Abstract
Objectives
We aimed to explore cytokine profile in patients as it relates to Coronavirus Disease 2019 (COVID-19) severity, and to establish a predictive cytokine score to discriminate severe from non-severe cases and provide a prognosis parameter for patients that will require intensive care unit (ICU) transfer.
Methods
Serum samples of 63 patients diagnosed with SARS-CoV-2 infection were collected early after hospital admission (day 0–3). Patients were categorized in five groups based on the clinical presentation, the PaO2/FiO2 ratio and the requirement of mechanical ventilation.
Results
Three cytokines, IL-6, IL-8 and IL-10, were markedly higher in severe forms (n = 44) than in non-severe forms (n = 19) (p < 0.005). A score combining levels of these three cytokines (IL-6*IL-8*IL-10) had the highest performance to predict severity: sensitivity of 86.4% (95% CI, 72.4–94.8) and specificity of 94.7% (95% CI, 74.0–99.9) for a cutoff value of 2068 pg/mL. Elevated levels of IL-6, IL-8 and IL-10 were also found in critically ill patients. The combination of IL-6*IL-10 serum levels allowed the highest predictability for ICU transfer: AUC of 0.898 (p < 0.0001).
Conclusion
The combinatorial IL-6*IL-8*IL-10 score at presentation was highly predictive of the progression to a severe form of the disease, and could contribute to improve patient triage and to adapt therapeutic strategy within clinical trials more accurately and efficiently.
Keywords: COVID-19, Inflammatory cytokines, Prediction, Disease severity, Intensive care unit, IL-6
Introduction
The novel SARS-CoV-2 has spread rapidly in most regions of the world, and COVID-19 was declared as a pandemic by the WHO on March 11, 2020 (Zhu et al., 2020a). Health care centers across the world had to adapt their practice to deal with the crisis by urgent mobilization of resources, especially in ICUs. In this pandemic situation with overcrowded emergency rooms and overwhelmed ICUs, early identification of patients with severe forms or needing intensive care has become crucial for optimal clinical management.
Previous studies have suggested a role of host immune response in the severity of the disease (Vabret et al., 2020). Cytokines are easily evaluable in serum and have been proposed as potential immunological biomarkers for predicting disease progression (Zhu et al., 2020b, Lucas et al., 2020).
The aim of the present study was to determine among a panel of cytokines which of them were possibly predictive of the development of a severe or critical issue when measured early during the course of the disease.
Methods
Sixty-three patients diagnosed with SARS-CoV-2 infection and admitted to Brugmann University Hospital (Brussels, Belgium) between March and May 2020 were prospectively enrolled in this study. Serum samples for all patients were collected at day 0, 1, 2 or 3 after hospital admission (mean day, 1.7). SARS-CoV-2 infection was confirmed by RT-PCR and/or chest-CT.
The SARS-CoV-2 infected patients were classified according to the severity of illness into five groups as follows (WHO, 2020):
-
(a)
Mild illness (n = 9): mild clinical symptoms,
-
(b)
Moderate illness (n = 10): lower airway respiratory symptoms or chest-CT scan finding of pneumonia without the need for supplemental oxygen,
-
(c)
Severe illness (n = 9): respiratory distress or SpO2 ≤ 93% or PaO2/FiO2 ≤ 300 mmHg,
-
(d)
Critical illness (n = 18): respiratory failure requiring mechanical ventilation or shock or end organ failure requiring ICU monitoring,
-
(e)
COVID-19 patients who died (n = 17).
The cytokine profile of 12 cytokines (IL-1b, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, IL-18, TNFα and INF-γ) was assessed in the different subgroups using an electrochemiluminescence plate-based assay (MSD, Meso Scale Discovery, MD, USA).
Performances of cytokines were compared to readily available inflammatory markers. C-reactive protein (CRP) levels were measured on a Cobas c702 Platform (Roche Diagnostics) and fibrinogen was determined using a Sysmex CS-5100 analyzer (Siemens Healthcare Diagnostics).
Results
During the study period, 63 patients were included (Table 1 ). Serum levels of IL-6 and IL-10 gradually increased with the severity of the disease course (Figure 2, Suppl). IL-6 and IL-10 levels were significantly higher in the critical group when compared to the mild group (IL-6 p < 0.0001, IL-10 p < 0.01) or the moderate group (IL-6 p < 0.01, IL-10 p < 0.05). Other cytokines did not discriminate between the five subgroups of patients based on severity of illness. Some cytokines were even undetectable in some patients (IL-1b, IL-2, IL-4, IL-12, IL-13).
Table 1.
