Abstract
The wound healing process is characterized by varied biological and molecular cascades including inflammation, tissue proliferation, and remodeling phase. To augment and maintain these cascades, an all-natural matrix system is proposed. Biocompatible biopolymers, sodium alginate and gelatin, were employed to prepare microfibers via extrusion-gelation into a physical crosslinking solution. Curcumin, an anti-inflammatory, anti-oxidant and wound healing agent, was loaded into the fibers as a natural bioactive compound. Curcumin-loaded composite microfibers and blank microfibers were fabricated using biopolymers such as sodium alginate and gelatin. The formulation batches were coded as A1G9-A10G0 according to the varied concentrations of sodium alginate and gelatin. The molecular transitions within the composite microfibers were characterized using FTIR and were further corroborated using molecular mechanics analysis. In mechanical properties tensile strength and elongation-at-break (extensibility) were ranging between 1.08 ± 0.01 to 3.53 ± 0.41 N/mm2 and 3.89 ± 0.18 to 0.61 ± 0.03%. The morphological analysis confirmed the formation and fabrication of the microfibers. In addition, physical evaluation including matrix degradation and entrapment efficiency was performed to give a comparative account of various formulations. The water uptake capacity of the blank and curcumin-loaded composite fibers was found to be in the range of 30.77 ± 2.17 to 100.00 ± 5.99 and 22.34 ± 1.11 to 56.34 ± 4.68, respectively. Composite microfibers presented a cumulative release of 85% in 72 h, confirming the prolonged release potential of the composite fibers. The drug release followed an anomalous (non-Fickian) release behavior asserting the role of degradation and diffusion. In an in vivo full-thickness cutaneous wound model, the composite microfibers provided higher degree of contraction 96.89 ± 3.76% as compared to the marketed formulation (Vicco turmeric cream). In conclusion, this all-natural, alginate–gelatin–curcumin composite has the potential to be explored as a cost-effective wound healing platform.
Keywords: Curcumin, Alginate, Gelatin, Fibers, Gelation, Wound healing
Introduction
Wound healing is a multiplex process of tissue regeneration initiated by the body in response to a traumatic injury involving opening up or missing some cellular structures (Bagher et al. 2020). For effective intervention and repair, the invading pathogens are removed from the site of damage. The site of wound is covered with a sterile dressing material to prevent infection and to augment the process of wound healing—a complex biological, chemical and mechanical process (Sorg et al. 2017). The three cascades which complete the cycle include inflammatory phase, proliferative phase and the tissue remodeling phase (Srivastav et al. 2018; Rajkumar et al. 2018). Topical application of an anti-inflammatory drug modality appears to be an obvious intervention.
Natural polyphenols may not only provide the anti-inflammatory action but also their anti-oxidant properties will certainly be beneficial against the oxidative damage (Pankongadisak et al. 2019; Hewlings et al. 2017). Curcumin, an active ingredient of the rhizome of Curcuma longa, plays a significant role in wound healing as an anti-inflammatory and radical scavenging agent (Akbik et al. 2014; Barchitta et al. 2019). It is a natural antibiotic which is employed for its anti-bacterial properties as well as used as an anti-oxidative, anti-inflammatory, antitumor, antimicrobial, anti-HIV and anti-carcinogenic agent (Varaprasad et al. 2020a, b, c). Despite having unique therapeutic potential of curcumin as an excellent wound healing agent, curcumin shows comparatively low physical and chemical stability (Kulkarni et al. 2020). This has led to the development of suitable carriers for curcumin to increase its stability and even bioavailability (Fereydouni et al. 2019; Kaur et al. 2019).
Fibrous systems, including microfibers and nanofibers, are considered as suitable carriers for drug delivery applications. Due to the soft-yet-flexible properties and porous morphology of the fibers, they are considered as an attractive option for wound dressings (Mutlu et al. 2018). The fibers also effectively absorb wound exudates. Previous research involving curcumin loaded fibrous structures (PVA electrospun fibrous mats) showed controlled drug release along with anti-bacterial activity against gram +ve and gram –ve bacteria (Mahmud et al. 2020). Another study involving chitosan-PVA membranes loaded with curcumin showed wound healing activity in a rat model (Abbas et al. 2019). In the present research, composite biopolymeric alginate-gelatin fibers were prepared for wound healing applications, as alginate has been used in the development of several wound dressing material in order to improve efficiency of wound healing (Varaprasad et al. 2020a, b, c; Afjoul et al. 2020). Gelatin is reported for wound healing applications as it enables fast migration of repaired cells along with providing antibacterial properties (Zahiri et al. 2020). The biopolymeric fibers were loaded with curcumin for potential wound repair. Ionotropic gelation is employed for the fabrication of fibers as there are too many variables in electrospinning, so it is impractical to produce ionically crosslinked fibers of alginate-alone using electrospinning. Alginate-alone rather forms a sheet instead of a mesh and post-spinning crosslinking of dried alginate fibers with Ca++ leads to surface crosslinking rather than bulk crosslinking. Curcumin sediments out with prolonged time and hence clogs the nozzle of the electrospinner (Bediako et al. 2020). In addition to the physicochemical, physicomechanical, and morphological analysis, the developed fibrous systems were tested in vivo for their therapeutic potential in a full-thickness wound model. The microfibers showed intricate molecular interaction profile within the alginate–gelatin matrix, presented anomalous drug release mechanism, and enhanced wound healing as compared to a commercial turmeric containing cream.
