Abstract
Pesticides are chemical substances intended for preventing or controlling pests. These are toxic substances which contaminate soil, water bodies and vegetative crops. Excessive use of pesticides may cause destruction of biodiversity. In plants, pesticides lead to oxidative stress, inhibition of physiological and biochemical pathways, induce toxicity, impede photosynthesis and negatively affect yield of crops. Increased production of reactive oxygen species like superoxide radicals, O−2 hydrogen peroxide, H2O2; singlet oxygen, O2; hydroxyl radical, OH−; and hydroperoxyl radical HO2−, causes damage to protein, lipid, carbohydrate and DNA within plants. Plant growth regulators (PGR) are recognized for promoting growth and development under optimal as well as stress conditions. PGR combat adverse effect by acting as chemical messenger and under complex regulation, enable plants to survive under stress conditions. PGR mediate various physiological and biochemical responses, thereby reducing pesticide-induced toxicity. Exogenous applications of PGRs, such as brassinosteroid, cytokinins, salicylic acid, jasmonic acid, etc., mitigate pesticide toxicity by stimulating antioxidant defense system and render tolerance towards stress conditions. They provide resistance against pesticides by controlling production of reactive oxygen species, nutrient homeostasis, increase secondary metabolite production, and trigger antioxidant mechanisms. These phytohormones protect plants against oxidative damage by activating mitogen-stimulated protein kinase cascade. Current study is based on reported research work that has shown the effect of PGR in promoting plant growth subjected to pesticide stress. The present review covers the aspects of pesticidal response of plants and evaluates the contribution of PGRs in mitigating pesticide-induced stress and increasing the tolerance of plants. Further, the study suggests the use of PGRs as a tool in mitigating effects of pesticidal stress together with improved growth and development.
Keywords: Antioxidant defense system, Nutrient homeostasis, Oxidative stress, Pesticides, Photosynthesis
Introduction
Any compound existing naturally or factitiously intended to terminate, restrain or mutate the life cycle of any pest is labeled as pesticide. Specific pests include weeds, microorganisms, insects, birds, rats and mice (Singh et al. 2018). Pesticides are categorized on the basis of their roles (insecticides, rodenticides, herbicides, fungicides etc.), chemical nature (organophosphates, organochlorine, carbamates, pyrethroids, triazines) amid all, organochlorines (OCs) are hazardous with maximum toxicity (Rani et al. 2017a, b), mobility (systemic and non-systemic), target rates (selective or broad spectrum) (Rodrigo et al. 2014). Further, they can also be classified on the basis of toxicity, mode of entry (Singh et al. 2018).
Pesticides have long-term and major role in agriculture, ensuring protection and increasing crop yield. Pesticides are used to obviate or limit the pest infection to maintain considerable agricultural production. First-generation pesticides used prior 1940 consisted arsenic, lead, nicotine, etc. were prevalently used for agriculture, and were relinquished due to toxicity and resistance towards pests. Soon after this era, synthetic pesticides evolved, termed as second-generation pesticides comprising, DDT (Dichlorodiphenyltrichloroethane), dieldrin, etc. (Handford et al. 2015).
Owing to limited agricultural land and rapidly growing population, there is a need to escalate the crop production to meet the demand of people and ensure food security. Consequently, initiatives are taken to scale up the crop yield by minimizing the crop loss from pest damage. In an effort to protect crops, pesticides are applied by humans to destroy the pests (Morillo and Villaverde 2017). On a global scale, it is estimated nearly 9000 breeds of insects, 50,000 species of plant pathogens, and 8000 types of weeds damage crops (Zhang et al. 2011). Approximately, insects cause 14% of loss, plant pathogens render 13% loss, and weeds lead to 13% loss in crop production (Pimentel 2009). Application of pesticides is needed to increase crop production. Substantially, one-third of the crop yields are produced by utilizing pesticides (Zhang 2018).
After-effects of pesticide on plants are dose-dependent causing disruption and alteration of various physiological and biochemical processes, ultimately reducing growth and productivity. Conversely, plants have ability to counteract pesticide stress via detoxification system. Among various approaches acquired by plants to compensate unfavorable impact of pesticide stress, PGR render signals to permit plants to endure under stressful conditions. PGR regulate signaling pathways and stimulate resistance in plants. They are one of the fundamental frameworks, incorporating metabolic reactions which are important for various processes of plant life, improving crop quality and yield (Varshney et al. 2015).
Quality and yield of crops are hampered by several pests. Pesticides are any substances or assortment of various substances intended for preventing or controlling pests. Indiscriminate use of pesticides has toxic effect on plants which compromise plants’ metabolism and may persist in various plant parts as pesticide residues (Sharma et al. 2018a, b). Pesticides absorbed by plants impair plant vigor and also induce threat to human beings and animals feeding on it (Ahmed et al. 2018). Detrimental effect is exerted on non-targeted plants by generating reactive oxygen species (Soares et al. 2018a). Reactive oxygen species are extremely reactive in nature, triggering oxidative damage of nucleic acids, lipids and proteins and that lead to variations in different biochemical and physiological processes of cell (Sharma et al. 2019). Use of pesticide involves various morphological, physiological, biochemical and molecular transformations in plants that have adverse impact on growth and development of plant and further result in resistance of pests towards pesticide (Shahzad et al. 2018).