Demographic and baseline characteristics of patients with COVID-19.
| Mild | Moderate | Severe | Critical | COVID who died | Total | |
|---|---|---|---|---|---|---|
| n | 9 | 10 | 9 | 18 | 17 | 63 |
| Age (median, years) | 81.7 | 73.4 | 69.9 | 61.8 | 78 | 72.7 |
| Age (min-max, years) | (32.4−88.2) | (18.6−92.6) | (30.8−92.2) | (36.5−78.2) | (60.2−95.9) | (18.6−95.9) |
| Gender | ||||||
| Female | 6 | 5 | 5 | 3 | 6 | 25 |
| Male | 3 | 5 | 4 | 15 | 11 | 38 |
| Comorbidity | ||||||
| Hypertension | 7/9 (78 %) | 5/10 (50 %) | 5/9 (55 %) | 10/18 (55 %) | 9/17 (53 %) | 36/63 (57 %) |
| Cardiovascular disease | 8/9 (89 %) | 5/10 (50 %) | 4/9 (44 %) | 6/18 (33 %) | 10/17 (59 %) | 33/63 (52 %) |
| Chronic respiratory disease | 3/9 (33 %) | 1/10 (10 %) | 2/9 (22 %) | 2/18 (11 %) | 3/17 (18 %) | 11/63 (17 %) |
| Diabetes | 1/9 (11 %) | 5/10 (50 %) | 2/9 (22 %) | 7/18 (39 %) | 5/17 (29 %) | 20/63 (32 %) |
| Obesity | 2/9 (22 %) | 4/10 (40 %) | 5/9 (55 %) | 5/18 (28 %) | 2/17 (12 %) | 18/63 (29 %) |
We further divided patients in two groups: severe versus non-severe and critically versus non-critically ill cases. IL-6 (p < 0.0001), IL-10 (p < 0.0001) and IL-8 (p < 0.0033), were significantly increased in severe cases (n = 44) versus non-severe cases (n = 19) (Figure 3A, Suppl), and in critically ill patients (n = 35) versus non-critically ill patients (n = 28) (IL-6 p < 0.0001, IL-10 p < 0.0001 and IL-8 p < 0.0337) (Figure 3B, Suppl).
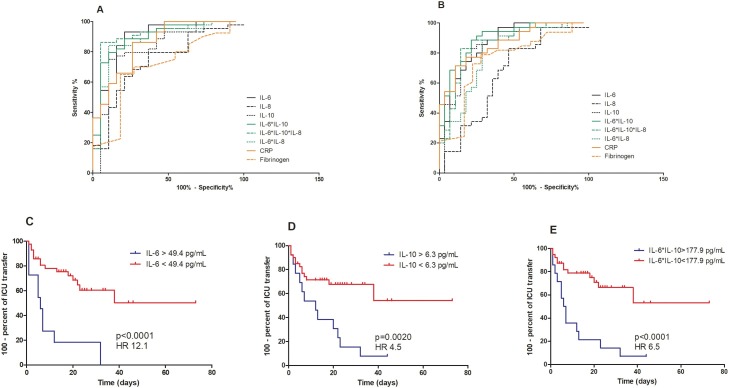
The AUC of IL-6, which was used for early prediction of the severity of COVID-19, was 0.885 (95% CI, 0.783−0.987, p < 0.0001) and was higher than the AUC obtained with conventional inflammatory markers (CRP, AUC 0.855 (95% CI, 0.753−0.958) and fibrinogen 0.701 (95% CI, 0.525−0.877)) Table 2 . Multiplication of levels of IL-6*IL-10*IL-8 into one score allowed an AUC reaching 0.904 (95% CI, 0.810−0.999). A cutoff value of the score at 2068 pg/mL showed high sensitivity (86.4%, 95% CI 72.7−94.8) and specificity (94.7%, 95% CI 74.0−99.9) (Figure 1 A).
Table 2.
ROC curve analysis of cytokines, CRP and fibrinogen levels.
