of the appearance of the metabolic disorders and are fully resolved within 12 to 36 h. In contrast, metabolic responses to respiratory disorders occur more slowly and may take up to several days to fully resolve as they require remodeling of acid-base handling mechanisms in the urinary system. The most rapid response—and the first line of defense of our body—to an acid-base disorder is given by chemical buffering which usually occurs within minutes.
In the following four sub-sections we will review the causes, consequences, and therapeutic paradigms for each of the four systemic acid-base disturbances. Several of these considerations are also relevant to the resolution of local acid-base disturbances and will be revisited in 4, 5. For a more complete and clinical perspective on these disturbances in isolation, and in combination, we refer the reader to reference [51].
3.2. Metabolic Acidosis
3.2.1. Causes and consequences
MAc is defined as acidemia due to a primary pathological deficit in [HCO3 −] rather than a physiological, compensatory lowering of [HCO3 −] in response to respiratory alkalosis [52]. The body can counter acid shifts in plasma pH in the short term by increasing respiratory drive to lower CO2 and, in the longer term, by increasing renal H+ excretion/HCO3 − generation. However, if these compensatory systems are defective or overwhelmed, MAc will result. For example, MAc can result from diet, chronic kidney disease, and diabetes (diabetic ketoacidosis) or can follow acute myocardial infarction (lactic acidosis), mutations in renal acid-base transporters (renal tubular acidosis, see Section 4.9), intoxication with compounds (e.g., aspirin), and diarrhea (loss of HCO3 −-rich secretions) [53], [54], [55], [56], [57]. Clinical manifestations vary depending on underlying cause, but generally include weakness, nausea, and flushed skin [58]. As we shall see, chronic MAc has severe consequences for long-term health.
3.2.2. Therapeutic paradigms
In cases where MAc is secondary to another disturbance such as in diabetes or diarrhea, treatment of the underlying disorder is the ultimate goal. However, for short term remediation of MAc, or for situations in which the primary defect is with acid-base homeostatic mechanisms, the typical course of action is ‘alkali therapy’ to address MAc by normalizing plasma pH. This can be achieved via two mechanisms.
3.2.2.1. Increasing base load
The simplest paradigm is administration of HCO3 − salts. A direct rise in plasma [HCO3 −] can be achieved either intravenously or by peritoneal dialysis. An indirect rise in plasma [HCO3 −] can be achieved by oral dosing; as the parietal cells of the stomach replace neutralized stomach acid, they also generate new HCO3 −, which is absorbed into circulation [59] (see also Fig. 9 ). There are however a number of caveats associated with HCO3 − administration [60]. One caveat is that the counter anion (usually Na+ or K+) may contribute to fluid retention or K+ imbalance. A second caveat is that the treatment has the potential to rapidly generate CO2. With oral administration this can manifest as bloating or even gastric rupture, whereas with intravenous administration, the CO2, if not effectively eliminated by the lungs, can enter cells causing a paradoxical intracellular acidification. A third caveat is that pH overshoot (i.e., overcompensation that creates its own pH disturbance) is possible if the dose is not well titrated. However, in practice, manifestation of the side effects associated with NaHCO3 administration is not a foregone conclusion [61], [62]. Alternative vehicles for intravenous alkali delivery such as Na2CO3 and CaCO3 produce less CO2 per neutralized H+ and impose less osmotic stress [63]. Carbicarb is a mixture of NaHCO3 and Na2CO3 that does not cause intracellular acidification [64], [65]. Citrate salts provide a gentler, indirect mean of raising HCO3 − as citrate is converted into HCO3 − in the liver. Alternative buffers such as THAM (tris-hydroxymethyl aminomethane aka Tris-base aka tromethamine aka trometamol) bind H+ without generating CO2 and the protonated product is readily cleared by the kidneys [66]. Furthermore, because a certain proportion of THAM is uncharged at physiological pH, it is cell permeable and can counter intracellular acidosis. Other HCO3 −-replacing bases include lactate and acetate [67], [68]. Finally, potential side effects can be ameliorated by administering buffers at a lower dose as part of an intravenous cocktail of buffers. For example, Tribonat is a mixture that includes NaHCO3, Na2HPO4, and sodium acetate [60], [67]. An added bonus of that mixture is that the inclusion of phosphate counters the hypophosphatemia associated with MAc.
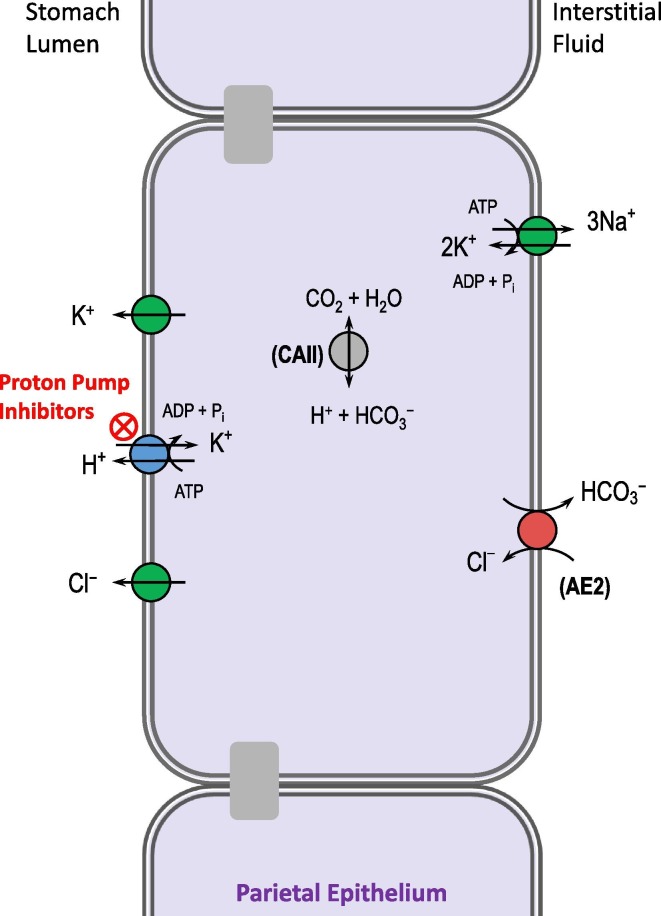
Fig. 9.
Targeting the stomach H+/K+-ATPase to treat acid-reflux disease. Proton pump inhibitors are widely used to reduce gastric acid secretion, as an alternative or adjunct strategy to neutralizing stomach acid with an antacid such as CaCO3. AE2: Cl–/HCO3– exchanger 2.
3.2.2.2. Lowering acid load
Dietary acid load is associated with lower serum HCO3 − [2], [69], [70] and thus there is scope for dietary correction of MAc by, for example, adherence to a very low protein [71] or otherwise “alkaline” diet [72]. pH imbalance in MAc can also be redressed by increasing H+ excretion. The thiazide diuretic hydrochlorothiazide increases H+ secretion by the renal collecting duct and has been used as an adjunct therapy with NaHCO3 for MAc [73]. Its role as a diuretic ought also to assist with excretion of the Na+ load associated with NaHCO3 treatment. Veverimer is an orally dosed H+ binding polymer that is in Phase III clinical trials at the time of writing for the treatment of MAc in the context of chronic kidney disease (ClinicalTrials.gov Identifier: NCT03710291). It binds H+ in the stomach for eventual excretion in the feces [59], [74], [75]. Moreover, the raising of gastric pH by veverimer prompts parietal cells to deposit HCO3 − into circulation, mimicking the alkaline tide associated with feeding. Another approach to counter MAc, is to increase cellular H+ consumption (and/or decrease lactic acid production) by metabolic means either by pyruvate administration or by stimulating pyruvate dehydrogenase using dichloroacetate (DCA) [76], [77].
3.3. Metabolic Alkalosis
3.3.1. Causes and consequences
MAlk is defined as alkalemia caused by a primary excess of HCO3 −. MAlk may follow volume depletion or hyperaldosteronism (promotes renal H+ secretion), vomiting (eliminates gastric acid, stimulating an alkaline tide), or the use of certain pharmaceuticals that mimic those responses (loop diuretics, antacids). Clinical manifestations can include confusion and tetany [58]. MAlk can also have a genetic cause. For example, Liddle Syndrome is associated with hyperactivity of the epithelial Na+ channel ENaC, the action of which promotes renal H+ secretion [78].
3.3.2. Therapeutic paradigms
Besides treatment of the underlying conditions, correction of MAlk has been achieved using the CA inhibitor ACZ (which by itself results in MAc [79]), by intravenous infusion of HCl [80], or (if MAlk follows loss of gastric acid) the use of H2-receptor agonists to prevent alkaline tide [81] (see Section 4.7.2).
3.4. Respiratory Acidosis
3.4.1. Causes and consequences
RAc is defined as acidemia with a plasma pCO2 > 45 mmHg at rest and at sea level [82]. It usually occurs when there is a disruption in the ventilatory system that causes a mismatch between the rate of CO2 removal and the rate of CO2 production, with consequent accumulation of CO2 into the blood (i.e., CO2 retention). This disruption can be caused by (i) inability of the lungs to remove the metabolically produced CO2 (i.e., reduced ventilation), (ii) defects in CO2 transport from tissue to lungs, and/or (iii) overproduction of CO2. Reduced ventilation can result from a depression of the respiratory center (e.g., due to sedative overdose or brain injury), airway obstruction (e.g., due to vomit aspiration or laryngospasm), neuromuscular disorders (e.g., due to Guillain-Barré syndrome) or restrictive defects of the chest (e.g., due to impaired functioning of the diaphragm)[83]. Defects of CO2 transport that lead to hypercapnia are less common and usually the result of reduced pulmonary perfusion in response, for example, to cardiac arrest or pulmonary embolism. Overproduction of CO2 is rarely the sole cause of RAc. In fact, under normal circumstances the body responds to increases in CO2 production by appropriately increasing ventilation in order to remove the excess CO2 and prevent hypercapnia. Situations in which the lungs are unable to match the increased CO2 production can occur in patients undergoing mechanical ventilation or with reduced respiratory reserve [82]. In fact, for therapeutic reasons, individuals on mechanical ventilation are often deliberately maintained in a state of ‘’permissive hypercapnia’’ (see Section 5.2). As for metabolic disturbances, RAc can be either acute or chronic. Acute RAc occurs when pCO2 rises very rapidly and the kidneys are unable to adequately increase HCO3 − production to compensate in such a short amount of time. Thus, only a very modest renal compensation occurs. On the other hand, during the longer timespan of chronic RAc (such as with chronic obstructive pulmonary disease, COPD), the kidneys are able to restore the acid-base balance by increasing acid excretion and HCO3 − production [84].
3.4.2. Therapeutic paradigms
Treatment is usually directed towards reversing the underlying cause and also at restoring adequate alveolar ventilation, which can be accomplished by endotracheal intubation with mechanical ventilation or positive pressure ventilation [85]. Because the sum of pCO2 and pO2 must be constant in the alveolar gas of patients breathing room air, hypercapnia leads to hypoxemia, a condition that can have consequences far more dangerous than those caused by hypercapnia [83]. Consequently, management of acute RAc is often also directed towards ensuring adequate oxygenation. Administration of O2 must be performed carefully because it may lead to increased CO2 retention, especially in patients with COPD [82]. Correction of hypercapnia in chronic RAc usually occurs slowly because rapid reduction of pCO2 can lead to overshoot alkalosis due to the renal compensation that increases [HCO3 −]. In the central nervous system (CNS), rapid alkalinization of the cerebrospinal fluid (CSF) can cause seizures and even coma [82].
The use of alkali therapy in RAc is controversial and indicated only in patients with acute hypercapnia and concurrent MAc [86]. Administration of NaHCO3 is contraindicated because it may increase CO2 production, reduce alveolar ventilation, and also cause a paradoxical acidosis in the CNS. As noted above for MAc, alterative alkali therapies such as Carbicarb that do not generate as much CO2 as NaHCO3 alone (see Section 3.2.2) may be preferable to correct pH in RAc. In patients with COPD, the CA inhibitor ACZ is sometimes used to stimulate respiration in order to improve oxygenation, reduce CO2 retention, and possibly remove the need for mechanical ventilation [87]. However, because CA is ubiquitous, the inhibitory effect of ACZ may impact a variety of tissues and have potential negative consequences on patients with pulmonary diseases. For this reason, the role of ACZ as a respiratory stimulant is controversial, especially in patients with severe COPD with or without hypercapnia [87]. Finally, CO2 can be de-gassed from blood using an extracorporeal CO2 removal (ECCO2R) device [88] or lowered by dialysis using a dialysate that has a low [HCO3 −] [89].
3.5. Respiratory Alkalosis
3.5.1. Causes and consequences
RAlk refers to alkalemia with a plasma pCO2 < 35 mmHg at rest and sea level [82]. It occurs when the ventilatory system does not work properly causing an increase in alveolar ventilation and/or reduced CO2 production with consequent CO2 depletion in the blood. Hyperventilation can result from stimulation of the respiratory centers (e.g., due to drugs or disorders of the CNS), hypoxemia or tissue hypoxia (e.g., due to high altitude), or lung diseases (e.g., pneumonia). Reduced CO2 production can result from a decrease in the basal metabolic rate (e.g., due to hypothermia) or in physical activity (e.g., due to muscle paralysis). Clinical manifestations can include rapid breathing and dizziness [58]. Although usually considered not life-threatening, severe RAlk can have serious consequences on the brain, lungs and the heart. Finally, hormone replacement therapy caused RAlk in a study of postmenopausal women [90].
3.5.2. Therapeutic paradigms
Treatment is usually directed towards correcting the underlying disorders. Abrupt correction of severe RAlk should be avoided because of the risks of cerebral and pulmonary reperfusion injury. ACZ is used in the prevention and treatment of RAlk associated with hyperventilation at high altitude (acute mountain sickness: AMS) in part because it enhances HCO3 − excretion in the urine, providing a compensatory lowering of pH [91].
4. Applications by Organ System
4.1. The Central Nervous System
4.1.1. The importance of acid-base balance
Neuronal activity presents a substantial challenge to local acid-base balance. Neurotransmitter-filled vesicles release H+ into the synaptic cleft [92], [93] (H+ themselves may be considered to be neurotransmitters [94]) and are removed from the synaptic cleft by H+-coupled neurotransmitter transporters such as the excitatory amino acid transporter EAAT1. GABA-activated anion channels in neurons and astrocytes release HCO3 − [95], [96], and the Ca2+/H+ exchange activity of the -PMCA- in neurons causes a rise in extracellular pH as it restores intracellular Ca2+ following an action potential [97]. The acid load that results from intensive neuronal firing can result in a drop in pHi that dampens neuronal activity: a mechanism that prevents excessive firing via effects upon pH-sensitive channels such as ASIC1a (Acid-sensing ion channel 1a) and NMDA (N-methyl-D-aspartate) receptors [98], [99], [100]. Conversely, alkalosis is associated with an increase in neuronal activity and seizures [101]. Neurons and astrocytes express numerous ABTs and CAs to maintain pH homeostasis and their importance is highlighted by the effects of their disruption [102]. For example, genetic disruptions in AE3 or NBCn2 are both associated with epilepsy [103], [104], although the mechanism is not simply related to effects of neuronal pHi on excitability as AE3 is an acid-loader while NBCn2 is an acid extruder and the outcomes of their deletion may depend on whether the neurons in question are excitatory or inhibitory. Several ABTs and CAs are expressed in the choroid plexus epithelia where their action supports the secretion of CSF. Genetic ablation of these transporters (e.g., NBCn2, NBCe2) in rodents is linked to reductions in ventricle fluid volume [105] while pharmacological inhibition of CAs results in reduction of intracranial pressure [106]. However, it is unclear whether these changes are accompanied by a fall in pH of the CSF.
Besides its role in determining pH, HCO3 − plays an important role in neuronal plasticity because the transmembrane gradients of Cl− and HCO3 − determine the reversal potential of GABA-activated channels and consequently whether GABAergic signals are depolarizing and excitatory or hyperpolarizing and inhibitory [107]. Changes in these gradients are important in two ways. Firstly, developmental changes in the gradient during central nervous system maturation promote the switch to inhibitory GABA signaling [108]. Secondly, activity-dependent changes in the gradient contribute to the pathophysiology of epilepsy by promoting a pathological switch to excitatory GABA signaling [107]. Neuronal Cl−–HCO3 − exchangers such as AE3 and NDCBE are likely to contribute to the status of these gradients [109]. Finally, mutations in endosomal NHE6 cause intellectual disability and are associated with defective synaptic remodeling [110].
Acidosis has a number of other consequences. For example in stroke, lactic acidosis is linked to ischemic damage [111]. In protein-aggregating neurodegenerative diseases, acidic pH promotes the aggregation of Alzheimer’s amyloid proteins [112]. A major genetic risk factor for Alzheimer’s Disease is incidence of the apolipoprotein E allelic variant ApoE4, which causes the epigenetic downregulation of NHE6 [113]. Loss of NHE6 from endosomes causes aberrant acidification and defective clearance of amyloid deposits [113]. Brain acid-base status also has consequences for mental health (see Section 5.1 Mental Health). The role of pH in the retina is considered in a later section (see Section 4.2 The Sensory Systems).
4.1.2. Therapeutic relevance of acid-base balance
The link between pH and neuronal excitability is exploited in the anticonvulsant value of inhaled 5% CO2 to induce hypercapnic acidosis [114]. Hypercapnia also has a neuroprotective role in stroke, by inhibiting caspase and other cytotoxic activities [115], and during reperfusion [116]. CA inhibitors are used as adjunct therapies for epilepsy [117] and have potential application for treatment of neuropathic pain [118], Alzheimer’s Disease [119], and cognitive disorders [120]. However, MAc is a side effect of systemic CA inhibition [121]. Lowered seizure thresholds in some strains of ABT-null mice suggest that ABTs may be potential targets for anticonvulsant therapy. However, the need for caution is shown by the observation that, at least in the case of NBCn2-null mice, a reduced seizure-threshold does not mean reduced neuronal excitability [122]. The role of ABTs and CAs in CSF secretion hints at the potential for targeting of these proteins to lower intracranial pressure in idiopathic intracranial hypertension (IIH). The use of CA inhibitors in patients with IIH produces some symptom relief, but the mechanism of action is uncertain [123].
Regarding therapies for neurodegenerative diseases, histone deacetylase inhibitors have shown potential to release NHE6 from its epigenetic restraints to restore amyloid protein processing in ApoE4 mice [113]. Another strategy that has been proposed to have potential to reverse amyloid deposition in Alzheimer’s Disease is the raising of brain pH [112].
Therapies that target the peripheral nervous system are discussed in the following section.
4.2. The Sensory Systems
4.2.1. The importance of acid-base balance
4.2.1.1. Sight
Most ocular tissues express one or more ABT or CA for the purpose of maintaining fluid and pH balance. Perhaps the most therapeutically tractable tissue is the ciliary body that employs CAII and a range of ABTs to secrete HCO3 −-containing aqueous humor into the anterior chamber [124], [125] (Fig. 5 ). This fluid leaks into the corneal stroma to flush out metabolic wastes and is returned to the anterior chamber by corneal endothelial cells which express a similar array of ABTs including NBCe1, MCT1, and the H+ channel SLC4A11 [126], [127]. Finally, the fluid is drained from the anterior chamber via the trabecular meshwork. Individuals with mutations in NBCe1 have band keratopathy, glaucoma, cataracts, and corneal edema linked to fluid/pH imbalance in the cornea, lens, and elsewhere [128]. Individuals with mutations in SLC4A11 also exhibit corneal edema [146]. ABTs and HCO3 − are also important for retinal function [129], [130], [131], as suggested by the link between NBCn1 mutation and progressive rod-cone dystrophy [132], or retinal degeneration in mice with defective expression of NBCe2 and MCTs [133], [134].
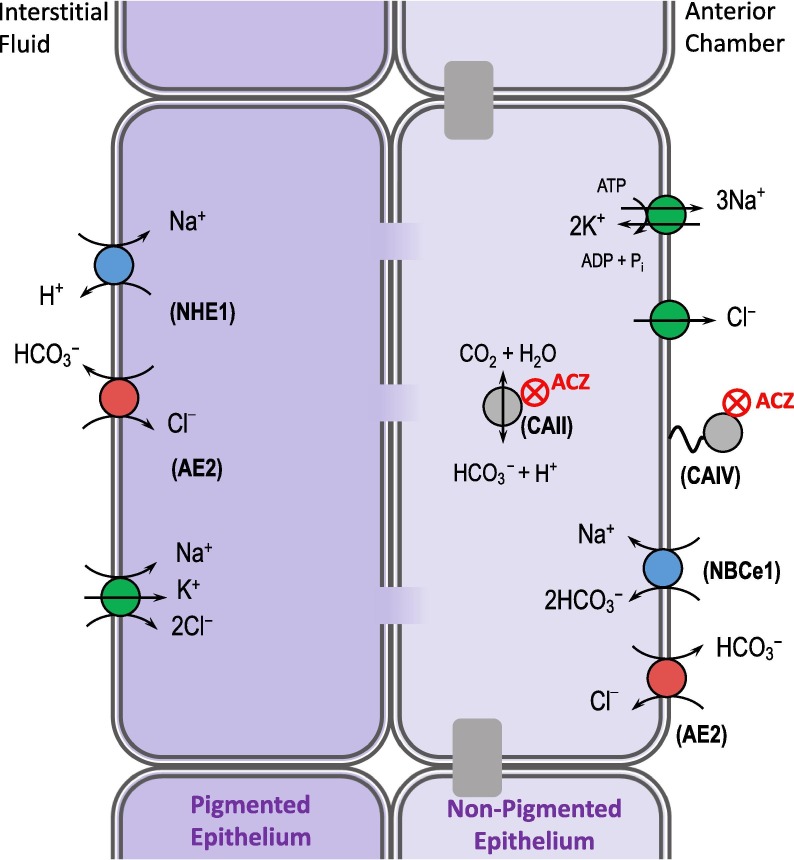
Fig. 5.
Targeting carbonic anhydrase to treat glaucoma. Glaucoma is retinal degeneration caused by increased intralocular pressure. Eye drops containing CA-inhibitors such as acetazolamide (ACZ) target CAs in the ciliary body and reduce the production of aqueous humor, lowering intraocular pressure. The ciliary body is a complex epithelial tissue comprised of two cell layers joined by gap junctions. A variety of ABTs and other transporters are required to move NaCl, which is followed by water, from the interstitial fluid into the anterior chamber of the eye. NHE1: Na+/H+ exchanger 1; AE2: Cl–/HCO3– exchanger 2; CAII: carbonic anhydrase II; CAIV: carbonic anhydrase IV; NBCe1: electrogenic Na+/HCO3− cotransporter.
4.2.1.2. Hearing and balance
Hearing loss is a symptom of several systemic diseases linked to defects in ABTs, including pendrin (Pendred syndrome [135]), the H+/K+-ATPase (distal renal tubular acidosis [136]), and SLC4A11 (Harboyan syndrome [137]). All of these ABTs are expressed in the inner ear where they help to maintain inner ear fluid pH and endocochlear potential [138]. Although a human correlate has not yet been reported, progressive hearing loss is also a feature of NBCn2-null mice [139]. Disruption of the H+-channel OTOP1 and the anion exchanger pendrin in mice is associated with malformation of the CaCO3 crystals (otoconia) that are essential for maintenance of balance [140], [141].
4.2.1.3. Taste
In addition to its role in the inner ear, OTOP1 is required for sour taste sensation [37].
4.2.1.4. Pain sensation
It is generally recommended to keep the pH of injected formulations close to physiological pH to avoid injection-site pain, with the added note that the inclusion of certain buffers may increase pain (hence new citrate-free formulations of adalimubab aka HUMIRA®) [142]. Low pHe exacerbates sensation of pain due to its effects on TRPV1 channel activation in nociceptive neurons. Furthermore, activation of these channels under acidotic conditions is associated with a drop in neuronal pHi that is mediated in part by a TRPV1-mediated H+ conductance [39]. Loss of pain sensation in children with Christianson Syndrome is associated with loss of NHE6 [143].
4.2.2. Therapeutic relevance of acid-base balance
4.2.2.1. Sight
CA inhibitors applied as eye drops have long been used to treat glaucoma by virtue of their ability to reduce the production of aqueous humor, although even their localized ophthalmic use has been documented to lead to the side-effect of systemic MAc in some prone individuals [144], [145]. Corneal edema that results from the expression of mutant misfolded SLC4A11 may be amenable to correction by small molecule folding chaperones [146]. NHE1 blockers are cytoprotective in a rat model of diabetic cataract formation and retinopathy [147]. Hypercapnia is protective against ischemia–reperfusion injury in the retina [148], as it is elsewhere in the central nervous system (see Section 4.1).
4.2.2.2. Hearing and balance
NaHCO3 solution is useful for softening and dispersing hardened ear wax [149]. However, we are unaware of any therapies specifically targeted to restoring the acid-base chemistry necessary for correct generation of endolymph or ostoconia. On a related topic there is one side effect of ear drops that pertains to acid-base balance. The acetic acid in some ear drops used to treat outer ear infection can be ototoxic because acetic acid can move across the round window into the inner ear, resulting in a drop in endocochlear potential (perhaps by acid inhibition of the Na+/K+-ATPase) as well as endolymph and perilymph pH [150].
4.2.2.3. Taste
We are unaware of any demonstrations of the usefulness of OTOP1 modulation in this area, but inhibitors of proteins that mediate bitter taste sensation have been used to mask bitter tastes, suggesting potential utility of OTOP1 blockers for masking sour tastes and increasing the palatability of sour-tasting medications [151], [152].
4.2.2.4. Pain sensation
Adjuvant NaHCO3 raises the pH of an injectable lidocaine solution and lowers perception of pain associated with lidocaine injection in one study, but the mechanism of the effect is uncertain [153]. See also Section 5.2 (Anesthesiology).
4.3. The Respiratory System
4.3.1. The importance of acid-base balance
Besides the increased respiratory drive to exhale CO2 in response to RAc [4] and the Bohr effect (see Section 4.4 The Circulatory System) the highest profile link between pH and respiration relates to the role of CFTR. Defects in CFTR are devastating because the Cl− and HCO3 − secretion that this channel normally mediates is a fundamental part of the mechanisms that drive fluid secretion in our bodies [154] (Fig. 6 ). The majority of deaths associated with CF are caused by respiratory failure [155]. In the lungs, fluid secretions are required to provide a moist surface for gas exchange, to liquefy mucus, and to flush inhaled particles and pathogens out towards the throat (mucociliary clearance). Besides the general importance of anion secretion, CFTR-mediated HCO3 − secretion plays a further role in pH homeostasis in the airway surface liquid (ASL); HCO3 − helps to unfold and hydrate mucus [156] and, by defending airway pH, has been hypothesized to promote a healthy local immune response to airway bacteria [157], [158]. HCO3 − secretion is modulated by epithelial H+ secretion, which is mediated by a host of acid-extruding transporters [159] (Fig. 6). Airway acidification is a feature of individuals with CF as well as those with asthma and tuberculosis [160], and is exacerbated by lactic acid production by airway pathogens and airway epithelia [161].
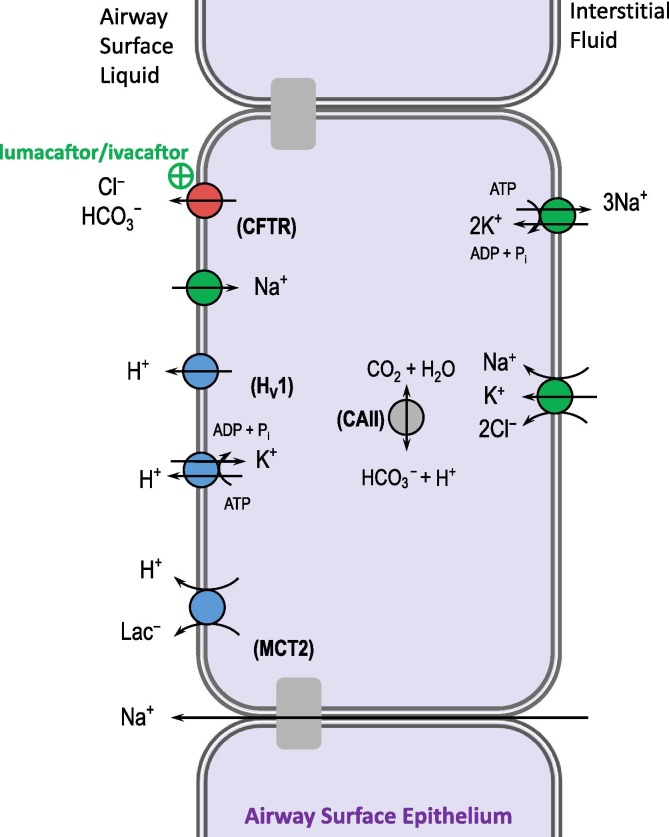
Fig. 6.
