Abstract
Hydrogels are a class of biomaterials used for a wide range of biomedical applications, including as a three-dimensional (3D) scaffold for cell culture that mimics the extracellular matrix (ECM) of native tissues. To understand the role of the ECM in the modulation of cardiac cell function, alginate was used to fabricate crosslinked gels with stiffness values that resembled embryonic (2.66 ± 0.84 kPa), physiologic (8.98 ± 1.29 kPa) and fibrotic (18.27 ± 3.17 kPa) cardiac tissues. The average pore diameter and hydrogel swelling were seen to decrease with increasing substrate stiffness. Cardiomyocytes cultured within soft embryonic gels demonstrated enhanced cell spreading, elongation, and network formation, while a progressive increase in gel stiffness diminished these behaviors. Cell viability decreased with increasing hydrogel stiffness. Furthermore, cells in fibrotic gels showed enhanced protein expression of the characteristic cardiac stress biomarker, Troponin-I, while reduced protein expression of the cardiac gap junction protein, Connexin-43, in comparison to cells within embryonic gels. The results from this study demonstrate the role that 3D substrate stiffness has on cardiac tissue formation and its implications in the development of complex matrix remodeling-based conditions, such as myocardial fibrosis.
Electronic supplementary material
The online version of this article (10.1007/s40204-020-00137-0) contains supplementary material, which is available to authorized users.
Keywords: Alginate, Cardiomyocytes, Elastic modulus, Cell viability, Scaffold stiffness
Introduction
Tissue stiffness is a dynamic biomechanical property that influences organismal development and physiology (Chen and Liu 2016; Stowers et al. 2015). As the extracellular matrix (ECM) undergoes remodeling during various biological processes, the mechanical properties of the environment in which living cells are embedded change accordingly. It is well established that variances in substrate stiffness can affect cellular migration, alignment, proliferation, morphology, and differentiation (Lee and Mooney 2001; Yang et al. 2017). In the human heart, the elastic modulus of the myocardium which develops from an embryonic value in the low kPa regime (2–4 kPa) (Engler et al. 2008) significantly increases beyond these values after cardiac fibrosis resulting from hypertrophy or myocardial infarction (Pandey et al. 2018). Biophysical characterization of the extracellular environment of cardiomyocytes has shown that extracellular stiffness is a major driving force for the maintenance of cardiomyocyte function and behavior such that deviations from ideal stiffness result in disruption of calcium transients, force generation, and alignment of sarcomeres during maturation (Jacot et al. 2008). A major challenge in the field is unraveling mechano-transduction pathways or the molecular mechanisms by which cells sense and respond to such mechano-biological signals. The overall objective of the present study is to develop systems that can be used to model the behavior of cardiomyocytes in progressively stiffer substrates representing healthy (embryonic and physiologic) and diseased (fibrotic) cardiac tissue states to study the underlying mechanisms involved in modulation of cardiomyocyte behavior and function.
Hydrogels are water-swollen polymeric networks that consist of crosslinked hydrophilic polymers that swell yet retain their structural fidelity in aqueous environments (Hosseinkhani et al. 2010). Such hydrogel-based biomaterials are often used to mimic the three-dimensional ECM scaffold found in native tissues, such as skin (Khalaji et al. 2020) and cardiac tissue (Hoffman 2012), because they are biocompatible, mostly biodegradable, and provide mechanical support to developing tissue. These highly porous and water-rich polymers provide an excellent means for cells to migrate and permit the effective distribution of nutrients to meet their metabolic needs (Nicodemus and Bryant 2008). This ability to swell under biological conditions makes both natural and synthetic hydrogels widely applicable for biomedical applications, such as tissue engineering (Anil Kumar et al. 2019a, b; Toosi et al. 2016), defect restoration (Toosi et al. 2019), wound healing (Qu et al. 2018; Cleetus et al. 2020), drug delivery (Cao et al. 2019; Narayanaswamy and Torchilin 2019; Farokhi et al. 2016), stem cell maturation and differentiation (Ghodsizadeh et al. 2014), and targeted cell-therapy (Joddar et al. 2018). Hydrogels possess a 3D-networked structure cross-linked through physical or chemical means (Huebsch et al. 2014). This insoluble cross-linked structure and high water content make them resemble natural soft tissue more than any other types of polymeric biomaterials. Hydrogel materials generally exhibit good biocompatibility and high permeability for oxygen, nutrients, and other water-soluble metabolites, making them attractive scaffolds for use in cell encapsulation (Joddar et al. 2018). Most hydrogels can be formed byphoto-polymerization, which can be carried out under mild conditions in the presence of living cells (Anil Kumar et al. 2019a, b). This allows homogeneous seeding of cells throughout the scaffold materials and formation of hydrogels in situ. Various elastic hydrogels with ~ 99% aqueous media can be formulated at physiological conditions with the maintenance of cell viability and function (Joddar et al. 2016; Lim and Sun 1980) while allowing the effective release of biomolecules and nutrients from the substates (Sharifzadeh and Hosseinkhani 2017).
Among these hydrogels, alginates have demonstrated extensive applicability as scaffolds for cell culture by ionic crosslinking in the presence of divalent Ca2+ ions (Lim and Sun 1980; Sun and Tan 2013). The widespread applicability of alginate hydrogels has been extended to culturing different cell types, both in vivo and in vitro (Andersen et al. 2015; Mondal et al. 2019). Alginate is a naturally derived polysaccharide from seaweed. Its linear molecular backbone is composed of (1,4)-linked β-d-mannuronate (M) and α-l-guluronate (G) residues in varying contents and distributions along the length of the polymer (Lee and Mooney 2012). Although the proportion of M to G residues varies, alginate can be more readily crosslinked as more G residues are present in the molecule along with divalent cations, such as Ca2+. Some of the well-known benefits of alginate hydrogels are gelation at physiological conditions, which permit transparency for microscopic evaluation and enable retention of homogenous, porous network formation that allows diffusion of nutrients and removal of waste (Drury and Mooney 2003).