| IL-6 | IL-8 | IL-10 | IL-6*IL-10 | IL-6*IL-10*IL-8 | IL-6*IL-8 | CRP | Fibrinogen | ||
|---|---|---|---|---|---|---|---|---|---|
| Non-severe vs severe group | AUC | 0,885 | 0,736 | 0,827 | 0,896 | 0,904 | 0,880 | 0,855 | 0,701 |
| 95% CI | 0,783−0,987 | 0,600−0,781 | 0,703−0,950 | 0,806−0,986 | 0,810−0,999 | 0,784−0,977 | 0,753−0,958 | 0,525−0,877 | |
| P value | <0,0001 | 0,003 | <0,0001 | <0,0001 | <0,0001 | <0,0001 | <0,0001 | 0,043 | |
| Sensitivity, % (95% CI) | 54,6 (38,9−69,6) | 43,2 (28,4−59,0) | 386 (24,4−54,5) | 72,7 (57,2−85,0) | 86,4 (72,7−94,8) | 56,8 (41,0−71,7) | 45,5 (30,4−61,2) | 65,0 (48,3−79,4) | |
| Specificity, % (95% CI) | 94,7 (74,0−99,9) | 89,5 (66,9−98,7) | 94,7 (74,0−99,9) | 94,7 (74,0−99,9) | 94,7 (74,0−99,9) | 94,7 (74,0−99,9) | 94,7 (74,0−99,9) | 81,8 (48,2−97,7) | |
| Positive-predictive value, % | 37,2 | 33,1 | 29,4 | 44,0 | 48,3 | 38,1 | 33,0 | 44,9 | |
| Negative-predictive value, % | 63,5 | 67,6 | 71,3 | 56,6 | 52,2 | 62,6 | 67,7 | 55,7 | |
| Non-critical vs critical group | AUC | 0,867 | 0,657 | 0,844 | 0,898 | 0,856 | 0,803 | 0,862 | 0,751 |
| 95% CI | 0,778−0,957 | 0,517−0,798 | 0,741−0,947 | 0,821−0,975 | 0,758−0,955 | 0,690−0,916 | 0,775−0,950 | 0,606−0,896 | |
| P value | <0,0001 | 0,033 | <0,0001 | <0,0001 | <0,0001 | <0,0001 | <0,0001 | 0,003 | |
| Sensitivity, % (95% CI) | 34,3 (19,1−52,2) | 14,3 (4,8−30,3) | 45,7 (28,8−63,3) | 51,4 (34,0−68,6) | 22,9 (10,4−40,1) | 28,6 (14,6−46,3) | 54,3 (36,7−71,2) | 57,6 (39,2−74,5) | |
| Specificity, % (95% CI) | 96,4 (81,7−99,9) | 96,4 (81,7−99,9) | 96,4 (81,7−99,9) | 96,4 (81,7−99,9) | 96,4 (81,7−99,9) | 96,4 (81,7−99,9) | 96,4 (81,7−99,9) | 83,3 (58,6−96,4) | |
| Positive-predictive value, % | 26,8 | 13,2 | 32,8 | 35,4 | 19,6 | 23,4 | 36,7 | 41,6 | |
| Negative-predictive value, % | 74,0 | 87,7 | 67,9 | 65,3 | 81,2 | 77,4 | 64,0 | 59,1 | |
AUC, area under the curve; CI, confidence interval.
Figure 1.
Predictive power of early cytokine measurement.
ROC curves of cytokines, CRP and fibrinogen levels to diagnose (A) non-severe versus severe and (B) critical versus non-critical patients with COVID-19.
Kaplan-Meier analyses of ICU transfer in patients with (C) low (n = 50) versus high IL-6 levels (n = 13), (D) low (n = 46) versus high IL-10 levels (n = 17) and (E) low (n = 44) versus high IL-6*IL-10 score (n = 19).
For diagnosis of critical cases, AUC of IL-6, IL-8 and IL-10 ranged from 0.657 to 0.867 with IL-6 showing the largest AUC (95% CI, 0.778−0.957, p < 0.0001). The combined measure of IL-6 and IL-10 increased the AUC to 0.898 (95% CI, 0.821−0.975, p < 0.0001) with a sensitivity of 51.4% (95% CI, 34.0−68.8) and a specificity of 96.4% (95% CI, 81.7−99.9) for a cutoff value of 178 pg/mL (Figure 1B). In comparison, performance of CRP and fibrinogen were lower (CRP, AUC 0.862 (95% CI, 0.775−0.950) and fibrinogen 0.751 (95% CI, 0.60−0.896)).
Next, we classified patients into two groups according to the optimal threshold of the cytokines obtained with the ROC curve analysis. Kaplan-Meier curves were constructed to assess the differences in ICU transfer between the high and low levels of cytokines. The follow-up time frame was calculated from the date of hospital admission to date of ICU transfer or death, or end of follow-up period. Patients with IL-6 or IL-10 higher than the optimal threshold (IL-6 > 49 pg/mL, IL-10 > 6 pg/mL) in the 3 first days of hospitalization were more likely to need ICU transfer (log-rank test, p < 0.0001 and p = 0.002, respectively) (Figure 1C, D).
Discussion
As previously described, we confirm that serum levels of pro-inflammatory and anti-inflammatory cytokines increase with severity of outcome of COVID-19 (Wilson et al., 2020), suggesting that the early inflammatory response could play a role in the severity of the disease, but also provides a potentially useful tool for early management of patients with severe forms.
The cytokine IL-6 has been the focus of several studies and has been proposed as a marker for disease prognosis in patients with COVID-19; higher levels correlated with severity, need for invasive ventilation, ICU admission and poor prognosis (Zhu et al., 2020b, Lucas et al., 2020, Chen et al., 2020b). While elevated levels of cytokines IL-8 and IL-10 have previously been associated with disease severity of COVID-19 (Chen et al., 2020a, Wan et al., 2020), their predictive power remains unclear.