Toxicity induced by pesticides
The indiscriminate use of pesticide manifests negative impact on various non-target species, for instance, nitrogen-fixing bacteria, phosphate-solubilizing bacteria (Ansari and Mahmood 2017) (Sarnaik et al. 2006), adversely affect the growth and productivity of crops upon accumulation within plant tissues, impede plant growth and production. Additionally, various pesticides tend to persist for long duration (upto years) in the environment, e.g., the half-life of γ-HCH is estimated to be 191 days in water (Kafilzadeh 2015). On that account, restriction has been imposed on numerous OCs, including DDT, throughout the world. As a result of persistence and bioaccumulation, they are prevalent and are detected in coastal ecosystem (Arienzo et al. 2013), wetlands (Main et al. 2014), fruits (Gui et al. 2019), birds (Addy-Orduna et al. 2019). Long-range transportation of pesticides via air or water results in widespread distribution (Rani et al. 2017a, b). Mode of action exerts toxic effect not only to specific organisms, but also to non-target species including human beings. Scilicet, deleterious impacts of pesticides, such as kitazin, hexaconazole and carbendazim, on dry matter accumulation, photosynthetic pigments, symbiotic features, nutrient uptake and seed yield of pea plants have been reported (Shahid et al. 2018). Uptake of pesticide into plant tissues varies with plants and chemicals, and can be greatly influenced by nature of chemical, environmental condition and mode of application. It is known that the penetration of pesticides into plant tissue is related to the physicochemical properties of active ingredient. Pesticides with systemic nature are taken up by roots or leaves and translocated to all parts of the plants (Simon-Delso et al. 2015). Imidacloprid is most widely used insecticide globally. It is neonicotinoid insecticide and is systemic in nature. IMI is utilized for wide variety of crops to control pests. It is translocated from soil to above ground aerial parts (usually leaves) through xylem. Later it routes to flowers, fruits and rest of tissues via phloem sap (Laurent and Rathahao 2003; Alsayeda et al. 2008). This insecticide blocks nicotinergic neuronal pathways by acting as an antagonist of nicotinyl acetylcholine receptor in insects (Hylemya antiqua Meig, Diabrotica balteata Lec and Agriotes sp.). IMI is primarily used for seed treatment, foliar spray in various crops and soil treatment (Sarkar et al. 2001). In Brassica juncea, imidacloprid application limits growth and development, reduces photosynthetic ability, generates oxidative stress, and stimulates enzymatic and non-enzymatic antioxidants resulting in deposition of IMI residues in plant parts. Application of imidacloprid results in phytotoxicity of plants, consequently impairing growth and chlorophyll content (Sharma et al. 2019). Glyphosate is a non-selective and broad-spectrum pesticide. It is eminently effective and widely used insecticide. It is primarily used to control competing and regenerating species on agricultural land, forests and gardens. Breakdown of glyphosate molecule generates glyphosate-free acids, thereby exerting phytotoxic effect. Glyphosate targets enzyme 5-enoylpyruvylshikimate-3-phosphate synthase. Foliar spray of glyphosate leads to penetration into cuticle and reach apoplast, further enters synplasts where phloem transport takes places (Dill et al. 2010). Restraining this enzyme leads to aggregation of shikimic acid and subsequent declines in biosynthesis of auxins, vitamins, aromatic amino acids and few vital metabolites synthesized by shikimate pathway, resulting in retarded growth and plant death (Sergiev et al. 2006). In recent years, concern about the effect of glyphosate on soil, water bodies and its accumulation pattern has emerged. The herbicidal effects are toxic to several soil microflora and vital arthropod predators of agroecosystem. It is also proven to pose a potential risk for non-target species (Spormann et al. 2019). Phenanthrene and pyrene are categorized under polycyclic aromatic hydrocarbons (PAHs). These are extremely persistent organic contaminants exhibiting mutagenic as well as teratogenic characteristics (Shahzad et al. 2018). Uptake of PAHs by plant leads to morphological alterations, such as growth inhibition, necrosis and chlorosis. Physiological alterations induced by PAHs are oxidative stress, transform antioxidant enzymes, DNA damage, prevent photosynthesis and cell death. Generation of reactive oxygen species (ROS) is due to oxidation of PAHs, leading to oxidative strain and cell damage (Ahammed et al. 2012a, b, c). Chlorothalonil (CHT) 2,4,5,6-tetrachloroisophthalonitrile exists as organic pollutant primarily used as fungicide with broad-spectrum and non-systemic properties. Polychlorinated biphenyls (PCBs) are broadly distributed organic toxicant, having ability to get accumulated and transported to far areas. Chlorpyrifos is an organophosphate compound, comprising acaricide and miticide. Since 1965, it is being utilized to control pests. Due to its toxicity, considerable reduction is observed in plant weight, lowers photosynthesis, decreases quantum efficacy of photosystem II and decline in photochemical quenching coefficient reveals detrimental effect on growth of seedlings subsequently elevating malondialdehyde levels (Shahzad et al. 2018).
Mechanism of plants to detoxify pesticides
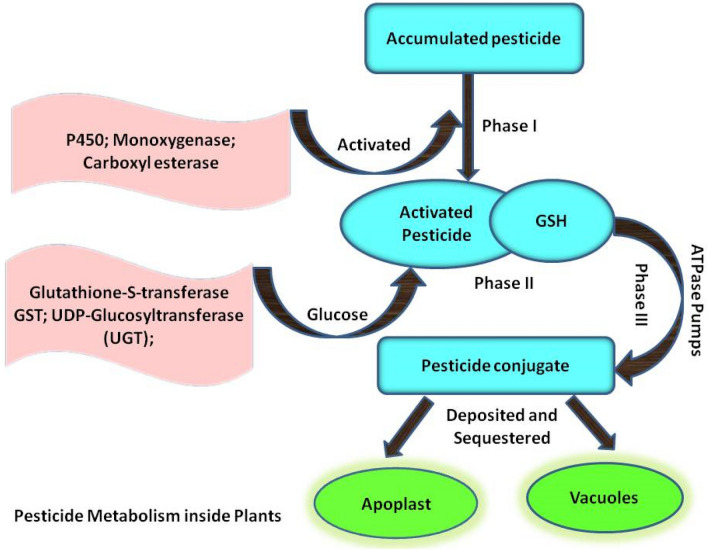
Existing as static life form, plants cannot circumvent stresses by relocation, rather they acquire an internal mechanism to assuage stressful conditions by various physiological and biochemical processes. Absorption of pesticide occurs via roots and leaves, further metabolized by intrinsic detoxification system or amassed in different parts of plant. The repercussions of pesticide use are production of ROS and adversely affecting growth and development of plant. Albeit, the internal mechanism – antioxidative defense system of plant is triggered to combat pesticide stress (Sharma et al. 2018a, b). Pesticides are metabolized by plants through enzyme-mediated pathways. This detoxification system is three-phased process. Phase I: activation of pesticide through hydrolysis, oxidation or reduction, induced by enzymes, such as carboxylesterases, cytochrome P450 monooxygenase, and peroxidase. Phase II: activated pesticide is conjugated with amino acids, glucose or glutathione. The conjugation is catalyzed by enzymes glutathione S-transferase and UDP–glycosyltransferase. Phase III: lastly, less toxic pesticide conjugates are carried to vacuoles/apoplasts or bind to cell wall/lignins (Fig. 1) (Sharma et al. 2019). Plants have in-built capability to break, transform and store pesticide, augmenting their intrinsic detoxification system would assist to guard plants as well as the ecosystem from pesticidal damage (Wang et al. 2017). Plant growth regulators play a fundamental role in perceiving and signaling varied stressful conditions including pesticide toxicity, thereby elicit various responses viz, modulate gene expression, activation of various stress signaling pathways in response to pesticide toxicity (Ali and Baek 2020) (Table 1).
Fig. 1.
Mechanism of Pesticide Detoxification: Pesticides are metabolized by plants through enzyme mediated pathways. This detoxification system is three phased process. Phase I: activation of pesticide is induced by enzymes carboxylesterases, cytochrome P450 and monooxygenase. Phase II: activated pesticide is conjugated with glutathione. The conjugation is catalyzed by enzymes glutathione S-transferase and UDP–glycosyltransferase. Phase III: lastly less toxic pesticide conjugates are carried to vacuoles/apoplasts (Modified after Shahzad et al. 2018)
Table 1.