Furthermore, cells can be extracted from these hydrogels using mild dissolution conditions that permit the retention of all native cell behavior and morphology (Andersen et al. 2015). However, these alginate gels are mechanically insufficient, towards their use as scaffolds to engineer robust biological tissues, such as cardiac tissue. In a previous study, we sought to address this challenge by encapsulation of multi-walled carbon nanotubes (MWCNT) serving as a reinforcing phase while being dispersed in a continuous phase of alginate (Joddar et al. 2016). Since the high doses of MWCNT were relatively cytotoxic, we next established an alternate method of dissolving CNT in surfactants for their homogenous dispersion, which also affords cytocompatibility (Alvarez-Primo et al. 2019). This and other such studies (Andersen et al. 2015; Yildirim et al. 2008) create the need for well-characterized alginates with high purity and consistent mechanical properties for cell encapsulation.
In this study, we adopted an alginate-based hydrogel system (Stowers et al. 2015) that was used to create scaffolds with biomechanical properties depicting various developmental and disease states in cardiac tissues, namely embryonic (soft), physiological (compliant/normal) and fibrotic (stiff) states. Human cardiomyocytes were cultured in these three distinct gels, and the resulting differences in morphology, intercellular network formation, and characteristic protein expression profile were compared. We hypothesized that cardiomyocytes in stiffer scaffolds would express altered behavior, including reduced cell spreading, elongation, and network formation when compared to cells cultured in softer scaffolds.
Results from this study reveal important information about the regulation of cardiomyocyte cell behavior in response to mechanical variances in the extracellular microenvironment. Basically by modulating the surrounding scaffold stiffness, we expected to identify and reveal important physiological changes in the cultured cardiomyocytes, which may be recapitulative of cardiac disease or fibrosis in vivo.
Materials and methods
Materials
MVG alginate (medium viscosity or MV, high guluronic or G content; Nova Matrix, Norway) was used to create three distinct gels of varying elastic moduli and was combined with Matrigel Matrix (Corning Inc., Corning, NY, USA) to provide points of cell adhesion within the crosslinked gels. According to the manufacturer, the alginate has greater than 60% G character. Calcium carbonate (CaCO3; Fisher Scientific, Fair Lawn, NJ, USA) and d-(+)-gluconic acid δ-lactone (GDL; Millipore Sigma, St. Louis, MO) were used as crosslinking agents. A buffer solution was created with sodium chloride (NaCl; Fisher Scientific, Fair Lawn, NJ, USA) and 1 M HEPES buffer solution (Fisher Scientific, Fair Lawn, NJ, USA).
Gel formulation
Alginate gels of varying stiffness were created, as described in Table 1. In brief, 1 mL of the gel was made by mixing 250 L Matrigel with 400 L of an alginate solution in Table 1 and vortexed to ensure homogeneity. All solutions were sterile-filtered, with the exception of the alginate solutions, which were autoclaved. After vortexing, 100 L of 300 mM NaCl in 1 mM HEPES buffer was then added to the alginate mixture and vortexed once again to further dissolve any unmixed powder. Meanwhile, another 100 L of the buffer was mixed separately with 50 L calcium carbonate 50 L GDL to create a dilute crosslinking solution. After seeding cardiomyocytes into the 24-well plate with the alginate (cell culture), the crosslinking solution is then pipetted into the well and mixed to ensure cell encapsulation by the crosslinked alginate gel. For the cell assays, the cell mixture represented 5% of the total gel volume, and all other solutions were sterile-filtered using 0.2 μm filters (Aerodisc LC 25 mm, PALL Life Sciences, Ann Arbor, MI, USA) prior to the gel preparation. Gels used for characterization were crosslinked without the addition of cells.
Table 1.
Concentrations of alginate, calcium carbonate, and GDL are shown for embryonic, physiologic, and fibrotic gels (from left to right)
| = | 400 L | + | 50 L | + | 50 L | + | 200 L | + | 250 L | + | 50 L | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Embryonic gel | = | 4% MVG alginate | + |
17.45 mg/mL (174 mM) CaCO3 |
+ | 62.21 mg/mL (348 mM) GDL | + | 300 mM NaCl + 1 mM HEPES buffer | + | Matrigel | + | Cell suspension |
| Physiologic gel | = | 7% MVG alginate | + |
45.35 mg/mL (453 mM) CaCO3 |
+ |
161.4 mg/mL (906 mM) GDL |
+ | 300 mM NaCl + 1 mM HEPES buffer | + | Matrigel | + | Cell suspension |
| Fibroticgel | = | 10% MVG alginate | + |
50 mg/mL (500 mM) CaCO3 |
+ |
177.9 mg/mL (998 mM) GDL |
+ | 300 mM NaCl + 1 mM HEPES buffer | + | Matrigel | + | Cell suspension |
Rheology
The mechanical stiffness of alginate gels was analyzed by conducting measurements on an Anton-Paar MCR 101 rheometer (Anton-Paar, Graz, Austria) with an 8 mm parallel plate configuration that performed oscillatory shear stress sweeps of 1% strain at frequencies from 0.5 to 50 Hz. The values for modulus were taken at 1.99 Hz for all groups, and the elastic modulus was determined by the storage and loss moduli obtained for all samples, using the formula (1):
| 1 |
In the above formula, G is the modulus of rigidity or shear modulus, E is the elastic modulus, and ν is the Poisson's ratio (Stowers et al. 2015). Before rheometric analysis, gel samples were processed by cutting a cylindrical punch of about 8 mm in diameter and 1 mm in thickness. The cylindrical cut-outs were allowed to swell in 1X PBS for 12 h before rheological testing.
Scanning electron microscopy (SEM) imaging and analysis of ultrastructure
Cross-sectional images of the lyophilized gel discs were collected using SEM, following published procedures (Alvarez-Primo et al. 2019). For SEM imaging, uniform-sized gel discs were lyophilized and sputter-coated with gold/palladium (2–3 min) in a sputter coater (Gatan Model 682 Precision etching coating system, Pleasantown, CA, USA) and visualized using SEM (S-4800, Hitachi, Japan) at voltages of 12–15 kV at varying magnifications. Collected images obtained were analyzed using Image J (Babiker et al. 2017) to determine their average pore diameter (µm) and how the variation in stiffness across the samples affected this parameter.