Materials and methods
Materials
Curcumin (CAS: 458-37-7) was purchased from Sigma-Aldrich, St. Louis, MO, USA. Sodium alginate (CAS: 9005-38-3), gelatin (CAS: 9000-70-8), and calcium chloride (CAS: 10035-04-8) were procured from Loba Chemie Mumbai, India. All other chemicals, solvent, and reagents used in the study were of analytical grade and were used as received.
Preparation of curcumin-loaded composite microfibers
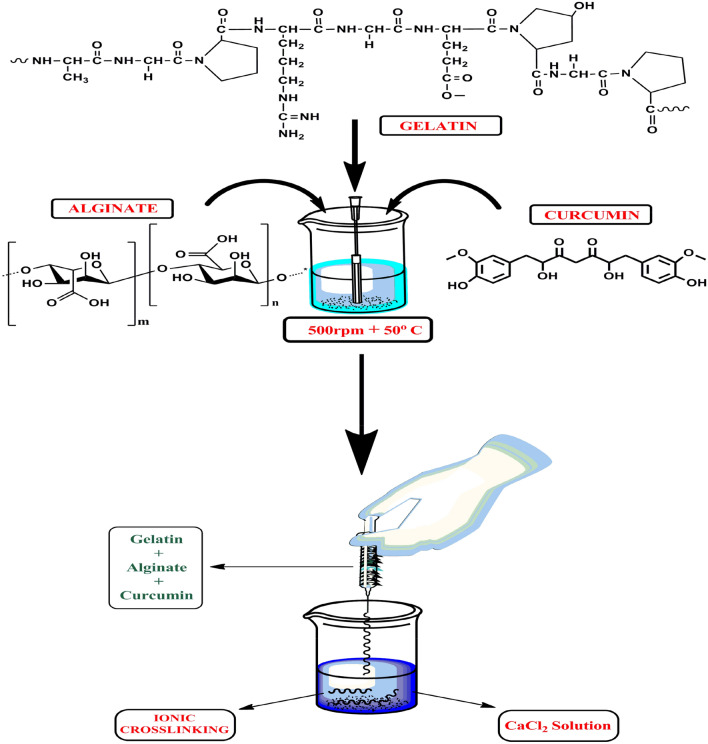
Ionotropic gelation method was used for the fabrication of curcumin-loaded, composite, alginate-gelatin microfibers. Briefly, sodium alginate:gelatin solutions were prepared in weight ratios as shown in (Table 1). An aqueous biopolymeric solution was prepared by solublizing alginate and gelatin by stirring for 30 min at 500 rpm. The biopolymeric solution was heated to 50° C for complete solubilisation of gelatin. Curcumin (50 mg) was first dissolved in 5 ml acetone and was then added slowly to the biopolymeric solution. The curcumin/alginate/gelatin mixture was then extruded (using a 22-gauge needle) into a beaker containing 1 wt% CaCl2. The fibers so formed by ionic crosslinking were washed with water and air dried. Blank alginate/gelatin fibers were also prepared using the above method with no drug added to the biopolymeric mixture as depicted in (Fig. 1) (Bediako et al. 2020).
Table 1.
Composition of curcumin-loaded composite microfibers
| Formulation | Sodium alginate (%w/w) | Gelatin (%w/w) | Curcumin (mg) |
|---|---|---|---|
| A9G1 | 90 | 10 | 50 |
| A8G2 | 80 | 20 | 50 |
| A7G3 | 70 | 30 | 50 |
| A6G4 | 60 | 40 | 50 |
| A5G5 | 50 | 50 | 50 |
| A4G6 | 40 | 60 | 50 |
| A3G7 | 30 | 70 | 50 |
| A2G8 | 20 | 80 | 50 |
| A1G9 | 10 | 90 | 50 |
| A10G0 | 100 | 0 | 50 |
Fig. 1.