Role of Plant Growth Regulators in recovery of plants under pesticide stress
| PGR | Pesticide | Negative impact of pesticide | Mechanism of alleviating pesticide-induced toxicity | Reference |
|---|---|---|---|---|
| Castasterone | Imidacloprid |
Generating oxidative stress Decreased plant growth Decline in chlorophyll content Down-regulation of key genes |
Enhanced content of chlorophyll, carotenoids, anthocyanins, xanthophylls Activation of antioxidative defense system Biosynthesis of organic acids and phenolics |
Sharma et al. (2019) |
| Cytokinin | Glyphosate |
Induced oxidative stress Decreased growth and pigment content |
Protective action by rendering hardiness in antioxidant defense system Increased levels of glutathione |
Sergiev et al. (2006) |
| EBL | Chlorothalonil |
Negative effect on soluble protein Raise malondialdehyde content |
Enhance osmoregulation Remarkably facilitate manifestation of antioxidant genes |
Wang et al. (2017) |
| EBL | Chlorpyrifos | Lowers photosynthesis and quantum yield of photosystem II |
Stimulate antioxidant defense system Improves pesticide degradation by switching on vital genes like P450 |
Xia et al. (2009) |
| EBL | Imidachloprid |
Hampers plant growth Lowers chlorophyll content Reduction in photosynthesis Lowers concentration of vigorous biomolecule |
Reduction in IMI toxicity Enhance plant growth Improves pigment storage Improves photosynthesis and stomatal modulation Promotes production of various phytochemicals |
Sharma et al. (2016, 2017a, b) |
| EBL | Phenanthrene and Pyrene |
Prevent photosynthesis Formation of H2O2, OH−, O2− Prevents seed germination Prevents pigment storage Deformation of leaves |
Ameliorate seed germination Improves morphological features Improves chlorophyll emission Augment plant biomass Enhance pesticide detoxification network |
(Ahammed et al. 2012a, 2012b, 2012c) |
| EBL | Polychlorinated biphenyl |
Reduction in biomass Lowers chlorophyll content Obstruct photosynthesis Inhibits stomatal conductance |
Enhances plant growth and development Mitigates photo inhibition Regulates oxidative stress Lowers lipid peroxidation |
Ahammed et al. (2013) |
| EBL | Terbutryn |
Decreases carbon dioxide assimilation Reduces quantum yield of photosystem II Lowers non photochemical quenching |
Increases plant biomass Enhances growth and development of plants Improves florescence and carbon dioxide assimilation |
Pinol and Simon (2009) |
| Jasmonic acid | Imidacloprid |
Production of ROS species leading oxidative burst Reduction in plant growth Reduced photosynthetic efficiency |
Modulates antioxidant defense system Reduction in oxidative stress Regulates biochemical and physiological processes |
Sharma et al. (2018a, b) |
| Salicylic acid | Glyphosate |
Obstructing EPSPS enzyme, leads to less carbon supply to important pathways Lowers the activity of nitrate reductase Enhanced MDA content, proline and ROS species Damages plant physiology |
Improves seedling height, growth and biomass Scavenges ROS species Increases carotenoids, chlorophyll and pigment content Boost the photosynthetic rate Increases protein content and synthesizes new proteins |
Singh et al. (2017) |
| Salicylic acid | Glyphosate |
Growth inhibition Accumulation of H2O2 O− |
Improved growth Lowered amount of H2O2 |
Spormann et al. (2019) |
| Salicylic acid | Napropamide |
Reduction in crop quality and yield Substantial formation of O−2, H2O2 and peroxides Oxidative damage in plasma membrane |
Improves plant tolerance Inhibits the accumulation of ROS species Protects cell membrane against lipid peroxidation Restores oxidative damage |
Cui et al. (2010) |
| Salicylic acid | Paraquat |
Induce oxidative damage by producing oxygen radicals Impedes photosynthesis, production cation radicals and inhibiting the production of NADPH Damages membrane stability |
Improved antioxidant capacity Inhibits pesticide penetration into cell Synthesizes proteins responsible for plant stress management Enhances stress tolerance by triggering detoxifying enzymes |
Ananieva et al. (2004) |
| Salicyclic acid | Thiram |
Oxidative stress Lowered total chlorophyll and carotenoids levels |
Decreade in H202, and MDA levels Increased photosynthetic pigments Regulation of antioxidant enzymes |
Yuzbasioglu and Dalyan (2019) |
PGRs: remedy for pesticide stress
Brassinosteroid
Brassinosteroids are intrinsic class of steroidal hormones necessary for plants growth and maturation. They regulate plant responses against plethora of abiotic and biotic stresses (Cui et al. 2010; Xie et al. 2011; Yang et al. 2011). Brassinosteroids induce resistance via stimulating antioxidant defense system and expediting pesticide metabolism. Biomass production inhibited by pesticides can remarkably be ameliorated via brassinosteroid treatment (Ahammed et al. 2012a). Extrinsic application of brassinosteroids enhances the rate of photosynthesis under abiotic stress conditions. It has be evinced that brassinosteroids-reduced polycyclic aromatic hydrocarbon incited phytotoxicity, consequently boosting photosynthesis and antioxidative defense mechanism (Ahmmed et al. 2012b). Brassinosteroids bind through BR-binding proteins, stabilizing heterodimer formation, stimulate receptor kinases and finally trigger brassinosteroids-mediated signaling cascade (Fellner 2003). As per reports, attenuation in pesticidal stress by expression of various stress-related genes including P450 and GST involved in metabolism of xenobiotic conjugates and activation of antioxidant system for cell protection against damage. Brassinosteroids abet in redox homeostasis and efficiently mitigate side effects of pesticide. Pesticide degradation is achieved by enhancing activity of GST through Respiratory burst oxidase homologue-dependent pathway and increasing metabolism of glutathione. Brassinosteroids are involved in cellular redox reaction and H2O2 production which lead to reduction in pesticide residues (Zhou et al. 2015). Alteration within the activities of antioxidative enzymes indicates abiotic stress. The activity of such enzymes accelerates during initial exposure of pesticides. Once pesticides lead to toxicity, the antioxidant enzyme activity drastically declines. Notably, exogenous application of brassinosteroids enhanced antioxidative enzyme activity and reduced MDA contents under pesticide stress. Improved activity of antioxidative defense system imparts tolerance under oxidative stress conditions. Brassinosteroids further promote ROS scavenging and hormonal homeostasis to combat pesticide-induced impediment in germination and seedling growth (Ahammed et al. 2012c). It is notified that brassinosteroids detoxify many pesticides including organophosphorus, carbamate and organochlorine in diverse plants, such as tea, cucumber, tomato, broccoli and rice (Zhou et al. 2015). In cucumber cultivars pesticides: chlorothalonil, chlorpyrifos, carbendazine and cypermethrin accumulate within tissues which were further sequestered via brassinosteroid treatment (Xia et al. 2009). Brassinosteroids strengthen antioxidative defense system that partakes in detoxification of pesticides under stress conditions (Sharma et al. 2012, 2013, 2015; Xia et al. 2009; Zhou et al. 2015). Terbutryn at higher dose reduced photosynthetic rate along with plant growth and fluorescence parameter in Vicia faba cultivars; however, exogenous application of brassinosteroids alleviates the aforementioned damage. Brassinosteroid treatment improved growth, chlorophyll fluorescence parameter and carbon dioxide absorption (Pinol and Simon 2009).