Swelling analysis
To account for the hydration and the swelling behavior of the gel scaffolds, samples were allowed to swell to equilibrium for up to 8 hin Dulbecco Modified Eagle’s Medium (DMEM without Ca2++, pH 7, 25 °C) following published protocols (Anil Kumar et al. 2019a, b). All gels samples were crosslinked and stored at − 80 °C (12 h) following which they were freeze-dried using a VirTis BenchTop Pro Freeze Dryer with Omnitronics (SP Scientific, Warminster, PA, USA). These dried samples were weighed (W0) and then immersed in DMEM, and the increase in weight was recorded periodically (Wt) at every 1 h interval for the total duration of this experiment. The swelling ratio was calculated using the following Eq. (2), where Ds was the degree of swelling, W0, and Wt were the weights of the samples in the dry and swollen states, respectively:
| 2 |
Cell culture in 3D gels
AC16 human cardiomyocyte cell lines (CM, Millipore cat no. SCC109) were cultured in Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 Ham (DMEM/F-12) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin as reported previously and henceforth referred to as the complete growth medium (Anil Kumar et al. 2019a, b). Originally, this cell line was derived from the fusion of primary adult human ventricular heart tissue cells with SV40 transformed, uridine auxotroph human fibroblasts, lacking mitochondrial DNA (Davidson et al. 2005). Following the use of a uridine-free medium as a selection for the removal of unfused fibroblasts, the remaining fused cells were subcloned and screened for the presence of SV40 large T-ag, β-myosin heavy chain (βMHC) and connexin-43 (Cx-43) (Davidson et al. 2005). Therefore, these cells are similar in their physiological behavior to most human primary cardiomyocytes. Media were changed every two days, and cells were passaged before reaching confluence. To confirm the viability of cells, post-encapsulation in gels after crosslinking, the CM were labeled with PKH26 red fluorescent membrane staining dye (Sigma), as done before (Anil Kumar et al. 2019a, b). For cell encapsulation studies, pre-stained cells were mixed with the alginate solution (1 × 106 cells/mL henceforth) before gelation to ensure a uniform distribution throughout the gels and crosslinked following procedure reported earlier. Alginate is not known to possess cell adhesive moieties; therefore, cell adhesion sites were introduced by the mixing of alginate solution with Matrigel (Stowers et al. 2015). These cell-gel constructs were cultured for 4 and 7 days (with complete growth medium, 37 °C, 5% CO2), respectively, after which these samples were imaged without fixing the cells in the gel samples using confocal fluorescence microscopy (Zeiss). Gelatin (0.1%, Gibco, Invitrogen, Carlsbad, CA) and Matrigel (Corning)-coated wells were used as controls for this experiment. For experimental samples, 1 × 106 cells/ well were seeded per well and in controls, 1 × 105 cells/ well were seeded in 24-well plates. At day 4 and day 7, images were acquired from the cells cultured within gels, and aspect ratio calculations were performed using an equation (Coakley et al. 2014) developed previously (Stowers et al. 2015):
| 3 |
Cell viability assay
All samples with cells were examined using a Live/Dead assay (Thermo Fisher Scientific, Waltham, USA) with Calcein AM that stained the live cells green, and Ethidium homodimer that stained the dead cells red, respectively. After 5 days of culture, the cell-laden constructs were subjected to the Live/Dead assay. Another separate sets of constructs were subjected to DAPI staining for the nuclei in stained in blue (Vector labs, VECTASHIELD® Antifade Mounting Medium with DAPI, Burlingame, CA). All Live/Dead stained cells and DAP-stained cells were then imaged using a Zeiss Axiovert Inverted Phase Contrast Fluorescent Microscope, to confirm the retention of viable (green) cells within the gel scaffolds and in non-gel controls. All images collected were analyzed using quantitative image analysis through Image J. The percentage of live or dead cells was obtained based on Eq. (4) as follows:
| 4 |
Cardiac connexin-43 and troponin-I expression
To understand how the gel stiffness modulates CM performance, AC16 cells were mixed in gels within 12-well plates, for a total of 48-h culture period and cultured in complete growth media. Following the completion of the experimental period for culture, protein extraction procedures followed by analysis occurred as described below. Cell-gel samples were taken out carefully from the wells rinsed in 1X phosphate-buffered saline (PBS), and cells extracted from the gels using 0.25% Trypsin–EDTA by the addition of 110 μL of protease and phosphatase inhibitors, using procedures described earlier (Anil Kumar et al. 2019a, b). The isolated AC16 cells were then sonicated and centrifuged to maximize protein quantification. Protein concentration was estimated on the Nanodrop for each sample, aliquot into individual tubes with a total of 25 μg of protein per tube. A total of 8 samples were then loaded onto a 10-well 12.5% polyacrylamide gel (Bio-Rad Mini Protean TGX stain-free) for separation of proteins and their subsequent analysis by western blotting. Primary antibodies were used to probe and detect cardiac Troponin-I (cTnI) and Connexin-43 (Cx-43) while the housekeeping gene β-actin and GAPDH served as controls (mouse monoclonal primary antibody, 1:200, Millipore-Sigma), respectively. The blots were imaged and analyzed using SysStat application.
Statistical analysis
All sets of experiments applied as n = 3 set of trials unless otherwise specified. Data are presented as the mean ± standard deviation. Statistical analysis was performed in each group using a one-way ANOVA and Tukey’s multiple comparison test. *p < 0.05 or lesser was deemed statistically significant.