Fabrication method of curcumin loaded composite microfibers
Characterization of curcumin-loaded composite fibers
The curcumin-loaded microfibers were evaluated for morphological characteristics using SEM analysis, entrapment efficiency, physicochemical evaluation via FTIR, molecular simulations and physical properties such as degradation study, water uptake, mechanical properties, in vitro release studies and in vivo animal studies.
Morphological analysis
Curcumin-loaded composite microfibers were mounted on a metal stub with the aid of a double-sided adhesive tape. The fibers were sputter coated with gold/palladium and the surface of the fibers was visualized under various magnifications (250 × and 500 ×) and at an accelerating voltage of 10 kV. The scanning electron microscopy was carried out on FEI Nova Nanolab 600 SEM (FEI, Hillsboro, Oregon, USA) and the obtained images were adjusted for clarity, brightness, and contrast using Paint.net (freeware raster graphics editor program) (Padilla et al. 2015).
Entrapment efficiency
The total amount of drug (curcumin) loaded in microfibers was determined in ternion using fiber samples (10 cm in length) and were weighed. The microfiber samples were placed in an aqueous (phosphate buffer saline)/ethanolic solvent (50:50; over 24 h) for maximal drug extraction from the microfibers. The fibers were crushed in mortar and pestle in the presence of the hydroalcoholic solvent and the mixture was filtered for further UV–Vis analysis (Perkin Elmer Lambda35) at 421 nm. The standard curve was plotted using standard solutions of curcumin in ethanol (Zahiri et al. 2019).
Physicochemical evaluation of curcumin-loaded composite microfibers
FTIR studies
To ascertain the molecular interactions between the constituent biopolymers, and the drug and biopolymers, ATR-FTIR spectroscopic analysis (IFS66/S, Alpha Bruker, Germany) was carried out on blank and drug-loaded composite microfibers. The analysis was carried out in the reflectance mode within the wave number region of 4000–500 cm−1 (Shababdoust et al. 2018).
Molecular simulations
The execution procedures of modeling and computational methods counting energy minimizations in Molecular Mechanics were performed (HyperChemLite 30, Gainesville, FL, USA) and Chimera UCSF). Alginate structures (4 saccharide units) and gelatin structures were constructed using natural bond angles. The models were primarily energy-minimized using the MM + Force Field algorithm and the molecular complex was assembled together with another by collateral disposition. The Polak–Ribiere Conjugate Gradient method until a RMS gradient (0.001 kcal/ mol) was perceived for further energy minimization (Bayomi et al. 2015; Kumar et al. 2018).
Physical properties of the developed fibers
Degradation study
To carry out the degradability analysis, the microfibers were mounted on aluminum foil and were put in phosphate buffer saline; PBS (pH of 7.4). The microfibers were incubated for 20 days and the temperature was maintained at 37 °C. After completion of 20 days, the microfibers were properly washed followed by drying in vacuum oven for 48 h. The SEM images were used for reporting morphological changes (Si et al. 2019).
Water uptake
Water uptake by the composite microfiber matrices was determined in phosphate-buffered saline (PBS) at pH 7.4. Briefly, the fibers were weighed (initial weight) and then immersed in 10 ml of PBS for 24 h before being carefully taken out with tweezers. The access water on the surface was soaked up with the aid of filter paper and the wet weight (final weight) was obtained (Golchin et al. 2019). The % water uptake was calculated as follows:
Mechanical properties
The mechanical properties of curcumin-loaded composite microfibers were analyzed using an uniaxial tensile machine (Instron5943, Canton, MA, USA) with a holding load cell capacity of 10 N and at a cross head speed of 5 mm/min. The samples of composite microfibers were cut into specified length to give a testing length of 5 cm and extension test procedure was executed at room temperature (Sharifah et al. 2017). The inherent tensile strength and elongation at break were then computed using the following equations:
In vitro drug release study
The in vitro drug release studies of the curcumin-loaded composite microfibers was performed in phosphate-buffered saline (PBS; pH 7.4) at 37 °C for 72 h. The microfibers (containing 5 mg of curcumin) were added to 50 ml of the PBS in a closed container and were placed in an orbital shaker. Subsequently, 3 ml aliquots were withdrawn at predetermined time intervals (0.5, 1, 2, 4, 8, 12, 24, 48 and 72 h) followed by replacement with fresh PBS to maintain a constant volume. The withdrawn samples were then analyzed for drug content at 421 nm. Kinetic studies were then performed to analyze the mechanism of drug release by fitting the release data in zero-order, first-order, Higuchi matrix, Hixson-Crowell, and Korsmeyer–Pappas models (Sun et al. 2013 and Wang et al. 2017).