24-epibrassinolide
24-epibrassinolide is a poly-hydroxylated steroid hormone. Epibrassinolides confer protection against pesticide stress by synthesizing array of phytochemicals in Brassica juncea. Treatment of 24-epibrassinolide induces various metabolic enzymes used for pesticide degradation. Pre-soak treatment of 24-epibrassinolide can remarkably regulate transcription of various antioxidants, defense-specific genes within tomato plant leaves, subjected to chlorpyrifos, thus indicating positive effect of 24-epibrassinolide in gene expression. 24-epibrassinolide is proven to accelerate transcription of chlorothalonil-degrading genes viz, P450 monooxygenase glutathione S-transferase (GST) and glutathione reductase (GR) (Wang et al. 2017). 24-epibrassinolide is considered to increase metabolism of chlorpyrifos, carbendazim, cypermethrin and chlorothalonil in Cucumis sativus L. by reducing the residual levels of pesticides (Xia et al. 2009). Exogenous application of brassinosteroids lowers organophosphorus, carbamate and organochlorine residues in broccoli, cucumber, rice, strawberry, tea and tomato. Co-application of brassinosteroid and chlorothalonil improved regulation of 301 genes which were observed through genome-wide microarray analysis (Zhou et al. 2015). 24-epibrassinolide stimulates cellulose synthesis, rendering biomass accumulation (Xie et al. 2011). Inhibition in biomass production may occur due to intrinsic metabolic alterations. Biomass yield is highly spurred by plant’s efficacy of Pn, which provides raw material for development and growth. And reduction in biomass under pesticidal stress may be due to obstruction of photosynthesis (Ahammed et al. 2012b). In Brassica juncea, imidacloprid residues within tissues were declined after exogenous application of 24-epibrassinolide and plants further rendered pesticides into less toxic form. Enhancement in the activities of antioxidant enzymes viz APOX, GPOX, GR, GST and POD along with glutathione contents was observed under imidacloprid stress. GST degrades pesticides into simple conjugates that can further be detoxified by other defense responses. GSH contents were remarkably increased and GSH1-2 gene expression was stimulated under pesticide toxicity (Sharma et al. 2016). In another study, the level of ROS viz O−2 and H2O2 crossed the threshold in presence of imidacloprid. And decline in ROS content was observed after application of 24-epibrassinolide. ROS generation is due to oxidative burst induced by pesticide stress which disrupts the antioxidant system. It was also demonstrated that up-regulation of RBO gene is accountable for formation of H2O2 under imidacloprid stress. Decline in O− after 24-epibrassionlide treatment occurred due to conversion of O−2 into H202 via SOD enzyme. Similarly, H2O2 levels also declined after 24-epibrassinolide treatment and it was due to conversion of H2O2 into H2O and oxygen via CAT enzyme (Sharma et al. 2017a, b). It is revealed that respiratory burst oxidase homologue 1 (RBOH1) is accountable for generation of H2O2 under pesticide toxicity (Zhou et al. 2015).
Castasterone
Castasterone is bioactive brassinosteroid; its treatment reduced reactive oxygen species (hydrogen peroxide and superoxide anion) accumulation in imidacloprid-exposed seedlings (Brassica juncea). Castasterone pre-treatment activates antioxidant defense system causing significant increase in enzymatic activity. Its pre-treatment also stimulates synthesis of citrate, fumarate, malate and succinate of Krebs cycle and phenolics. In addition, there is up-regulation in gene expression of chief enzymes entailed in Krebs cycle (CS, SUCLG1, SDH, FH), pigment metabolism (CHLASE, PSY, CHS), ROS generation (RBO), carbon fixation (RUBISCO), phenolic biosynthesis (PAL), antioxidative enzymes (SOD, CAT, POD, DHAR, GR, GST), and pesticide detoxification system (CXE,P450, NADH) (Sharma et al. 2019). Castasterone supplementation enhanced seedling growth in Brassica juncea subjected to imidacloprid. This phytohormone plays a vital role in modulating cell division, expansion and synthesis of cellulose (Gonzalez-Garcia et al. 2011; Xie et al. 2011). Castasterone modulates the CHLASE expression which assists in restoring biosynthesis of chlorophyll under stress conditions (Kaur et al. 2017). Under imidacloprid stress, CHLASE expression, and various pigments, such as xanthophylls carotenoids and anthocyanins, improved by application of castasterone. These pigments exhibit strong antioxidative properties, thereby palliating pesticide stress (Sharma et al. 2016b). Castasterone up-regulates the PSY and CHS gene expressions which encode for enzymes phytoene synthase and chalcone synthase, respectively, essential for synthesis of carotenoids, xanthophylls and anthocyanin correspondingly (Yuan et al. 2015).
Salicylic acid
Salicylic acid is a phenolic substance, having function in plant growth, like germination, flowering, chlorophyll biosynthesis and vegetative growth. Pesticide toxicity is mitigated by employing plants with exogenous salicylic acid. Salicylic acid treatment improves pigment content, increases photosynthesis, leads to antioxidant enzyme activity and lowers the effect of residual pesticides in rice, maize, sunflower and wheat. Application of salicylic acid in tomato plant elevated glutathione S-transferase (GST) activity by up-regulating expression of GST1, GST2, GST3 and P450 under pesticide stress condition (Yuzbasioglu et al. 2019). Salicylic acid improves antioxidant activity in plants and increases tolerance towards oxidative stress (Fernandes et al. 2018). Deposition of salicylates within plant tissue indicates defensive mechanism towards stress condition. Salicylic acid treatment moderates effect of glyphosate generated on growth and H2O2 amount. Salicyclic acid promotes non-protein thiol production and enhances activity of ascorbate peroxidase (APX), catalase (CAT), glutathione S-transferase and superoxide dismutase (SOD) in barley plants (Spormann et al. 2019). Salicylic acid restores the damage caused by thiram in tomato cultivars by ameliorating photosynthetic pigment contents, antioxidant defense system, abating oxidative injuries and detoxifying the pesticide (Yuzbasioglu et al. 2019). Application of salicylic acid in low doses augments H2O2 levels that act as a messenger during stress signaling. The prompt increase in oxidative ions triggers the antioxidative defense mechanism (Horvath et al. 2007). Salicylic acid supplementation remarkably restores the structure of cell organelles including nuclear membrane, chloroplast and cell membrane in Oryza sativa cultivars under pesticide stress (Wang et al. 2016). Exogenous application of salicylic acid reduces the lipid peroxidation and H2O2 formation under glyphosate-induced stress condition. Additionally, salicylic acid also modulates the antioxidant defense system in tomato cultivars subjected to thiram toxicity (Singh et al. 2017). Another study reveals that salicylic acid supplementation decreased the activity of CAT, while increasing the activity of POX and GR enzymes in barley cultivars under paraquat stress (Ananieva et al. 2004). It was reported that salicylic acid can provide resistance via boosting GST activity in rapeseed plants exposed to napropamide stress (Cui et al. 2010).