Results and discussion
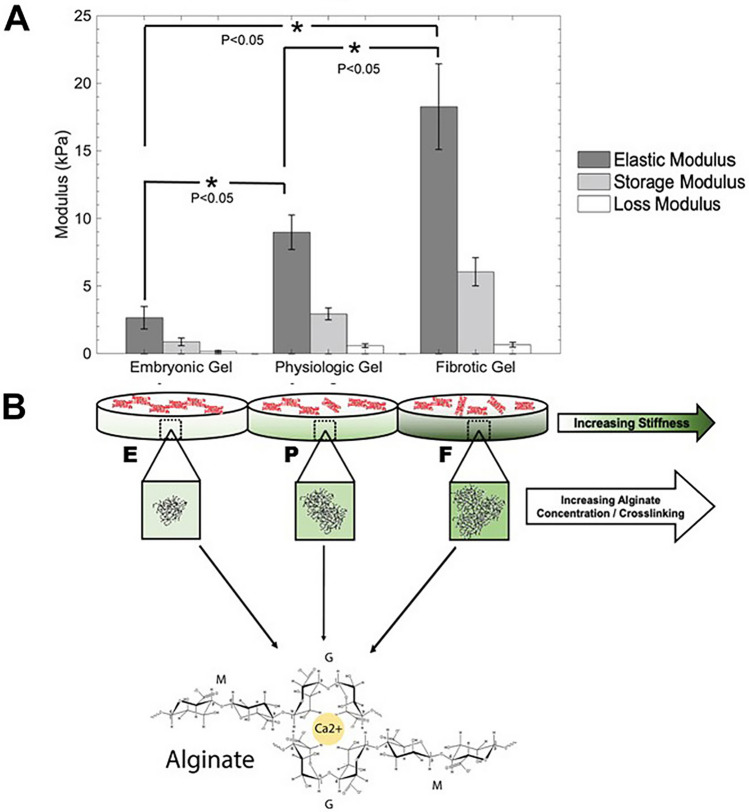
Following the exposure to Ca2+ ions, alginate reacts immediately, resulting in non-homogenous crosslinking and gel formation. To better control this abrupt and instantaneous crosslinking, we adopted an ionic gelling mechanism that acts by the introduction of Ca2+ ions directly into the alginate matrix at a controlled rate (Schmitt et al. 2015). Thus, homogeneously crosslinked alginate gels were made using insoluble CaCO3 providing a source of calcium, and GDL, that is known to hydrolyze upon dissolution in water to release calcium ions, following published guidelines from another study (Fig. 1) (Stowers et al. 2015). When the GDL is slowly hydrolyzed, it results in dropping pH of the solution and, subsequently, a release of Ca2+ ions from the CaCO3 into the solution. We allowed a time-delayed release of calcium ions, resulting in the formation of homogenous alginate gels within 1 h. Alginate gel formulation was optimized to yield characteristic stiffness by testing 4–10% (w/v) MVG alginate solutions against 10–50 mg/mL CaCO3 (0.17–0.5 mM) solutions. It is well known that stiffness or elastic modulus of ionic crosslinked polymers is known to rise proportionately with increasing content of the basic monomer and the density of the crosslinking ions (Liu and Huglin 1995). After initial testing and rheological analysis of the crosslinked gels (Supplementary Fig. 1), three formulations were selected to represent ‘embryonic’, ‘physiologic’, and ‘fibrotic’, respectively, which are referenced in Table 1.
Fig. 1.
Shown in a are the rheological properties of alginate gels, including elastic-, storage- and loss-modulus. In b, a schematic depiction the corresponding gels of increasing mechanical stiffness made by increasing the concentration of both the biopolymer and calcium crosslinking solution is included. p values are all significant. *0.009; **0.008; ***0.034
It was found that these relevant stiffness values can be achieved using this type of alginate polymer, especially due to the high guluronate content that makes the gels more readily cross-linkable. However, the molar concentration of GDL was always 3.5 times that of CaCO3 to maintain a neutral pH. This range of concentration for CaCO3 and GDL has been reported to be well tolerated by cells in other studies (Stowers et al. 2015). Since it is not known how the CM would specifically interact with these crosslinked gels, we opted to work with extremely low concentrations of Ca2+ ions compared with published literature, which suggests the use of 50 mM CaCO3 for crosslinking and gel formation occur within 30 min (Schmitt et al. 2015).
For cell encapsulation studies, cells were mixed with the alginate solution before gelation to ensure a uniform distribution throughout the gel. Optimized gels showed viscoelastic behavior, and the storage modulus was greater than the loss modulus for all hydrogel compositions. Furthermore, the average elastic moduli for the optimized embryonic, physiologic, and fibrotic gels were 2.66 ± 0.84, 8.98 ± 1.29, and 18.27 ± 3.17 kPa, respectively (Fig. 1a), which were all significantly different from one another (p < 0.05). However, based on others published approaches to create physiological and fibrotic scaffolds, the ~ 2-fold difference in the scaffold stiffness in these two cases may not be optimal for studying their effects on varying cell behavior and function across these two scaffolds (Williams et al. 2015). In future studies, we will focus on further enhancing the stiffness of the fibrotic scaffolds to coax cell behavior that may be expected by culturing atop of extremely stiff substrates.
The analysis of electron micrographs revealed a highly porous interconnected architecture for all three gels permissive towards cell culture applications (Dewavrin et al. 2014), as can be seen by representative images in Fig. 2a–c. The average pore diameter (end-to-end length) was determined to be 51.2 ± 2.8 μm, 38.4 ± 15.9 μm, and 32.3 ± 9.1 μm for the embryonic, physiologic, and fibrotic gels, respectively, as represented in Fig. 2d and were all significantly different among the group. As shown by these results, the average pore diameter decreases with stiffness, which is also reported by another group (Annabi et al. 2010). Figure 2e depicts the swelling behavior of the embryonic, physiologic, and fibrotic scaffolds in DMEM. The embryonic scaffolds were found to swell to a greater extent, whereas the fibrotic scaffolds appeared to swell the least. Since swelling behavior is dependent on the extent of polymer crosslinking (Khan and Ranjha 2014; Oh et al. 1998), these results could imply that the fibrotic scaffolds were crosslinked to a greater degree due to their increased alginate and CaCO3 content, with respect to the physiological and embryonic scaffolds. However, the fibrotic scaffolds were observed to start disintegrating after the 48 h mark. The embryonic scaffolds were relatively more stable and were noted to undergo further swelling. The physiologic scaffolds demonstrated the greatest stability in terms of reaching equilibrium and maintaining a stable swelling ratio without dissociating into smaller constituents. The swelling data were analyzed through a Two-Step Anova and corroborated with a Post Hoc Honestly Significant Difference Tukey test, pointing towards a very highly significant difference in swelling ratio (p < 0.01) observed in the embryonic tissue. Although the physiologic and fibrotic scaffolds posed almost no statistical difference between their swelling rates, their degradation was also noted in a similar manner, for which it was determined that the physiologic scaffolds maintained higher integrity in comparison to the embryonic and fibrotic scaffolds, by maintaining greater stability for longer periods of time, after reaching equilibrium in DMEM.
Fig. 2.
SEM images a–c are representatives captured to reveal their ultrastructure. d Shows the average pore size of each gel depicted in a–c. e Depicts the swelling behavior of the three different gels in DMEM
Unlike covalently crosslinked hydrogels, alginate crosslinks are reversible and when exposed to aqueous media (Delaney et al. 2010). During swelling, it leads to the leaching out of the Ca2++ ions into the culture media from the scaffolds initiating their disintegration. Since the fibrotic scaffolds had the highest concentration of Ca2++ ions present in the scaffold, when these ions are slowly released into the aqueous environment, the scaffold starts to disintegrate. This was evidenced in the fibrotic scaffolds after 48 h, which contained the highest concentration of alginate compared to the other two types of gels studied. It is anticipated that the physiologic and embryonic scaffolds, which contain lower concentrations of alginate and Ca2++ ions, respectively, may resist the disintegration for a slightly longer period corresponding to the slower rate of leaching of Ca2++ ions from the scaffold to the surrounding aqueous environment.