Enhancing fluid secretion in the lungs. The cystic fibrosis transmembrane regulator CFTR promotes the movement of HCO3−-containing fluid onto the airway surface to promote mucociliary clearance and lung health. Drugs such as lumacaftor/ivacaftor rescue this function in some individuals with cystic fibrosis (CF) by helping misfolded CFTR molecules to function normally. Alternative pH-based strategies have been suggested as adjunct CF therapy, such as blockade of the many H+-secreting acid-base transporters . Hv1: voltage-gated H+-channel; MCT2: mocarboxylate transporter 2; CAII: carbonic anhydrase II.
4.3.2. Therapeutic relevance of acid-base balance
The new, personalized CF therapies have focused on stimulation of defective CFTR to restore fluid secretion [162] (e.g., lumacaftor/ivacaftor, see Section 2.2.7), but are targeted to individuals with specific CF genotypes and thus alternative general therapies are still required. Strategies focused on correcting ASL pH include inhalation of nebulized bases such as NaHCO3 [163], [164] and THAM [165] as well as block of airway H+ secretion using H+/K+-ATPase inhibitors [166]. All of these strategies result in improvements in ASL pH and some also improve mucus viscosity and/or pathogen clearance. An in vitro study suggests that MCT2 blockade could also be protective of ASL pH in individuals with CF [161] by reducing epithelial H+ secretion. A newly described paracellular pathway for HCO3 − secretion by CF airway epithelia might also be amenable to therapeutic modulation [167].
4.4. The Circulatory System
4.4.1. The importance of acid-base balance
4.4.1.1. Heart
MAc is associated with reduced cardiac contractility. This phenomenon is explained by diverse mechanistic elements such as the pH-dependence of the channels and transporters that regulate Ca2+ handling in myocytes as well as the dampening effect of acidosis on the responsiveness of the contractile apparatus to Ca2+ [168]. Whether the heart rate is lowered by acidosis is harder to predict because of the complex effects of acidosis upon the sympathoadrenal system [169]. Intracellular acidosis in myocardial infarction after a period of ischemia, is countered, during reperfusion, by the action of acid-extruders such as NBCs and NHEs [170]. However, the accompanying Na+ load can be sufficient to reverse the action of the 3Na+/2Ca2+ exchanger, raising [Ca2+]i and increasing susceptibility to ventricular arrhythmias [171], [172]. Paradoxically, the loss of NBCe1 function can also result in Ca2+ overload because compensatory acid-extrusion mediated by NBCn1 and NHE1 imposes double the Na+ load per HCO3 − equivalent; a mechanism proposed to promote hypertrophy of cardiomyocytes in spontaneously hypertensive rats [173]. CA activity is also pro-hypertrophic [174]. On the other hand, the action of the acid-loading anion exchange AE3 is considered to be protective against hypertrophy [175]. Finally, NHE1 action in the mitochondria is proposed to contribute to mitochondrial damage in the diseased heart [176].
4.4.1.2. Vasculature
Typically, acidosis causes arterial vessels to dilate resulting in a fall in peripheral resistance, while veins may constrict [169]. It is perhaps then no surprise that numerous blood pressure traits are linked to polymorphisms in ABT genes [177]. At least at the level of the vascular response of arteries, NBCs and NHEs are required for normal vascular smooth muscle contractility and sensitivity to vasodilators [177]. However, blood pressure is a complex trait that is not determined by vascular response alone, so explanation of these linkages is not simple. Another important aspect is that MAc inhibits progression of vascular calcification [178].
4.4.1.3. Red blood cells
The Bohr effect describes the influence of pH and pCO2 upon the oxygen carrying capacity of hemoglobin. In systemic capillaries, metabolically produced CO2 enters the red blood cells (RBCs) where it is hydrolyzed into HCO3 − and H+ by the action of CAII. The newly produced HCO3 − is then extruded by AE1, causing a fall in RBC pHi which, describing the Bohr effect, reduces the Hb-O2 binding affinity, promoting O2 release from Hb to tissue (Fig. 7 A). The reverse process occurs in the pulmonary capillaries. Here, as CO2 leaves, RBC pHi rises thereby favoring O2 binding to Hb (Fig. 7B). Thus, acidosis enhances O2 delivery into tissues, but diminishes O2 loading in the lungs [169]. This relationship between pH and gas exchange is partly sensitized by the content of the hemoglobin-regulating molecule 2,3-DPG (diphosphoglyceric acid) in RBCs, a parameter which itself is pH-dependent; 2,3-DPG levels increase with chronic acidosis promoting O2 release [179].
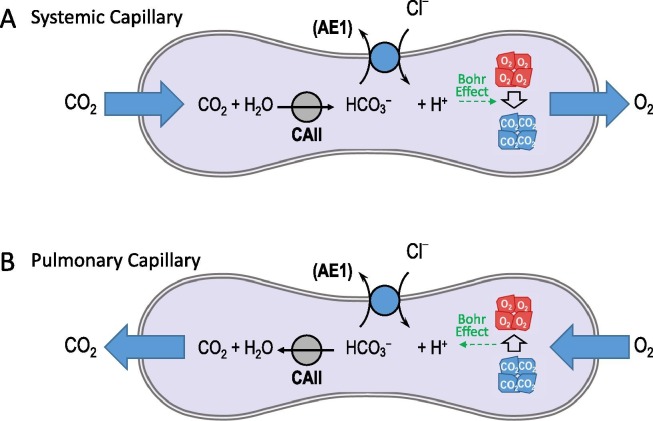
Fig. 7.
The Bohr Effect. The action of the Cl–/HCO3– exchanger AE1 and cytosolic carbonic anhydrase II (CAII) promote O2 release in systemic capillaries (panel A) and CO2 release in pulmonary capillaries because of the pH-dependence of the affinity of hemoglobin for O2 (the Bohr effect).
4.4.2. Therapeutic relevance of acid-base balance
4.4.2.1. Heart
Exogenous expression of skeletal muscle CAIII in mouse cardiomyocytes enhances defense of pHi and preserves cardiac function during MAc [180]. NaHCO3 is used to counter lactic acidosis in cardiac arrest and during prolonged cardiopulmonary resuscitation, but aside from its value at normalizing pre-existing MAc or hyperkalemia (acidosis promotes cellular K+ release), compelling data that this treatment improves outcomes are lacking [181], [182], [183]. NHE1 blockers have shown promise as cardioprotective agents in reperfusion injury [184] and likely act by targeting both plasma membrane and mitochondrial NHE1 [185], [186]. Although the NHE1 blocker cariporide caused serious side-effects in clinical trials (see Section 2.2.2), alternative approaches are available. For example, a microRNA that lowers NHE1 expression protects cardiomyocytes from apoptosis during prolonged endoplasmic reticulum stress [187]. In addition, antibodies and drugs that block NBCs have also demonstrated cardioprotective properties in animal models of ischemia reperfusion injury [188], [189]. Just as blockade of acid-extruders is cardioprotective, so too is the stimulation of the acid loader AE3. This has been achieved in cell models using the glycoside sasanqua saponin [190], an extract from a herb used in traditional Chinese medicine.
4.4.2.2. Vasculature
We are unaware of any reports of acid-base based therapies for blood pressure that directly target the vasculature, but a discussion of diuretics for lowering blood pressure in congestive heart failure is provided in Section 4.9 The Urinary System. Some alkali-containing therapies may enhance progression of vascular calcification [191] while use of the CA blocker ACZ has therapeutic value in calcifying disease [178], [192], perhaps by lowering pH.
4.4.2.3. Red blood cells
One study has cautioned the use of NaHCO3 in congestive heart failure because, in the face of adaptively elevated 2,3-DPG levels, a sudden rise in pH could result in a maladaptive increase in Hb-O2 affinity and risk of myocardial ischemia [193].
4.5. The Muscular System
4.5.1. The importance of acid-base balance
In skeletal muscles, the build-up of lactic acid during intense exercise correlates with muscle weakness and self-limiting fatigue. However, the contribution of lactic acidosis to those symptoms may not be as direct or major as once thought [194], [195]. Generalized acidosis may contribute to weakness via alterations in neuromuscular drive [196] and/or a decreased driving force for lactate efflux [197]. Regardless, acidosis promotes degradation of muscle protein [198]. A high estimated dietary acid load has been associated with frailty in elderly Japanese women [199]. Recovery from lactic acidosis is mediated by MCTs, NBCs, and NHEs [200], while CAIII specifically has been shown to play a role in defense from muscle fatigue [201].
4.5.2. Therapeutic relevance of acid-base balance
Many studies suggest the utility of NaHCO3 for improving exercise performance. For example, induction of MAlk by ingestion of oral NaHCO3 solutions has been shown to improve exercise endurance [202] and reduce perception of effort [203] in limited trials. However, taking a broader view of the field, the results of trials that link pH and exercise performance are deemed inconclusive due to inconsistent methodology and subgroup effects [204], [205]. It has also been suggested that any competitive benefits that could be gained from NaHCO3 administration, from an athletic viewpoint, may be outweighed by gastrointestinal side effects such as bloating [206]. Away from the arena, HCO3 − administration or a reduced dietary acid load could have value in maintaining muscle mass in older adults [207], [208].
4.6. The Skeletal System
4.6.1. The importance of acid-base balance
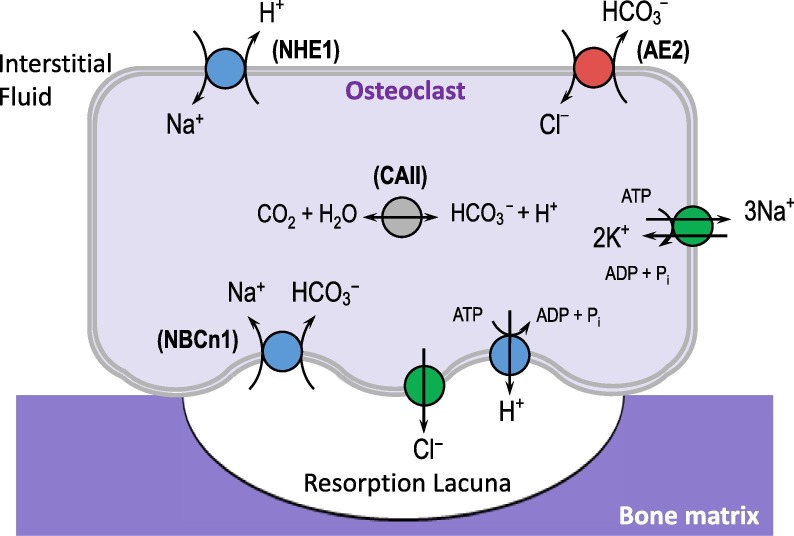
Mineralized material is eroded by acids as is evident in the case of tooth enamel, which is subject to demineralization by dietary acids (see Section 5.4 Oral Health). However acidosis also inhibits bone growth by inhibiting osteoblasts, stimulating the activity of bone-resorbing osteoclasts [209], and influencing hormonal axes [198], [210] (see also Section 4.11 about the effects of pH on the endocrine system). Accordingly, serum [HCO3 −] positively correlates with bone mineral density (BMD) [211] and negatively correlates with levels of serum parathyroid hormone (which promotes bone resorption) [212]. At a local level, the process of bone remodeling, as well as the hormonal mobilization of Ca2+ and Pi from bone, requires that osteoclasts secrete H+ onto the bone surface. These cells express intracellular CAII to generate H+ and HCO3 −, an apical v-type H+-ATPase to secrete H+ onto the bone surface, and basolateral AE2 to export HCO3 − and defend osteoclast pHi from alkalosis during H+ secretion (Fig. 8 ). Mutations in the v-type ATPase and CAII disable bone resorption by osteoclasts and are associated with increased bone density and osteopetrosis in humans [213], [214].
Fig. 8.
The role of acid-base transporters and carbonic anhydrases (CAs) in bone remodeling. Osteoclasts secrete acid onto the bone surface to resorb minerals during bone growth/remodeling and in response to hormonal requirements for release of mineralized Ca2+ and phosphate. One report suggested an off-target bone-sparing effect of CA inhibitors, used to treat glaucoma, in a group of post-menopausal women. NHE1: Na+/H+ exchanger 1; AE2: Cl–/HCO3– exchanger 2; CAII: carbonic anhydrase II; NBCn1: electroneutral Na+/HCO3− cotransporter.
4.6.2. Therapeutic relevance of acid-base balance
The acid-base regulating proteins of osteoclasts are amenable to pharmaceutical modulation and their blockade ought to be protective of osteoporosis. For example, AE2 may be a useful target for increasing BMD because BMD is elevated in AE2-null mice and cattle (reviewed in ref.[215]). Regarding CAII, one study showed a fortuitous bone-sparing effect in post-menopausal women who were chronic users of CA-inhibitors for glaucoma treatment [216]. Another study showed a paradoxical, but therapeutically valuable, BMD-lowering effect of CA inhibition in three children with sclerosing bone dysplasias. In these children, osteoclasts are already defective so the predominant effect of CA-inhibition is induction of chronic MAc which promotes bone resorption [217]. The therapeutic utility of PPIs to treat osteoporosis is negated by their negative influence on intestinal Ca2+ absorption [218]. In fact, several studies link PPI use with fracture susceptibility and low BMD (reviewed in refs. [219], [220]). Because of these side effects, PPIs are used with caution in some groups who may take them as antacid therapy [219], [220] (and see next section).
4.7. The Upper digestive System
4.7.1. The importance of acid-base balance
The three major health-related aspects of acid-base in this system are the roles of salivary HCO3 − in defense of enamel (which are discussed in Section 5.4 Oral Health), gastric acid secretion, and peptic ulceration with Helicobacter pylori. Gastric acid is required to activate digestive enzymes, stimulate downstream secretory processes, and to kill ingested pathogens. It is secreted by parietal cells using similar transport mechanisms employed by osteoclasts (described in the previous section). Thus, the secretion of acid across the apical membrane is mediated by a H+/K+-ATPase and is balanced by the extrusion of HCO3 − into the plasma via AE2 (the alkaline tide associated with feeding [59], [221], see Fig. 9). Stomach epithelia are protected from acid injury by a mucus lining. The pathogenic bacterium H.pylori is able to survive in gastric acid because it can take up urea from its environment, via a H+-gated urea channel, and convert it into ammonia (NH3) to neutralize acid in its immediate environment [222]. In the vicinity of the mucus layer, this action causes H.pylori to raise mucus pH, lowering its viscoelasticity, promoting bacterial infiltration, and ultimately resulting in inflammation, ulceration [223], and risk of gastric cancer [224]. Another condition, gastroesophageal reflux disease, is caused by reflux of gastric acid into the esophagus and can cause heartburn and, in severe cases, can lead to esophageal damage. Salivary HCO3 − plays an important role in esophageal acid defense [225], [226], [227] by neutralizing gastric acid [228]. Conversely, the action of NHE1 in esophageal epithelia may exacerbate the damage, perhaps by indirectly stimulating pro-apoptotic pathways [229].
4.7.2. Therapeutic relevance of acid-base balance
Acid reflux symptoms can be relieved by neutralizing gastric acid with antacids, which at their simplest are just bicarbonate or carbonate salts (e.g., TUMS® is calcium carbonate). However, an early antacid regimen for peptic ulcers, based on administration of milk and CaCO3 and still observed in the modern age in self-medicating individuals, results in adverse outcomes: the so-called ‘milk-alkali syndrome’ characterized by MAlk and hypercalcemia [230], [231]. An alternative approach to lowering gastric acidity is to use PPIs or H2-receptor agonists which dampen the signaling pathways that stimulate H+ secretion. H2 agonists, in addition, therapeutically lower the activity of esophageal NHE1 [232]. Some over-the-counter formulations combine these drugs with an antacid to lower the dose of each and minimize side effects of each such as bloating (from gastric CO2 generation) and osteoporosis (from chronic inhibition of intestinal Ca2+ reabsorption, see Section 4.6). Orally-dosed acid-chelators such as veverimer, also raise gastric acid pH [233] but have not been tested as a therapy for heartburn. The achlorhydric phenotype of Ae2-null mice suggests that AE2 blockage may have potential as a therapeutic target [234]. PPIs in combination with antibiotics are used to treat H.pylori infections: it has been proposed that raising stomach pH permits faster bacterial growth, potentiating the effects of antibiotics that act on dividing bacteria [235]. Inhibitors of the urease and H+-gated urea transporter of H.pylori are other potential therapeutic modalities that remain in development [236].
4.8. The Lower digestive System
4.8.1. The importance of acid-base balance
The exocrine pancreas secretes a HCO3 −-rich fluid that is vital for neutralizing gastric juices (chyme) passing into the duodenum. The alkaline pH of pancreatic juice holds digestive enzymes such as amylase and lipase in an inactive state until the secreted fluid is neutralized in the duodenal lumen by chyme, preventing damage to the pancreatic ducts. In CF, duodenal hyperacidity also holds pancreatic enzymes in an inactive state, but without the neutralization of chyme, they are not even active in the duodenum leading to malabsorption of nutrients such as lipids [237].
All along the intestine, HCO3 −-containing fluid secretions are required to promote gastric motility. The loss of this fluid in feces represents a substantial acid load. Consequently, CFTR mutations result in intestinal blockage [238] and secretory diarrhea can result in MAc [239]. Balancing the secretory processes, NHE3 and SLC26A3 act together to promote fluid reabsorption (Fig. 10 ). Accordingly, downregulation of intestinal NHE3 by the enterotoxigenic bacteria (E.coli and C.difficile) and inactivating genetic defects in NHE3 and SLC26A3 are all associated with hypersecretion and diarrhea [240], [241], [242], [243]. On the subject of gut microbiota, intestinal pH can both influence and be influenced by the composition of gut microbiome [244]. In individuals with insufficient intestine to absorb nutrients (short bowel syndrome), unabsorbed carbohydrates promote the growth of lactic acid-producing bacteria, which can lead to d-lactic acidosis [245]. Finally, the absorption of many nutrients depends on the action of H+-coupled ABTs (e.g., the H+-coupled oligopeptide transporters of the SLC15 family [246]), which in turn require the presence of acid extruders such as NBCe1 to maintain epithelial pH during H+-coupled nutrient absorption. Indeed, NBCe1-null mice exhibit defective nutrient absorption, which contributes to their general failure to thrive [247].
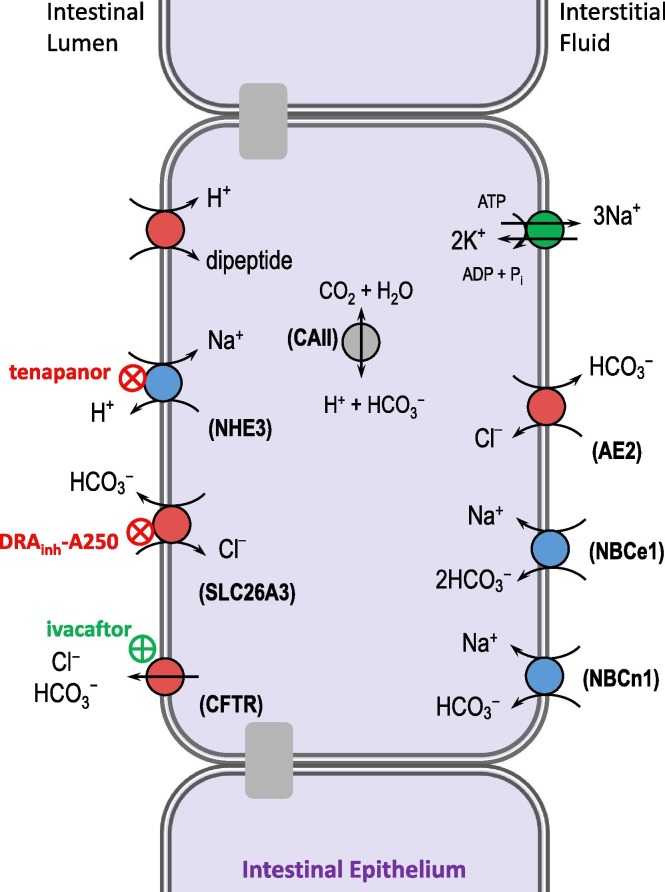
Fig. 10.
Targeting intestinal acid-base transporters to treat constipation. Intestinal fluid absorption is promoted by the combined action of the Na+/H+ exchanger 3 (NHE3) and the Cl–/HCO3– exchanger (SLC26A3), which perform the net uptake of NaCl, and therefore water. Inhibition of either, to reduce fluid absorption from the intestinal lumen is a useful therapy for irritable bowel syndrome with constipation. Ivacaftor is similarly useful in cystic fibrosis by restoring intestinal fluid secretion. CFTR: cystic fibrosis transmembrane regulator; CAII: carbonic anhydrase II; AE2: Cl–/HCO3– exchanger 2; NBCe1: electrogenic Na+/HCO3− cotransporter; NBCn1: electroneutral Na+/HCO3− cotransporter.
4.8.2. Therapeutic relevance of acid-base balance
The ability of small molecule inhibitors of NHE3 (tenapanor) and SLC26A3 (the 4,8-dimethylcoumarin drug “DRAinh-A250”) to reduce intestinal fluid absorption makes them valuable therapies for irritable bowel syndrome with constipation and for relief of constipation in CF [248], [249]. The CFTR corrector ivacaftor improves intestinal HCO3 − secretion and nutrient absorption in individuals with CF [250]. The mode of action of the anti-constipation drug linaclotide (LINZESS®: a guanylate cyclase C receptor agonist) encompasses both paradigms by promoting the cGMP-mediated reduction of NHE3 [251] and activation of CFTR activities [252].
4.9. The Urinary System
4.9.1. The importance of acid-base balance
4.9.1.1. Nephron function
The kidneys are vital to whole body pH balance (see Section 2 Acid-Base Homeostasis). It is the kidneys that generate HCO3 −, reabsorb HCO3 − from the glomerular filtrate to prevent its loss in urine, and excrete H+ in the form of NH4 + or titratable acids such as phosphate [253] (Fig. 2). Thus, it is no surprise that defects in renal transport mechanisms result in MAc. These acidifying diseases can be acquired or genetic. Fanconi syndrome is a degeneration of the proximal tubule, while renal tubular acidosis (RTA [254]) can result from mutations in acid-base transporters such as NBCe1 (type II proximal RTA: pRTA), H+-ATPase or AE1 (type I distal RTA: dRTA), CAII (type III RTA), or disruption of H+ secretion due to hypoaldosteronism (type IV dRTA). Chronic kidney disease (CKD) is also associated with MAc [255], and MAc itself promotes progression of CKD (see below).
MAlk is a common finding in CF patients. CF-model mice are less capable of defending against HCO3 − loads than their wild-type counterparts, due to downregulation of the renal HCO3 −-secreting anion exchanger pendrin [256] and presumably loss of direct CFTR-mediated HCO3 − secretion.
Concurrently, acid-base status has profound influence on kidney function. The various mechanisms that allow the kidneys to increase acid excretion in response to acute increases in acid load (e.g., ammoniagenesis and the renal endocrine response to acidosis) can be maladaptive in chronic MAc, leading to inflammation and fibrosis [257]. It is perhaps then not coincidental that low serum [HCO3 −] is linked to a higher risk of chronic kidney disease in both adults and children [258], [259]. An additional set of renal pathologies follows the integration of acid/base and salt/water handling by the nephron. For example, states and conditions of increased sodium reabsorption by the proximal tubule (e.g., volume contraction) or the collecting duct (e.g., hyperactivity of the epithelial Na+ channel ENaC in Liddle’s syndrome) result in MAlk (see Section 3.3 Metabolic Alkalosis) while hyperkalemia can cause MAc [260].
4.9.1.2. Stone formation
Urinary pH can influence stone formation which can lead to inflammation and obstructie kidney injury. A high urinary pH can cause the formation of calcium oxalate or calcium phosphate crystals, while a low urinary pH promotes uric acid crystallization [261]. Urinary pH can be modified by uropathogenic bacteria. Urease-expressing bacteria generate NH3, which can substantially raise urinary pH, promoting deposition of struvite and apatite crystals [262]. Besides the consequences of stone formation in the urinary tract, these deposits can cause the encrustation and blockage of indwelling catheters [263]. It is interesting to recall that the pathogenic action of another bacterium, H.pylori, in the stomach also depends on urease action (see Section 4.7).
4.9.2. Therapeutic relevance of acid-base balance
4.9.2.1. Nephron function
Many studies point to the value of correcting MAc for preserving the function of the failing kidney and slowing CKD progression [264], [265], [266]. We outlined corrective strategies based around alkali therapy in Section 3.2.2), but there is an additional prophylactic value in emergency settings. NaHCO3 infusion is protective against the kidney damage that can result from traumatic rhabdomyolysis (due to a crush injury), preventing development of MAc and tempering the renal toxicity of myoglobin [267], [268].
Another consideration related to therapies is that a number of drugs cause metabolic acid-base disturbances because they are nephrotoxic [269] or incidentally interfere with the kidneys' ability to excrete acid [55]. For example, CA inhibitors such as ACZ that are used as diuretics, due to their ability to interfere with fluid reabsorption, also cause MAc [270]. On the other hand, loop diuretic use can cause MAlk [271]. Another example is penicillin antibiotics which, acting as significant non-reabsorbed anions in the collecting duct lumen, promote hypersecretion of K+ and H+, resulting in hypokalemia and MAlk [272], [273].
Finally for this section, the ability of CF kidneys to secrete excess HCO3 − is restored by treatment with the CFTR-restoring drug-cocktail lumacaftor/ivacaftor (ORKAMBI®) [274].
4.9.2.2. Stone formation
Both citrate and low pH discourage the formation of calcium precipitates [275]. Thus, ingesting lemon juice, which raises urinary citrate while lowering urinary pH, decreases the propensity to form kidney stones and catheter-blocking deposits [276]. Dietary supplementation with citrate salts is also effective for this purpose because, despite resulting in a rise in urinary pH, the accompanying rise in urinary citrate increases the pH of crystal nucleation to an even higher pH value [277], [278]. Urinary pH can also exert a meaningful influence on drug excretion as discussed later (see Section 6.5).
4.10. The Reproductive System
4.10.1. The importance of acid-base balance
At this point in our review we have presented ample evidence that ABTs are necessary to sustain life, and now we will see that they are also necessary to create new life. In the male reproductive tract, H+ secretion by clear cells in the tail of the epididymis is required to maintain an acidic luminal pH for storage of sperm [279]. HCO3 − secretion along the length of epididymis is necessary to functionally activate sperm before ejaculation [280] and prevent their inactivation by the acidic vaginal environment (discussed later). Indeed low levels of HCO3 − are associated with lowered sperm motility [281]. In the female reproductive tract, endometrial epithelial cells further secrete a HCO3 −-rich fluid that is necessary for sperm capacitation and fertilization [282]. Furthermore, the secretory phase of the uterine cycle is associated with a dramatic rise in the pH of the oviduct lumen, corresponding with a level of HCO3 − that is sufficient to promote thinning of mucus during ovulation, favoring sperm mobility [283], and to promote dispersal of the egg-surrounding corona cells to allow fertilization [284]. HCO3 − is even a prerequisite for the acrosome reaction [285], by virtue of its ability to stimulate soluble adenylyl cyclase to produce cAMP and initiate requisite signaling cascades [286]. Finally, once fertilization has occurred, acidification of uterine fluid is a necessary prerequisite for embryo implantation [287]. Numerous ABTs are involved in these processes; for example, loss of AE2, SLC26A3, CFTR, or NHE8 are all associated with infertility or reduced fertility in male mice [282], [288], [289], [290]. The acidic pH of the vagina noted earlier is caused by the metabolic activity of lactobacilli and serves to defend against sexually transmitted disease pathogens. Loss of the acidity in bacterial vaginosis is associated with increased susceptibility to infection by sexually-transmitted diseases [291].
With regard to ultimate reproductive success, maternal-fetal acid-base balance is an important determinant of perinatal outcomes [292]. For example, obstructed labor has poor outcomes due to intermittent hypoxia and lactic acidosis [293].