Cytokinins
Cytokinins comprise a family of PGR, essential for promoting growth and development of plants. They are involved in regulation of cell division. Systematic use of exogenous cytokinin not only boosts plant growth and development but also has a role in mitigating stress. Cytokinin, exhibits defensive response in maize plants, by causing hardiness within antioxidant defense system. Treatment with phenylurea cytokinin led tolerance towards glyphosate toxicity. It plays protective role by promoting hardiness in antioxidant defense system of Zea mays plants (Sergiev et al. 2006). Glyphosate declines the growth and pigments in Zea mays, also leading to lipid peroxidation, further causing oxidative stress. Cytokinin acts as herbicide safeners, a group of synthetic compounds with great potential to protect plants against damage induced by herbicides (Farago et al. 1994). Cytokinin leads to enhancement in GSH content, thereby opposing the oxidative damage imposed by glyphosate. It also activates the antioxidative enzymes in maize cultivars. It has been observed that H2O2 level increased after the treatment of cytokinin. Both H2O2 and GSH assist in regulation of antioxidant defense system (Foyer et al. 1997). Moreover, improvement in the levels of ascorbic acid and α-tocopherol was demonstrated after exogenous application of cytokinin (kinetin) (Zhang and Schmidt 2000).
Jasmonic acid
Jasmonates are group of phytohormones, including jasmonic acid and methyl jasmonates, conferring systemic resistance in plants against biotic and abiotic stresses. (Yan et al. 2015). Jasmonic acid plays essential role in countering environmental stress besides inducing growth and development of plants (Wasternack and Hause 2013; Wasternack 2014). Phytotoxic response induced by imazapic in tobacco plants extenuates by exogenous application of jasmonic acid. Treatment of jasmonic acid induces antioxidant response and resistance towards stress condition. Pesticides lead to formation of reactive oxygen species in plants due to which cell death occurs by peroxidation of cell membrane lipids, enzyme inhibition, damage in genetic material and protein oxidation, in such stressful condition, jasmonic acid acts as a protective agent. Phytotoxic effect induced by imazapic in tomato cultivars was palliated via jasmonic acid treatment. Enhancement in chlorophyll content by exogenous application of jasmonic acid in imazapic exposed tomato cultivar was observed (Kaya and Doganlar 2016). Reduction in total chlorophyll content is due to oxidative stress mediated by pesticides. Chlorophyllase enzyme level elevates during stress condition which results in impairment of chlorophyll (Santos 2004; Sakuraba et al. 2014). In another study, the CHLASE were over-expressed in presence of imidacloprid, which was further down-regulated via jasmonic acid application in Brassica juncea cultivars. And jasmonic acid-induced regulation of CHLASE gene resulted in revival of chlorophyll level under imidacloprid toxicity. Furthermore, few secondary metabolites including phenolics and anthocyanins partake in protecting photosynthetic pigments. It was well indicated that total phenols and anthocyanin content were elevated by supplementing jasmonic acid in imidacloprid subjected Brassica juncea plants, suggesting their role in chlorophyll pigment protection against pesticide toxicity (Sharma et al. 2018a, b). Carotenoids are involved in protective processes against oxidative injuries, while protecting chlorophylls against photo-oxidation (Li et al. 2010). With the application of jasmonic acid, the carotenoid contents were increased in presence of imazapic in tomato cultivars. It was also observed that without the treatment of jasmonic acid, the carotenoids levels were elevated in presence of pesticides, and that could be to resistance exhibited by plants against pesticide (Kaya and Doganlar 2016). Malondialdehyde is the final product of lipid peroxidation process, being an imperative indicator of oxidative stress (Smirnoff 1993). The findings reveal that malondialdehyde content declines by the application of jasmonic acid in plants’ subjected stress. It is suggested that jasmonic acid hinders lipid peroxidation and scavenges free radicals, thereby lowering malondialdehyde content in plants exposed to stress conditions. Antioxidant defense system is crucial for protecting cell components against ROS-induced cell damage, where jasmonic acid prevents oxidative stress via enhancing antioxidant enzyme activity, such as that of CAT and APX in plants (Qiu et al. 2014). Glutathione is one such compound which plays essential role in normal as well as during stress conditions by detoxifying xenobiotic compounds (Marrs 1996). Exogenous application of jasmonic acid significantly elevated glutathione content and its activity exposed to pesticides (Kaya and Doganlar 2016; Kaya and Yigit 2014; Bi et al. 2012). In plants, RUBISCO is associated in modulating physiological processes during stress conditions (Perdomo et al. 2017). However, after application of jasmonic acid, the expression of RUBISCO is up-regulated, suggesting the probable role of this phytohormone in stimulating growth under stress conditions. In imidacloprid-exposed plants, the amendment of jasmonic acid resulted in significant improvement in seedling growth of Brassica juncea. Moreover, in absence of any stress inducer, the jasmonic acid exhibits no remarkable difference in seedling growth, it can be because jasmonic acid-responsive genes do not express in absence of stress inducers (Sharma et al. 2018a, b). Jasmonic acid genes express and lead to transcription regulating physiological processes in presence of stressful conditions (Wasternack and Hause 2013).
Conclusion and future perspectives
Pesticides induce toxicity to plants by producing reactive oxygen species, increasing chlorophyllase activity and breakdown of chlorophyll molecules. Plant growth regulators play a crucial role in pesticide detoxification by mitigating toxicity, protecting and imparting resistance to plants in response to pesticide toxicity. Seed treatment with various plant growth regulators can proficiently recuperate plant growth by controlling the plant cellular processes. The roles of different plant growth regulators in plant defense mechanism and in growth protection render a direct way of attenuating the stresses. In the presence of pesticide toxicity, plant growth regulators induce tolerance primarily by activating plants defense mechanism, which involves the enzymatic antioxidants and non-enzymatic antioxidants. In future outlook, PGR will contribute to plant protection and decline pesticide residues in food crops. Additionally, exploring imperative secondary metabolites and stress signaling pathways will assist in understanding precise mechanism of plant response towards pesticide stress. Further, crosstalk studies will explicate molecular mechanism of plant growth regulators in growth- and defense-related processes. Additionally, studies will also elucidate important secondary metabolites and stress signaling pathways for pesticide stress management in plants. Withal, to ensure cost-effectiveness, PGPR (plant growth-promoting rhizobacteria) and other microbial consortium can be incorporated in the soil. Inoculating microbial culture promotes synthesis of PGR which will lead to effective detoxification and degradation of pesticides.
Acknowledgement
I would like to thank authors for their useful suggestions and Lovely Professional University for providing us platform to work.