After 4 and 7 days, fluorescent images of cells forming networks within the gels were acquired. Shown in Fig. 3a are images acquired from samples at day 4 (top row) and day 7 of culture (bottom row). From a comparison of both rows in Fig. 3a, cell proliferation in all three conditions was confirmed. However, in the entire alginate/Matrigel composite gels (Fig. 3a), we observed elongated cells in the embryonic gels that became more rounded with increased stiffening as was the case for cells in physiologic and fibrotic gels. This observation was confirmed in Fig. 3b, where the average aspect ratio of cells in each gel sample, is depicted. The aspect ratio for cells cultured in the embryonic gels was the highest (p = 0.02), followed by the other two gels, respectively. However, there was no significant difference between the aspect ratios for cells cultured in the physiologic and the fibrotic gels.
Fig. 3.
Shown in a are cells cultured within the 3D gels and on 2D Matrigel controls. The cells were pre-stained with PKH26 (red) to reveal network formation in these cultures. Also, the magnified insets depict the elongated cell morphology within the embryonic gels, which became gradually rounded as the gels were progressively stiffened from the physiologic to the fibrotic gels. Shown in b is the average cellular aspect ratio (width/height) for cells cultured within the gels. The aspect ratio is maximum in the case of cells cultured within the embryonic gels and is close to the value of 1 (implying rounded cells) as the gels were stiffened
Shown in Fig. 4a and Supplementary Fig. 2 are bright field images from all cell-gel samples acquired at day 7, which clearly depict the network formation among proliferating cell cultures. For quantification purposes, a network was defined as a coupled pattern within a group of adjacent cells as established by others (Stowers et al. 2015). The average number of cellular networks per unit area of the sample imaged was calculated and represented in Fig. 4b. The embryonic gels contained a significantly higher number of networks, which proportionately decreased as the gel stiffness was increased. Magnified insets of these networks from all gels are depicted in Supplementary Fig. 3. In embryonic gels, the cells were capable of enzymatic degradation or mechanical deformation of the matrix to form extensions into the gels. As the gels were progressively stiffened, the cells’ ability to remodel the matrix was diminished, and the cells appeared predominantly rounded, since the matrix was relatively stiffer to deform or because the degradation sites were obstructed by the introduction of additional crosslinks (Stowers et al. 2015). The cellular network behavior confirmation is provided by the representative image that clearly shows networks established by human AC16 cells when cultured atop Matrigel-coated 2D wells (Supplementary Fig. 2). However, when Matrigel was excluded from the gels, no networks were evident after 5 days of culture in all gels, although the cells retained viability and depicted cluster formation (Supplementary Fig. 4).
Fig. 4.
Shown in a are phase-contrast images of cellular networks cultured within the 3D gels and on 2D Matrigel Controls (Supplementary Fig. 2). It is evident that the average number of cellular networks per unit area is significantly reduced as the gels were progressively stiffened. Shown in b is the average number of cellular networks within all the gels. P values are all significant
Cell viability determined with the Live/Dead assay revealed an increasing number of dead cells, and a decreasing number of live cells as the stiffness of the alginate scaffold increased as seen in Fig. 5. Embryonic gels had 89 ± 10% live cells with 11 ± 10% dead cells, physiologic gels had 71 ± 15% live cells with 29 ± 15% dead cells, and fibrotic gels had 61 ± 13 live cells with 39 ± 13% dead cells within each scaffold, respectively, as represented in Fig. 5a–f. There was a significant difference (p < 0.05) between the number of live to dead cells in both the embryonic and physiologic-mimicking scaffolds; however, no such significance was seen in the fibrotic scaffold. Furthermore, there was a significant difference in the percentage of viable and dead cells between the embryonic and fibrotic scaffolds, as illustrated in Fig. 5g. The inverse trend in cell viability with respect to matrix stiffness can possibly be attributed to the diffusion barriers of nutrients and oxygen caused by the increased polymeric network associated with stiffer scaffolds as suggested by the decreased porosity results in Fig. 2 and elucidated by others (Sawyer et al. 2016). Implications of this data can be extrapolated to explain why grafts in the zone of dense collagen deposition after myocardial infarction lead to reduced cell engraftment compared to softer grafts (Liang et al. 2019). The live cell count was noted to decrease proportionately with increasing scaffold stiffness as has been validated by other’s published works (Sawyer et al. 2016). To further study the effects of scaffold stiffness on the expression of cardiac-specific markers, Cx-43 and cTnI, cells were probed for these proteins using a western blot (Fig. 6) in the softest and stiffest of the gels. Cx-43 is found at gap junction channels between neighboring cardiomyocytes. The protein plays a role in enhancing intercellular communication, electrical coupling, and promote synchronous contractility of the heart (You et al. 2011). The amount of Cx-43 was observed to be 1.4 times higher in softer gels and compared to stiffer ones (Fig. 6a), suggesting cardiomyogenesis is promoted in softer microenvironments leading to enhanced cellular communication and network formation as corroborated by the observed network formations seen in Fig. 4. Amplification of Cx-43 in softer 3D substrates emphasizes hydrogel stiffness as an important factor for the optimization of tissue-engineered cardiac constructs within hydrogels that are often used to mimic 3D environments of native tissues in-vitro (Anil Kumar et al. 2019a, b; Li and Guan 2011).
Fig. 5.
Cell viability was assessed by performing the live/dead assay on cells within the embryonic, physiologic, and fibrotic scaffolds after 5 days of culture. a, c, e Depict the live cells stained by Calcein AM (green), and b, d, f Represent the dead cells stained by Ethidium homodimer (red) for the embryonic, physiologic and fibrotic scaffolds, respectively. The number of live to dead cells in both the embryonic and physiologic scaffolds was found to be statistically significant (p < 0.05), unlike the fibrotic scaffolds. g Depicts the percentage of live and dead cells for each of the three scaffolds
Fig. 6.