4.10.2. Therapeutic relevance of acid-base balance
Because vaginal acidity tends to dampen sperm motility, vaginal douching with NaHCO3 improves fertility [294], [295]. Conversely, vaginal acidification is contraceptive and prophylactic. The spermicidal properties of lemon juice have long been appreciated [296]. Phexxi™ (formerly known as ACIDFORM: [297]), is the most recent of a series of acidic contraceptive gels. Phexxi™ is a vaginally-applied gel of lactic acid, citric acid, and potassium bitartrate that is indicated by the FDA to prevent pregnancy [298]. An alternative, ‘BufferGel’, utilizes an acidic polymer for the same purpose [291]. By lowering vaginal pH these products also confer microbicidal benefits [291]. Finally, some ABT-targeted drugs interfere with fertility: CA inhibitors, for example, prevent dispersal of corona cells [299] and PPIs inhibit sperm motility [300].
Finally, with regard to childbirth, peri-operative NaHCO3 infusion has been proposed as a possible measure to improve outcomes in obstructed labor [293].
4.11. The Endocrine System and Metabolism
4.11.1. The importance of acid-base balance
Many metabolic reactions that consume or generate acids and bases can, in disease, result in MAc. The liver makes important contributions to acid-base balance by consuming lactate (countering lactic acidosis), generating albumin (a weak acid: hypoalbuminemia is associated with MAlk) and producing keto acids (contributing to diabetic ketoacidosis) [301]. Furthermore, acid-base status impacts the activity of the enzymes that constitute several key metabolic pathways. For example, acidosis inhibits glycolysis [302] and lipolysis [303] but stimulates gluconeogenesis [304], [305]. The ability of disturbed acid-base balance to interfere with glycemic control is further evidenced by the following observations: (i) acidosis and alkalosis both lower glucose-stimulated insulin release from pancreatic islets [306], (ii) the HCO3 − content of plasma correlates with insulin solubility [307], (iii) acidosis is associated with decreased insulin sensitivity [308], [309]. This latter observation is due in part to the pH-sensitivity of the interaction between insulin and its receptor: a bell-shaped pH-dependence that exhibits strongest binding at pH ~ 8.0 [310]. In fact, insulin can also influence ABT action. For example, insulin promotes renal NBCe1 activity. In type 2 diabetes the resulting pathological increase in renal fluid absorption caused by NBCe1 upregulation is thought to contribute to hypertension [311].
Other hormones that influence acid-base balance include secretin (increases pancreatic HCO3 − secretion in response to duodenal acidity [312]), angiotensin II and aldosterone (increases renal acid excretion in acidosis [84], [313]), and parathyroid hormone (increases renal acid excretion and excretion of urinary-buffer phosphate in acidosis [212]). Interestingly, licorice can cause MAlk because one of its constituent compounds (glycyrrhizic acid) indirectly causes overstimulation of the aldosterone receptor [314]. Hormones whose levels are pathologically altered in acidosis include aldosterone and endothelin (increased [315], [316]), cortisone (increased [317]), and IGF-1 (decreased [318]).
Metabolic reprogramming of cancer cells in the hypoxic and acidotic tumor environment is discussed further in a later section (see Section 5.6 Oncology.) The influence and therapeutic relevance of pH upon endocrine and metabolic aspects of heart function, muscle mass, and bone growth have been discussed in earlier Sections (4.4, 4.5, 4.6).
4.11.2. Therapeutic relevance of acid-base balance
The link between dietary acid load and development of insulin sensitivity has made dietary control an appealing target for lowering the incidence of type 2 diabetes, although overwhelming evidence of efficacy is currently lacking [308]. Because of the pH-dependence of insulin solubility, the dissolution time of administered insulin is slower in plasma from diabetics with DKA than in otherwise normal plasma, suggesting at face value that combined insulin-bicarbonate therapy might be valuable [319]. However, such treatment in practice may be of limited value and has been linked to development of cerebral edema in children [320]. The influence of pH on drug solubility is discussed in more detail in Section 6.2.
4.12. The Immune System
4.12.1. The importance of acid-base balance
pH plays a role in all aspects of the immune response. First we will enumerate some broadly applicable effects: (i) The cytosolic alkalinization that promotes cytoskeletal rearrangement during neutrophil spreading (the morphological change that is important for capillary adhesion and extravasation) requires NHE1 action [321]. (ii) Inflammatory sites are usually acidic due to the metabolic activities of invading bacteria and neutrophils, an environment that promotes the production of proinflammatory cytokines [322] (see also Section 4.3). (iii) During bacterial killing, H+ efflux into the phagosome is necessary for charge compensation during the respiratory burst that produces cytotoxic superoxide anions. This action is mediated by the voltage-gated H+ channel HV1 [323]. (iv) Some of the generated superoxide is converted into cytotoxic hypochlorous acid (HOCl) which is able to diffuse back into the neutrophil cytoplasm. Thus, the action of phagosomal HV1, together with the action of NHE1 in the plasma membrane, defends the neutrophil cytoplasm from acidosis which would otherwise dampen NADPH oxidase activity [321], [324], [325].
Although the pH sensitivity of individual immune cell types are well characterized in vitro (e.g., acidosis decreases leukocyte and neutrophil mobility [326], promotes complement activation [327], modulates expression of inflammatory mediators [326]), there are many subtleties to these effects. For example, the type of acidosis, type of cell, activation state of the cell, and effects on phagocytic activity versus migration may all influence the effect of acid-base disturbance on the immune response mediated by a given cell type [328]. Thus, for example, acidosis enhances bacterial killing by neutrophils [329] and leukocytes [330] but not by macrophages [331]. Although it is complicated to tease out a set of concerted mechanisms, in general, systemic acidosis is associated with compromised immune function [328], [332], [333].
The link between pH and disturbed immune response is demonstrated by the following example. Single nucleotide polymorphisms in the acid-loading protein AE2 are linked to progression of primary biliary cholangitis. A mechanism is suggested by studies of Ae2-null mice in which loss of AE2 from cytotoxic T cells causes a pHi increase that promotes their proliferation, activation and survival, amplifying the autoimmune response against damaged liver cells. Furthermore, type IV dRTA is linked to systemic lupus erythematosus, although the causal relationship is unclear [334], [335].
4.12.2. Therapeutic relevance of acid-base balance
Several paradigms have been proposed to suppress a pathological immune response. Oral NaHCO3 dosing in rats stimulates an anti-inflammatory response; this effect could be harnessed to prevent tissue damage in autoimmune diseases such as rheumatoid arthritis [336]. One preliminary study in mice even suggests that oral NaHCO3 dosing could be useful to suppress peanut allergy [337]. Regarding the pharmacological aspect, MCT1 inhibitors act as immunosuppressors by interfering with the disposal of lactate during T-cell activation [338]. Finally, the NHE1 blocker cariporide suppresses the systemic immune response to burn injury in rats, although the mechanism is unclear [339].
On the other hand, knowledge of acid-base balance can be exploited to promote an immune response. It has been suggested that blocking AE2 to promote a stronger cytotoxic T-cell response could be useful for treatment of chronic infections [333]. Considerations about the role of pH in cancer immunotherapy are included in Section 5.6.
5. Other Applications by Clinical Specialty
5.1. Mental Health
5.1.1. The importance of acid-base balance
Neuronal activity is pH dependent (see Section 4.1) and there is emerging evidence that agents of acid-base balance can influence behavior and progression of neuropsychiatric disorders. Inhalation of CO2 (despite its general dampening action on neuronal excitability, see Section 4.1.2) invokes anxiety and panic, and the influence of CO2 is exacerbated in individuals with panic disorders [340]. Studies in mice indicate that lowering brain pH triggers the action of the acid-sensing ion channel ASIC1a in the regions of the brain responsible for stress, fear, and social behavior [341], [342]. The acquisition of fear-related freezing behavior in mice is enhanced by ASIC1a overexpression [343] and dampened by ASIC1a disruption [342]. In humans, decreased brain pH is also associated with schizophrenia, bipolar disorder, and autism spectrum disorder [344]. One study even suggests that a high dietary acid load correlates with incidence of emotional problems and hyperactivity in young children, but causality could not be established as it could not be discounted that behavior influenced dietary habits [345]. In terms of the linkage between ABTs and neuropsychiatric disorders, SLC4A4 is a biomarker (both in terms of incidence of a specific single nucleotide polymorphism and in terms of reduced expression determined by microarray) of suicide ideation and completion, especially with bipolar disorder [346]. Although the mechanistic details of the linkage are unknown, the role of NBCe1 in controlling of brain pH is likely to be relevant. Perhaps also of tangential relevance, due to its impact upon systemic acid-base balance [347], is that estimated glomerular filtration rate in CKD, which typically correlates with ability to excrete acid, is inversely correlated to depressive symptoms and suicide ideation [348].
5.1.2. Therapeutic relevance of acid-base balance
The anxiety response to CO2 inhalation is a useful clinical test to follow the effectiveness of treatments for panic disorders [349]. The response itself can be quelled by ACZ [350] but, as this is just a model of panic disorder, the use of CA blockers to treat actual panic disorders is unclear. The above-mentioned studies of ASIC1a-null mice suggest that ASIC1a inhibition could be therapeutic for panic disorder. Regarding the link between SLC4A4 and suicide, it is unclear how modulating NBCe1 activity might influence depression, although detection of the biomarker could help to identify high risk individuals and thereby inform therapeutic strategies [346]. Finally, it has been suggested that antipsychotic medications could contribute to lactic acidosis and be partly responsible for decreased pH in the brains of individuals with schizophrenia [351].
5.2. Anesthesiology
5.2.1. The importance of acid-base balance
As mentioned previously, plasma pH depends on adequate ventilation to exhaust CO2 thus mechanical ventilation can induce respiratory acid-base disturbances. Another important aspect for consideration in this section is that the bioavailability of anesthetic agents can be influenced by acid-base status.
5.2.2. Therapeutic relevance of acid-base balance
When using mechanical ventilation, two important considerations, related to acid-base balance, must be raised. The first consideration relates to low-flow anesthesia or closed-circuit rebreathing systems that return exhaled anesthetic gas mixtures. In these systems CO2 must be removed from the recirculated air. For this purpose, CO2 scrubbers are used to adsorb CO2. The archetypal scrubber is soda lime, a mixture of NaOH and Ca(OH)2, which reacts with carbonic acid to form an insoluble CaCO3 precipitate (although a number of other technologies are available [352], [353], see also Section 3.4.2). The choice of technology can be important as many CO2 scrubbers can have undesirable reactions with anesthetic gases, producing toxins such as carbon monoxide [354]. The second consideration is for individuals in which low-tidal-volume ventilation is indicated, such as those with acute respiratory distress syndrome or COPD. These individuals may be deliberately under-ventilated to prevent mechanical stress on the lungs. Thus, patients are maintained in a state of compensated RAc called “permissive hypercapnia”. Under some circumstances, such as in critically ill patients, this hypercapnic state may be protective due to its anti-inflammatory influence [355] (see also Section 4.12 The Immune System).
Acid-base balance concerns are not limited to inhaled anesthetics. Prolonged infusion with the intravenously administered anesthetic propofol can cause severe lactic acidosis [356]. The influence of pH on the pharmacokinetics of drugs in general is discussed in Section 6.
5.3. Surgery
5.3.1. The importance of acid-base balance
Perioperative interventions have the potential to disturb acid-base homeostasis with negative consequences for outcome. Post-operative MAc is a well described but complex phenomenon related to issues including lactate accumulation in poorly perfused tissues and hyperchloremic acidosis due to dilution/displacement of HCO3 −-containing plasma by infused saline [357], [358], [359], [360]. Pre-operative acidosis has also been described and has been linked to stress and fasting [357]. Post-operative MAlk has also been described following general surgery and has been linked to the infusion of citrate-buffered plasma [361], [362]. Peri-operative MAlk is linked to the removal of stomach acid by nasogastric suction. Poor outcomes have been associated with both post-operative MAc [363] and MAlk [361], although this is not a universal finding [357].
On a related theme, the usefulness of stored blood for transfusion can be compromised by numerous storage lesions including low pH that follows anaerobic metabolism by stored cells [364], [365]. Finally, an acidic tissue environment appears to favor natural wound healing, yet alkaline pH favors the success of skin grafts [366].
5.3.2. Therapeutic relevance of acid-base balance
Altering the chemistry of infused fluids is the obvious strategy to counter post-operative acid-base disturbances. With specific regard to post-operative MAc, dichloroacetate treatment tempered the pathological rise in lactate following liver transplantation, although no effect on outcome was observed [367]. Other treatments for MAc are discussed in Section 3.2.2. Treatments for MAlk are discussed in Section 3.3.2. Finally, a study in mice suggests that raising the pH of stored blood enhances RBC survival after transfusion [368]. The pH-dependence of wound healing suggests that therapeutic maintenance of the pH of the wound or graft could aid healing [366].
5.4. Oral Health
5.4.1. The importance of acid-base balance
HCO3 − is a major buffer in stimulated saliva [369], defending oral pH against acidic foods and drinks and against those acids produced from sugars by acidophilic bacteria in the oral cavity. Acid defense protects enamel from erosion and oral pH is also an important determinant of a healthy oral microbiome; low salivary flow and pH generally encourage the presence of pathogenic and cariogenic bacteria (e.g., [370], [371]). As a consequence, low salivary pH, [HCO3 −], and/or buffer capacity are predictors of cavity formation [372], [373], [374]. Salivary pH is lowered in many groups of individuals such as patients undergoing chemotherapy for head/neck cancer [370], cocaine users [375], or tobacco smokers [376]. Low salivary pH is also described in individuals with diseases such as CF [377], Sjögren’s syndrome [378], and juvenile idiopathic arthritis [379]. Not all studies report a major impact on dental health in these cases as compensating factors may be in play [379]. ABTs and CAs play important roles in salivary secretion as well as in enamel formation [312], [380]. For example, defective dentition is a feature of some individuals with NBCe1 mutations [381] and appears not to be a secondary consequence of acidemia [382].
5.4.2. Therapeutic relevance of acid-base balance
Salivary pH is a useful biomarker for oral health [383] and the usefulness of baking soda in oral hygiene was first suggested as long ago as 1911 [384]. NaHCO3 delivery either as a mouth wash [385], mucoadhesive spray [386] or sugar-free gum [387] raises salivary pH and, in some cases, may lower colonization of acidophilic bacteria. In individuals undergoing chemotherapy for leukemia, use of a NaHCO3 mouthwash lowered susceptibility to mouth ulcers [388]. In smokers a similar treatment reduced levels of the inflammatory biomarker IL-1β [376]. NaHCO3-containing dentifrices have been shown to be effective at neutralizing plaque pH [389], enhancing plaque removal [390], and inhibiting formation of caries [391]. pH-stabilizing resins used in restorative dentistry have also been suggested to be useful cariostatic by releasing OH− [392]. Finally, reducing dietary intake of sugars is also beneficial to oral pH because it limits the acidification that can be caused by acidophilic bacteria. Hence sugar-free gum potentiates the effect of stimulated saliva secretion at raising plaque pH [393].
5.5. Infectious Disease
5.5.1. The importance of acid-base balance
We have dealt with various aspects of acid-base-related medical microbiology in earlier sections (see sections on 4.3, 4.7, 4.8, 4.10, 4.12, 5.4). In this section we will confine our considerations to viruses and parasites, using influenza and malaria as examples.
5.5.1.1. Influenza
Viral infection results in intracellular acidification due to the increased glycolytic rate of infected cells and the release of H+ from the endosomes of infected cells (via the viral M2 H+-channel). Both of these mechanisms are necessary to support viral replication. In defending pHi against the increased acid load, infected cells extrude H+, creating a concomitant acidification of pHe at the cell surface [394].
5.5.1.2. Malaria
There are multiple acid-base-related aspects to the malarial lifecycle and its pathological impact. First of all, mosquitos are attracted by CO2 , and CO2 sensitizes them to human odors [395]. Secondly, the erythrocytic phase of malaria infection requires that Plasmodium invades RBCs; one of the surface antigens that Plasmodium exploits for host-cell recognition and invasion is the AE1-glycophorin A complex. Consequently, Ae1-null mice are immune to infection [396] as are individuals with an AE1 defect that causes the abnormal red cell morphology (South East Asian Ovalocytosis, SAO) [397]. Finally, malarial infection causes numerous metabolic disturbances such as MAc that, in part, follows the tissue hypoxia and hyperlactemia caused by blocked microvasculature. MAc is a strong prognosticator of fatal outcome in infected individuals [398], [399].
5.5.2. Therapeutic relevance of acid-base balance
5.5.2.1. Influenza
M2 H+-channel blockers have potential to target influenza strains that are resistant to currently available antiviral treatments [400]. On the other hand, the use of PPIs may increase susceptibility to viral infection in the gastrointestinal tract by neutralizing the stomach acidity that typically destroys viral particles [401].
5.5.2.2. Malaria
Lactic acidosis in malaria can be ameliorated by dichloroacetate treatment [402]. Malarial resistance in individuals with SAO suggests that transfusions with SAO blood may be a useful therapy for individuals infected with drug-resistant Plasmodium [403]. Finally, the parasite’s own ABTs are a potential target for antimalarial action [404].
5.6. Oncology
5.6.1. The importance of acid-base balance
The rapid proliferation of cancerous cells is associated with the Warburg effect (also known as ‘aerobic glycolysis’): a shift from aerobic to anaerobic metabolism even in the presence of oxygen [405], [406]. In this state, cells increase their ATP production by increasing their rate of glucose uptake with consequent-increased lactate and H+ production. Because of their high metabolic rates, such cells would tend to have a much lower pHi than normal cells. However, cancer cells are able to maintain near-normal pHi by upregulating acid-extruding ABTs such as NBCe1, NBCn1, NHE1, H+-ATPase, and MCT4 to facilitate the removal of H+, as well as extracellular CAs (CAIX, CAXII) to facilitate the removal of CO2 [407], [408], [409], [410], [411] (Fig. 11 ). Aquaporins (AQPs)—which can serve as a conduit for transmembrane movements of CO2 [412]—also promote tumor growth and survival, but it is not clear that promotion of CO2 removal is a major part of their pathological importance [407], [413], [414]. As shown in cancer cell-lines [415], acidosis can also increase the drive on the TCA cycle (promoting ATP production for H+ extrusion) and the pentose phosphate pathway (promoting NADPH production to counter ROS, promoting cell survival) [415]. The combined action of these processes results in a drastic reduction in local pHe. These changes allow cancer cells both to outcompete neighboring non-cancer cells and to mobilize and spread to other parts of the body. Metastasis and tumor survival are further enhanced by acid-dependent remodeling of the extracellular matrix [416] and suppression of anti-tumor immune responses [417] (see also Section 4.12 The Immune System). It is noteworthy that a high level of net endogenous acid production was associated with higher mortality in breast-cancer recurrence in a cohort of early stage breast cancer survivors [418]. Moreover, acid-treatment of melanoma cells selects for more invasive phenotypes [419] and the extent of upregulation of various ABTs and CAs can be an adverse prognostic factor (e.g., [420], [421], [422]).
Fig. 11.
The role of acid-base transporters (ABTs) and carbonic anhydrases (CAs) in cancer. Numerous acid-base handling proteins are upregulated in rapidly proliferating tumor cells to help them dispose of metabolic acids and create an acidic microenvironment that disadvantages non-tumor cells in their vicinity. As most of these ABTs and CAs are gainfully expressed elsewhere in the body, therapies in development are focused on blocking those rarer targets that are preferentially expressed in the hypoxic tumor environment such as the monocarboxylate transporter MCT4 and CAIX. Other approached include exploiting the acidic milieu of the tumor for the targeted delivery of chemotherapeutic drugs (see Section 6.6). NBCe1: electrogenic Na+/HCO3− cotransporter; NBCn1: electroneutral Na+/HCO3− cotransporter; NHE1: Na+/H+ exchanger 1; pHe: extracellular pH.
5.6.2. Therapeutic relevance of acid-base balance
The importance of acid-base balance in cancer has suggested that targeting tumor pH could be a valuable adjuvant therapy. The three main therapeutic modalities discussed below are: (i) interfering with the ability of cancer cells to defend their pHi, (ii) interfering with the ability of cancer cells to create an acidic extracellular environment, and (iii) bolstering the immune response in an acidic tumor microenvironment.
5.6.2.1. Interfering with pHi defense
Blocking the defense of pHi by cancer cells can be achieved by inhibiting acid-extruding ABTs and/or CAs [410], [423]. The value of these approaches is demonstrated in diverse studies that report anything from delayed tumor-growth in NBCn1-null mice [424] to positive clinical outcomes with adjuvant use of -PPIs [425]. However, reports from clinical trials are currently sparse. Given the potential for side effects due to the physiological importance of these proteins in all organ systems, much attention has focused on inhibiting those proteins, such as CAIX, which are specifically upregulated in cancer cells (in response to hypoxia) and which are not abundantly expressed elsewhere [426]. Another approach, the combinatorial use of blockers, allows for a lower dose of each and thus fewer undesired effects on non-tumor cells. The use of a combination of five compounds, each of which targets a different ABT/CA, revealed effectiveness at reducing intracellular brain tumor acidification in mice and the consequent activation of the pro-apoptotic marker caspase-3 in tumor cells, with little negative effect on non-tumor cells [427]. Interestingly, the potency of such approaches is enhanced by glucose loading, which increases the acid-load in these glycolytic tumor cells [428]. Finally, as different ABTs/CAs are upregulated in different tumor types, generic approaches are also valuable to consider. For example, a number of anticancer drugs, such as salinomycin, that are not known to specifically target ABTs or CAs, also promote cellular acidification [423].
5.6.2.2. Interfering with extracellular acidification
Anecdotal evidence suggests that interfering with extracellular acidification might be achieved by dietary means [429]. Indeed, oral NaHCO3 supplementation can raise tumor pHe and inhibit metastasis in mice [430], but the overall consequence is complex, with one recent study suggesting that such therapy may confoundingly promote tumor proliferation [431]. However, the addition of adjuvant NaHCO3 to a chemotherapeutic agent that was fed directly into a hepatic tumor via catherization of tumor-feeding arteries (TILA-TACE: targeting intra-tumoral lactic acidosis with trans-arterial chemoembolization) was associated with markedly improved outcomes [432]. In short, there is currently insufficient data to show that lowering dietary acid load improves outcomes, except by virtue of the association of such diets with general well-being [433] and the role of alkalinization in enhancing the safety of certain chemotherapeutic drugs [434]. The acidic tumor microenvironment can also be exploited to promote local drug delivery as discussed in Section 6.6.
5.6.2.3. Bolstering antitumor immune response
The acidic tumor microenvironment promotes an anti-inflammatory T cell response (see Section 5.6.1), but the deletion of AE2 promotes a pro-inflammatory T cell response (see Section 4.12.1). Therefore inhibition of T cell AE2 could be a valuable paradigm for enhancing a cytotoxic immune response [435].
6. pH-dependent Aspects of Pharmaceutical Therapy
6.1. Introduction
In this section, we will focus on the influence of pH upon pharmacokinetic properties of drugs. We will also consider how all of these pH-related properties can be harnessed to therapeutic advantage, particularly in diseases associated with acid-base imbalance. Oral drug delivery is the most common and convenient method used to administer drugs whereby they can either directly access their targets or be absorbed into circulation to reach their intended targets. Thus, oral drug delivery will serve as our paradigm for discussion. However, these concepts are also relevant to other, parenteral, methods of drug delivery such as inhalation of nebulized substances and injection into subcutaneous, intramuscular, or intravenous compartments.
Gastrointestinal (GI) pH is one of the major determinants of oral drug bioavailability as it affects various properties including solubility and dissolution rate, stability, and absorbability. pH varies drastically along the GI tract [436], [437], [438]. Starting from a value of ~ 1.5 in the stomach, it rises to ~ 6.0 in the duodenum, reaches ~ 7.4 in the terminal ileum, and falls again to ~ 6.7 in the colon [438]. An additional consideration is that these values can vary with age, presence of food, diseases, and also by the co-administration of other drugs [439], [440], [441], [442]. Finally, we note that the influence of pH on bioavailability could, in pH-disturbed states, result in underdose or overdose.
6.2. The Influence of pH upon Drug Solubility and Dissolution
Most drugs are weak acids and bases. The relationship between the drug ionization constant (pKa) and the pH of the environment to which the drug is exposed (hereafter referred to as the environmental pH) is a critical determinant of drug solubility and dissolution rate in aqueous compartments [443]. This relationship determines the ratio of the concentration of the unionized (i.e., uncharged) form (U) to the concentration of the ionized (i.e., charged) form (I) of the drug and is described by the Henderson-Hasselbalch equation.
For a weakly acidic drug (HA, HA A− + H+), the Henderson-Hasselbalch equation states that
| (2) |
Rearranging Eq. (2), the ratio of the unionized form vs the ionized form is
| (3) |
Thus, according to Eq. (3),
-
1.
if pH < pKa (i.e., 10pKa−pH > 1), the unionized form U (i.e., the acidic neutral form HA) prevails;
-
2.
if pH > pKa (i.e., 10pKa−pH < 1) , the ionized form I (i.e., the conjugate weak base A−) prevails.
Similarly, for a weakly basic drug (B, BH+ B + H+), the Henderson-Hasselbalch equation states that
| (4) |
and
| (5) |
Now, according to Eq. (5),
-
3.
if pH < pKa (i.e., 10pH−pKa < 1), the ionized form I (i.e., the acidic conjugate weak base BH+) prevails;
-
4.
if pH > pKa (i.e., 10pKa−pH > 1) , the unionized form U (i.e., the basic neutral for B) prevails.
Thus, because the ionized form I is more water-soluble than the unionized form (due to better solvation between the ionized form I and the dipole of the water molecule), weakly acidic drugs have higher solubility at high pH whereas weakly basic drugs have higher solubility at low pH. This means that weakly acidic drugs tend to dissolve more easily in the intestine whereas weakly basic drugs tend to dissolve more easily in the stomach. Because drug solubility is pH-dependent, the dissolution profile of the drug (i.e., the process by which a solid drug dissolves into solution) may also be pH-dependent [440], [443]. Drug solubility and dissolution may be enhanced by several techniques including (i) chemical derivatization to alter drug pKa, (ii) administration of the drug in ionic form (as a salt) rather than as a free acid or base, or (iii) alteration of environmental pH by the adjuvant use of acids or bases to better match the pKa of the drug [444], [445], [446]. In some cases, it may be desirable to lower the solubility and dissolution of parenterally-administered drugs in order to prolong their half-life.
As we discuss in the next section, the influence of drug pKa and environmental pH on drug ionization has important ramifications for drug absorption and distribution.
6.3. The Influence of pH upon Drug Absorption and Distribution
Besides solubility in aqueous solvent, the ionization state of a drug can influence its solubility in the lipid phase (e.g., drug pKa can be modified to increase drug polarity and hence reduce drug lipophilicity) and therefore affect its ability to permeate cell membranes [444], [447].
Assuming that passive diffusion (i.e., transmembrane concentration gradient is the driving force) is the mechanism by which a drug moves across a membrane and that only uncharged ions can freely diffuse across the membrane, we can turn again our attention to the Henderson-Hasselbalch equation to describe the importance of drug pKa and environmental pH to drug absorption and distribution between body compartments. We note that, according to point 1) above, the Henderson-Hasselbalch equation predicts that weak acids tend to be absorbed in an acidic environment (e.g., in the stomach). Similarly, according to point 4) above, the Henderson-Hasselbalch equation predicts that weak bases tend to be absorbed in a basic environment (e.g., in the small intestine).
In 1957, Shore and coworkers proposed the pH partition hypothesis (first introduced by Jacobs in 1940 [448]) to describe the influence of pKa and pH upon the gastric secretion of a variety of intravenously-administered weakly acidic and basic drugs [449]. The results of their experiments could be explained with their theoretical model of drug absorption across a lipoid barrier (i.e., the gastric mucosa) that separates two compartments (i.e., the stomach lumen and the blood) with different pH and permeable only to the unionized form, which was assumed at equilibrium. They found that the extent to which a drug moves between compartments (the GI tract and blood) depends on the value of the drug pKa and the environmental pH values of the two compartments, as explained below using Eqs. (3), (5) as a starting point.