Abbreviations
- DDT
Dichlorodiphenyltrichloroethane
- IMI
Imidacloprid
- OCs
Organochlorines
- PAHs
Polycyclic aromatic hydrocarbons
- PGR
Plant growth regulator
- ROS
Reactive oxygen species
Author contributions
SJ prepared the manuscript and DK reviewed the manuscript. RS, RB and PA corrected major revisions after receiving suggestions from reviewers. All the authors checked, edited and approved the manuscript prior to its re submission.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributor Information
Sadaf Jan, Email: sadafjan0705@gmail.com.
Rattandeep Singh, Email: rattan.19383@lpu.co.in.
Renu Bhardwaj, Email: renubhardwaj82@gmail.com.
Parvaiz Ahmad, Email: parvaizbot@yahoo.com.
Dhriti Kapoor, Email: dhriti405@gmail.com, Email: dhriti405@gmail.com.
References
- Addy-Orduna LM, Brodeur JC, Mateo R. Oral acute toxicity of imidacloprid, thiamethoxam and clothianidin in eared doves: a contribution for the risk assessment of neonicotinoids in birds. Sci Total Environ. 2019;650:1216–1223. doi: 10.1016/j.scitotenv.2018.09.112. [DOI] [PubMed] [Google Scholar]
- Ahammed GJ, Gao CJ, Ogweno JO, Zhou YH, Xia XJ, Mao WH, Shi K, Yu JQ. Brassinosteroids induce plant tolerance against phenanthrene by enhancing degradation and detoxification in Solanum lycopersicum L. Ecotox Environ Safe. 2012;80:28–36. doi: 10.1016/j.ecoenv.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Ahammed GJ, Yuan HL, Ogweno JO, Zhou YH, Xia XJ, Mao WH, Shi K, Yu JQ. Brassinosteroid alleviates phenanthrene and pyrene phytotoxicity by increasing detoxification activity and photosynthesis in tomato. Chemosphere. 2012;86(5):546–555. doi: 10.1016/j.chemosphere.2011.10.038. [DOI] [PubMed] [Google Scholar]
- Ahammed GJ, Zhang S, Shi K, Zhou YH, Yu JQ. Brassinosteroid improves seed germination and early development of tomato seedling under phenanthrene stress. Plant Growth Regul. 2012;68(1):87–96. [Google Scholar]
- Ahammed GJ, Ruan YP, Zhou J, Xia XJ, Shi K, Zhou YH, Yu JQ. Brassinosteroid alleviates polychlorinated biphenyls-induced oxidative stress by enhancing antioxidant enzymes activity in tomato. Chemosphere. 2013;90(11):2645–2653. doi: 10.1016/j.chemosphere.2012.11.041. [DOI] [PubMed] [Google Scholar]
- Ahmed A, Shamsi A, Bano B. Deciphering the toxic effects of iprodione, a fungicide and malathion, an insecticide on thiol protease inhibitor isolated from yellow Indian mustard seeds. Environ Toxicol Pharmacol. 2018;61:52–60. doi: 10.1016/j.etap.2018.05.019. [DOI] [PubMed] [Google Scholar]
- Ali M, Baek KH. Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int J Mol Sci. 2020;21(2):621. doi: 10.3390/ijms21020621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsayeda H, Pascal-Lorber S, Nallanthigal C, Debrauwer L, Laurent F. Transfer of the insecticide [14 C] imidacloprid from soil to tomato plants. Environ Chem Lett. 2008;6(4):229–234. [Google Scholar]
- Ananieva EA, Christov KN, Popova LP. Exogenous treatment with salicylic acid leads to increased antioxidant capacity in leaves of barley plants exposed to paraquat. J Plant Physiol. 2004;161(3):319–328. doi: 10.1078/0176-1617-01022. [DOI] [PubMed] [Google Scholar]
- Ansari RA, Mahmood I. Optimization of organic and bio-organic fertilizers on soil properties and growth of pigeon pea. Sci Hortic. 2017;226:1–9. [Google Scholar]
- Arienzo M, Masuccio AA, Ferrara L. Evaluation of sediment contamination by heavy metals, organochlorinated pesticides, and polycyclic aromatic hydrocarbons in the Berre coastal lagoon (southeast France) Arch Environ Con Tox. 2013;65(3):396–406. doi: 10.1007/s00244-013-9915-3. [DOI] [PubMed] [Google Scholar]
- Bi YF, Miao SS, Lu YC, Qiu CB, Zhou Y, Yang H. Phytotoxicity, bioaccumulation and degradation of isoproturon in green algae. J Hazard Mater. 2012;243:242–249. doi: 10.1016/j.jhazmat.2012.10.021. [DOI] [PubMed] [Google Scholar]
- Cui J, Zhang R, Wu GL, Zhu HM, Yang H. Salicylic acid reduces napropamide toxicity by preventing its accumulation in rapeseed (Brassica napus L.) Arch Environ Con Tox. 2010;59(1):100–108. doi: 10.1007/s00244-009-9426-4. [DOI] [PubMed] [Google Scholar]
- Dill GM, Sammons RD, Feng PC, Kohn F, Kretzmer K, Mehrsheikh A, Haupfear EA. Glyphosate: discovery, development, applications, and properties. Dev Manage. 2010;1:1–33. [Google Scholar]
- Farago S, Brunold C, Kreuz K. Herbicide safeners and glutathione metabolism. Physiol Plant. 1994;91(3):537–542. [Google Scholar]
- Fellner M. Recent progress in brassinosteroid researchhormone perception and signal transductionBrassinosteroid. Dordrecht: Springer; 2003. [Google Scholar]
- Fernandes RB, De Godoy KF, Malavazi I, Anschau V, Chaves DB, Filho A, Miyamoto S, Netto LES. Identification and characterization of reduction agents of 1-Cys peroxiredoxins from Aspergillus fumigatus, a human opportunistic pathogen. Free Radic Biol Med. 2018;120:S155–S156. [Google Scholar]
- Foyer CH, Lopez-Delgado H, Dat J, Scott IM. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant. 1997;100(2):241–254. [Google Scholar]
- González-García MP, Vilarrasa-Blasi J, Zhiponova M, Divol F, Mora-García S, Russinova E, Caño-Delgado AI. Brassinosteroids control meristem size by promoting cell cycle progression in Arabidopsis roots. Development. 2011;138(5):849–859. doi: 10.1242/dev.057331. [DOI] [PubMed] [Google Scholar]
- Gui T, Jia GF, Xu J, Ge SJ, Long XF, Zhang YP, Hu DY. Determination of the residue dynamics and dietary risk of thiamethoxam and its metabolite clothianidin in citrus and soil by LC-MS/MS. J Environ Sci Heal B. 2019;54(4):326–335. doi: 10.1080/03601234.2019.1571361. [DOI] [PubMed] [Google Scholar]
- Handford CE, Elliott CT, Campbell K. A review of the global pesticide legislation and the scale of challenge in reaching the global harmonization of food safety standards. Integr Environ Assess Manag. 2015;11(4):525–536. doi: 10.1002/ieam.1635. [DOI] [PubMed] [Google Scholar]
- Horváth E, Szalai G, Janda T. Induction of abiotic stress tolerance by salicylic acid signaling. J Plant Growth Regul. 2007;26(3):290–300. [Google Scholar]