Western blot analysis for cardiac biomarkers Cx-43 and Troponin are displayed between the softest (embryonic) and stiffest (fibrotic) gels in our study. Intercellular Connexin-43 (a) found in gap junctions between neighboring cardiomyocytes is expressed more in softer environments, while cTnI expression increases in stiffer gels (b)
Enhanced cTnI expression was observed in cells within fibrotic gels as compared to those cultured in soft embryonic gels by a factor of 1.35, as graphically illustrated in Fig. 6b. The cTnI protein is specific to cardiac tissue and commonly used as a marker for myocardial damage (Sharma et al. 2004). Other studies have also demonstrated cTnI is discharged from viable cardiomyocytes by stimulation of stretch-responsive integrins (Hessel et al. 2008). Thus, intensified cTnI expressed in our system can be due to a combination of both factors.
Cell viability was seen to decrease with increasing scaffold stiffness (Fig. 6), which could cause the upregulation of the cTnI stress biomarker due to the smaller pore size within the scaffold that would allow cell migration and gas exchange. Enhancement of cTnI can also be attributed to the high stretch-responsive mechanical stimulation of cellular integrins that interact with the stiff mechanical environment of the scaffold.
We adopted a dynamically tunable system using alginate hydrogels previously developed by Stowers et al. to study how it affects cellular morphology and network formation in cardiac myocytes (Stowers et al. 2015). The formation of cellular networks within tissue-engineered cardiac constructs is important for successful organ replacement therapy because this ensures the maintenance of cardiac electrophysiology (Cui et al. 2017).
Reconstruction of complex three-dimensional (Coakley et al. 2014) tissues, such as liver and heart, remains difficult. To effectively create vascularized tissue-engineered cardiac constructs, we must understand how cardiomyocytes interact with the ECM to form cellular networks and cross-connections. ECM interactions are important for the proliferation, migration, and differentiation of various types of cardiac cells, including endothelial cells (Valiente-Alandi et al. 2016). Such a rationale served as a fundamental basis for this study.
The variable gel stiffness’s resulted from the guluronic regions in the alginate backbone that were physically crosslinked in the presence of divalent cations and the variable calcium concentration. Cell viability was maintained across all gels, and changes in cell morphology were revealed in response to stiffening, making this platform well suited for 3D cell encapsulation experiments. A well-organized cellular and vascular network are essential for permitting metabolic and nutrient exchange throughout engineered 3D tissues (Kang et al. 2018). Specialized techniques have been adopted by many to promote the formation of such cell-based networks, including the use of cardiac cell progenitors (Wu et al. 2008), stem cells (Kusuma et al. 2013), and advanced fabrication techniques (Wang et al. 2019). In all of these approaches combined, the role of the stiffness of the underlying ECM is not well outlined. In this study, we have demonstrated that matrix stiffness has a specific and important role in the formation of such cardiac cell networks, which are essential toward cardiomyocyte function and behavior and modulation of disease pathology. This study forms the basis for the utilization of a dynamically tunable substrate to study how the upregulation of matrix ECM stiffness can result in loss of cell function, as may be the case in cardiac fibrosis or cardiomyopathy. The system is simple yet unique as it allows reversible stiffening or softening to model cell behavior as needed. In the future, we will attempt to modulate the stiffness of these alginate substrates in real time to study their effects on cardiac cell cultures, including a combination of cardiomyocytes, cardiac fibroblasts, and endothelial cells.
Conclusion
We have developed an alginate gel system that represents embryonic, physiologic and fibrotic matrices by exhibiting the range of matrix stiffness that is common to these tissue states in vivo. Our 3D system recapitulates the features of others reported works on 2D models reporting on the stiffness of the fetal, adult, and fibrotic heart and their effects, respectively (Pandey et al. 2018). Our system is cell compatible and is responsive to modeling dynamic phenomena, such as tissue fibrosis, to isolate the effects of matrix stiffening. We used this system to demonstrate the regulation of CM morphology by matrix stiffness in all gels. In summary, the soft embryonic gels permitted extensive cell spreading, elongation, and network formation. They further showed greater expression of Cx-43 and reduced cTnI expression. On the contrary, the stiffer gels showed the progressively lesser extent of cell spreading, elongation, and network formation. The stiffest gel showed reduced Cx-43 and enhanced cTnI expression. In the future, we will explore the ability to dynamically control the gel stiffness, both spatially and temporally. Thus, it will be possible to translate in vitro outcomes to more relevant in vivo disease models while maintaining the hydrogel system composition constant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Matthew Alonzo acknowledges the Eloise E. and Patrick B. Wieland fellowship at UTEP and the Gates Millennium Scholarship Program. Fabian Alvarez Primo acknowledges the Eloise E. and Patrick B. Wieland fellowship and the Dissertation Completion Fellowship at UTEP. Monica Delgado acknowledges the Gates Millennium Scholarship Program. We acknowledge the technical assistance received from Dr. Armando Varela for kindly assisting us with the confocal microscopy. We are grateful to Moinak Joddar, freshman at UT Cockrell School of Engineering for his help with formatting of the references.