We demonstrate in this study the predictive ability of IL-6, IL-8 and IL-10 to diagnose and anticipate severe or critical evolution of COVID-19. IL-6 had higher performance to predict the severe or critical outcome of COVID-19 than cytokines IL-8 and IL-10 but also than the conventional inflammatory markers CRP and fibrinogen. Interestingly, we also show that combining the levels of these three cytokines (multiplication of levels of IL-6*IL-10*IL-8) into one score allowed a better discrimination among the severe and non-severe groups than the use of isolated cytokine levels. A cutoff value of the score at 2068 pg/mL showed the best performance with high sensitivity and specificity. For predicting critical forms of COVID-19, the combined detection of cytokines IL-6 and IL-10 (multiplication of levels of IL-6*IL-10) was the most performant.
To our knowledge, this study is the first to demonstrate the prediction ability of the combination of cytokines for the need to transfer COVID-19 patients to the ICU. This is of high clinical relevance as the score could help early adequate triaging of patients to optimize hospital workflow and resource planning, which were particularly problematic during the pandemic situation. This early identification of a particular inflammatory profile could also help physicians to better select patients who might benefit from an early-adapted anti-inflammatory treatment.
The present study has several limitations. First, the sample size is relatively small. Second, data from negative controls is lacking due to the government’s policy to restrict the flow of people to hospital. Further studies at larger scale are needed to corroborate the predictive power of cytokines to assess disease severity.
In summary, cytokines are easily obtained in clinical practice and can be used to accurately predict outcome in patients with COVID-19. We propose a combinatorial analysis of three cytokines (IL-6, IL-8 and IL-10) to predict disease progression with higher performance than conventional inflammatory markers.
Conflict of interest
The authors declare no conflicts of interests.
Funding source
CN received a grant from the Brugmann Foundation.
Ethical approval
The study was approved by the Ethical Committee of the Brugmann Hospital (CE2020/63) and informed consent was obtained from each patient. The study was registered on clinicaltrials.gov as NCT04346017.
Acknowledgements
We thank Asma Benslimane and Thao Tran Thi Thanh for their technical support. ND is a post-doctorate clinical master specialist of the F.R.S-FNRS.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.10.003.
Appendix A. Supplementary data
The following are Supplementary data to this article:
Cytokine profile in COVID-19 patients related to the severity of the disease. Results of cytokine levels measured between day 0 and day 3 after hospital admission are presented as medians ± inter-quartile ranges (IQR). Comparison between the groups was assessed by nonparametric Kruskal-Wallis analysis of variance, followed by a Dunn’s multiple comparison test. *p < 0.05; **p < 0.01; ***p < 0.005.
Comparison of cytokine profile in SARS-CoV-2 infected patients. Comparison of cytokine profile measured between day 0 and day 3 after hospital admission in (A) non-severe versus severe and (B) non-critically ill versus critically ill patients. Comparisons were assessed by the two-tailed nonparametric Mann-Whitney test. *p < 0.05; **p < 0.01; ***p < 0.005. NS, non-severe group (mild and moderate cases); S, severe group (severe and critical cases); NC, non-critical group (mild, moderate and severe cases); C, critical group.
References
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunological features of severe and moderate Coronavirus Disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C., Wong P., Klein H., Castro T.B.R., Silva J., Sundaram M. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020 doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M. Immunology of COVID-19: current state of the science. Immunity. 2020;52(6):910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br J Haematol. 2020;189(3):428–437. doi: 10.1111/bjh.16659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.G., Simpson L.J., Ferreira A.-M., Rustagi A., Roque J.A., Asuni A. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight. 2020;5(17):e140289. doi: 10.1172/jci.insight.140289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance. WHO/2019-nCoV/clinical/2020.4. [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Cai T., Fan L., Lou K., Hua X., Huang Z. et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332–333. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cytokine profile in COVID-19 patients related to the severity of the disease. Results of cytokine levels measured between day 0 and day 3 after hospital admission are presented as medians ± inter-quartile ranges (IQR). Comparison between the groups was assessed by nonparametric Kruskal-Wallis analysis of variance, followed by a Dunn’s multiple comparison test. *p < 0.05; **p < 0.01; ***p < 0.005.
Comparison of cytokine profile in SARS-CoV-2 infected patients. Comparison of cytokine profile measured between day 0 and day 3 after hospital admission in (A) non-severe versus severe and (B) non-critically ill versus critically ill patients. Comparisons were assessed by the two-tailed nonparametric Mann-Whitney test. *p < 0.05; **p < 0.01; ***p < 0.005. NS, non-severe group (mild and moderate cases); S, severe group (severe and critical cases); NC, non-critical group (mild, moderate and severe cases); C, critical group.