- Jx C, Zhou Yh, Jg D, Xj X, Kai S, Chen Sc Yu, Jq, Role of nitric oxide in hydrogen peroxide-dependent induction of abiotic stress tolerance by brassinosteroids in cucumber. Plant Cell Environ. 2011;34(2):347–358. doi: 10.1111/j.1365-3040.2010.02248.x. [DOI] [PubMed] [Google Scholar]
- Kafilzadeh F. Assessment of organochlorine pesticide residues in water, sediments and fish from Lake Tashk. Iran Achiev Life Sci. 2015;9(2):107–111. [Google Scholar]
- Kaur R, Yadav P, Sharma A, Thukral AK, Kumar V, Kohli SK, Bhardwaj R. Castasterone and citric acid treatment restores photosynthetic attributes in Brassica juncea L. under Cd (II) toxicity. Ecotox Environ Safe. 2017;145:466–475. doi: 10.1016/j.ecoenv.2017.07.067. [DOI] [PubMed] [Google Scholar]
- Kaya A, Doganlar ZB. Exogenous jasmonic acid induces stress tolerance in tobacco (Nicotiana tabacum) exposed to imazapic. Ecotox Environ Safe. 2016;124:470–479. doi: 10.1016/j.ecoenv.2015.11.026. [DOI] [PubMed] [Google Scholar]
- Kaya A, Yigit E. The physiological and biochemical effects of salicylic acid on sunflowers (Helianthus annuus) exposed to flurochloridone. Ecotox Environ Safe. 2014;106:232–238. doi: 10.1016/j.ecoenv.2014.04.041. [DOI] [PubMed] [Google Scholar]
- Laurent FM, Rathahao E. Distribution of [14C] imidacloprid in sunflowers (Helianthus annuus L.) following seed treatment. J Agr Food Chem. 2003;51(27):8005–8010. doi: 10.1021/jf034310n. [DOI] [PubMed] [Google Scholar]
- Li G, Wan S, Zhou J, Yang Z, Qin P. Leaf chlorophyll fluorescence, hyperspectral reflectance, pigments content, malondialdehyde and proline accumulation responses of castor bean (Ricinus communis L.) seedlings to salt stress levels. Ind Crop Prod. 2010;31(1):13–19. [Google Scholar]
- Main AR, Headley JV, Peru KM, Michel NL, Cessna AJ, Morrissey CA. Widespread use and frequent detection of neonicotinoid insecticides in wetlands of Canada’s Prairie Pothole Region. PLoS ONE. 2014;9:3. doi: 10.1371/journal.pone.0092821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs KA. The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Biol. 1996;47(1):127–158. doi: 10.1146/annurev.arplant.47.1.127. [DOI] [PubMed] [Google Scholar]
- Morillo E, Villaverde J. Advanced technologies for the remediation of pesticide-contaminated soils. Sci Total Environ. 2017;586:576–597. doi: 10.1016/j.scitotenv.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Perdomo JA, Capó-Bauçà S, Carmo-Silva E, Galmés J. Rubisco and rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit. Front Plant Sci. 2017;8:490. doi: 10.3389/fpls.2017.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel D. Integrated pest management: innovation-development process. Dordrecht: Springer; 2009. Pesticides and pest control; pp. 83–87. [Google Scholar]
- Piñol R, Simón E. Effect of 24-epibrassinolide on chlorophyll fluorescence and photosynthetic CO 2 assimilation in Vicia faba plants treated with the photosynthesis-inhibiting herbicide terbutryn. J Plant Growth Regul. 2009;28(2):97–105. [Google Scholar]
- Qiun ZB, Guo JL, Zhu AJ, Zhang L, Zhang MM. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol Environ Safe. 2014;104:202–208. doi: 10.1016/j.ecoenv.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Rani M, Shanker U, Jassal V. Recent strategies for removal and degradation of persistent & toxic organochlorine pesticides using nanoparticles: a review. J Environ Manage. 2017;190:208–222. doi: 10.1016/j.jenvman.2016.12.068. [DOI] [PubMed] [Google Scholar]
- Rani M, Shanker U, Jassal V. Recent strategies for removal and degradation of persistent and toxic organochlorine pesticides using nanoparticles: a review. J Environ Manage. 2017;190:208–222. doi: 10.1016/j.jenvman.2016.12.068. [DOI] [PubMed] [Google Scholar]
- Rodrigo MA, Oturan N, Oturan MA. Electrochemically assisted remediation of pesticides in soils and water: a review. Chem Rev. 2014;114(17):8720–8745. doi: 10.1021/cr500077e. [DOI] [PubMed] [Google Scholar]
- Sakuraba Y, Kim D, Kim YS, Hörtensteiner S, Paek NC. Arabidopsis STAYGREEN-LIKE (SGRL) promotes abiotic stress-induced leaf yellowing during vegetative growth. FEBS Lett. 2014;588(21):3830–3837. doi: 10.1016/j.febslet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- Santos CV. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci Hortic. 2004;103(1):93–99. [Google Scholar]
- Sarkar MA, Roy S, Kole RK, Chowdhury A. Persistence and metabolism of imidacloprid in different soils of West Bengal. Pest Manag Sci. 2001;57(7):598–602. doi: 10.1002/ps.328. [DOI] [PubMed] [Google Scholar]
- Sarnaik SS, Kanekar PP, Raut VM, Taware SP, Chavan KS, Bhadbhade BJ. Effect of application of different pesticides to soybean on the soil microflora. J Environ Biol. 2006;37(2):423–426. [PubMed] [Google Scholar]
- Sergiev IG, Alexieva VS, Ivanov SV, Moskova II, Karanov EN. The phenylurea cytokinin 4PU-30 protects maize plants against glyphosate action. Pestic Biochem Physiol. 2006;85(3):139–146. [Google Scholar]
- Shahid M, Ahmed B, Khan MS. Evaluation of microbiological management strategy of herbicide toxicity to greengram plants. Biocatal Agric Biotechnol. 2018;14:96–108. [Google Scholar]
- Shahzad B, Tanveer M, Che Z, Rehman A, Cheema SA, Sharma A, Song H. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: a review. Ecotox Environ Safe. 2018;147:935–944. doi: 10.1016/j.ecoenv.2017.09.066. [DOI] [PubMed] [Google Scholar]
- Sharma I, Bhardwaj R, Pati PK. Mitigation of adverse effects of chlorpyrifos by 24-epibrassinolide and analysis of stress markers in a rice variety Pusa Basmati-1. Ecotox environ safe. 2012;85:72–81. doi: 10.1016/j.ecoenv.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Sharma I, Bhardwaj R, Pati PK. Stress modulation response of 24-epibrassinolide against imidacloprid in an elite indica rice variety Pusa Basmati-1. Pest Biochem Phys. 2013;105(2):144–153. [Google Scholar]