This study was funded by the NIH 1SC2HL134642-01 and NSF (CBET 1927628). The authors also acknowledge support for materials and supplies for this project obtained from NSF-MRI (DMR 1826268). The research reported in this article was also supported by the National Institute of General Medical Sciences of the National Institutes of Health under Linked Award Numbers RL5GM118969, TL4GM118971, and UL1GM118970. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with ethical standards
Conflict of interest
There are no conflicts to declare.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alvarez-Primo F, Anil Kumar S, Manciu FS, Joddar B. Fabrication of surfactant-dispersed HiPco single-walled carbon nanotube-based alginate hydrogel composites as cellular products. Int J Mol Sci. 2019;20:4802. doi: 10.3390/ijms20194802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen T, Auk-Emblem P, Dornish M. 3D cell culture in alginate hydrogels. Microarrays. 2015;4:133–161. doi: 10.3390/microarrays4020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anil Kumar S, Matthew Alonzo M, Allen SC, Abelseth L, Thakur V, Akimoto J, Ito Y, Willerth S, Suggs L, Chattopadhyay M, Joddar B. A visible light-cross-linkable, fibrin–gelatin-based bioprinted construct with human cardiomyocytes and fibroblasts. ACS Biomater Sci Eng. 2019;5:4551–4563. doi: 10.1021/acsbiomaterials.9b00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anil Kumar S, Allen SC, Tasnim N, Akter T, Shinhye Park S, Kumar A, Chattopadhyay M, Ito Y, Suggs LJ, Joddar B. The applicability of furfuryl-gelatin as a novel bioink for tissue engineering applications. J Biomed Mater Res Part B Appl Biomater. 2019;107(2):314–323. doi: 10.1002/jbm.b.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annabi N, Mithieux SM, Weiss AS, Dehghani F. Cross-linked open-pore elastic hydrogels based ontropoelastin, elastin and high pressure CO2. Biomaterials. 2010;31:1655–1665. doi: 10.1016/j.biomaterials.2009.11.051. [DOI] [PubMed] [Google Scholar]
- Babiker NE, Gassum A, Abdelraheem NE, Arbab MAR, Aldeaf SAH, El-Sheikh MAA, Musa HH. The progress of stem cells in the treatment of diabetes mellitus type 1. Prog Stem Cell. 2017;4:175–188. doi: 10.15419/psc.v4i01.184. [DOI] [Google Scholar]
- Cao M, Wang Y, Hu X, Gong H, Li R, Cox H, Zhang J, Waigh TA, Xu H, Lu JR. Reversible thermoresponsive peptide–PNIPAM hydrogels for controlled drug delivery. Biomacromol. 2019;20:3601–3610. doi: 10.1021/acs.biomac.9b01009. [DOI] [PubMed] [Google Scholar]
- Chen F-M, Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci. 2016;53:86–168. doi: 10.1016/j.progpolymsci.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleetus CM, Primo FA, Fregoso G, Raveendran NL, Noveron JC, Spencer CT, Ramana CH, Joddar B. Alginate hydrogels with embedded ZnO nanoparticles for wound healing therapy. Int J Nanomed. 2020;15:5097–5111. doi: 10.2147/IJN.S255937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coakley MF, Hurt DE, Weber N, Mtingwa M, Fincher EC, Alekseyev V, Chen DT, Yun A, Gizaw M, Swan J, Yoo TS, Huyen Y. The NIH 3D print exchange: a public resource for bioscientific and biomedical 3D prints. 3D Print Addit Manuf. 2014;1:137–140. doi: 10.1089/3dp.2014.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Nowicki M, Fisher JP, Zhang LG. 3D bioprinting for organ regeneration. Adv Healthc Mater. 2017;6:1601118. doi: 10.1002/adhm.201601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MM, Nesti C, Palenzuela L, Walker WF, Hernandez E, Protas L, Hirano M, Issac ND. Novel cell lines derived from adult human ventricular cardiomyocytes. J Mol Cell Cardiol. 2005;39:133–147. doi: 10.1016/j.yjmcc.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Delaney JT, Liberski AR, Perelaer J, Schubert US. Reactive inkjet printing of calcium alginate hydrogel porogens—a new strategy to open-pore structured matrices with controlled geometry. Soft Matter. 2010;6:866–869. doi: 10.1039/B922888H. [DOI] [Google Scholar]
- Dewavrin J-Y, Hamzavi N, Shim V, Raghunath M. Tuning the architecture of three-dimensional collagen hydrogels by physiological macromolecular crowding. Acta Biomater. 2014;10:4351–4359. doi: 10.1016/j.actbio.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/S0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhi M, Mottaghitalab F, Shokrgozar ML, Ou KL, Mao C, Hosseinkhani H. Importance of dual delivery systems for bone tissue engineering. J Control Release. 2016;225:152–169. doi: 10.1016/j.jconrel.2016.01.033. [DOI] [PubMed] [Google Scholar]
- Ghodsizadeh A, Hosseinkhani H, Piryaei A, Pournasr B, Najarasl M, Hiraoka Y, Baharvand H. Galactosylated collagen matrix enhanced in vitro maturation of human embryonic stem cell-derived hepatocyte-like cells. Biotechnol Lett. 2014;36(5):1095–1106. doi: 10.1007/s10529-014-1454-0. [DOI] [PubMed] [Google Scholar]
- Hessel M, Atsma DE, van der Valk EJ, Bax WH, Schalij MJ, van der Laarse A. Release of cardiac troponin I from viable cardiomyocytes is mediated by integrin stimulation. Pflüg Arch Eur J Physiol. 2008;455:979–986. doi: 10.1007/s00424-007-0354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AS. Hydrogels for biomedical applications. Adv Drug Del Rev. 2012;64:18–23. doi: 10.1016/j.addr.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Hosseinkhani H, Hosseinkhani M, Hattori S, Matsouka R, Kawaguchi M. Micro and nano-scale in vitro 3D culture system for cardiac stem cells. J Biomed Mater Res Part A. 2010;94(1):1–8. doi: 10.1016/j.matdes.2020.108794. [DOI] [PubMed] [Google Scholar]
- Huebsch N, Kearney CJ, Zhao X, Kim J, Cezar CA, Suo Z, Mooney DJ. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc Nat Acad Sci. 2014;111(27):9762–9767. doi: 10.1073/pnas.1405469111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J. 2008;95:3479–3487. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joddar B, Garcia E, Casas A, Stewart CM. Development of functionalized multi-walled carbon-nanotube-based alginate hydrogels for enabling biomimetic technologies. Sci Rep. 2016;6:1–12. doi: 10.1038/srep32456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joddar B, Tasnim N, Thakur V, Kumar A, McCallum RW, Chattopadhyay M. Delivery of mesenchymal stem cells from gelatin-alginate hydrogels to stomach lumen for treatment of gastroparesis. Bioengineering. 2018;5(1):12. doi: 10.3390/bioengineering5010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B, Shin J, Ji Park H, Rhyou C, Kang D, Lee SJ, Yoon YS, Cho SW, Lee H. High-resolution acoustophoretic 3D cell patterning to construct functional collateral cylindroids for ischemia therapy. Nat Commun. 2018;9:1–13. doi: 10.1038/s41467-018-07823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaji S, Golshan Ebrahimi N, HosseinkhaniH, Enhancement of biocompatibility of PVA/HTCC blend polymer with collagen for skin care application. Int J Polym Mater Polym Biomater. 2020 doi: 10.1080/00914037.2020.1725761. [DOI] [Google Scholar]