6.3.1. In the case of a weakly acidic drug
The ratio of the total concentration of the drug in blood ([TA]Bl = [HA]Bl + [A−] Bl = [U]Bl + [I]Bl) and the total concentration of the drug in the GI tract ([TA]GI = [HA]GI + [A−]GI = [U]GI + [I]GI) is
| (6) |
Fig. 12 A illustrates the distribution of a hypothetical weakly acidic drug (pKa = 3.4) between the GI tract (e.g., the stomach with a hypothetical pHGI = 1.4) and the blood (pHBl = 7.4). Note that these numerical values are for illustrative purposes and were chosen to make the calculations easier. This example illustrates that at equilibrium the ratio [TA]Bl/[TA]GI = (107.4−3.4 + 1)/(101.4−3.4 + 1) ≅ 104. Thus, the pH partition hypothesis suggests that a weakly acidic drug is more concentrated in the more basic compartment (‘ion trapping’). That is to say that weakly acidic drugs such as aspirin (pKa = 3.5) can be effectively absorbed from the stomach.
Fig. 12.
The influence of pH and pKa on drug distribution. Theoretical distribution of a hypothetical weakly acidic drug (pKa = 3.4, panel ‘A’) and a hypothetical weakly basic drug (pKa = 8.4, panel ‘B’) between two aqueous compartments with different pH (GI tract at pH = 1.4 and blood at pH = 7.4). Assuming that only the unionized form U (HA in panel ‘A’ and B in panel ‘B’) can cross the membrane and that U is equilibrated across the plasma membrane, panel ‘A’ shows that a weakly acidic drug is more concentrated in the alkaline compartment. This result suggests that weakly acidic drugs tend to be absorbed from the more acidic compartment to the more basic compartment (blue arrows). Panel ‘B’ shows that a weakly basic drug is more concentrated in the acidic compartment, indicating that weakly basic drugs are poorly absorbed in an acidic compartment (blue arrows). Weakly basic drugs are in fact poorly absorbed from the stomach. GI: gastrointestinal; Bl: blood. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
6.3.2. In the case of a weakly basic drug
The ratio of the total concentration of the drug in blood ([TB]Bl = [B]Bl + [BH+] Bl = [U]Bl + [I]Bl) and the total concentration of the drug in the GI tract ([TB]GI = [B]GI + [BH+]GI = [U]GI + [I]GI) is
| (7) |
Fig. 12B illustrates the distribution of a hypothetical weakly basic drug (pKa = 8.4) between the GI tract (e.g., the stomach with a hypothetical pHGI = 1.4) and the blood (pHBl = 7.4). Note that these numerical values are for illustrative purposes and were chosen to make the calculations easier. This example illustrates that at equilibrium the ratio [TB]Bl/[TB]GI = (108.4−7.4 + 1)/(108.4−1.4 + 1) ≅ 10−6. Thus, the pH partition hypothesis suggests that a weakly basic drug is more concentrated in the more acidic compartment. That is to say that the model predicts that weakly basic drugs (e.g., propranolol) should be poorly absorbed from the stomach and absorbed to a greater extent in the small intestine (e.g., pH = 6.4) where the ratio [TB]Bl/[TB]GI = (108.4−7.4 + 1)/(108.4−6.4 + 1) ≅ 0.1. In practice, an alternative route of administration may be preferable for weakly basic drugs.
In practice, the model has several limitations because the pH partition hypothesis ignores other substantial influences upon drug absorption. For example, the assumption of equilibrium is unrealistic in such a dynamic system. Furthermore, even weakly acidic drugs can be substantially absorbed in the small intestine because of the large luminal surface area that it presents [450]. Finally, the pH-partition hypothesis does not consider the mechanism by which drugs can move across epithelial layers. Today we know that both the transcellular and paracellular pathways play important roles in the absorption and elimination of both charged and uncharged drug forms [451]. Low-specificity membrane transporter proteins that contribute to transcellular drug transport around the body include organic anion transporters (OATs), organic cation transporters (OCTs), some MCTs, and members of the ABC transporter superfamily, such as the P-glycoprotein transporters (P-gp) [452], [453], [454], [455], [456]. In some cases, the transporters themselves may be pH-sensitive or coupled to the transport of acids and bases.
The importance of such considerations is exemplified by lowered absorption of weakly basic drugs in individuals with an unusually high stomach pH such as those taking PPIs or individuals with achlorhydria [457]. For example, the dissolution and absorption rate of the weakly basic antifungal agent ketoconazole, which is soluble only at pH lower than 3, can be enhanced by co-administration of an acidic, carbonated beverage [457]. A related example, albeit related to absorption of drugs by bacteria, is that the adjuvant use of NaHCO3 enhances the in vitro potency of antibiotics by interfering with the proton motive force that drives antibiotic efflux from bacteria [458].
As we will see in Section 6.5, considerations of drug solubility and transepithelial movement also influence drug elimination in urine by the kidneys.
6.4. The Influence of pH upon Drug Stability
When developing drugs for oral delivery it is important to account for the adverse acidic environment of the stomach, which can cause instability and rapid degradation of drugs before they reach the small intestine for absorption. This could happen because the polymers used for the tablet coating may be susceptible to pH. For this reason, carriers for oral drug delivery are tested for pH-sensitivity and endurance in acidic environment. Acid-resistant polymers that only dissolve above certain pH values are sometimes used as enteric or gastro-resistant coatings [459], [460], [461], [462]. This is the case, for example, for oral delivery of insulin [463], [464].
6.5. The Influence of pH upon Drug Elimination
The pH partition hypothesis provides the theoretical framework for understanding how urinary pH influences renal drug excretion; acidification of urine favors elimination of weakly basic drugs (because their absorption is reduced) whereas alkalinization of urine favors elimination of weakly acidic drugs. For example, urine acidification via administration of ammonium chloride increases elimination of the weakly basic drug amphetamine [465], whereas urine alkalinization via intravenous administration of NaHCO3 enhances elimination of acidic drugs like salicylic acid (i.e., aspirin) and can be helpful in the management of drug poisoning like aspirin intoxication [465], [466], [467]. As we considered earlier, partitioning is just one aspect of drug distribution. Many OATs and OCTs [468] are expressed in nephron epithelia where their action is vital for delivering drugs from the peritubular capillaries into the nephron lumen for excretion in urine and for delivering diuretics (e.g., furosemide) to their therapeutic targets in the nephron lumen. The direct secretion of these drugs into the nephron lumen is necessary because many such drugs are substantially bound to albumin in circulation and are not effectively filtered into the nephron lumen at the glomerulus.
6.6. Exploiting pH for Targeted Drug Delivery
Acidity can be harnessed to target drug release to acidic environments such as the stomach or pathological acidotic microenvironments such as tumors. One example under development is a gastro-floating matrix tablet that contains adjuvant NaHCO3 with the drug, produces CO2 upon reaction with gastric acid causing the dosage form to remain buoyant in the stomach for prolonged release [469]. Another example is the use of pH-sensitive vehicles such as micelles that could release cytotoxic chemotherapeutic agents only in the acidic tumor environment [470]. Similarly, as suggested by the disparity between the usefulness of an anticancer drug that was identified in an in vitro screening performed at neutral pH and its value in vivo in the acidic tumor microenvironment [471], it is possible that some drugs may be inactive in circulation and may not be activated until they reach an acidic environment.
7. Concluding Remarks
ABTs and CAs play major roles in a variety of pathologies and provide an array of potential therapeutic targets, but many are currently lacking specific or safe drugs. The application of epigenetic modulators and other genetic tools to alter their expression is an underexplored area of research. It is interesting to note that, among the many treatment paradigms for diverse acid disturbances, NaHCO3 administration is a common thread. Its low cost and ready availability have prompted its nickname “enemy of the pharmaceutical industry.” However, despite the promise of numerous limited trials, robust evidence in favor of its broad effectiveness in many fields is currently lacking. This is perhaps due to a lack of appreciation of subgroup effects or a lack of standardization among trails. Nonetheless, research into the therapeutic importance of balancing pH remains robust and promises the delivery of many more effective treatments in the coming years.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The work of BNQ and MDP was supported by National Institute of Health (NIH) grant R01-EY028580. The work of RO was supported by NIH grant K01-DK107787.
References
- 1.Astrup P., Severinghaus J.W. 1st ed. Munksgaard; Denmark: 1986. The history of blood gases, acids and bases. [Google Scholar]
- 2.Amodu A., Abramowitz M.K. Dietary Acid, Age, and Serum Bicarbonate Levels among Adults in the United States. CJASN. 2013;8:2034–2042. doi: 10.2215/CJN.03600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alam I., Alam I., Paracha P.I., Pawelec G. Higher estimates of daily dietary net endogenous acid production (NEAP) in the elderly as compared to the young in a healthy, free-living elderly population of Pakistan. Clin. Interv. Aging. 2012;7:565–573. doi: 10.2147/CIA.S37158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Putnam R.W., Filosa J.A., Ritucci N.A. Cellular mechanisms involved in CO(2) and acid signaling in chemosensitive neurons. Am. J. Physiol., Cell Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- 5.Occhipinti R., Boron W.F. Mathematical modeling of acid-base physiology. Prog. Biophys. Mol. Biol. 2015;117:43–58. doi: 10.1016/j.pbiomolbio.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boron W.F. Acid Base Physiology. In: Boron W.F., Boulpaep E.L., editors. Medical Physiology. A Cellular and Molecular Approach. 3rd ed., Elsevier Saunders; Philadelphia, PA: 2017. pp. 628–646. [Google Scholar]
- 7.Castro D., Keenaghan M. Arterial Blood Gas. StatPearls. 2020 [PubMed] [Google Scholar]
- 8.Lim E., Miyamura J., Chen J.J. Racial/Ethnic-Specific Reference Intervals for Common Laboratory Tests: A Comparison among Asians, Blacks, Hispanics, and White. Hawaii J. Med. Public Health. 2015;74:302–310. [PMC free article] [PubMed] [Google Scholar]
- 9.Crews D.C., Banerjee T., Wesson D.E., Morgenstern H., Saran R., Burrows N.R., Williams D.E., Powe N.R. Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team, Race/Ethnicity, Dietary Acid Load, and Risk of End-Stage Renal Disease among US Adults with Chronic Kidney Disease. Am. J. Nephrol. 2018;47:174–181. doi: 10.1159/000487715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foley R.N., Wang C., Ishani A., Collins A.J. NHANES III: Influence of Race on GFR Thresholds and Detection of Metabolic Abnormalities. JASN. 2007;18:2575–2582. doi: 10.1681/ASN.2006121411. [DOI] [PubMed] [Google Scholar]
- 11.Rodacki M., Pereira J.R.D., Nabuco de Oliveira A.M., Barone B., Mac Dowell R., Perricelli P., Bravo M.T., de Oliveira M.M., Brum J.D., Belem L.C., de Ornellas P.G., Berardo R.S., Luescher J., Campos L., de M. Vangelotti A., Kupfer R., Zajdenverg L., Milech A., Paulo de Oliveira J.E. Ethnicity and young age influence the frequency of diabetic ketoacidosis at the onset of type 1 diabetes. Diabetes Res. Clin. Pract. 2007;78:259–262. doi: 10.1016/j.diabres.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Nyenwe E., Loganathan R., Blum S., Ezuteh D., Erani D., Palace M., Ogugua C. Admissions for diabetic ketoacidosis in ethnic minority groups in a city hospital. Metab. Clin. Exp. 2007;56:172–178. doi: 10.1016/j.metabol.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Harris A.N., Lee H.-W., Fang L., Verlander J.W., Weiner I.D. Differences in acidosis-stimulated renal ammonia metabolism in the male and female kidney. Am. J. Physiol. Renal Physiol. 2019;317:F890–F905. doi: 10.1152/ajprenal.00244.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frassetto L.A., Morris R.C., Sebastian A. Effect of age on blood acid-base composition in adult humans: role of age-related renal functional decline. Am. J. Physiol. 1996;271:F1114–1122. doi: 10.1152/ajprenal.1996.271.6.F1114. [DOI] [PubMed] [Google Scholar]
- 15.Salameh A.I., Ruffin V.A., Boron W.F. Effects of metabolic acidosis on intracellular pH responses in multiple cell types. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307:R1413–1427. doi: 10.1152/ajpregu.00154.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park H.J., Lyons J.C., Ohtsubo T., Song C.W. Acidic environment causes apoptosis by increasing caspase activity. Br J Cancer. 1999;80:1892–1897. doi: 10.1038/sj.bjc.6690617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nedergaard M., Goldman S.A., Desai S., Pulsinelli W.A. Acid-induced death in neurons and glia. J. Neurosci. 1991;11:2489–2497. doi: 10.1523/JNEUROSCI.11-08-02489.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flinck M., Kramer S.H., Pedersen S.F. Roles of pH in control of cell proliferation. Acta Physiol (Oxf). 2018;223 doi: 10.1111/apha.13068. [DOI] [PubMed] [Google Scholar]
- 19.Lee J.Y., Onanyan M., Garrison I., White R., Crook M., Alexeyev M.F., Kozhukhar N., Pastukh V., Swenson E.R., Supuran C.T., Stevens T. Extrinsic acidosis suppresses glycolysis and migration while increasing network formation in pulmonary microvascular endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2019;317:L188–L201. doi: 10.1152/ajplung.00544.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engevik A.C., Kaji I., Goldenring J.R. The Physiology of the Gastric Parietal Cell. Physiol. Rev. 2020;100:573–602. doi: 10.1152/physrev.00016.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blake-Palmer K.G., Karet F.E. Cellular physiology of the renal H+ATPase. Curr. Opin. Nephrol. Hypertens. 2009;18:433–438. doi: 10.1097/MNH.0b013e32832e9c58. [DOI] [PubMed] [Google Scholar]
- 22.Daugirdas J.T., Arrieta J., Ye M., Flores G., Battle D.C. Intracellular acidification associated with changes in free cytosolic calcium. Evidence for Ca2+/H+ exchange via a plasma membrane Ca(2+)-ATPase in vascular smooth muscle cells. J. Clin. Invest. 1995;95:1480–1489. doi: 10.1172/JCI117819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strand D.S., Kim D., Peura D.A. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver. 2017;11:27–37. doi: 10.5009/gnl15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donowitz M., Ming Tse C., Fuster D. SLC9/NHE gene family, a plasma membrane and organellar family of Na+/H+ exchangers. Mol. Aspects Med. 2013;34:236–251. doi: 10.1016/j.mam.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondapalli K.C., Prasad H., Rao R. An inside job: how endosomal Na+/H+ exchangers link to autism and neurological disease. Front Cell Neurosci. 2014;8 doi: 10.3389/fncel.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su Y.R., Menon A.G. Epithelial sodium channels and hypertension. Drug Metab. Dispos. 2001;29:553–556. [PubMed] [Google Scholar]
- 27.Chang H.B., Gao X., Nepomuceno R., Hu S., Sun D. Na(+)/H(+) exchanger in the regulation of platelet activation and paradoxical effects of cariporide. Exp. Neurol. 2015;272:11–16. doi: 10.1016/j.expneurol.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avkiran M., Cook A.R., Cuello F. Targeting Na+/H+ exchanger regulation for cardiac protection: a RSKy approach? Curr Opin Pharmacol. 2008;8:133–140. doi: 10.1016/j.coph.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Sinagra E., Rossi F., Raimondo D., Conoscenti G., Anderloni A., Guarnotta V., Maida M. Tenapanor for the treatment of irritable bowel syndrome with constipation. Expert Rev Clin Pharmacol. 2020;13:473–479. doi: 10.1080/17512433.2020.1762570. [DOI] [PubMed] [Google Scholar]
- 30.Halestrap A.P. The SLC16 gene family – Structure, role and regulation in health and disease. Mol. Aspects Med. 2013;34:337–349. doi: 10.1016/j.mam.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Contreras-Baeza Y., Sandoval P.Y., Alarcón R., Galaz A., Cortés-Molina F., Alegría K., Baeza-Lehnert F., Arce-Molina R., Guequén A., Flores C.A., Martín A.S., Barros L.F. Monocarboxylate transporter 4 (MCT4) is a high affinity transporter capable of exporting lactate in high-lactate microenvironments. J. Biol. Chem. 2019;294:20135–20147. doi: 10.1074/jbc.RA119.009093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith D.E., Clémençon B., Hediger M.A. Proton-coupled oligopeptide transporter family SLC15: Physiological, pharmacological and pathological implications. Mol Aspects Med. 2013;34:323–336. doi: 10.1016/j.mam.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanai Y., Clémençon B., Simonin A., Leuenberger M., Lochner M., Weisstanner M., Hediger M.A. The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol. Aspects Med. 2013;34:108–120. doi: 10.1016/j.mam.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Beloueche-Babari M., Casals Galobart T., Delgado-Goni T., Wantuch S., Parkes H.G., Tandy D., Harker J.A., Leach M.O. Monocarboxylate transporter 1 blockade with AZD3965 inhibits lipid biosynthesis and increases tumour immune cell infiltration. Br. J. Cancer. 2020;122:895–903. doi: 10.1038/s41416-019-0717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeCoursey T.E. The Voltage-Gated Proton Channel: A Riddle, Wrapped in a Mystery, inside an Enigma. Biochemistry. 2015;54:3250–3268. doi: 10.1021/acs.biochem.5b00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myers E.J., Marshall A., Jennings M.L., Parker M.D. Mouse Slc4a11 expressed in Xenopus oocytes is an ideally selective H+/OH- conductance pathway that is stimulated by rises in intracellular and extracellular pH. Am. J. Physiol., Cell Physiol. 2016;311:C945–C959. doi: 10.1152/ajpcell.00259.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tu Y.-H., Cooper A.J., Teng B., Chang R.B., Artiga D.J., Turner H.N., Mulhall E.M., Ye W., Smith A.D., Liman E.R. An evolutionarily conserved gene family encodes proton-selective ion channels. Science. 2018;359:1047–1050. doi: 10.1126/science.aao3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quade B.N., Marshall A., Parker M.D. pH dependence of the Slc4a11-mediated H+ conductance is influenced by intracellular lysine residues and modified by disease-linked mutations. Am. J. Physiol., Cell Physiol. 2020;319:C359–C370. doi: 10.1152/ajpcell.00128.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hellwig N., Plant T.D., Janson W., Schäfer M., Schultz G., Schaefer M. TRPV1 acts as proton channel to induce acidification in nociceptive neurons. J. Biol. Chem. 2004;279:34553–34561. doi: 10.1074/jbc.M402966200. [DOI] [PubMed] [Google Scholar]
- 40.Hug M.J., Tamada T., Bridges R.J. CFTR and bicarbonate secretion by [correction of to] epithelial cells. News Physiol. Sci. 2003;18:38–42. doi: 10.1152/nips.01412.2002. [DOI] [PubMed] [Google Scholar]
- 41.Han Y., Shewan A.M., Thorn P. HCO3- Transport through Anoctamin/Transmembrane Protein ANO1/TMEM16A in Pancreatic Acinar Cells Regulates Luminal pH. J. Biol. Chem. 2016;291:20345–20352. doi: 10.1074/jbc.M116.750224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fatima-Shad K., Barry P.H. Anion permeation in GABA- and glycine-gated channels of mammalian cultured hippocampal neurons. Proc. Biol. Sci. 1993;253:69–75. doi: 10.1098/rspb.1993.0083. [DOI] [PubMed] [Google Scholar]
- 43.Parker M.D., Boron W.F. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol. Rev. 2013;93:803–959. doi: 10.1152/physrev.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ducoudret O., Diakov A., Müller-Berger S., Romero M.F., Frömter E. The renal Na-HCO3-cotransporter expressed in Xenopus laevis oocytes: inhibition by tenidap and benzamil and effect of temperature on transport rate and stoichiometry. Pflugers Arch. 2001;442:709–717. doi: 10.1007/s004240100594. [DOI] [PubMed] [Google Scholar]
- 45.Shao X., Kao L., Aduladze N., Kurtz I. Stoichiometry and inhibitory pharmacology of electrogenic sodium bicarbonate cotransporter NBC4c (NBCe2-C) expressed in HEK-293 cells. FASEB J. 2009;23:800.2. [Google Scholar]
- 46.Alper S.L., Sharma A.K. The SLC26 Gene Family of Anion Transporters and Channels. Mol Aspects Med. 2013;34:494–515. doi: 10.1016/j.mam.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamprecht G., Baisch S., Schoenleber E., Gregor M. Transport properties of the human intestinal anion exchanger DRA (down-regulated in adenoma) in transfected HEK293 cells. Pflugers Arch. 2005;449:479–490. doi: 10.1007/s00424-004-1342-x. [DOI] [PubMed] [Google Scholar]
- 48.D’Ambrosio K., De Simone G., Supuran C.T. Chapter 2 - Human Carbonic Anhydrases: Catalytic Properties, Structural Features, and Tissue Distribution. In: Supuran C.T., De Simone G., editors. Carbonic Anhydrases as Biocatalysts. Elsevier; Amsterdam: 2015. pp. 17–30. [DOI] [Google Scholar]
- 49.Occhipinti R., Boron W.F. Role of Carbonic Anhydrases and Inhibitors in Acid-Base Physiology: Insights from Mathematical Modeling. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20153841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.K. Farzam, M. Abdullah, Acetazolamide, in: StatPearls, StatPearls Publishing, Treasure Island (FL), 2020. http://www.ncbi.nlm.nih.gov/books/NBK532282/ (accessed September 6, 2020).
- 51.Rose B.D., Post T.W. 5th ed. McGraw-Hill; 2001. Clinical physiology of acid-base and electrolyte disorders. [Google Scholar]
- 52.Raphael K.L. Metabolic Acidosis in CKD: Core Curriculum 2019. Am. J. Kidney Dis. 2019;74:263–275. doi: 10.1053/j.ajkd.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 53.Rennke H.G., Denker B.M. Lippincott Williams & Wilkins; 2007. Renal Pathophysiology: The Essentials. [Google Scholar]
- 54.Laing C.M., Toye A.M., Capasso G., Unwin R.J. Renal tubular acidosis: developments in our understanding of the molecular basis. Int. J. Biochem. Cell Biol. 2005;37:1151–1161. doi: 10.1016/j.biocel.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 55.A.Q.T. Pham, L.H.R. Xu, O.W. Moe, Drug-Induced Metabolic Acidosis, F1000Res. 4 (2015). https://doi.org/10.12688/f1000research.7006.1. [DOI] [PMC free article] [PubMed]
- 56.Kraut J.A., Madias N.E. Metabolic acidosis: pathophysiology, diagnosis and management. Nat Rev Nephrol. 2010;6:274–285. doi: 10.1038/nrneph.2010.33. [DOI] [PubMed] [Google Scholar]
- 57.Carnauba R.A., Baptistella A.B., Paschoal V., Hübscher G.H. Diet-Induced Low-Grade Metabolic Acidosis and Clinical Outcomes: A Review. Nutrients. 2017;9 doi: 10.3390/nu9060538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lazenby R.B. 4th Edition. Lippincott Williams & Wilkins; 2011. Handbook of Pathophysiology. [Google Scholar]
- 59.Niv Y., Fraser G.M. The alkaline tide phenomenon. J. Clin. Gastroenterol. 2002;35:5–8. doi: 10.1097/00004836-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Bjerneroth G. Tribonat–a comprehensive summary of its properties. Crit. Care Med. 1999;27:1009–1013. doi: 10.1097/00003246-199905000-00047. [DOI] [PubMed] [Google Scholar]
- 61.Goldsmith D.J., Forni L.G., Hilton P.J. Bicarbonate therapy and intracellular acidosis. Clin. Sci. 1997;93:593–598. doi: 10.1042/cs0930593. [DOI] [PubMed] [Google Scholar]
- 62.Susantitaphong P., Sewaralthahab K., Balk E.M., Jaber B.L., Madias N.E. Short- and Long-Term Effects of Alkali Therapy in Chronic Kidney Disease: A Systematic Review. Am J Nephrol. 2012;35:540–547. doi: 10.1159/000339329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mason T.G., Kraut J.A. Treatment of Acidified Blood Using Reduced Osmolarity Mixed-Base Solutions. Front Physiol. 2016;7:625. doi: 10.3389/fphys.2016.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kucera R.R., Shapiro J.I., Whalen M.A., Kindig N.B., Filley G.F., Chan L. Brain pH effects of NaHCO3 and Carbicarb in lactic acidosis. Crit. Care Med. 1989;17:1320–1323. doi: 10.1097/00003246-198912000-00015. [DOI] [PubMed] [Google Scholar]
- 65.Sun J.H., Filley G.F., Hord K., Kindig N.B., Bartle E.J. Carbicarb: an effective substitute for NaHCO3 for the treatment of acidosis. Surgery. 1987;102:835–839. [PubMed] [Google Scholar]
- 66.Kallet R.H., Jasmer R.M., Luce J.M., Lin L.H., Marks J.D. The Treatment of Acidosis in Acute Lung Injury with Tris-Hydroxymethyl Aminomethane (THAM) Am J Respir Crit Care Med. 2000;161:1149–1153. doi: 10.1164/ajrccm.161.4.9906031. [DOI] [PubMed] [Google Scholar]
- 67.Neavyn M.J., Boyer E.W., Bird S.B., Babu K.M. Sodium Acetate as a Replacement for Sodium Bicarbonate in Medical Toxicology: a Review. J Med Toxicol. 2013;9:250–254. doi: 10.1007/s13181-013-0304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwartz W.B., Waters W.C. Lactate versus bicarbonate. A reconsideration of the therapy of metabolic acidosis. Am. J. Med. 1962;32:831–834. doi: 10.1016/0002-9343(62)90029-3. [DOI] [PubMed] [Google Scholar]
- 69.Scialla J.J., Appel L.J., Astor B.C., Miller E.R., Beddhu S., Woodward M., Parekh R.S., Anderson C.A.M. Estimated net endogenous acid production and serum bicarbonate in African Americans with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:1526–1532. doi: 10.2215/CJN.00150111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ikizler H.O., Zelnick L., Ruzinski J., Curtin L., Utzschneider K.M., Kestenbaum B., Himmelfarb J., de Boer I.H. Dietary Acid Load is Associated With Serum Bicarbonate but not Insulin Sensitivity in Chronic Kidney Disease. J Ren Nutr. 2016;26:93–102. doi: 10.1053/j.jrn.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Di Iorio B.R., Di Micco L., Marzocco S., De Simone E., De Blasio A., Sirico M.L., Nardone L. Very Low-Protein Diet (VLPD) Reduces Metabolic Acidosis in Subjects with Chronic Kidney Disease: The “Nutritional Light Signal” of the Renal Acid Load. Nutrients. 2017;9 doi: 10.3390/nu9010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goraya N., Munoz-Maldonado Y., Simoni J., Wesson D.E. Fruit and Vegetable Treatment of Chronic Kidney Disease-Related Metabolic Acidosis Reduces Cardiovascular Risk Better than Sodium Bicarbonate. Am. J. Nephrol. 2019;49:438–448. doi: 10.1159/000500042. [DOI] [PubMed] [Google Scholar]
- 73.Donckerwolcke R.A., van Stekelenburg G.J., Tiddens H.A. Therapy of bicarbonate-losing renal tubular acidosis. Arch. Dis. Child. 1970;45:774–779. doi: 10.1136/adc.45.244.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wesson D.E., Mathur V., Tangri N., Stasiv Y., Parsell D., Li E., Klaerner G., Bushinsky D.A. Long-term safety and efficacy of veverimer in patients with metabolic acidosis in chronic kidney disease: a multicentre, randomised, blinded, placebo-controlled, 40-week extension. Lancet. 2019;394:396–406. doi: 10.1016/S0140-6736(19)31388-1. [DOI] [PubMed] [Google Scholar]
- 75.C. Brady, E.R. Chemaly, J.W. Lohr, M.D. Parker, Veverimer: an advance in base therapy for metabolic acidosis, 2020. (2020). http://atm.amegroups.com/article/view/42806. [DOI] [PMC free article] [PubMed]
- 76.Zhou F.Q. Pyruvate in the correction of intracellular acidosis: a metabolic basis as a novel superior buffer. Am. J. Nephrol. 2005;25:55–63. doi: 10.1159/000084141. [DOI] [PubMed] [Google Scholar]
- 77.Stacpoole P.W., Wright E.C., Baumgartner T.G., Bersin R.M., Buchalter S., Curry S.H., Duncan C.A., Harman E.M., Henderson G.N., Jenkinson S. A controlled clinical trial of dichloroacetate for treatment of lactic acidosis in adults. The Dichloroacetate-Lactic Acidosis Study Group. N. Engl. J. Med. 1992;327:1564–1569. doi: 10.1056/NEJM199211263272204. [DOI] [PubMed] [Google Scholar]
- 78.Gennari F.J. Pathophysiology of metabolic alkalosis: a new classification based on the centrality of stimulated collecting duct ion transport. Am. J. Kidney Dis. 2011;58:626–636. doi: 10.1053/j.ajkd.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 79.Moviat M., Pickkers P., van der Voort P.H., van der Hoeven J.G. Acetazolamide-mediated decrease in strong ion difference accounts for the correction of metabolic alkalosis in critically ill patients. Crit. Care. 2006;10:R14. doi: 10.1186/cc3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guffey J.D., Haas C.E., Crowley A., Connor K.A., Kaufman D.C. Hydrochloric Acid Infusion for the Treatment of Metabolic Alkalosis in Surgical Intensive Care Unit Patients. Ann Pharmacother. 2018;52:522–526. doi: 10.1177/1060028018754389. [DOI] [PubMed] [Google Scholar]
- 81.Barton C.H., Vaziri N.D., Ness R.L., Saiki J.K., Mirahmadi K.S. Cimetidine in the Management of Metabolic Alkalosis Induced by Nasogastric Drainage. Arch Surg. 1979;114:70–74. doi: 10.1001/archsurg.1979.01370250072015. [DOI] [PubMed] [Google Scholar]
- 82.N.E. Madias, H.J. Adrogué, Respiratory Acid–Base Disorders, in: Seldin and Giebisch’s The Kidney, 5th ed., Philadelphia, PA, 2013: pp. 2113–2138.