- Sharma I, Bhardwaj R, Pati PK. Exogenous application of 28-homobrassinolide modulates the dynamics of salt and pesticides induced stress responses in an elite rice variety Pusa Basmati-1. J Plant Growth Regul. 2015;34(3):509–518. [Google Scholar]
- Sharma A, Kumar V, Singh R, Thukral AK, Bhardwaj R. Effect of seed pre-soaking with 24-epibrassinolide on growth and photosynthetic parameters of Brassica juncea L. in imidacloprid soil. Ecotox Environ Safe. 2016;133:195–201. doi: 10.1016/j.ecoenv.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kumar V, Bhardwaj R, Thukral AK. Seed pre-soaking with 24-epibrassinolide reduces the imidacloprid pesticide residues in green pods of Brassica juncea L. Toxicol Environ Chem. 2017;99(1):95–103. [Google Scholar]
- Sharma A, Thakur S, Kumar V, Kesavan AK, Thukral AK, Bhardwaj R. 24-epibrassinolide stimulates imidacloprid detoxification by modulating the gene expression of Brassica juncea L. BMC Plant Biol. 2017;17(1):56. doi: 10.1186/s12870-017-1003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Kumar V, Kumar R, Shahzad B, Thukral AK, Bhardwaj R. Brassinosteroid-mediated pesticide detoxification in plants: a mini-review. Cogent Food Agric. 2018;4(1):1436212. [Google Scholar]
- Sharma A, Kumar V, Yuan H, Kanwar MK, Bhardwaj R, Thukral AK, Zheng B. Jasmonic acid seed treatment stimulates insecticide detoxification in Brassica juncea L. Front plant sci. 2018;9:1609. doi: 10.3389/fpls.2018.01609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Yuan H, Kumar V, Ramakrishnan M, Kohli SK, Kaur R, Thukral AK, Bhardwaj R, Zheng B. Castasterone attenuates insecticide induced phytotoxicity in mustard. Ecotox Environ Safe. 2019;179:50–61. doi: 10.1016/j.ecoenv.2019.03.120. [DOI] [PubMed] [Google Scholar]
- Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, Goulson D. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res. 2015;22(1):5–34. doi: 10.1007/s11356-014-3470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Singh NB, Singh A, Hussain I. Exogenous application of salicylic acid to alleviate glyphosate stress in Solanum lycopersicum. Int J Veg Sci. 2017;23(6):552–566. [Google Scholar]
- Singh NS, Sharma R, Parween T, Patanjali PK. Modern Age Environmental Problems and their Remediation. Cham: Springer; 2018. Pesticide contamination and human health risk factor; pp. 49–68. [Google Scholar]
- Smirnoff N. Tansley Review No. 52. The role of active oxygen in the response of plants to water deficit and desiccation. New phytol. 1993;1:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- Soares C, Spormann S, Fidalgo F. Salicylic acid improves the performance of the enzymatic antioxidant system of barley exposed to glyphosate. Free Radic Biol Med. 2018;120:S157. [Google Scholar]
- Spormann S, Soares C, Fidalgo F. Salicylic acid alleviates glyphosate-induced oxidative stress in Hordeum vulgare L. J Environ Manage. 2019;241:226–234. doi: 10.1016/j.jenvman.2019.04.035. [DOI] [PubMed] [Google Scholar]
- Varshney S, Khan MIR, Masood A, Per TS, Rasheed F, Khan NA. Contribution of plant growth regulators in mitigation of herbicidal stress. J Plant Biochem Physiol. 2015;3:2. [Google Scholar]
- Wang J, Lv M, Islam F, Gill RA, Yang C, Ali B, Zhou W. Salicylic acid mediates antioxidant defense system and ABA pathway related gene expression in Oryza sativa against quinclorac toxicity. Ecotox Environ Safe. 2016;133:146–156. doi: 10.1016/j.ecoenv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Wang Z, Jiang Y, Peng X, Xu S, Zhang H, Gao J, Xi Z. Exogenous 24-epibrassinolide regulates antioxidant and pesticide detoxification systems in grapevine after chlorothalonil treatment. Plant Growth Regul. 2017;81(3):455–466. [Google Scholar]
- Wasternack C. Action of jasmonates in plant stress responses and development—applied aspects. Biotechnol Adv. 2014;32(1):31–39. doi: 10.1016/j.biotechadv.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot. 2013;111(6):1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XJ, Zhang Y, Wu JX, Wang JT, Zhou YH, Shi K, Yu YL, Yu JQ. Brassinosteroids promote metabolism of pesticides in cucumber. J Agric Food Chem. 2009;57(18):8406–8413. doi: 10.1021/jf901915a. [DOI] [PubMed] [Google Scholar]
- Xie L, Yang C, Wang X. Brassinosteroids can regulate cellulose biosynthesis by controlling the expression of CESA genes in Arabidopsis. J exp bot. 2011;62(13):4495–4506. doi: 10.1093/jxb/err164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zhang W, Chen J, Li X. Methyl jasmonate alleviates cadmium toxicity in Solanum nigrum by regulating metal uptake and antioxidative capacity. Biol Plant. 2015;59(2):373–381. [Google Scholar]
- Yang CJ, Zhang C, Lu YN, Jin JQ, Wang XL. The mechanisms of brassinosteroids' action: from signal transduction to plant development. Mol plant. 2011;4(4):588–600. doi: 10.1093/mp/ssr020. [DOI] [PubMed] [Google Scholar]
- Yuan LB, Peng ZH, Zhi TT, Zho Z, Liu Y, Zhu Q, Ren CM. Brassinosteroid enhances cytokinin-induced anthocyanin biosynthesis in Arabidopsis seedlings. Biol plantarum. 2015;59(1):99–105. [Google Scholar]
- Yüzbaşıoğlu E, Dalyan E. Salicylic acid alleviates thiram toxicity by modulating antioxidant enzyme capacity and pesticide detoxification systems in the tomato (Solanum lycopersicum Mill.) Plant Physiol Biochem. 2019;135:322–330. doi: 10.1016/j.plaphy.2018.12.023. [DOI] [PubMed] [Google Scholar]
- Zhang W. Global pesticide use: profile, trend, cost/benefit and more. Proc Int Acad Ecol Environ Sci. 2018;8(1):1. [Google Scholar]
- Zhang X, Schmidt RE. Hormone-containing products’ impact on antioxidant status of tall fescue and creeping bentgrass subjected to drought. Crop Sci. 2000;40(5):1344–1349. [Google Scholar]
- Zhang W, Jiang F, Ou J. Global pesticide consumption and pollution: with China as a focus. Proc Int Acad Ecol Environ Sci. 2011;1(2):125. [Google Scholar]
- Zhou Y, Xia X, Yu G, Wang J, Wu J, Wang M, Yang Y, Shi K, Yu Y, Chen Z, Gan J. Brassinosteroids play a critical role in the regulation of pesticide metabolism in crop plants. Sci Rep. 2015;5:9018. doi: 10.1038/srep09018. [DOI] [PMC free article] [PubMed] [Google Scholar]