- Khan S, Ranjha NM. Effect of degree of cross-linking on swelling and on drug release of low viscous chitosan/poly(vinyl alcohol) hydrogels. Polym Bull. 2014;71:2133–2158. doi: 10.1007/s00289-014-1178-2. [DOI] [Google Scholar]
- Kusuma S, Shen YI, Hanjaya-Putra D, Mali P, Cheng L, Gerecht S. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc Natl Acad Sci. 2013;110:12601–12606. doi: 10.1073/pnas.1306562110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1880. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiZ GJ. Hydrogels for cardiac tissue engineering. Polymers. 2011;3:740–761. doi: 10.3390/polym3020740. [DOI] [Google Scholar]
- Liang J, Huang W, Jiang L, Paul C, Li X, Wang Y. Concise review: reduction of adverse cardiac scarring facilitates pluripotent stem cell-based therapy for myocardial infarction. Stem Cells. 2019;37:844–854. doi: 10.1002/stem.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huglin MB. Effective crosslinking densities and elastic moduli of some physically crosslinked hydrogels. Polymer. 1995;36:1715–1718. doi: 10.1016/0032-3861(95)99018-P. [DOI] [Google Scholar]
- Mondal A, Gebeyehu A, Miranda M, Bahadur D, Patel N, Ramakrishnan S, Rishi A, Singh M. Characterization and printability of sodium alginate-gelatin hydrogel for bioprinting NSCLC co-culture. Sci Rep. 2019;9:1–12. doi: 10.1038/s41598-019-55034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanaswamy R, Torchilin VP. Hydrogels and their applications in targeted drug delivery. Molecules. 2019;24:603. doi: 10.3390/molecules24030603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:149–165. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KS, Oh JS, Choi HS, Bae YC. Effect of cross-linking density on swelling behavior of NIPA gel particles. Macromolecules. 1998;31:7328–7335. doi: 10.1021/ma971554y. [DOI] [Google Scholar]
- Pandey P, Hawkws W, Hu J, Megone WV, Gautrot J, Anilkumar N, Zhang M, Hirvonen L, Cox S, Ehler E, Hone J, Sheetz M, Iskatsch T. Cardiomyocytes sense matrix rigidity through a combination of muscle and non-muscle myosin contractions. Dev Cell. 2018;44(326–336):e323. doi: 10.1016/j.devcel.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J, Zhou X, Liang Y, Zhang T, Ma PX, Guo B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 2018;183:185–199. doi: 10.1016/j.biomaterials.2018.08.044. [DOI] [PubMed] [Google Scholar]
- Sawyer S, Oest M, Margulies B, Soman P. Behavior of encapsulated saos-2 cells within gelatin methacrylate hydrogels. J Tissue Sci Eng. 2016 doi: 10.4172/2157-7552.1000173. [DOI] [Google Scholar]
- Schmitt A, Rodel P, Anamur C, Seeliger C, Imhoff AB, Herbst E, Vogt S, Griensven MV, Winter G, Engert J. Calcium alginate gels as stem cell matrix-making paracrine stem cell activity available for enhanced healing after surgery. PLoS ONE. 2015;10:e0118937–e0118937. doi: 10.1371/journal.pone.0118937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifzadeh G, Hosseinkhani H. Biomolecule-responsive hydrogels in medicine. Adv Healthc Mater. 2017;6(24):1700801. doi: 10.1002/adhm.201700801. [DOI] [PubMed] [Google Scholar]
- Sharma S, Jackson P, Makan J. Cardiac troponins. J Clin Pathol. 2004;57:1025–1026. doi: 10.1136/jcp.2003.015420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers RS, Allen SC, Suggs LJ. Dynamic phototuning of 3D hydrogel stiffness. Proc Natl Acad Sci. 2015;112:1953–1958. doi: 10.1073/pnas.1421897112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Tan H. Alginate-based biomaterials for regenerative medicine applications. Materials. 2013;6:1285–1309. doi: 10.3390/ma6041285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toosi S, Naderi-Meshkin H, Kalalinia F, Hosseinkhani H, Heirani-Tabasi A, Havakhah S, Nekuei S, Jafarian AH, Rezari F, Peivandi MT, Mesgarani H, Behravan J. Bone defect healing is induced by collagen sponge/polyglycolic acid. J Mater Sci Mater Med. 2019;30(3):33. doi: 10.1007/s10856-019-6235-9. [DOI] [PubMed] [Google Scholar]
- Toosi S, Naderi-Meshkin H, Kalalinia F, Peivandi MT, Hosseinkhani H, Bahrami AR, Heirani-Tabasi A, Mirahmadi M, Behravan J. PGA-incorporated collagen: toward a biodegradable composite scaffold for bone-tissue engineering. J Biomed Mater Res Part A. 2016;104(8):2020–2028. doi: 10.1002/jbm.a.35736. [DOI] [PubMed] [Google Scholar]
- Valiente-Alandi I, Schafer AE, Blaxall BC. Extracellular matrix-mediated cellular communication in the heart. J Mol Cell Cardiol. 2016;91:228–237. doi: 10.1016/j.yjmcc.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Mithieux SM, Weiss AS. Fabrication techniques for vascular and vascularized tissue engineering. Adv Healthc Mater. 2019;8:1900742. doi: 10.1002/adhm.201900742. [DOI] [PubMed] [Google Scholar]
- Williams C, Budina E, Stoppel WL, Sulivan KE, Emani S, Emani SM, Black LD. Cardiac extracellular matrix–fibrin hybrid scaffolds with tunable properties for cardiovascular tissue engineering. Acta Biomater. 2015;14:84–95. doi: 10.1016/j.actbio.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM, Chien KR, Mummery C. Origins and fates of cardiovascular progenitor cells. Cell. 2008;132:537–543. doi: 10.1016/j.cell.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang K, Gu X, Leong KW. Biophysical regulation of cell behavior—cross talk between substrate stiffness and nanotopography. Engineering. 2017;3:36–54. doi: 10.1016/J.ENG.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim ED, Yin X, Nair K, Sun W. Fabrication, characterization, and biocompatibility of single-walled carbon nanotube-reinforced alginate composite scaffolds manufactured using freeform fabrication technique. J Biomed Mater Res Part B Appl Biomater. 2008;87:406–414. doi: 10.1002/jbm.b.31118. [DOI] [PubMed] [Google Scholar]
- You J-O, Rafat M, Ye GJ, Auguste DT. Nanoengineering the heart: conductive scaffolds enhance connexin 43 expression. Nano Lett. 2011;11:3643–3648. doi: 10.1021/nl201514a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.