- 83.Madias N.E., Adrogué H.J. National Kidney Foundation Primer on Kidney Diseases. 7th ed., Elsevier; Philadelphia, PA: 2018. Respiratory Acidosis and Alkalosis; pp. 152–159. [Google Scholar]
- 84.Skelton L.A., Boron W.F., Zhou Y. Acid-base transport by the renal proximal tubule. J. Nephrol. 2010;23(Suppl 16):S4–18. [PMC free article] [PubMed] [Google Scholar]
- 85.D. Rawat, P. Modi, S. Sharma, Hypercapnea, in: StatPearls, StatPearls Publishing, Treasure Island (FL), 2020. http://www.ncbi.nlm.nih.gov/books/NBK500012/. [PubMed]
- 86.Adrogué H.J., Madias N.E. Alkali therapy for respiratory acidosis: a medical controversy. Am. J. Kidney Dis. 2020;75:265–271. doi: 10.1053/j.ajkd.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 87.Adamson R., Swenson E.R. Acetazolamide use in severe chronic obstructive pulmonary disease. Pros and Cons, Annals ATS. 2017;14:1086–1093. doi: 10.1513/AnnalsATS.201701-016FR. [DOI] [PubMed] [Google Scholar]
- 88.Arazawa D.T., Kimmel J.D., Finn M.C., Federspiel W.J. Acidic sweep gas with carbonic anhydrase coated hollow fiber membranes synergistically accelerates CO2 removal from blood. Acta Biomater. 2015;25:143–149. doi: 10.1016/j.actbio.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cove M.E., Vu L.H., Ring T., May A.G., Federspiel W.J., Kellum J.A. A Proof of Concept Study, Demonstrating Extracorporeal Carbon Dioxide Removal Using Hemodialysis with a Low Bicarbonate Dialysate. ASAIO J. 2019;65:605–613. doi: 10.1097/MAT.0000000000000879. [DOI] [PubMed] [Google Scholar]
- 90.Orr-Walker B.J., Horne A.M., Evans M.C., Grey A.B., Murray M.A., McNeil A.R., Reid I.R. Hormone replacement therapy causes a respiratory alkalosis in normal postmenopausal women. J. Clin. Endocrinol. Metab. 1999;84:1997–2001. doi: 10.1210/jcem.84.6.5797. [DOI] [PubMed] [Google Scholar]
- 91.Leaf D.E., Goldfarb D.S. Mechanisms of action of acetazolamide in the prophylaxis and treatment of acute mountain sickness. J. Appl. Physiol. 2007;102:1313–1322. doi: 10.1152/japplphysiol.01572.2005. [DOI] [PubMed] [Google Scholar]
- 92.Sinning A., Hübner C.A. Minireview: pH and synaptic transmission. FEBS Lett. 2013;587:1923–1928. doi: 10.1016/j.febslet.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 93.Münster-Wandowski A., Zander J.-F., Richter K., Ahnert-Hilger G. Co-existence of Functionally Different Vesicular Neurotransmitter Transporters. Front. Synaptic Neurosci. 2016;8 doi: 10.3389/fnsyn.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Du J., Reznikov L.R., Price M.P., Zha X., Lu Y., Moninger T.O., Wemmie J.A., Welsh M.J. Protons are a neurotransmitter that regulates synaptic plasticity in the lateral amygdala. Proc. Natl. Acad. Sci. U.S.A. 2014;111:8961–8966. doi: 10.1073/pnas.1407018111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grover L.M., Lambert N.A., Schwartzkroin P.A., Teyler T.J. Role of HCO3- ions in depolarizing GABAA receptor-mediated responses in pyramidal cells of rat hippocampus. J. Neurophysiol. 1993;69:1541–1555. doi: 10.1152/jn.1993.69.5.1541. [DOI] [PubMed] [Google Scholar]
- 96.Ma B.-F., Xie M.-J., Zhou M. Bicarbonate efflux via GABAA receptors depolarizes membrane potential and inhibits two-pore domain potassium channels of astrocytes in rat hippocampal slices. Glia. 2012;60:1761–1772. doi: 10.1002/glia.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Makani S., Chesler M. Rapid rise of extracellular pH evoked by neural activity is generated by the plasma membrane calcium ATPase. J. Neurophysiol. 2010;103:667–676. doi: 10.1152/jn.00948.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chesler M. Regulation and Modulation of pH in the Brain. Physiol. Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 99.Ziemann A.E., Schnizler M.K., Albert G.W., Severson M.A., Howard M.A., Welsh M.J., Wemmie J.A. Seizure termination by acidosis depends on ASIC1a. Nat. Neurosci. 2008;11:816–822. doi: 10.1038/nn.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Traynelis S.F., Cull-Candy S.G. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990;345:347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- 101.Helmy M.M., Tolner E.A., Vanhatalo S., Voipio J., Kaila K. Brain alkalosis causes birth asphyxia seizures, suggesting therapeutic strategy. Ann. Neurol. 2011;69:493–500. doi: 10.1002/ana.22223. [DOI] [PubMed] [Google Scholar]
- 102.Zhao H., Carney K.E., Falgoust L., Pan J.W., Sun D., Zhang Z. Emerging roles of Na+/H+ exchangers in epilepsy and developmental brain disorders. Prog Neurobiol. 2016;138–140:19–35. doi: 10.1016/j.pneurobio.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gurnett C.A., Veile R., Zempel J., Blackburn L., Lovett M., Bowcock A. Disruption of sodium bicarbonate transporter SLC4A10 in a patient with complex partial epilepsy and mental retardation. Arch. Neurol. 2008;65:550–553. doi: 10.1001/archneur.65.4.550. [DOI] [PubMed] [Google Scholar]
- 104.Sander T., Toliat M.R., Heils A., Leschik G., Becker C., Rüschendorf F., Rohde K., Mundlos S., Nürnberg P. Association of the 867Asp variant of the human anion exchanger 3 gene with common subtypes of idiopathic generalized epilepsy. Epilepsy Res. 2002;51:249–255. doi: 10.1016/s0920-1211(02)00152-3. [DOI] [PubMed] [Google Scholar]
- 105.Christensen H.L., Nguyen A.T., Pedersen F.D., Damkier H.H. Na+ dependent acid-base transporters in the choroid plexus; insights from slc4 and slc9 gene deletion studies. Front Physiol. 2013;4:304. doi: 10.3389/fphys.2013.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Uldall M., Botfield H., Jansen-Olesen I., Sinclair A., Jensen R. Acetazolamide lowers intracranial pressure and modulates the cerebrospinal fluid secretion pathway in healthy rats. Neurosci. Lett. 2017;645:33–39. doi: 10.1016/j.neulet.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 107.Staley K.J., Soldo B.L., Proctor W.R. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- 108.Ben-Ari Y. The GABA excitatory/inhibitory developmental sequence: a personal journey. Neuroscience. 2014;279:187–219. doi: 10.1016/j.neuroscience.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 109.Irie T., Hara M., Yasukura T., Minamino M., Omori K., Matsuda H., Inoue K., Inagaki C. Chloride concentration in cultured hippocampal neurons increases during long-term exposure to ammonia through enhanced expression of an anion exchanger. Brain Res. 1998;806:246–256. doi: 10.1016/s0006-8993(98)00700-8. [DOI] [PubMed] [Google Scholar]
- 110.Gao A.Y.L., Ilie A., Chang P.K.Y., Orlowski J., McKinney R.A. A Christianson syndrome-linked deletion mutation (Δ287ES288) in SLC9A6 impairs hippocampal neuronal plasticity. Neurobiol. Dis. 2019;130 doi: 10.1016/j.nbd.2019.104490. [DOI] [PubMed] [Google Scholar]
- 111.Hakim A.M., Shoubridge E.A. Cerebral acidosis in focal ischemia. Cerebrovasc Brain Metab Rev. 1989;1:115–132. [PubMed] [Google Scholar]
- 112.Atwood C.S., Moir R.D., Huang X., Scarpa R.C., Bacarra N.M., Romano D.M., Hartshorn M.A., Tanzi R.E., Bush A.I. Dramatic aggregation of Alzheimer abeta by Cu(II) is induced by conditions representing physiological acidosis. J. Biol. Chem. 1998;273:12817–12826. doi: 10.1074/jbc.273.21.12817. [DOI] [PubMed] [Google Scholar]
- 113.Prasad H., Rao R. Amyloid clearance defect in ApoE4 astrocytes is reversed by epigenetic correction of endosomal pH. PNAS. 2018;115:E6640–E6649. doi: 10.1073/pnas.1801612115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tolner E.A., Hochman D.W., Hassinen P., Otáhal J., Gaily E., Haglund M.M., Kubová H., Schuchmann S., Vanhatalo S., Kaila K. 5% CO2 is a potent, fast acting inhalation anticonvulsant. Epilepsia. 2011;52:104–114. doi: 10.1111/j.1528-1167.2010.02731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Deng R.-M., Liu Y.-C., Li J.-Q., Xu J.-G., Chen G. The role of carbon dioxide in acute brain injury. Med Gas Res. 2020;10:81–84. doi: 10.4103/2045-9912.285561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fan Y.-Y., Shen Z., He P., Jiang L., Hou W., Shen Y., Zhang X.-N., Hu W.-W., Chen Z. A novel neuroprotective strategy for ischemic stroke: transient mild acidosis treatment by CO2 inhalation at reperfusion. J Cereb Blood Flow Metab. 2014;34:275–283. doi: 10.1038/jcbfm.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reiss W.G., Oles K.S. Acetazolamide in the treatment of seizures. Ann Pharmacother. 1996;30:514–519. doi: 10.1177/106002809603000515. [DOI] [PubMed] [Google Scholar]
- 118.Carta F., Di Cesare Mannelli L., Pinard M., Ghelardini C., Scozzafava A., McKenna R., Supuran C.T. A class of sulfonamide carbonic anhydrase inhibitors with neuropathic pain modulating effects. Bioorg. Med. Chem. 2015;23:1828–1840. doi: 10.1016/j.bmc.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 119.Provensi G., Carta F., Nocentini A., Supuran C.T., Casamenti F., Passani M.B., Fossati S. A New Kid on the Block? Carbonic Anhydrases as Possible New Targets in Alzheimer’s Disease. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20194724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Blandina P., Provensi G., Passsani M.B., Capasso C., Supuran C.T. Carbonic anhydrase modulation of emotional memory. Implications for the treatment of cognitive disorders. J. Enzyme Inhib. Med. Chem. 2020;35:1206–1214. doi: 10.1080/14756366.2020.1766455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sinha A., Oo P., Asghar M.U., Cheema H.A., Mehta S.S., Leinwand J.C., Janga K. Type II Renal Tubular Acidosis Secondary to Topiramate: A Review. Cureus. 2018;10 doi: 10.7759/cureus.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sinning A., Liebmann L., Hübner C.A. Disruption of Slc4a10 augments neuronal excitability and modulates synaptic short-term plasticity. Front Cell Neurosci. 2015;9:223. doi: 10.3389/fncel.2015.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eftekhari S., Westgate C.S.J., Uldall M.S., Jensen R.H. Preclinical update on regulation of intracranial pressure in relation to idiopathic intracranial hypertension. Fluids and Barriers of the CNS. 2019;16:35. doi: 10.1186/s12987-019-0155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shahidullah M., To C.-H., Pelis R.M., Delamere N.A. Studies on bicarbonate transporters and carbonic anhydrase in porcine nonpigmented ciliary epithelium. Invest. Ophthalmol. Vis. Sci. 2009;50:1791–1800. doi: 10.1167/iovs.08-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Counillon L., Touret N., Bidet M., Peterson-Yantorno K., Coca-Prados M., Stuart-Tilley A., Wilhelm S., Alper S.L., Civan M.M. Na+/H+ and CI-/HCO3-antiporters of bovine pigmented ciliary epithelial cells. Pflugers Arch. 2000;440:667–678. doi: 10.1007/s004240000302. [DOI] [PubMed] [Google Scholar]
- 126.Patel S.P., Parker M.D. SLC4A11 and the Pathophysiology of Congenital Hereditary Endothelial Dystrophy. Biomed Res. Int. 2015;2015 doi: 10.1155/2015/475392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nehrke K. H(OH), H(OH), H(OH): a holiday perspective. Focus on “Mouse Slc4a11 expressed in Xenopus oocytes is an ideally selective H+/OH- conductance pathway that is stimulated by rises in intracellular and extracellular pH”. Am. J. Physiol., Cell Physiol. 2016;311:C942–C944. doi: 10.1152/ajpcell.00309.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Suzuki M., Seki G., Yamada H., Horita S., Fujita T. Functional Roles of Electrogenic Sodium Bicarbonate Cotransporter NBCe1 in Ocular Tissues. Open Ophthalmol J. 2012;6:36–41. doi: 10.2174/1874364101206010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dreffs A., Henderson D., Dmitriev A.V., Antonetti D.A., Linsenmeier R.A. Retinal pH and Acid Regulation During Metabolic Acidosis. Curr. Eye Res. 2018;43:902–912. doi: 10.1080/02713683.2018.1458882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Warren T.J., Van Hook M.J., Supuran C.T., Thoreson W.B. Sources of protons and a role for bicarbonate in inhibitory feedback from horizontal cells to cones in Ambystoma tigrinum retina. J. Physiol. (Lond.) 2016;594:6661–6677. doi: 10.1113/JP272533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Duda T., Wen X.-H., Isayama T., Sharma R.K., Makino C.L. Bicarbonate Modulates Photoreceptor Guanylate Cyclase (ROS-GC) Catalytic Activity. J. Biol. Chem. 2015;290:11052–11060. doi: 10.1074/jbc.M115.650408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ahn J., Chiang J., Gorin M.B. Novel mutation in SLC4A7 gene causing autosomal recessive progressive rod-cone dystrophy. Ophthalmic Genet. 2020;41:386–389. doi: 10.1080/13816810.2020.1783691. [DOI] [PubMed] [Google Scholar]
- 133.Kao L., Kurtz L.M., Shao X., Papadopoulos M.C., Liu L., Bok D., Nusinowitz S., Chen B., Stella S.L., Andre M., Weinreb J., Luong S.S., Piri N., Kwong J.M.K., Newman D., Kurtz I. Severe neurologic impairment in mice with targeted disruption of the electrogenic sodium bicarbonate cotransporter NBCe2 (Slc4a5 gene) J. Biol. Chem. 2011;286:32563–32574. doi: 10.1074/jbc.M111.249961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Han J.Y.S., Kinoshita J., Bisetto S., Bell B.A., Nowak R.A., Peachey N.S., Philp N.J. Role of monocarboxylate transporters in regulating metabolic homeostasis in the outer retina: Insight gained from cell-specific Bsg deletion. FASEB J. 2020;34:5401–5419. doi: 10.1096/fj.201902961R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bizhanova A., Kopp P. Genetics and phenomics of Pendred syndrome. Mol. Cell. Endocrinol. 2010;322:83–90. doi: 10.1016/j.mce.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 136.Stover E.H., Borthwick K.J., Bavalia C., Eady N., Fritz D.M., Rungroj N., Giersch A.B.S., Morton C.C., Axon P.R., Akil I., Al-Sabban E.A., Baguley D.M., Bianca S., Bakkaloglu A., Bircan Z., Chauveau D., Clermont M.-J., Guala A., Hulton S.A., Kroes H., Volti G.L., Mir S., Mocan H., Nayir A., Ozen S., Soriano J.R., Sanjad S.A., Tasic V., Taylor C.M., Topaloglu R., Smith A.N., Karet F.E. Novel ATP6V1B1 and ATP6V0A4 mutations in autosomal recessive distal renal tubular acidosis with new evidence for hearing loss. J. Med. Genet. 2002;39:796–803. doi: 10.1136/jmg.39.11.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Desir J., Abramowicz M. Congenital hereditary endothelial dystrophy with progressive sensorineural deafness (Harboyan syndrome) Orphanet J Rare Dis. 2008;3:28. doi: 10.1186/1750-1172-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Peters T.A., Monnens L.A.H., Cremers C.W.R.J., Curfs J.H.A.J. Genetic disorders of transporters/channels in the inner ear and their relation to the kidney. Pediatr. Nephrol. 2004;19:1194–1201. doi: 10.1007/s00467-004-1626-6. [DOI] [PubMed] [Google Scholar]
- 139.Huebner A.K., Maier H., Maul A., Nietzsche S., Herrmann T., Praetorius J., Hübner C.A. Early Hearing Loss upon Disruption of Slc4a10 in C57BL/6 Mice. J. Assoc. Res. Otolaryngol. 2019;20:233–245. doi: 10.1007/s10162-019-00719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hurle B., Ignatova E., Massironi S.M., Mashimo T., Rios X., Thalmann I., Thalmann R., Ornitz D.M. Non-syndromic vestibular disorder with otoconial agenesis in tilted/mergulhador mice caused by mutations in otopetrin 1. Hum Mol Genet. 2003;12:777–789. doi: 10.1093/hmg/ddg087. [DOI] [PubMed] [Google Scholar]
- 141.Dror A.A., Politi Y., Shahin H., Lenz D.R., Dossena S., Nofziger C., Fuchs H., Hrabé de Angelis M., Paulmichl M., Weiner S., Avraham K.B. Calcium oxalate stone formation in the inner ear as a result of an Slc26a4 mutation. J. Biol. Chem. 2010;285:21724–21735. doi: 10.1074/jbc.M110.120188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Usach I., Martinez R., Festini T., Peris J.-E. Subcutaneous Injection of Drugs: Literature Review of Factors Influencing Pain Sensation at the Injection Site. Adv Ther. 2019;36:2986–2996. doi: 10.1007/s12325-019-01101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Petitjean H., Fatima T., Mouchbahani-Constance S., Davidova A., Ferland C.E., Orlowski J., Sharif-Naeini R. Loss of SLC9A6/NHE6 impairs nociception in a mouse model of Christianson Syndrome. Pain. 2020 doi: 10.1097/j.pain.0000000000001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hoffmanová I., Sánchez D. Metabolic acidosis and anaemia associated with dorzolamide in a patient with impaired renal function. Br J Clin Pharmacol. 2018;84:796–799. doi: 10.1111/bcp.13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Capino A.C., Dannaway D.C., Miller J.L. Metabolic Acidosis with Ophthalmic Dorzolamide in a Neonate. J Pediatr Pharmacol Ther. 2016;21:256–259. doi: 10.5863/1551-6776-21.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alka K., Casey J.R. Ophthalmic Nonsteroidal Anti-Inflammatory Drugs as a Therapy for Corneal Dystrophies Caused by SLC4A11 Mutation. Invest. Ophthalmol. Vis. Sci. 2018;59:4258–4267. doi: 10.1167/iovs.18-24301. [DOI] [PubMed] [Google Scholar]
- 147.Lupachyk S., Stavniichuk R., Komissarenko J.I., Drel V.R., Obrosov A.A., El-Remessy A.B., Pacher P., Obrosova I.G. Na+/H+-exchanger-1 inhibition counteracts diabetic cataract formation and retinal oxidative-nitrative stress and apoptosis. Int. J. Mol. Med. 2012;29:989–998. doi: 10.3892/ijmm.2012.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lin L.-T., Chen J.-T., Tai M.-C., Chen Y.-H., Chen C.-L., Pao S.-I., Hsu C.R., Liang C.-M. Protective effects of hypercapnic acidosis on Ischemia–reperfusion-induced retinal injury. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0211185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Piromchai P., Laohakittikul C., Khunnawongkrit S., Srirompotong S. Cerumenolytic Efficacy of 2.5% Sodium Bicarbonate Versus Docusate Sodium: A Randomized, Controlled Trial. Otol. Neurotol. 2020;41 doi: 10.1097/MAO.0000000000002672. [DOI] [PubMed] [Google Scholar]
- 150.Ikeda K., Morizono T. The preparation of acetic acid for use in otic drops and its effect on endocochlear potential and pH in inner ear fluid. Am. J. Otolaryngol. 1989;10:382–385. doi: 10.1016/0196-0709(89)90032-X. [DOI] [PubMed] [Google Scholar]
- 151.Fotsing J.R., Darmohusodo V., Patron A.P., Ching B.W., Brady T., Arellano M., Chen Q., Davis T.J., Liu H., Servant G., Zhang L., Williams M., Saganich M., Ditschun T., Tachdjian C., Karanewsky D.S. Discovery and Development of S6821 and S7958 as Potent TAS2R8 Antagonists. J. Med. Chem. 2020;63:4957–4977. doi: 10.1021/acs.jmedchem.0c00388. [DOI] [PubMed] [Google Scholar]
- 152.Slack J.P., Brockhoff A., Batram C., Menzel S., Sonnabend C., Born S., Galindo M.M., Kohl S., Thalmann S., Ostopovici-Halip L., Simons C.T., Ungureanu I., Duineveld K., Bologa C.G., Behrens M., Furrer S., Oprea T.I., Meyerhof W. Modulation of Bitter Taste Perception by a Small Molecule hTAS2R Antagonist. Curr. Biol. 2010;20:1104–1109. doi: 10.1016/j.cub.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Parham S.M., Pasieka J.L. Effect of pH modification by bicarbonate on pain after subcutaneous lidocaine injection. Can J Surg. 1996;39:31–35. [PMC free article] [PubMed] [Google Scholar]
- 154.Saint-Criq V., Gray M.A. Role of CFTR in epithelial physiology. Cell Mol Life Sci. 2017;74:93–115. doi: 10.1007/s00018-016-2391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Flume P.A. Pulmonary complications of cystic fibrosis. Respir Care. 2009;54:618–627. doi: 10.4187/aarc0443. [DOI] [PubMed] [Google Scholar]
- 156.Chen E.Y.T., Yang N., Quinton P.M., Chin W.-C. A new role for bicarbonate in mucus formation. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;299:L542–549. doi: 10.1152/ajplung.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Massip-Copiz M.M., Santa-Coloma T.A. Extracellular pH and lung infections in cystic fibrosis. Eur. J. Cell Biol. 2018;97:402–410. doi: 10.1016/j.ejcb.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 158.Shah V.S., Meyerholz D.K., Tang X.X., Reznikov L., Abou Alaiwa M., Ernst S.E., Karp P.H., Wohlford-Lenane C.L., Heilmann K.P., Leidinger M.R., Allen P.D., Zabner J., McCray P.B., Ostedgaard L.S., Stoltz D.A., Randak C.O., Welsh M.J. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351:503–507. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fischer H., Widdicombe J.H. Mechanisms of Acid and Base Secretion by the Airway Epithelium. J Membr Biol. 2006;211:139–150. doi: 10.1007/s00232-006-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.J. Hunt, B. Gaston, Airway pH homeostasis in asthma and other inflammatory lung diseases, in: Therapeutic Targets in Airway Inflammation, CRC Press, 2013: pp. 63–80.
- 161.Garnett J.P., Kalsi K.K., Sobotta M., Bearham J., Carr G., Powell J., Brodlie M., Ward C., Tarran R., Baines D.L. Hyperglycaemia and Pseudomonas aeruginosa acidify cystic fibrosis airway surface liquid by elevating epithelial monocarboxylate transporter 2 dependent lactate-H+ secretion. Sci Rep. 2016;6:37955. doi: 10.1038/srep37955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Clancy J.P., Cotton C.U., Donaldson S.H., Solomon G.M., VanDevanter D.R., Boyle M.P., Gentzsch M., Nick J.A., Illek B., Wallenburg J.C., Sorscher E.J., Amaral M.D., Beekman J.M., Naren A.P., Bridges R.J., Thomas P.J., Cutting G., Rowe S., Durmowicz A.G., Mense M., Boeck K.D., Skach W., Penland C., Joseloff E., Bihler H., Mahoney J., Borowitz D., Tuggle K.L. CFTR modulator theratyping: Current status, gaps and future directions. J. Cyst. Fibros. 2019;18:22–34. doi: 10.1016/j.jcf.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stigliani M., Manniello M.D., Zegarra-Moran O., Galietta L., Minicucci L., Casciaro R., Garofalo E., Incarnato L., Aquino R.P., Del Gaudio P., Russo P. Rheological Properties of Cystic Fibrosis Bronchial Secretion and in Vitro Drug Permeation Study: The Effect of Sodium Bicarbonate. J Aerosol Med Pulm Drug Deliv. 2016;29:337–345. doi: 10.1089/jamp.2015.1228. [DOI] [PubMed] [Google Scholar]
- 164.Gomez C.C.S., Parazzi P.L.F., Clinckspoor K.J., Mauch R.M., Pessine F.B.T., Levy C.E., Peixoto A.O., Ribeiro M.Â.G.O., Ribeiro A.F., Conrad D., Quinton P.M., Marson F.A.L., Ribeiro J.D. Safety, Tolerability, and Effects of Sodium Bicarbonate Inhalation in Cystic Fibrosis. Clin Drug Investig. 2020;40:105–117. doi: 10.1007/s40261-019-00861-x. [DOI] [PubMed] [Google Scholar]
- 165.Alaiwa M.H.A., Launspach J.L., Sheets K.A., Rivera J.A., Gansemer N.D., Taft P.J., Thorne P.S., Welsh M.J., Stoltz D.A., Zabner J. Repurposing tromethamine as inhaled therapy to treat CF airway disease. JCI Insight. 2016;1 doi: 10.1172/jci.insight.87535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Delpiano L., Thomas J.J., Yates A.R., Rice S.J., Gray M.A., Saint-Criq V. Esomeprazole Increases Airway Surface Liquid pH in Primary Cystic Fibrosis Epithelial Cells. Front Pharmacol. 2018;9:1462. doi: 10.3389/fphar.2018.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Thornell I.M., Rehman T., Pezzulo A.A., Welsh M.J. Paracellular bicarbonate flux across human cystic fibrosis airway epithelia tempers changes in airway surface liquid pH. J. Physiol. (Lond.). 2020 doi: 10.1113/JP280120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Orchard C.H., Kentish J.C. Effects of changes of pH on the contractile function of cardiac muscle. Am. J. Physiol. 1990;258:C967–981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- 169.Mitchell J.H., Wildenthal K., Johnson R.L. The effects of acid-base disturbances on cardiovascular and pulmonary function. Kidney Int. 1972;1:375–389. doi: 10.1038/ki.1972.48. [DOI] [PubMed] [Google Scholar]
- 170.Ten Hove M., Nederhoff M.G.J., Van Echteld C.J.A. Relative contributions of Na+/H+ exchange and Na+/HCO3- cotransport to ischemic Nai+ overload in isolated rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H287–292. doi: 10.1152/ajpheart.01102.2003. [DOI] [PubMed] [Google Scholar]
- 171.Nagai T., Anzai T., Kaneko H., Anzai A., Mano Y., Nagatomo Y., Kohsaka S., Maekawa Y., Kawamura A., Yoshikawa T., Ogawa S. Impact of systemic acidosis on the development of malignant ventricular arrhythmias after reperfusion therapy for ST-elevation myocardial infarction. Circ. J. 2010;74:1808–1814. doi: 10.1253/circj.cj-10-0229. [DOI] [PubMed] [Google Scholar]
- 172.Bai J., Yin R., Wang K., Zhang H. Mechanisms Underlying the Emergence of Post-acidosis Arrhythmia at the Tissue Level: A Theoretical Study. Front Physiol. 2017;8 doi: 10.3389/fphys.2017.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Orlowski A., Ciancio M.C., Caldiz C.I., De Giusti V.C., Aiello E.A. Reduced sarcolemmal expression and function of the NBCe1 isoform of the Na+-HCO3− cotransporter in hypertrophied cardiomyocytes of spontaneously hypertensive rats: role of the renin-angiotensin system. Cardiovasc. Res. 2014;101:211–219. doi: 10.1093/cvr/cvt255. [DOI] [PubMed] [Google Scholar]
- 174.Alvarez B.V., Johnson D.E., Sowah D., Soliman D., Light P.E., Xia Y., Karmazyn M., Casey J.R. Carbonic anhydrase inhibition prevents and reverts cardiomyocyte hypertrophy. J. Physiol. (Lond.) 2007;579:127–145. doi: 10.1113/jphysiol.2006.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Al Moamen N.J., Prasad V., Bodi I., Miller M.L., Neiman M.L., Lasko V.M., Alper S.L., Wieczorek D.F., Lorenz J.N., Shull G.E. Loss of the AE3 anion exchanger in a hypertrophic cardiomyopathy model causes rapid decompensation and heart failure. J. Mol. Cell. Cardiol. 2011;50:137–146. doi: 10.1016/j.yjmcc.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Alvarez B.V., Villa-Abrille M.C. Mitochondrial NHE1: a newly identified target to prevent heart disease. Front Physiol. 2013;4 doi: 10.3389/fphys.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Boedtkjer E., Aalkjaer C. Disturbed acid-base transport: an emerging cause of hypertension. Front Physiol. 2013;4 doi: 10.3389/fphys.2013.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang S., Xu J., Feng Y., Zhang J., Cui L., Zhang H., Bai Y. Extracellular acidosis suppresses calcification of vascular smooth muscle cells by inhibiting calcium influx via L-type calcium channels. Clin. Exp. Hypertens. 2018;40:370–377. doi: 10.1080/10641963.2017.1384482. [DOI] [PubMed] [Google Scholar]
- 179.Bellingham A.J., Detter J.C., Lenfant C. Regulatory mechanisms of hemoglobin oxygen affinity in acidosis and alkalosis. J Clin Invest. 1971;50:700–706. doi: 10.1172/JCI106540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Feng H.-Z., Jin J.-P. Transgenic expression of carbonic anhydrase III in cardiac muscle demonstrates a mechanism to tolerate acidosis. Am. J. Physiol., Cell Physiol. 2019;317:C922–C931. doi: 10.1152/ajpcell.00130.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ahn S., Kim Y.-J., Sohn C.H., Seo D.W., Lim K.S., Donnino M.W., Kim W.Y. Sodium bicarbonate on severe metabolic acidosis during prolonged cardiopulmonary resuscitation: a double-blind, randomized, placebo-controlled pilot study. J Thorac Dis. 2018;10:2295–2302. doi: 10.21037/jtd.2018.03.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Levy M.M. An evidence-based evaluation of the use of sodium bicarbonate during cardiopulmonary resuscitation. Crit Care Clin. 1998;14:457–483. doi: 10.1016/s0749-0704(05)70011-7. [DOI] [PubMed] [Google Scholar]
- 183.Aschner J.L., Poland R.L. Sodium bicarbonate: basically useless therapy. Pediatrics. 2008;122:831–835. doi: 10.1542/peds.2007-2400. [DOI] [PubMed] [Google Scholar]
- 184.Karmazyn M. NHE-1: still a viable therapeutic target. J. Mol. Cell. Cardiol. 2013;61:77–82. doi: 10.1016/j.yjmcc.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 185.Teshima Y., Akao M., Jones S.P., Marbán E. Cariporide (HOE642), a selective Na+-H+ exchange inhibitor, inhibits the mitochondrial death pathway. Circulation. 2003;108:2275–2281. doi: 10.1161/01.CIR.0000093277.20968.C7. [DOI] [PubMed] [Google Scholar]
- 186.Toda T., Kadono T., Hoshiai M., Eguchi Y., Nakazawa S., Nakazawa H., Higashijima N., Ishida H. Na+/H+ exchanger inhibitor cariporide attenuates the mitochondrial Ca2+ overload and PTP opening. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3517–3523. doi: 10.1152/ajpheart.00483.2006. [DOI] [PubMed] [Google Scholar]
- 187.Kim J.O., Kwon E.J., Song D.W., Lee J.S., Kim D.H. miR-185 inhibits endoplasmic reticulum stress-induced apoptosis by targeting Na+/H+ exchanger-1 in the heart. BMB Rep. 2016;49:208–213. doi: 10.5483/bmbrep.2016.49.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Khandoudi N., Albadine J., Robert P., Krief S., Berrebi-Bertrand I., Martin X., Bevensee M.O., Boron W.F., Bril A. Inhibition of the cardiac electrogenic sodium bicarbonate cotransporter reduces ischemic injury. Cardiovasc. Res. 2001;52:387–396. doi: 10.1016/s0008-6363(01)00430-8. [DOI] [PubMed] [Google Scholar]
- 189.De Giusti V.C., Orlowski A., Villa-Abrille M.C., de Cingolani G.E.C., Casey J.R., Alvarez B.V., Aiello E.A. Antibodies against the cardiac sodium/bicarbonate co-transporter (NBCe1) as pharmacological tools. Br. J. Pharmacol. 2011;164:1976–1989. doi: 10.1111/j.1476-5381.2011.01496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Qiu L.-Y., Duan G.-L., Yan Y.-F., Li Y.-Y., Wang H., Xiao L., Liao Z.-P., Chen H.-P. Sasanquasaponin induces increase of Cl-/HCO3- exchange of anion exchanger 3 and promotes intracellular Cl- efflux in hypoxia/reoxygenation cardiomyocytes. Mol Med Rep. 2017;16:2953–2961. doi: 10.3892/mmr.2017.6882. [DOI] [PubMed] [Google Scholar]
- 191.Fernandez-Fernandez B., Martin-Cleary C., Ortiz A. Bicarbonate therapy, phosphate binders, and risk for vascular calcification. Kidney Int. 2014;86:1056. doi: 10.1038/ki.2014.191. [DOI] [PubMed] [Google Scholar]
- 192.Finer G., Price H.E., Shore R.M., White K.E., Langman C.B. Hyperphosphatemic familial tumoral calcinosis: response to acetazolamide and postulated mechanisms. Am. J. Med. Genet. A. 2014;164A:1545–1549. doi: 10.1002/ajmg.a.36476. [DOI] [PubMed] [Google Scholar]
- 193.Bersin R.M., Chatterjee K., Arieff A.I. Metabolic and hemodynamic consequences of sodium bicarbonate administration in patients with heart disease. Am. J. Med. 1989;87:7–14. doi: 10.1016/s0002-9343(89)80476-0. [DOI] [PubMed] [Google Scholar]
- 194.Fitts R.H. Cellular mechanisms of muscle fatigue. Physiol. Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- 195.Stackhouse S.K., Reisman D.S., Binder-Macleod S.A. Challenging the role of pH in skeletal muscle fatigue. Phys Ther. 2001;81:1897–1903. [PubMed] [Google Scholar]
- 196.Cairns S.P. Lactic acid and exercise performance : culprit or friend? Sports Med. 2006;36:279–291. doi: 10.2165/00007256-200636040-00001. [DOI] [PubMed] [Google Scholar]
- 197.Lindinger M.I., McKelvie R.S., Heigenhauser G.J. K+ and Lac- distribution in humans during and after high-intensity exercise: role in muscle fatigue attenuation? J. Appl. Physiol. 1995;78:765–777. doi: 10.1152/jappl.1995.78.3.765. [DOI] [PubMed] [Google Scholar]
- 198.Mitch W.E. Metabolic and clinical consequences of metabolic acidosis. J. Nephrol. 2006;19(Suppl 9):S70–75. [PubMed] [Google Scholar]
- 199.Kataya Y., Murakami K., Kobayashi S., Suga H., Sasaki S. Three-generation Study of Women on Diets and Health Study Group, Higher dietary acid load is associated with a higher prevalence of frailty, particularly slowness/weakness and low physical activity, in elderly Japanese women. Eur J Nutr. 2018;57:1639–1650. doi: 10.1007/s00394-017-1449-4. [DOI] [PubMed] [Google Scholar]
- 200.Juel C. Regulation of pH in human skeletal muscle: adaptations to physical activity. Acta Physiol (Oxf). 2008;193:17–24. doi: 10.1111/j.1748-1716.2008.01840.x. [DOI] [PubMed] [Google Scholar]
- 201.Feng H.-Z., Jin J.-P. Carbonic Anhydrase III Is Expressed in Mouse Skeletal Muscles Independent of Fiber Type-Specific Myofilament Protein Isoforms and Plays a Role in Fatigue Resistance. Front Physiol. 2016;7:597. doi: 10.3389/fphys.2016.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Egger F., Meyer T., Such U., Hecksteden A. Effects of Sodium Bicarbonate on High-Intensity Endurance Performance in Cyclists: A Double-Blind. Randomized Cross-Over Trial, PLOS ONE. 2014;9 doi: 10.1371/journal.pone.0114729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Macutkiewicz D., Sunderland C. Sodium bicarbonate supplementation does not improve elite women’s team sport running or field hockey skill performance. Physiol Rep. 2018;6 doi: 10.14814/phy2.13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hadzic M., Eckstein M.L., Schugardt M. The Impact of Sodium Bicarbonate on Performance in Response to Exercise Duration in Athletes: A Systematic Review. J Sports Sci Med. 2019;18:271–281. [PMC free article] [PubMed] [Google Scholar]
- 205.Siegler J.C., Marshall P.W.M., Bishop D., Shaw G., Green S. Mechanistic Insights into the Efficacy of Sodium Bicarbonate Supplementation to Improve Athletic Performance. Sports Med Open. 2016;2 doi: 10.1186/s40798-016-0065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Freis T., Hecksteden A., Such U., Meyer T. Effect of sodium bicarbonate on prolonged running performance: A randomized, double-blind, cross-over study. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0182158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Frassetto L., Morris R.C., Sebastian A. Potassium bicarbonate reduces urinary nitrogen excretion in postmenopausal women. J. Clin. Endocrinol. Metab. 1997;82:254–259. doi: 10.1210/jcem.82.1.3663. [DOI] [PubMed] [Google Scholar]
- 208.Granic A., Sayer A.A., Robinson S.M. Dietary Patterns, Skeletal Muscle Health, and Sarcopenia in Older Adults. Nutrients. 2019;11 doi: 10.3390/nu11040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bushinsky D.A., Frick K.K. The effects of acid on bone. Curr. Opin. Nephrol. Hypertens. 2000;9:369–379. doi: 10.1097/00041552-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 210.Challa A., Krieg R.J., Thabet M.A., Veldhuis J.D., Chan J.C. Metabolic acidosis inhibits growth hormone secretion in rats: mechanism of growth retardation. Am. J. Physiol. 1993;265:E547–553. doi: 10.1152/ajpendo.1993.265.4.E547. [DOI] [PubMed] [Google Scholar]
- 211.Chen W., Melamed M.L., Abramowitz M.K. Serum Bicarbonate and Bone Mineral Density in US Adults. Am J Kidney Dis. 2015;65:240–248. doi: 10.1053/j.ajkd.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bichara M., Mercier O., Borensztein P., Paillard M. Acute metabolic acidosis enhances circulating parathyroid hormone, which contributes to the renal response against acidosis in the rat. J Clin Invest. 1990;86:430–443. doi: 10.1172/JCI114729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Frattini A., Orchard P.J., Sobacchi C., Giliani S., Abinun M., Mattsson J.P., Keeling D.J., Andersson A.K., Wallbrandt P., Zecca L., Notarangelo L.D., Vezzoni P., Villa A. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat. Genet. 2000;25:343–346. doi: 10.1038/77131. [DOI] [PubMed] [Google Scholar]
- 214.Sly W.S., Whyte M.P., Sundaram V., Tashian R.E., Hewett-Emmett D., Guibaud P., Vainsel M., Baluarte H.J., Gruskin A., Al-Mosawi M. Carbonic anhydrase II deficiency in 12 families with the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. N. Engl. J. Med. 1985;313:139–145. doi: 10.1056/NEJM198507183130302. [DOI] [PubMed] [Google Scholar]
- 215.Parker M.D. Mouse Models of SLC4-linked Disorders of HCO3- Transporter Dysfunction. Am. J. Physiol., Cell Physiol. 2018;314:C569–C588. doi: 10.1152/ajpcell.00301.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pierce W.M., Nardin G.F., Fuqua M.F., Sabah-Maren E., Stern S.H. Effect of chronic carbonic anhydrase inhibitor therapy on bone mineral density in white women. J. Bone Miner. Res. 1991;6:347–354. doi: 10.1002/jbmr.5650060406. [DOI] [PubMed] [Google Scholar]
- 217.González-Rodríguez J.D., Luis-Yanes M.I., Inglés-Torres E., Arango-Sancho P., Cabrera-Sevilla J.E., Duque-Fernández M.R., Gil-Sánchez S., García-Nieto V.M. Can acetazolamide be used to treat diseases involving increased bone mineral density? Intractable Rare Dis Res. 2016;5:284–289. doi: 10.5582/irdr.2016.01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yang Y.-X. Chronic proton pump inihibitor therapy and calcium metabolism. Curr Gastroenterol Rep. 2012;14:473–479. doi: 10.1007/s11894-012-0290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nassar Y., Richter S. Proton-pump Inhibitor Use and Fracture Risk: An Updated Systematic Review and Meta-analysis. J Bone Metab. 2018;25:141–151. doi: 10.11005/jbm.2018.25.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liu J., Li X., Fan L., Yang J., Wang J., Sun J., Wang Z. Proton pump inhibitors therapy and risk of bone diseases: An update meta-analysis. Life Sci. 2019;218:213–223. doi: 10.1016/j.lfs.2018.12.058. [DOI] [PubMed] [Google Scholar]
- 221.Kopic S., Murek M., Geibel J.P. Revisiting the parietal cell. Am. J. Physiol., Cell Physiol. 2010;298:C1–C10. doi: 10.1152/ajpcell.00478.2009. [DOI] [PubMed] [Google Scholar]
- 222.Weeks D.L., Eskandari S., Scott D.R., Sachs G. A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science. 2000;287:482–485. doi: 10.1126/science.287.5452.482. [DOI] [PubMed] [Google Scholar]
- 223.Celli J.P., Turner B.S., Afdhal N.H., Keates S., Ghiran I., Kelly C.P., Ewoldt R.H., McKinley G.H., So P., Erramilli S., Bansil R. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. PNAS. 2009;106:14321–14326. doi: 10.1073/pnas.0903438106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wroblewski L.E., Peek R.M., Wilson K.T. Helicobacter pylori and Gastric Cancer: Factors That Modulate Disease Risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Skoczylas T., Yandrapu H., Poplawski C., Asadi M., Wallner G., Sarosiek J. Salivary bicarbonate as a major factor in the prevention of upper esophageal mucosal injury in gastroesophageal reflux disease. Dig. Dis. Sci. 2014;59:2411–2416. doi: 10.1007/s10620-014-3099-1. [DOI] [PubMed] [Google Scholar]
- 226.Dosedělová V., Ďurč P., Dolina J., Konečný Š., Foret F., Kubáň P. Analysis of bicarbonate, phosphate and other anions in saliva by capillary electrophoresis with capacitively coupled contactless conductivity detection in diagnostics of gastroesophageal reflux disease. Electrophoresis. 2020;41:116–122. doi: 10.1002/elps.201900319. [DOI] [PubMed] [Google Scholar]
- 227.Yandrapu H., Marcinkiewicz M., Poplawski C., Han K., Zbroch T., Goldin G., Sarosiek I., Namiot Z., Sarosiek J. Role of Saliva in Esophageal Defense: Implications in Patients With Nonerosive Reflux Disease. Am J Med Sci. 2015;349:385–391. doi: 10.1097/MAJ.0000000000000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Helm J.F., Dodds W.J., Hogan W.J., Soergel K.H., Egide M.S., Wood C.M. Acid neutralizing capacity of human saliva. Gastroenterology. 1982;83:69–74. [PubMed] [Google Scholar]
- 229.Cao L., Yuan Z., Liu M., Stock C. (Patho-)Physiology of Na+/H+ Exchangers (NHEs) in the Digestive System. Front Physiol. 2020;10 doi: 10.3389/fphys.2019.01566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Medarov B.I. Milk-Alkali Syndrome. Mayo Clin Proc. 2009;84:261–267. doi: 10.4065/84.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Caruso J.B., Patel R.M., Julka K., Parish D.C. Health-Behavior Induced Disease: Return of the Milk-Alkali Syndrome. J Gen Intern Med. 2007;22:1053–1055. doi: 10.1007/s11606-007-0226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Siddique I., Khan I. Regulation of Na/H exchanger-1 in gastroesophageal reflux disease: possible interaction of histamine receptor. Dig. Dis. Sci. 2003;48:1832–1838. doi: 10.1023/a:1025503318409. [DOI] [PubMed] [Google Scholar]
- 233.Tricida 2018 Annual Report and Proxy, Tricida. (n.d.). https://ir.tricida.com/financial-information/annual-reports (accessed March 9, 2020).
- 234.Gawenis L.R., Ledoussal C., Judd L.M., Prasad V., Alper S.L., Stuart-Tilley A., Woo A.L., Grisham C., Sanford L.P., Doetschman T., Miller M.L., Shull G.E. Mice with a targeted disruption of the AE2 Cl-/HCO3- exchanger are achlorhydric. J. Biol. Chem. 2004;279:30531–30539. doi: 10.1074/jbc.M403779200. [DOI] [PubMed] [Google Scholar]
- 235.Scott D.R., Sachs G., Marcus E.A. The role of acid inhibition in Helicobacter pylori eradication. F1000Res. 2016;5 doi: 10.12688/f1000research.8598.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hassan S.T.S., Šudomová M. The Development of Urease Inhibitors: What Opportunities Exist for Better Treatment of Helicobacter pylori Infection in Children? Children (Basel). 2017;4 doi: 10.3390/children4010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wilschanski M., Novak I. The Cystic Fibrosis of Exocrine Pancreas. Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.van der Doef H.P.J., Kokke F.T.M., van der Ent C.K., Houwen R.H.J. Intestinal Obstruction Syndromes in Cystic Fibrosis: Meconium Ileus, Distal Intestinal Obstruction Syndrome, and Constipation. Curr Gastroenterol Rep. 2011;13:265–270. doi: 10.1007/s11894-011-0185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gennari F.J., Weise W.J. Acid-Base Disturbances in Gastrointestinal Disease. CJASN. 2008;3:1861–1868. doi: 10.2215/CJN.02450508. [DOI] [PubMed] [Google Scholar]
- 240.Wedenoja S., Pekansaari E., Höglund P., Mäkelä S., Holmberg C., Kere J. Update on SLC26A3 mutations in congenital chloride diarrhea. Hum. Mutat. 2011;32:715–722. doi: 10.1002/humu.21498. [DOI] [PubMed] [Google Scholar]
- 241.Hecht G., Hodges K., Gill R.K., Kear F., Tyagi S., Malakooti J., Ramaswamy K., Dudeja P.K. Differential regulation of Na+/H+ exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G370–378. doi: 10.1152/ajpgi.00432.2003. [DOI] [PubMed] [Google Scholar]
- 242.Hayashi H., Szászi K., Coady-Osberg N., Furuya W., Bretscher A.P., Orlowski J., Grinstein S. Inhibition and redistribution of NHE3, the apical Na+/H+ exchanger, by Clostridium difficile toxin B. J. Gen. Physiol. 2004;123:491–504. doi: 10.1085/jgp.200308979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Janecke A.R., Heinz-Erian P., Yin J., Petersen B.-S., Franke A., Lechner S., Fuchs I., Melancon S., Uhlig H.H., Travis S., Marinier E., Perisic V., Ristic N., Gerner P., Booth I.W., Wedenoja S., Baumgartner N., Vodopiutz J., Frechette-Duval M.-C., De Lafollie J., Persad R., Warner N., Tse C.M., Sud K., Zachos N.C., Sarker R., Zhu X., Muise A.M., Zimmer K.-P., Witt H., Zoller H., Donowitz M., Müller T. Reduced sodium/proton exchanger NHE3 activity causes congenital sodium diarrhea. Hum. Mol. Genet. 2015;24:6614–6623. doi: 10.1093/hmg/ddv367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Walker A.W., Duncan S.H., McWilliam Leitch E.C., Child M.W., Flint H.J. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 2005;71:3692–3700. doi: 10.1128/AEM.71.7.3692-3700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kowlgi N.G., Chhabra L. D-lactic acidosis: an underrecognized complication of short bowel syndrome. Gastroenterol Res Pract. 2015;2015 doi: 10.1155/2015/476215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Thwaites D.T., Anderson C.M.H. H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine. Exp. Physiol. 2007;92:603–619. doi: 10.1113/expphysiol.2005.029959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yu Q., Liu X., Liu Y., Riederer B., Li T., Tian D.-A., Tuo B., Shull G., Seidler U. Defective small intestinal anion secretion, dipeptide absorption, and intestinal failure in suckling NBCe1-deficient mice. Pflugers Arch. 2016;468:1419–1432. doi: 10.1007/s00424-016-1836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Haggie P.M., Cil O., Lee S., Tan J.-A., Rivera A.A., Phuan P.-W., Verkman A.S. SLC26A3 inhibitor identified in small molecule screen blocks colonic fluid absorption and reduces constipation. JCI Insight. 2018;3 doi: 10.1172/jci.insight.121370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chey W.D., Lembo A.J., Rosenbaum D.P. Efficacy of Tenapanor in Treating Patients With Irritable Bowel Syndrome With Constipation: A 12-Week, Placebo-Controlled Phase 3 Trial (T3MPO-1) Am. J. Gastroenterol. 2020;115:281–293. doi: 10.14309/ajg.0000000000000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gelfond D., Heltshe S., Ma C., Rowe S.M., Frederick C., Uluer A., Sicilian L., Konstan M., Tullis E., Roach R.N.C., Griffin K., Joseloff E., Borowitz D. Impact of CFTR Modulation on Intestinal pH, Motility, and Clinical Outcomes in Patients With Cystic Fibrosis and the G551D Mutation. Clin Transl Gastroenterol. 2017;8 doi: 10.1038/ctg.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.McHugh D.R., Cotton C.U., Moss F.J., Vitko M., Valerio D.M., Kelley T.J., Hao S., Jafri A., Drumm M.L., Boron W.F., Stern R.C., McBennett K., Hodges C.A. Linaclotide improves gastrointestinal transit in cystic fibrosis mice by inhibiting sodium/hydrogen exchanger 3, American Journal of Physiology-Gastrointestinal and Liver. Physiology. 2018;315:G868–G878. doi: 10.1152/ajpgi.00261.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ahsan M.K., Tchernychev B., Kessler M.M., Solinga R.M., Arthur D., Linde C.I., Silos-Santiago I., Hannig G., Ameen N.A. Linaclotide activates guanylate cyclase-C/cGMP/protein kinase-II-dependent trafficking of CFTR in the intestine. Physiol Rep. 2017;5 doi: 10.14814/phy2.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Giebisch G., Windhager E. Transport of acids and bases. In: Boron W.F., Boulpaep E.L., editors. Medical Physiology. A Cellular and Molecular Approach, 2nd updated, Elsevier Saunders, Philadelphia, PA; 2012. pp. 851–865. [Google Scholar]
- 254.Alexander R.T., Bitzan M. Renal Tubular Acidosis. Pediatr. Clin. North Am. 2019;66:135–157. doi: 10.1016/j.pcl.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 255.Raphael K.L. Metabolic Acidosis and Subclinical Metabolic Acidosis in CKD. JASN. 2018;29:376–382. doi: 10.1681/ASN.2017040422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Varasteh Kia M., Barone S., McDonough A.A., Zahedi K., Xu J., Soleimani M. Downregulation of the Cl-/HCO3-Exchanger Pendrin in Kidneys of Mice with Cystic Fibrosis: Role in the Pathogenesis of Metabolic Alkalosis. Cell. Physiol. Biochem. 2018;45:1551–1565. doi: 10.1159/000487691. [DOI] [PubMed] [Google Scholar]
- 257.Wesson D.E., Buysse J.M., Bushinsky D.A. Mechanisms of Metabolic Acidosis-Induced Kidney Injury in Chronic Kidney Disease. JASN. 2020;31:469–482. doi: 10.1681/ASN.2019070677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Brown D.D., Roem J., Ng D.K., Reidy K.J., Kumar J., Abramowitz M.K., Mak R.H., Furth S.L., Schwartz G.J., Warady B.A., Kaskel F.J., Melamed M.L. Low Serum Bicarbonate and CKD Progression in Children. Clin J Am Soc Nephrol. 2020;15:755–765. doi: 10.2215/CJN.07060619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Shah S.N., Abramowitz M., Hostetter T.H., Melamed M.L. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am. J. Kidney Dis. 2009;54:270–277. doi: 10.1053/j.ajkd.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Harris A.N., Grimm P.R., Lee H.-W., Delpire E., Fang L., Verlander J.W., Welling P.A., Weiner I.D. Mechanism of Hyperkalemia-Induced Metabolic Acidosis. J. Am. Soc. Nephrol. 2018;29:1411–1425. doi: 10.1681/ASN.2017111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Moe O.W. Kidney stones: pathophysiology and medical management. The Lancet. 2006;367:333–344. doi: 10.1016/S0140-6736(06)68071-9. [DOI] [PubMed] [Google Scholar]
- 262.Griffith D.P., Musher D.M., Itin C. Urease. The primary cause of infection-induced urinary stones, Invest Urol. 1976;13:346–350. [PubMed] [Google Scholar]
- 263.Burr R.G., Nuseibeh I.M. Urinary catheter blockage depends on urine pH, calcium and rate of flow. Spinal Cord. 1997;35:521–525. doi: 10.1038/sj.sc.3100424. [DOI] [PubMed] [Google Scholar]
- 264.Mahajan A., Simoni J., Sheather S.J., Broglio K.R., Rajab M.H., Wesson D.E. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010;78:303–309. doi: 10.1038/ki.2010.129. [DOI] [PubMed] [Google Scholar]
- 265.de Brito-Ashurst I., Varagunam M., Raftery M.J., Yaqoob M.M. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J. Am. Soc. Nephrol. 2009;20:2075–2084. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Goraya N., Wesson D.E. Clinical evidence that treatment of metabolic acidosis slows the progression of chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2019;28:267–277. doi: 10.1097/MNH.0000000000000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hunter J.D., Gregg K., Damani Z. Rhabdomyolysis, Contin Educ Anaesth Crit Care. Pain. 2006;6:141–143. doi: 10.1093/bjaceaccp/mkl027. [DOI] [Google Scholar]
- 268.Moore K.P., Holt S.G., Patel R.P., Svistunenko D.A., Zackert W., Goodier D., Reeder B.J., Clozel M., Anand R., Cooper C.E., Morrow J.D., Wilson M.T., Darley-Usmar V., Roberts L.J. A Causative Role for Redox Cycling of Myoglobin and Its Inhibition by Alkalinization in the Pathogenesis and Treatment of Rhabdomyolysis-induced Renal Failure. J. Biol. Chem. 1998;273:31731–31737. doi: 10.1074/jbc.273.48.31731. [DOI] [PubMed] [Google Scholar]
- 269.Hall A.M., Bass P., Unwin R.J. Drug-induced renal Fanconi syndrome. QJM. 2014;107:261–269. doi: 10.1093/qjmed/hct258. [DOI] [PubMed] [Google Scholar]
- 270.Wile D. Diuretics: a review. Ann. Clin. Biochem. 2012;49:419–431. doi: 10.1258/acb.2011.011281. [DOI] [PubMed] [Google Scholar]
- 271.Galla J.H. Metabolic Alkalosis. JASN. 2000;11:369–375. doi: 10.1681/ASN.V112369. [DOI] [PubMed] [Google Scholar]
- 272.Lipner H.I., Ruzany F., Dasgupta M., Lief P.D., Bank N. The behavior of carbenicillin as a nonreabsorbable anion. J. Lab. Clin. Med. 1975;86:183–194. [PubMed] [Google Scholar]
- 273.Brunner F.P., Frick P.G. Hypokalaemia, Metabolic Alkalosis, and Hypernatraemia due to “Massive” Sodium Penicillin Therapy. Br Med J. 1968;4:550–552. doi: 10.1136/bmj.4.5630.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Berg P., Svendsen S.L., Sorensen M.V., Larsen C.K., Andersen J.F., Jensen-Fangel S., Jeppesen M., Schreiber R., Cabrita I., Kunzelmann K., Leipziger J. Impaired Renal HCO3- Excretion in Cystic Fibrosis. J. Am. Soc. Nephrol. 2020;31:1711–1727. doi: 10.1681/ASN.2020010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Pak C.Y. Citrate and renal calculi: an update. Miner Electrolyte Metab. 1994;20:371–377. [PubMed] [Google Scholar]
- 276.Penniston K.L., Steele T.H., Nakada S.Y. Lemonade Therapy Increases Urinary Citrate and Urine Volumes in Patients with Recurrent Calcium Oxalate Stone Formation. Urology. 2007;70:856–860. doi: 10.1016/j.urology.2007.06.1115. [DOI] [PubMed] [Google Scholar]
- 277.Pak C.Y., Fuller C., Sakhaee K., Preminger G.M., Britton F. Long-term treatment of calcium nephrolithiasis with potassium citrate. J. Urol. 1985;134:11–19. doi: 10.1016/s0022-5347(17)46962-x. [DOI] [PubMed] [Google Scholar]
- 278.Khan A., Housami F., Melotti R., Timoney A., Stickler D. Strategy to control catheter encrustation with citrated drinks: a randomized crossover study. J. Urol. 2010;183:1390–1394. doi: 10.1016/j.juro.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 279.Brown D., Smith P.J., Breton S. Role of V-ATPase-rich cells in acidification of the male reproductive tract. J. Exp. Biol. 1997;200:257–262. doi: 10.1242/jeb.200.2.257. [DOI] [PubMed] [Google Scholar]
- 280.Tajima Y., Okamura N., Sugita Y. The activating effects of bicarbonate on sperm motility and respiration at ejaculation. Biochim. Biophys. Acta. 1987;924:519–529. doi: 10.1016/0304-4165(87)90168-1. [DOI] [PubMed] [Google Scholar]
- 281.Okamura N., Tajima Y., Ishikawa H., Yoshii S., Koiso K., Sugita Y. Lowered levels of bicarbonate in seminal plasma cause the poor sperm motility in human infertile patients. Fertil. Steril. 1986;45:265–272. doi: 10.1016/s0015-0282(16)49166-1. [DOI] [PubMed] [Google Scholar]
- 282.Chan H.C., Shi Q.X., Zhou C.X., Wang X.F., Xu W.M., Chen W.Y., Chen A.J., Ni Y., Yuan Y.Y. Critical role of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Mol. Cell. Endocrinol. 2006;250:106–113. doi: 10.1016/j.mce.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 283.Muchekehu R.W., Quinton P.M. A new role for bicarbonate secretion in cervico-uterine mucus release. J Physiol. 2010;588:2329–2342. doi: 10.1113/jphysiol.2010.187237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Maas D.H., Storey B.T., Mastroianni L. Hydrogen ion and carbon dioxide content of the oviductal fluid of the rhesus monkey (Macaca mulatta) Fertil. Steril. 1977;28:981–985. [PubMed] [Google Scholar]
- 285.Lee M.A., Storey B.T. Bicarbonate is essential for fertilization of mouse eggs: mouse sperm require it to undergo the acrosome reaction. Biol. Reprod. 1986;34:349–356. doi: 10.1095/biolreprod34.2.349. [DOI] [PubMed] [Google Scholar]
- 286.Jin S.-K., Yang W.-X. Factors and pathways involved in capacitation: how are they regulated? Oncotarget. 2016;8:3600–3627. doi: 10.18632/oncotarget.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Xiao S., Li R., El Zowalaty A.E., Diao H., Zhao F., Choi Y., Ye X. Acidification of uterine epithelium during embryo implantation in mice. Biol Reprod. 2017;96:232–243. doi: 10.1095/biolreprod.116.144451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Oberheide K., Puchkov D., Jentsch T.J. Loss of the Na+/H+ exchanger NHE8 causes male infertility in mice by disrupting acrosome formation. J. Biol. Chem. 2017;292:10845–10854. doi: 10.1074/jbc.M117.784108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Höglund P., Hihnala S., Kujala M., Tiitinen A., Dunkel L., Holmberg C. Disruption of the SLC26A3-mediated anion transport is associated with male subfertility. Fertil. Steril. 2006;85:232–235. doi: 10.1016/j.fertnstert.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 290.Medina J.F., Recalde S., Prieto J., Lecanda J., Saez E., Funk C.D., Vecino P., van Roon M.A., Ottenhoff R., Bosma P.J., Bakker C.T., Elferink R.P.J.O. Anion exchanger 2 is essential for spermiogenesis in mice. Proc. Natl. Acad. Sci. U.S.A. 2003;100:15847–15852. doi: 10.1073/pnas.2536127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Zeitlin L., Hoen T.E., Achilles S.L., Hegarty T.A., Jerse A.E., Kreider J.W., Olmsted S.S., Whaley K.J., Cone R.A., Moench T.R. Tests of Buffergel for contraception and prevention of sexually transmitted diseases in animal models. Sex Transm Dis. 2001;28:417–423. doi: 10.1097/00007435-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 292.Omo-Aghoja L. Maternal and Fetal Acid-Base Chemistry: A Major Determinant of Perinatal Outcome. Ann Med Health Sci Res. 2014;4:8–17. doi: 10.4103/2141-9248.126602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Musaba M.W., Barageine J.K., Ndeezi G., Wandabwa J.N., Weeks A. Effect of preoperative bicarbonate infusion on maternal and perinatal outcomes of obstructed labour in Mbale Regional Referral Hospital: a study protocol for a randomised controlled trial. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Everhardt E., Dony J.M., Jansen H., Lemmens W.A., Doesburg W.H. Improvement of cervical mucus viscoelasticity and sperm penetration with sodium bicarbonate douching. Hum. Reprod. 1990;5:133–137. doi: 10.1093/oxfordjournals.humrep.a137056. [DOI] [PubMed] [Google Scholar]
- 295.Ansari A.H., Gould K.G., Ansari V.M. Sodium bicarbonate douching for improvement of the postcoital test. Fertil. Steril. 1980;33:608–612. [PubMed] [Google Scholar]
- 296.Suthutvoravut S., Kamyarat O. Spermicidal effects of lemon juice and juices from other natural products. Agriculture and Natural Resources. 2016;50:133–138. doi: 10.1016/j.anres.2015.09.004. [DOI] [Google Scholar]
- 297.Bayer L.L., Jensen J.T. ACIDFORM: a review of the evidence. Contraception. 2014;90:11–18. doi: 10.1016/j.contraception.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 298.Thomas M.A., Chappell B.T., Maximos B., Culwell K.R., Dart C., Howard B. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2 doi: 10.1016/j.conx.2020.100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Stambaugh R., Noriega C., Mastroianni L. Bicarbonate ion; the corona cell dispersing factor of rabbit tubal fluid. J. Reprod. Fertil. 1969;18:51–58. doi: 10.1530/jrf.0.0180051. [DOI] [PubMed] [Google Scholar]
- 300.Escoffier J., Arnaud B., Kaba M., Hograindleur J.P., Le Blévec E., Martinez G., Stévant I., Ray P.F., Arnoult C., Nef S. Pantoprazole, a proton-pump inhibitor, impairs human sperm motility and capacitation in vitro. Andrology. 2020 doi: 10.1111/andr.12855. [DOI] [PubMed] [Google Scholar]
- 301.Scheiner B., Lindner G., Reiberger T., Schneeweiss B., Trauner M., Zauner C., Funk G.-C. Acid-base disorders in liver disease. J. Hepatol. 2017;67:1062–1073. doi: 10.1016/j.jhep.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 302.Trivedi B., Danforth W.H. Effect of pH on the kinetics of frog muscle phosphofructokinase. J. Biol. Chem. 1966;241:4110–4112. [PubMed] [Google Scholar]
- 303.Fredholm B.B., Hjemdahl P. Inhibition by acidosis of adenosine 3’,5’-cyclic monophosphate accumulation and lipolysis in isolated rat fat cells. Acta Physiol. Scand. 1976;96:160–169. doi: 10.1111/j.1748-1716.1976.tb10185.x. [DOI] [PubMed] [Google Scholar]
- 304.Beech J.S., Iles R.A., Cohen R.D. Bicarbonate in the treatment of metabolic acidosis: effects on hepatic intracellular pH, gluconeogenesis, and lactate disposal in rats. Metab. Clin. Exp. 1993;42:341–346. doi: 10.1016/0026-0495(93)90084-2. [DOI] [PubMed] [Google Scholar]
- 305.Goodman A.D., Fuisz R.E., Cahill G.F. Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966;45:612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Hyder A., Laue C., Schrezenmeir J. Effect of extracellular pH on insulin secretion and glucose metabolism in neonatal and adult rat pancreatic islets. Acta Diabetol. 2001;38:171–178. doi: 10.1007/s592-001-8075-9. [DOI] [PubMed] [Google Scholar]
- 307.Lougheed W.D., Fischer U., Perlman K., Albisser A.M. A physiological solvent for crystalline insulin. Diabetologia. 1981;20:51–53. doi: 10.1007/BF00253817. [DOI] [PubMed] [Google Scholar]
- 308.Della Guardia L., Thomas M.A., Cena H. Insulin Sensitivity and Glucose Homeostasis Can Be Influenced by Metabolic Acid Load. Nutrients. 2018;10 doi: 10.3390/nu10050618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Williams R.S., Heilbronn L.K., Chen D.L., Coster A.C.F., Greenfield J.R., Samocha-Bonet D. Dietary acid load, metabolic acidosis and insulin resistance - Lessons from cross-sectional and overfeeding studies in humans. Clin Nutr. 2016;35:1084–1090. doi: 10.1016/j.clnu.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 310.Waelbroeck M. The pH dependence of insulin binding. A quantitative study. J. Biol. Chem. 1982;257:8284–8291. [PubMed] [Google Scholar]
- 311.Nakamura M., Satoh N., Suzuki M., Kume H., Homma Y., Seki G., Horita S. Stimulatory effect of insulin on renal proximal tubule sodium transport is preserved in type 2 diabetes with nephropathy. Biochem. Biophys. Res. Commun. 2015;461:154–158. doi: 10.1016/j.bbrc.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 312.Lee M.G., Ohana E., Park H.W., Yang D., Muallem S. Molecular Mechanism of Pancreatic and Salivary Glands Fluid and HCO3− Secretion. Physiol Rev. 2012;92:39–74. doi: 10.1152/physrev.00011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Wagner C.A. Effect of mineralocorticoids on acid-base balance. Nephron Physiol. 2014;128:26–34. doi: 10.1159/000368266. [DOI] [PubMed] [Google Scholar]
- 314.Mumford E., Unwin R.J., Walsh S.B. Liquorice, Liddle, Bartter or Gitelman-how to differentiate? Nephrol. Dial. Transplant. 2019;34:38–39. doi: 10.1093/ndt/gfy199. [DOI] [PubMed] [Google Scholar]
- 315.Wesson D.E., Simoni J. Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int. 2010;78:1128–1135. doi: 10.1038/ki.2010.348. [DOI] [PubMed] [Google Scholar]
- 316.Pallini A., Hulter H.N., Muser J., Krapf R. Role of endothelin-1 in renal regulation of acid-base equilibrium in acidotic humans. Am. J. Physiol. Renal Physiol. 2012;303:F991–999. doi: 10.1152/ajprenal.00309.2012. [DOI] [PubMed] [Google Scholar]
- 317.Esche J., Shi L., Sánchez-Guijo A., Hartmann M.F., Wudy S.A., Remer T. Higher diet-dependent renal acid load associates with higher glucocorticoid secretion and potentially bioactive free glucocorticoids in healthy children. Kidney Int. 2016;90:325–333. doi: 10.1016/j.kint.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 318.Brüngger M., Hulter H.N., Krapf R. Effect of chronic metabolic acidosis on the growth hormone/IGF-1 endocrine axis: New cause of growth hormone insensitivity in humans. Kidney Int. 1997;51:216–221. doi: 10.1038/ki.1997.26. [DOI] [PubMed] [Google Scholar]
- 319.Fischer U., Lougheed W.D., Albisser A.M. In vivo bicarbonate deficiency and insulin dissolution. ASAIO Trans. 1989;35:26–29. [PubMed] [Google Scholar]
- 320.Rose K.L., Pin C.L., Wang R., Fraser D.D. Combined Insulin and Bicarbonate Therapy Elicits Cerebral Edema in a Juvenile Mouse Model of Diabetic Ketoacidosis. Pediatr. Res. 2007;61:301–306. doi: 10.1203/pdr.0b013e318030d193. [DOI] [PubMed] [Google Scholar]
- 321.Demaurex N., Downey G.P., Waddell T.K., Grinstein S. Intracellular pH regulation during spreading of human neutrophils. J. Cell Biol. 1996;133:1391–1402. doi: 10.1083/jcb.133.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Rajamäki K., Nordström T., Nurmi K., Åkerman K.E.O., Kovanen P.T., Öörni K., Eklund K.K. Extracellular Acidosis Is a Novel Danger Signal Alerting Innate Immunity via the NLRP3 Inflammasome. J Biol Chem. 2013;288:13410–13419. doi: 10.1074/jbc.M112.426254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.DeCoursey T.E. The intimate and controversial relationship between voltage-gated proton channels and the phagocyte NADPH oxidase. Immunol. Rev. 2016;273:194–218. doi: 10.1111/imr.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Morgan D., Capasso M., Musset B., Cherny V.V., Ríos E., Dyer M.J.S., DeCoursey T.E. Voltage-gated proton channels maintain pH in human neutrophils during phagocytosis. Proc. Natl. Acad. Sci. U.S.A. 2009;106:18022–18027. doi: 10.1073/pnas.0905565106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Fukushima T., Waddell T.K., Grinstein S., Goss G.G., Orlowski J., Downey G.P. Na+/H+ exchange activity during phagocytosis in human neutrophils: role of Fcgamma receptors and tyrosine kinases. J. Cell Biol. 1996;132:1037–1052. doi: 10.1083/jcb.132.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Kellum J.A., Song M., Li J. Science review: Extracellular acidosis and the immune response: clinical and physiologic implications. Crit Care. 2004;8:331–336. doi: 10.1186/cc2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Peake P.W., Pussell B.A., Mackinnon B., Charlesworth J.A. The effect of pH and nucleophiles on complement activation by human proximal tubular epithelial cells. Nephrol. Dial. Transplant. 2002;17:745–752. doi: 10.1093/ndt/17.5.745. [DOI] [PubMed] [Google Scholar]
- 328.Erra Díaz F., Dantas E., Geffner J. Unravelling the Interplay between Extracellular Acidosis and Immune Cells. Mediators Inflamm. 2018 (2018). doi: 10.1155/2018/1218297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Beachy J.C., Weisman L.E. Acute asphyxia affects neutrophil number and function in the rat. Crit. Care Med. 1993;21:1929–1934. doi: 10.1097/00003246-199312000-00022. [DOI] [PubMed] [Google Scholar]
- 330.Leblebicioglu B., Lim J.S., Cario A.C., Beck F.M., Walters J.D. pH changes observed in the inflamed gingival crevice modulate human polymorphonuclear leukocyte activation in vitro. J. Periodontol. 1996;67:472–477. doi: 10.1902/jop.1996.67.5.472. [DOI] [PubMed] [Google Scholar]
- 331.Riemann A., Wußling H., Loppnow H., Fu H., Reime S., Thews O. Acidosis differently modulates the inflammatory program in monocytes and macrophages, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1862 (2016):72–81. doi: 10.1016/j.bbadis.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 332.Lardner A. The effects of extracellular pH on immune function. J. Leukoc. Biol. 2001;69:522–530. doi: 10.1189/jlb.69.4.522. [DOI] [PubMed] [Google Scholar]
- 333.Concepcion A.R., Salas J.T., Sarvide S., Sáez E., Ferrer A., López M., Portu A., Banales J.M., Hervás-Stubbs S., Oude Elferink R.P.J., Prieto J., Medina J.F. Anion exchanger 2 is critical for CD8(+) T cells to maintain pHi homeostasis and modulate immune responses. Eur. J. Immunol. 2014;44:1341–1351. doi: 10.1002/eji.201344218. [DOI] [PubMed] [Google Scholar]
- 334.Porteous H., Morgan N., Lanfranco J., Garcia-Buitrago M., Young L., Lenz O. Systemic lupus erythematosus associated with type 4 renal tubular acidosis: a case report and review of the literature. J Med Case Reports. 2011;5:114. doi: 10.1186/1752-1947-5-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Koul P.A., Wahid A., Shah B.A. Systemic lupus erythematosus with distal renal tubular acidosis presenting as hypokalemic paralysis with respiratory failure. Saudi J Kidney Dis Transpl. 2003;14:190–193. [PubMed] [Google Scholar]
- 336.Ray S.C., Baban B., Tucker M.A., Seaton A.J., Chang K.C., Mannon E.C., Sun J., Patel B., Wilson K., Musall J.B., Ocasio H., Irsik D., Filosa J.A., Sullivan J.C., Marshall B., Harris R.A., O’Connor P.M. Oral NaHCO3 Activates a Splenic Anti-Inflammatory Pathway: Evidence That Cholinergic Signals Are Transmitted via Mesothelial Cells. J. Immunol. 2018;200:3568–3586. doi: 10.4049/jimmunol.1701605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Bowman C.C., Selgrade M.K. Sodium Bicarbonate Facilitates Low-dose Oral Tolerance To Peanut In Mice. Journal of Allergy and Clinical Immunology. 2008;121:S96–S97. doi: 10.1016/j.jaci.2007.12.385. [DOI] [Google Scholar]
- 338.Murray C.M., Hutchinson R., Bantick J.R., Belfield G.P., Benjamin A.D., Brazma D., Bundick R.V., Cook I.D., Craggs R.I., Edwards S., Evans L.R., Harrison R., Holness E., Jackson A.P., Jackson C.G., Kingston L.P., Perry M.W.D., Ross A.R.J., Rugman P.A., Sidhu S.S., Sullivan M., Taylor-Fishwick D.A., Walker P.C., Whitehead Y.M., Wilkinson D.J., Wright A., Donald D.K. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat. Chem. Biol. 2005;1:371–376. doi: 10.1038/nchembio744. [DOI] [PubMed] [Google Scholar]
- 339.Yang X., Bai H., Cai W., Liu J., Wang Y., Xu Y., Li J., Zhou Q., Han J., Zhu X., Dong M., Hu D. Inhibition of Na+/H+ exchanger 1 by cariporide alleviates burn-induced multiple organ injury. J. Surg. Res. 2013;185:797–804. doi: 10.1016/j.jss.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 340.Griez E.J.L., Lousberg H., van den Hout M.A. CO2 vulnerability in panic disorder. Psychiatry Res. 1987;20:87–95. doi: 10.1016/0165-1781(87)90001-1. [DOI] [PubMed] [Google Scholar]
- 341.Lebow M.A., Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry. 2016;21:450–463. doi: 10.1038/mp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wemmie J.A., Askwith C.C., Lamani E., Cassell M.D., Freeman J.H., Welsh M.J. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J. Neurosci. 2003;23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Wemmie J.A., Coryell M.W., Askwith C.C., Lamani E., Leonard A.S., Sigmund C.D., Welsh M.J. Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3621–3626. doi: 10.1073/pnas.0308753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Hagihara H., Catts V.S., Katayama Y., Shoji H., Takagi T., Huang F.L., Nakao A., Mori Y., Huang K.-P., Ishii S., Graef I.A., Nakayama K.I., Shannon Weickert C., Miyakawa T. Decreased Brain pH as a Shared Endophenotype of Psychiatric Disorders. Neuropsychopharmacology. 2018;43:459–468. doi: 10.1038/npp.2017.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Bühlmeier J., Harris C., Koletzko S., Lehmann I., Bauer C.-P., Schikowski T., von Berg A., Berdel D., Heinrich J., Hebebrand J., Föcker M., Standl M., Libuda L. Dietary Acid Load and Mental Health Outcomes in Children and Adolescents: Results from the GINIplus and LISA Birth Cohort Studies. Nutrients. 2018;10 doi: 10.3390/nu10050582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Niculescu A.B., Levey D.F., Phalen P.L., Le-Niculescu H., Dainton H.D., Jain N., Belanger E., James A., George S., Weber H., Graham D.L., Schweitzer R., Ladd T.B., Learman R., Niculescu E.M., Vanipenta N.P., Khan F.N., Mullen J., Shankar G., Cook S., Humbert C., Ballew A., Yard M., Gelbart T., Shekhar A., Schork N.J., Kurian S.M., Sandusky G.E., Salomon D.R. Understanding and predicting suicidality using a combined genomic and clinical risk assessment approach. Mol Psychiatry. 2015;20:1266–1285. doi: 10.1038/mp.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Kraut J.A., Madias N.E. Metabolic Acidosis of CKD: An Update. Am. J. Kidney Dis. 2016;67:307–317. doi: 10.1053/j.ajkd.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 348.Jhee J.H., Lee E., Cha M.-U., Lee M., Kim H., Park S., Yun H.-R., Jung S.-Y., Kee Y.K., Yoon C.-Y., Han S.H., Yoo T.-H., Kang S.-W., Park J.T. Prevalence of depression and suicidal ideation increases proportionally with renal function decline, beginning from early stages of chronic kidney disease. Medicine (Baltimore). 2017;96 doi: 10.1097/MD.0000000000008476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Valença A.M., Nardi A.E., Nascimento I., Zin W.A., Versiani M. Carbon dioxide test as an additional clinical measure of treatment response in panic disorder. Arq Neuropsiquiatr. 2002;60:358–361. doi: 10.1590/s0004-282x2002000300003. [DOI] [PubMed] [Google Scholar]
- 350.Mathew R.J., Wilson W.H., Tant S. Responses to hypercarbia induced by acetazolamide in panic disorder patients. Am J Psychiatry. 1989;146:996–1000. doi: 10.1176/ajp.146.8.996. [DOI] [PubMed] [Google Scholar]
- 351.Halim N.D., Lipska B.K., Hyde T.M., Deep-Soboslay A., Saylor E.M., Herman M.M., Thakar J., Verma A., Kleinman J.E. Increased lactate levels and reduced pH in postmortem brains of schizophrenics: medication confounds. J. Neurosci. Methods. 2008;169:208–213. doi: 10.1016/j.jneumeth.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Holloway A.M. Possible alternatives to soda lime. Anaesth Intensive Care. 1994;22:359–362. doi: 10.1177/0310057X9402200405. [DOI] [PubMed] [Google Scholar]
- 353.B.S. Freeman, Absorption of carbon dioxide, in: Anesthesiology Core Review, 1.0, McGraw-Hill, 2014: pp. 45–48.
- 354.Baum J.A., Woehlck H.J. Interaction of inhalational anaesthetics with CO2 absorbents. Best Practice & Research Clinical Anaesthesiology. 2003;17:63–76. doi: 10.1053/bean.2003.0269. [DOI] [PubMed] [Google Scholar]
- 355.Curley G., Contreras M.M.B., Nichol A.D., Higgins B.D., Laffey J.G. Hypercapnia and acidosis in sepsis: a double-edged sword? Anesthesiology. 2010;112:462–472. doi: 10.1097/ALN.0b013e3181ca361f. [DOI] [PubMed] [Google Scholar]
- 356.Choi Y.J., Kim M.C., Lim Y.J., Yoon S.Z., Yoon S.M., Yoon H.R. Propofol Infusion Associated Metabolic Acidosis in Patients Undergoing Neurosurgical Anesthesia: A Retrospective Study. J Korean Neurosurg Soc. 2014;56:135–140. doi: 10.3340/jkns.2014.56.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Lawton T.O., Quinn A., Fletcher S.J. Perioperative metabolic acidosis: The Bradford Anaesthetic Department Acidosis Study. J Intensive Care Soc. 2019;20:11–17. doi: 10.1177/1751143718772792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Skellett S., Mayer A., Durward A., Tibby S.M., Murdoch I.A. Chasing the base deficit: hyperchloraemic acidosis following 0.9% saline fluid resuscitation. Arch. Dis. Child. 2000;83:514–516. doi: 10.1136/adc.83.6.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Park C.-M., Chun H.-K., Jeon K., Suh G.Y., Choi D.W., Kim S. Factors related to post-operative metabolic acidosis following major abdominal surgery. ANZ Journal of Surgery. 2014;84:574–580. doi: 10.1111/j.1445-2197.2012.06235.x. [DOI] [PubMed] [Google Scholar]
- 360.Waters J.H., Miller L.R., Clack S., Kim J.V. Cause of metabolic acidosis in prolonged surgery. Crit. Care Med. 1999;27:2142–2146. doi: 10.1097/00003246-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 361.Okusawa S., Aikawa N., Abe O. Postoperative metabolic alkalosis following general surgery: its incidence and possible etiology. Jpn J Surg. 1989;19:312–318. doi: 10.1007/BF02471407. [DOI] [PubMed] [Google Scholar]
- 362.Bıçakçı Z., Olcay L. Citrate metabolism and its complications in non-massive blood transfusions: association with decompensated metabolic alkalosis+respiratory acidosis and serum electrolyte levels. Transfus. Apher. Sci. 2014;50:418–426. doi: 10.1016/j.transci.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 363.Silva J., Oliveira A., Marti Y., Gonzaga T., Ferreira A., Maia V.P., Rezend E. Outcome of surgical patients who present acidosis postoperatively. Crit Care. 2011;15:P64. doi: 10.1186/cc10212. [DOI] [Google Scholar]
- 364.Oyet C., Okongo B., Onyuthi R.A., Muwanguzi E. Biochemical changes in stored donor units: implications on the efficacy of blood transfusion. J. Blood. Med. 2018;9:111–115. doi: 10.2147/JBM.S163651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Zubair A.C. Clinical impact of blood storage lesions. Am. J. Hematol. 2010;85:117–122. doi: 10.1002/ajh.21599. [DOI] [PubMed] [Google Scholar]
- 366.Schneider L.A., Korber A., Grabbe S., Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch. Dermatol. Res. 2007;298:413–420. doi: 10.1007/s00403-006-0713-x. [DOI] [PubMed] [Google Scholar]
- 367.Shangraw R.E., Lohan-Mannion D., Hayes A., Moriarty R.M., Fu R., Robinson S.T. Dichloroacetate stabilizes the intraoperative acid-base balance during liver transplantation. Liver Transpl. 2008;14:989–998. doi: 10.1002/lt.21485. [DOI] [PubMed] [Google Scholar]
- 368.Chang A.L., Kim Y., Seitz A.P., Schuster R.M., Pritts T.A. pH modulation ameliorates the red blood cell storage lesion in a murine model of transfusion. J. Surg. Res. 2017;212:54–59. doi: 10.1016/j.jss.2016.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Lilienthal B. An analysis of the buffer systems in saliva. J. Dent. Res. 1955;34:516–530. doi: 10.1177/00220345550340040701. [DOI] [PubMed] [Google Scholar]
- 370.Müller V.J., Belibasakis G.N., Bosshard P.P., Wiedemeier D.B., Bichsel D., Rücker M., Stadlinger B. Change of saliva composition with radiotherapy. Arch. Oral Biol. 2019;106 doi: 10.1016/j.archoralbio.2019.104480. [DOI] [PubMed] [Google Scholar]
- 371.Ayars G.H., Altman L.C., Fretwell M.D. Effect of decreased salivation and pH on the adherence of Klebsiella species to human buccal epithelial cells. Infect Immun. 1982;38:179–182. doi: 10.1128/iai.38.1.179-182.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Kaur A., Kwatra K.S., Kamboj P. Evaluation of non-microbial salivary caries activity parameters and salivary biochemical indicators in predicting dental caries. J Indian Soc Pedod Prev Dent. 2012;30:212–217. doi: 10.4103/0970-4388.105013. [DOI] [PubMed] [Google Scholar]
- 373.González-Aragón Pineda A.E., García Pérez A., García-Godoy F. Salivary parameters and oral health status amongst adolescents in Mexico. BMC Oral Health. 2020;20:190. doi: 10.1186/s12903-020-01182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Pyati S.A., Naveen Kumar R., Kumar V., Praveen Kumar N.H., Parveen Reddy K.M. Salivary Flow Rate, pH, Buffering Capacity, Total Protein, Oxidative Stress and Antioxidant Capacity in Children with and without Dental Caries. J Clin Pediatr Dent. 2018;42:445–449. doi: 10.17796/1053-4625-42.6.7. [DOI] [PubMed] [Google Scholar]
- 375.Woyceichoski I.E.C., Costa C.H., de Araújo C.M., Brancher J.A., Resende L.G., Vieira I., de Lima A.A.S. Salivary buffer capacity, pH, and stimulated flow rate of crack cocaine users. J Investig Clin Dent. 2013;4:160–163. doi: 10.1111/j.2041-1626.2012.00126.x. [DOI] [PubMed] [Google Scholar]
- 376.Hamza S.A., Wahid A., Afzal N., Asif S., Imran M.F., Khurshid Z., Bokhari S.A.H. Effect of Sodium Bicarbonate Mouth Wash on Salivary pH and Interleukin-1β Levels among Smokers. Eur J Dent. 2020;14:260–267. doi: 10.1055/s-0040-1709896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Pawlaczyk-Kamieńska T., Borysewicz-Lewicka M., Batura-Gabryel H. Salivary Biomarkers and Oral Microbial Load in Relation to the Dental Status of Adults with Cystic Fibrosis. Microorganisms. 2019;7 doi: 10.3390/microorganisms7120692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Pedersen A.M.L., Bardow A., Nauntofte B. Salivary changes and dental caries as potential oral markers of autoimmune salivary gland dysfunction in primary Sjögren’s syndrome. BMC Clinical Pathology. 2005;5:4. doi: 10.1186/1472-6890-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Kobus A., Kierklo A., Zalewska A., Kuźmiuk A., Szajda S.D., Ławicki S., Bagińska J. Unstimulated salivary flow, pH, proteins and oral health in patients with Juvenile Idiopathic Arthritis. BMC Oral Health. 2017;17:94. doi: 10.1186/s12903-017-0386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Lacruz R.S., Smith C.E., Kurtz I., Hubbard M.J., Paine M.L. New paradigms on the transport functions of maturation-stage ameloblasts. J. Dent. Res. 2013;92:122–129. doi: 10.1177/0022034512470954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Myers E.J., Yuan L., Felmlee M.A., Lin Y.-Y., Jiang Y., Pei Y., Wang O., Li M., Xing X.-P., Marshall A., Xia W.-B., Parker M.D. A novel mutant Na+ /HCO3- cotransporter NBCe1 in a case of compound-heterozygous inheritance of proximal renal tubular acidosis. J. Physiol. (Lond.) 2016;594:6267–6286. doi: 10.1113/JP272252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Salerno E.E., Patel S.P., Marshall A., Marshall J., Alsufayan T., Mballo C.S.A., Quade B.N., Parker M.D. Extrarenal Signs of Proximal Renal Tubular Acidosis Persist in Nonacidemic Nbce1b/c-Null Mice. J. Am. Soc. Nephrol. 2019;30:979–989. doi: 10.1681/ASN.2018050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Baliga S., Muglikar S., Kale R. Salivary pH: A diagnostic biomarker. J Indian Soc Periodontol. 2013;17:461–465. doi: 10.4103/0972-124X.118317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Ciancio S.G. Baking soda dentifrices and oral health. J Am Dent Assoc. 2017;148:S1–S3. doi: 10.1016/j.adaj.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 385.Chandel S., Khan M.A., Singh N., Agrawal A., Khare V. The effect of sodium bicarbonate oral rinse on salivary pH and oral microflora: A prospective cohort study. Natl J Maxillofac Surg. 2017;8:106–109. doi: 10.4103/njms.NJMS_36_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Abbate G.M., Levrini L., Caria M.P. Salivary pH after a glucose rinse: effect of a new mucoadhesive spray (Cariex) based on sodium bicarbonate and xylitol. J Clin Dent. 2014;25:71–75. [PubMed] [Google Scholar]
- 387.Ballal R.K., Bhat S.S., Ramdas S.S., Ballal S. Effect of Chewing Bicarbonate-containing Sugar-free Gum on the Salivary pH: An in vivo Study. Int J Clin Pediatr Dent. 2016;9:35–38. doi: 10.5005/jp-journals-10005-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Choi S.-E., Kim H.-S. Sodium Bicarbonate Solution versus Chlorhexidine Mouthwash in Oral Care of Acute Leukemia Patients Undergoing Induction Chemotherapy: A Randomized Controlled Trial. Asian Nurs Res (Korean Soc Nurs Sci). 2012;6:60–66. doi: 10.1016/j.anr.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 389.Blake-Haskins J.C., Gaffar A., Volpe A.R., Bánóczy J., Gintner Z., Dombi C. The effect of bicarbonate/fluoride dentifrices on human plaque pH. J Clin Dent. 1997;8:173–177. [PubMed] [Google Scholar]
- 390.Putt M.S., Milleman K.R., Ghassemi A., Vorwerk L.M., Hooper W.J., Soparkar P.M., Winston A.E., Proskin H.M. Enhancement of plaque removal efficacy by tooth brushing with baking soda dentifrices: results of five clinical studies. J Clin Dent. 2008;19:111–119. [PubMed] [Google Scholar]
- 391.Tanzer J.M., McMahon T., Grant L. Bicarbonate-based powder and paste dentifrice effects on caries. Clin Prev Dent. 1990;12:18–21. [PubMed] [Google Scholar]
- 392.Chacko Y., Lakshminarayanan L. pH stabilizing properties of a posterior light cured resin composite: an in vivo study. Oper Dent. 2001;26:219–222. [PubMed] [Google Scholar]
- 393.Campus G., Cagetti M.G., Sacco G., Solinas G., Mastroberardino S., Lingström P. Six months of daily high-dose xylitol in high-risk schoolchildren: a randomized clinical trial on plaque pH and salivary mutans streptococci. Caries Res. 2009;43:455–461. doi: 10.1159/000264682. [DOI] [PubMed] [Google Scholar]
- 394.Liu H., Maruyama H., Masuda T., Honda A., Arai F. The Influence of Virus Infection on the Extracellular pH of the Host Cell Detected on Cell Membrane. Front Microbiol. 2016;7 doi: 10.3389/fmicb.2016.01127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Dekker T., Geier M., Cardé R.T. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J. Exp. Biol. 2005;208:2963–2972. doi: 10.1242/jeb.01736. [DOI] [PubMed] [Google Scholar]
- 396.Baldwin M.R., Li X., Hanada T., Liu S.-C., Chishti A.H. Merozoite surface protein 1 recognition of host glycophorin A mediates malaria parasite invasion of red blood cells. Blood. 2015;125:2704–2711. doi: 10.1182/blood-2014-11-611707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Rosanas-Urgell A., Lin E., Manning L., Rarau P., Laman M., Senn N., Grimberg B.T., Tavul L., Stanisic D.I., Robinson L.J., Aponte J.J., Dabod E., Reeder J.C., Siba P., Zimmerman P.A., Davis T.M.E., King C.L., Michon P., Mueller I. Reduced risk of Plasmodium vivax malaria in Papua New Guinean children with Southeast Asian ovalocytosis in two cohorts and a case-control study. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Day N.P., Phu N.H., Mai N.T., Chau T.T., Loc P.P., Chuong L.V., Sinh D.X., Holloway P., Hien T.T., White N.J. The pathophysiologic and prognostic significance of acidosis in severe adult malaria. Crit. Care Med. 2000;28:1833–1840. doi: 10.1097/00003246-200006000-00025. [DOI] [PubMed] [Google Scholar]
- 399.Dondorp A.M., Chau T.T.H., Phu N.H., Mai N.T.H., Loc P.P., Chuong L.V., Sinh D.X., Taylor A., Hien T.T., White N.J., Day N.P.J. Unidentified acids of strong prognostic significance in severe malaria. Crit. Care Med. 2004;32:1683–1688. doi: 10.1097/01.ccm.0000132901.86681.ca. [DOI] [PubMed] [Google Scholar]
- 400.Jalily P.H., Duncan M.C., Fedida D., Wang J., Tietjen I. Put a cork in it: Plugging the M2 viral ion channel to sink influenza. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Charpiat B., Bleyzac N., Tod M. Proton Pump Inhibitors are Risk Factors for Viral Infections: Even for COVID-19? Clin Drug Investig. 2020 doi: 10.1007/s40261-020-00963-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Krishna S., Agbenyega T., Angus B.J., Bedu-Addo G., Ofori-Amanfo G., Henderson G., Szwandt I.S., O’Brien R., Stacpoole P.W. Pharmacokinetics and pharmacodynamics of dichloroacetate in children with lactic acidosis due to severe malaria. QJM. 1995;88:341–349. [PubMed] [Google Scholar]
- 403.Jajosky R.P., Jajosky A.N., Jajosky P.G. Can exchange transfusions using red blood cells from donors with Southeast Asian ovalocytosis prevent or ameliorate cerebral malaria in patients with multi-drug resistant Plasmodium falciparum? Transfus. Apher. Sci. 2017;56:865–866. doi: 10.1016/j.transci.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 404.Spillman N.J., Allen R.J.W., McNamara C.W., Yeung B.K.S., Winzeler E.A., Diagana T.T., Kirk K. Na(+) regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe. 2013;13:227–237. doi: 10.1016/j.chom.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Liberti M.V., Locasale J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.DeBerardinis R.J., Chandel N.S. We need to talk about the Warburg effect. Nat Metab. 2020;2:127–129. doi: 10.1038/s42255-020-0172-2. [DOI] [PubMed] [Google Scholar]
- 407.Parks S.K., Chiche J., Pouyssegur J. pH control mechanisms of tumor survival and growth. J. Cell. Physiol. 2011;226:299–308. doi: 10.1002/jcp.22400. [DOI] [PubMed] [Google Scholar]
- 408.Gorbatenko A., Olesen C.W., Boedtkjer E., Pedersen S.F. Regulation and roles of bicarbonate transporters in cancer. Front Physiol. 2014;5:130. doi: 10.3389/fphys.2014.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Fliegel L. Role of pH Regulatory Proteins and Dysregulation of pH in Prostate Cancer. Rev. Physiol. Biochem. Pharmacol. 2020 doi: 10.1007/112_2020_18. [DOI] [PubMed] [Google Scholar]
- 410.McIntyre A., Hulikova A., Ledaki I., Snell C., Singleton D., Steers G., Seden P., Jones D., Bridges E., Wigfield S., Li J.-L., Russell A., Swietach P., Harris A.L. Disrupting Hypoxia-Induced Bicarbonate Transport Acidifies Tumor Cells and Suppresses Tumor Growth. Cancer Res. 2016;76:3744–3755. doi: 10.1158/0008-5472.CAN-15-1862. [DOI] [PubMed] [Google Scholar]
- 411.Mboge M.Y., Mahon B.P., McKenna R., Frost S.C. Carbonic Anhydrases: Role in pH Control and Cancer. Metabolites. 2018;8 doi: 10.3390/metabo8010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Boron W.F. Sharpey-Schafer lecture: gas channels. Exp. Physiol. 2010;95:1107–1130. doi: 10.1113/expphysiol.2010.055244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Hulikova A., Swietach P. Rapid CO2 permeation across biological membranes: implications for CO2 venting from tissue. FASEB J. 2014;28:2762–2774. doi: 10.1096/fj.13-241752. [DOI] [PubMed] [Google Scholar]
- 414.Dajani S., Saripalli A., Sharma-Walia N. Water transport proteins–aquaporins (AQPs) in cancer biology. Oncotarget. 2018;9:36392–36405. doi: 10.18632/oncotarget.26351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.LaMonte G., Tang X., Chen J.L.-Y., Wu J., Ding C.-K.C., Keenan M.M., Sangokoya C., Kung H.-N., Ilkayeva O., Boros L.G., Newgard C.B., Chi J.-T. Acidosis induces reprogramming of cellular metabolism to mitigate oxidative stress. Cancer Metab. 2013;1:23. doi: 10.1186/2049-3002-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Boedtkjer E., Pedersen S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020;82:103–126. doi: 10.1146/annurev-physiol-021119-034627. [DOI] [PubMed] [Google Scholar]
- 417.Bohn T., Rapp S., Luther N., Klein M., Bruehl T.-J., Kojima N., Aranda Lopez P., Hahlbrock J., Muth S., Endo S., Pektor S., Brand A., Renner K., Popp V., Gerlach K., Vogel D., Lueckel C., Arnold-Schild D., Pouyssegur J., Kreutz M., Huber M., Koenig J., Weigmann B., Probst H.-C., von Stebut E., Becker C., Schild H., Schmitt E., Bopp T. Tumor immunoevasion via acidosis-dependent induction of regulatory tumor-associated macrophages. Nat. Immunol. 2018;19:1319–1329. doi: 10.1038/s41590-018-0226-8. [DOI] [PubMed] [Google Scholar]
- 418.Wu T., Hsu F.-C., Pierce J.P. Increased Acid-Producing Diet and Past Smoking Intensity Are Associated with Worse Prognoses among Breast Cancer Survivors: A Prospective Cohort Study. J Clin Med. 2020;9 doi: 10.3390/jcm9061817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Moellering R.E., Black K.C., Krishnamurty C., Baggett B.K., Stafford P., Rain M., Gatenby R.A., Gillies R.J. Acid treatment of melanoma cells selects for invasive phenotypes. Clin. Exp. Metastasis. 2008;25:411–425. doi: 10.1007/s10585-008-9145-7. [DOI] [PubMed] [Google Scholar]
- 420.Gunawan I., Hatta M., Benyamin A.F., Islam A.A. The Hypoxic Response Expression as a Survival Biomarkers in Treatment-Naive Advanced Breast Cancer. Asian Pac. J. Cancer Prev. 2020;21:629–637. doi: 10.31557/APJCP.2020.21.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Wang H., Long X., Wang D., Lou M., Zou D., Chen R., Nian W., Zhou Q. Increased expression of Na+/H+ exchanger isoform 1 predicts tumor aggressiveness and unfavorable prognosis in epithelial ovarian cancer. Oncol Lett. 2018;16:6713–6720. doi: 10.3892/ol.2018.9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Li Z., Wu Q., Sun S., Wu J., Li J., Zhang Y., Wang C., Yuan J., Sun S. Monocarboxylate transporters in breast cancer and adipose tissue are novel biomarkers and potential therapeutic targets. Biochem. Biophys. Res. Commun. 2018;501:962–967. doi: 10.1016/j.bbrc.2018.05.091. [DOI] [PubMed] [Google Scholar]
- 423.Harguindey S., Stanciu D., Devesa J., Alfarouk K., Cardone R.A., Polo Orozco J.D., Devesa P., Rauch C., Orive G., Anitua E., Roger S., Reshkin S.J. Cellular acidification as a new approach to cancer treatment and to the understanding and therapeutics of neurodegenerative diseases. Semin. Cancer Biol. 2017;43:157–179. doi: 10.1016/j.semcancer.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 424.Lee S., Axelsen T.V., Andersen A.P., Vahl P., Pedersen S.F., Boedtkjer E. Disrupting Na+, HCO3−-cotransporter NBCn1 (Slc4a7) delays murine breast cancer development. Oncogene. 2016;35:2112–2122. doi: 10.1038/onc.2015.273. [DOI] [PubMed] [Google Scholar]
- 425.Wang B.-Y., Zhang J., Wang J.-L., Sun S., Wang Z.-H., Wang L.-P., Zhang Q.-L., Lv F.-F., Cao E.-Y., Shao Z.-M., Fais S., Hu X.-C. Intermittent high dose proton pump inhibitor enhances the antitumor effects of chemotherapy in metastatic breast cancer. J. Exp. Clin. Cancer Res. 2015;34:85. doi: 10.1186/s13046-015-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Marks I.S., Gardeen S.S., Kurdziel S.J., Nicolaou S.T., Woods J.E., Kularatne S.A., Low P.S. Development of a Small Molecule Tubulysin B Conjugate for Treatment of Carbonic Anhydrase IX Receptor Expressing Cancers. Mol. Pharm. 2018;15:2289–2296. doi: 10.1021/acs.molpharmaceut.8b00139. [DOI] [PubMed] [Google Scholar]
- 427.Albatany M., Ostapchenko V.G., Meakin S., Bartha R. Brain tumor acidification using drugs simultaneously targeting multiple pH regulatory mechanisms. J. Neurooncol. 2019;144:453–462. doi: 10.1007/s11060-019-03251-7. [DOI] [PubMed] [Google Scholar]
- 428.Pivovarova A.I., MacGregor G.G. Glucose-dependent growth arrest of leukemia cells by MCT1 inhibition: Feeding Warburg’s sweet tooth and blocking acid export as an anticancer strategy. Biomed. Pharmacother. 2018;98:173–179. doi: 10.1016/j.biopha.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 429.Alessandra L. Manipulating pH in Cancer Treatment: Alkalizing Drugs and Alkaline Diet. J Complement Med Alt Healthcare. 2017;2 [Google Scholar]
- 430.Robey I.F., Baggett B.K., Kirkpatrick N.D., Roe D.J., Dosescu J., Sloane B.F., Hashim A.I., Morse D.L., Raghunand N., Gatenby R.A., Gillies R.J. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69:2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Voss N.C.S., Dreyer T., Henningsen M.B., Vahl P., Honoré B., Boedtkjer E. Targeting the Acidic Tumor Microenvironment: Unexpected Pro-Neoplastic Effects of Oral NaHCO3 Therapy in Murine Breast Tissue. Cancers (Basel). 2020;12 doi: 10.3390/cancers12040891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.M. Chao, H. Wu, K. Jin, B. Li, J. Wu, G. Zhang, G. Yang, X. Hu, A nonrandomized cohort and a randomized study of local control of large hepatocarcinoma by targeting intratumoral lactic acidosis, ELife. 5 (n.d.). https://doi.org/10.7554/eLife.15691. [DOI] [PMC free article] [PubMed]
- 433.Zick S.M., Snyder D., Abrams D.I. Pros and Cons of Dietary Strategies Popular Among Cancer Patients. Oncology (Williston Park, N.Y.) 2018;32:542–547. [PubMed] [Google Scholar]
- 434.Hamano H., Mitsui M., Zamami Y., Takechi K., Nimura T., Okada N., Fukushima K., Imanishi M., Chuma M., Horinouchi Y., Izawa-Ishizawa Y., Kirino Y., Nakamura T., Teraoka K., Ikeda Y., Fujino H., Yanagawa H., Tamaki T., Ishizawa K. Irinotecan-induced neutropenia is reduced by oral alkalization drugs: analysis using retrospective chart reviews and the spontaneous reporting database. Support Care Cancer. 2019;27:849–856. doi: 10.1007/s00520-018-4367-y. [DOI] [PubMed] [Google Scholar]
- 435.Celay J., Lozano T., Concepcion A.R., Beltrán E., Rudilla F., García-Barchino M.J., Robles E.F., Rabal O., de Miguel I., Panizo C., Casares N., Oyarzabal J., Prieto J., Medina J.F., Lasarte J.J., Martínez-Climent J.Á. Targeting the anion exchanger 2 with specific peptides as a new therapeutic approach in B lymphoid neoplasms. Haematologica. 2018;103:1065–1072. doi: 10.3324/haematol.2017.175687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Nugent S., Kumar D., Rampton D., Evans D. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut. 2001;48:571–577. doi: 10.1136/gut.48.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Evans D.F., Pye G., Bramley R., Clark A.G., Dyson T.J., Hardcastle J.D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29:1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Hua S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract - Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Effinger A., O’Driscoll C.M., McAllister M., Fotaki N. Impact of gastrointestinal disease states on oral drug absorption – implications for formulation design – a PEARRL review. J. Pharm. Pharmacol. 2019;71:674–698. doi: 10.1111/jphp.12928. [DOI] [PubMed] [Google Scholar]
- 440.Abuhelwa A.Y., Williams D.B., Upton R.N., Foster D.J.R. Food, gastrointestinal pH, and models of oral drug absorption. Eur. J. Pharm. Biopharm. 2017;112:234–248. doi: 10.1016/j.ejpb.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 441.Bai J.P.F., Burckart G.J., Mulberg A.E. Literature Review of Gastrointestinal Physiology in the Elderly, Pediatric Patients, and in Patients with Gastrointestinal Diseases. J. Pharm. Sci. 2016;105:476–483. doi: 10.1002/jps.24696. [DOI] [PubMed] [Google Scholar]
- 442.Mitra A., Parrott N., Miller N., Lloyd R., Tistaert C., Heimbach T., Ji Y., Kesisoglou F. Prediction of pH-Dependent Drug-Drug Interactions for Basic Drugs Using Physiologically Based Biopharmaceutics Modeling: Industry Case Studies. J. Pharm. Sci. 2020;109:1380–1394. doi: 10.1016/j.xphs.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 443.Pobudkowska A., Domańska U. Study of pH-dependent drugs solubility in water. CI&CEQ. 2014;20:115–126. doi: 10.2298/CICEQ120531116P. [DOI] [Google Scholar]
- 444.Charifson P.S., Walters W.P. Acidic and Basic Drugs in Medicinal Chemistry: A Perspective. J. Med. Chem. 2014;57:9701–9717. doi: 10.1021/jm501000a. [DOI] [PubMed] [Google Scholar]
- 445.Savjani K.T., Gajjar A.K., Savjani J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012;2012 doi: 10.5402/2012/195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.D.H. Barich, M.T. Zell, E.J. Munson, Physicochemical Properties, Formulation, and Drug Delivery, in: Drug Delivery, John Wiley & Sons, Ltd, 2016: pp. 35–48. https://doi.org/10.1002/9781118833322.ch3.
- 447.Waring M.J., Johnstone C. A quantitative assessment of hERG liability as a function of lipophilicity. Bioorg. Med. Chem. Lett. 2007;17:1759–1764. doi: 10.1016/j.bmcl.2006.12.061. [DOI] [PubMed] [Google Scholar]
- 448.Jacobs M.H. Some aspects of cell permeability to weak electrolytes. Cold Spring Harbor Symp Quant Biol. 1940;8:30–39. [Google Scholar]
- 449.Shore P.A., Brodie B.B., Hogben C.A.M. The gastric secretion of drugs: a pH partition hypothesis. J Pharmacol Exp Ther. 1957;119:361–369. [PubMed] [Google Scholar]
- 450.Helander H.F., Fändriks L. Surface area of the digestive tract - revisited. Scand. J. Gastroenterol. 2014;49:681–689. doi: 10.3109/00365521.2014.898326. [DOI] [PubMed] [Google Scholar]
- 451.Salama N.N., Eddington N.D., Fasano A. Tight junction modulation and its relationship to drug delivery. Adv. Drug Deliv. Rev. 2006;58:15–28. doi: 10.1016/j.addr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 452.Wagner D.J., Hu T., Wang J. Polyspecific Organic Cation Transporters and their Impact on Drug Intracellular Levels and Pharmacodynamics. Pharmacol Res. 2016;111:237–246. doi: 10.1016/j.phrs.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Andreev E., Brosseau N., Carmona E., Mes-Masson A.-M., Ramotar D. The human organic cation transporter OCT1 mediates high affinity uptake of the anticancer drug daunorubicin. Sci. Rep. 2016;6:20508. doi: 10.1038/srep20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Nigam S.K., Bush K.T., Martovetsky G., Ahn S.-Y., Liu H.C., Richard E., Bhatnagar V., Wu W. The Organic Anion Transporter (OAT) Family: A Systems Biology Perspective. Physiol Rev. 2015;95:83–123. doi: 10.1152/physrev.00025.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Wessler J.D., Grip L.T., Mendell J., Giugliano R.P. The P-Glycoprotein Transport System and Cardiovascular Drugs. J Am Coll Cardiol. 2013;61:2495–2502. doi: 10.1016/j.jacc.2013.02.058. [DOI] [PubMed] [Google Scholar]
- 456.Kim R.B. Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug Metab. Rev. 2002;34:47–54. doi: 10.1081/DMR-120001389. [DOI] [PubMed] [Google Scholar]
- 457.Mitra A., Kesisoglou F. Impaired Drug Absorption Due to High Stomach pH: A Review of Strategies for Mitigation of Such Effect To Enable Pharmaceutical Product Development. Mol. Pharmaceutics. 2013;10:3970–3979. doi: 10.1021/mp400256h. [DOI] [PubMed] [Google Scholar]
- 458.Farha M.A., French S., Stokes J.M., Brown E.D. Bicarbonate Alters Bacterial Susceptibility to Antibiotics by Targeting the Proton Motive Force. ACS Infect Dis. 2018;4:382–390. doi: 10.1021/acsinfecdis.7b00194. [DOI] [PubMed] [Google Scholar]
- 459.El-Sherbiny I.M., Salama A., Sarhan A.A. Ionotropically cross-linked pH-sensitive IPN hydrogel matrices as potential carriers for intestine-specific oral delivery of protein drugs. Drug Dev. Ind. Pharm. 2011;37:121–130. doi: 10.3109/03639045.2010.495754. [DOI] [PubMed] [Google Scholar]
- 460.Koetting M.C., Guido J.F., Gupta M., Zhang A., Peppas N.A. pH-responsive and enzymatically-responsive hydrogel microparticles for the oral delivery of therapeutic proteins: Effects of protein size, crosslinking density, and hydrogel degradation on protein delivery. J. Control. Release. 2016;221:18–25. doi: 10.1016/j.jconrel.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Yu M., Wu J., Shi J., Farokhzad O.C. Nanotechnology for protein delivery: Overview and perspectives. J. Control. Release. 2016;240:24–37. doi: 10.1016/j.jconrel.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Thakral S., Thakral N.K., Majumdar D.K. Eudragit: a technology evaluation. Expert Opin Drug Deliv. 2013;10:131–149. doi: 10.1517/17425247.2013.736962. [DOI] [PubMed] [Google Scholar]
- 463.Yoshida K., Sato H., Takahashi S., Anzai J. Preparation of Layer-by-layer Thin Films Containing Insulin and its pH-Sensitive Decomposition. Polym. J. 2008;40:90–91. doi: 10.1295/polymj.PJ2007126. [DOI] [Google Scholar]
- 464.Lowman A.M., Morishita M., Kajita M., Nagai T., Peppas N.A. Oral delivery of insulin using pH-responsive complexation gels. J. Pharm. Sci. 1999;88:933–937. doi: 10.1021/js980337n. [DOI] [PubMed] [Google Scholar]
- 465.Ornillo C., Harbord N. Fundaments of Toxicology—Approach to the Poisoned Patient. Advances in Chronic Kidney Disease. 2020;27:5–10. doi: 10.1053/j.ackd.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 466.Mirrakhimov A.E., Ayach T., Barbaryan A., Talari G., Chadha R., Gray A. The Role of Sodium Bicarbonate in the Management of Some Toxic Ingestions. Int J Nephrol. 2017;2017 doi: 10.1155/2017/7831358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Minich D., Bland J. Acid-alkaline balance: role in chronic disease and detoxification. Altern. Ther. Health Med. 2007 [PubMed] [Google Scholar]
- 468.Ito S., Ando H., Ose A., Kitamura Y., Ando T., Kusuhara H., Sugiyama Y. Relationship Between the Urinary Excretion Mechanisms of Drugs and Their Physicochemical Properties. JPharmSci. 2013;102:3294–3301. doi: 10.1002/jps.23599. [DOI] [PubMed] [Google Scholar]
- 469.Shim B.H., Kim D.K., Ye J.S., Lee J. Gastro-floating matrices designed for simultaneous improvement of floating and drug release capabilities: an in vitro case study of matrices loaded with ciprofloxacin hydrochloride. J. Drug Delivery Sci. Technol. 2011;21:279–281. doi: 10.1016/S1773-2247(11)50038-3. [DOI] [Google Scholar]
- 470.Gao G.H., Li Y., Lee D.S. Environmental pH-sensitive polymeric micelles for cancer diagnosis and targeted therapy. J Control Release. 2013;169:180–184. doi: 10.1016/j.jconrel.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 471.Pellegrini P., Serviss J.T., Lundbäck T., Bancaro N., Mazurkiewicz M., Kolosenko I., Yu D., Haraldsson M., D’Arcy P., Linder S., De Milito A. A drug screening assay on cancer cells chronically adapted to acidosis. Cancer Cell International. 2018;18:147. doi: 10.1186/s12935-018-0645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]