Wound healing study
Twelve healthy 8-week-old (220–250 g) Wistar rats (male) were divided randomly into three groups (control group, microfiber group, and the marketed product group) with four rats in each group. The animals were anesthetized with intraperitoneal injection of the mixture of 30 mg/kg ketamine and 4 mg/kg xylazine followed by removal of hair from the dorsal surface of upper back of each rat. Then a 1 cm of full-thickness wound was created by excising the dermis and epidermis. Curcumin-loaded composite microfiber dressings (A5G5) were applied to cover the wound beds as the microfiber group while Vicco turmeric cream was applied as the commercial product. The wound dressings were changed regularly (every 4 days) and the wound lesion size were captured at a close and fixed distance at 0, 3, 7, and 14 days (Saraswathy et al. 2012; Niranjan et al. 2019). The wound closure rate was then calculated using the following formula:
Results and discussion
Characterization of curcumin-loaded composite fiber
The curcumin-loaded microfibers were characterized for morphological characteristics using SEM analysis and entrapment efficiency. The physicochemical properties were evaluated via FTIR, molecular simulations and physical properties such as degradation study, water uptake, mechanical properties, in vitro release studies
and in vivo animal studies.
Morphological analysis
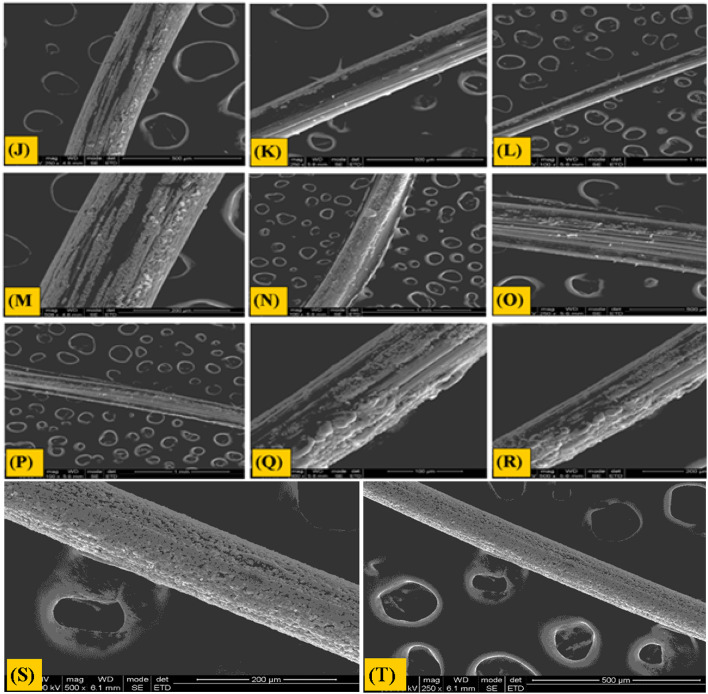
The surface morphology of alginate/gelatin composite fibers was studied using SEM. The diameter of the fibers was measured between 200 and 400 µm. Some crystal deposition could be seen on the surface of the fibers which could be due to the deposition of calcium chloride crystals after diffusion to the surface while drying. The surfaces and sections of two batches (AG10 and AG30) revealed a smooth and uniformly homogeneous morphology which clearly indicated inflated miscibility and uniform homogeneity in between alginate and gelatin as shown in (Fig. 2).
Fig. 2.
Morphological analysis of different batches at magnification value (a) is 500 × of batch A1G9 (b) is 250 × of batch A1G9 (c) is 497 × of Batch A2G8 (d) is 250 × of batch A2G8 (e) is 500 × of batch A3G7 (f) is 250 × of batch A3G7 (g) is 500 × of batch A4G6 (h) is 250 × of batch A4G6 (i) is 500 × of batch A5G5 (j) is 250 × of batch A5G5 (k) is 500 × of batch A6G4 (l) is 250 × of batch A6G4 (m) is 500 × of batch A7G3 (n) is 250 × of batch A7G3 (o) is 500 × of batch A8G2 (p) is 250 × of batch A8G2 (q) is 500 × of batch A9G1 (r) is 250 × of batch A9G1 (s) is 500 × of batch A10G0 and (t) is 250 × of batch A10G0
Entrapment efficiency
The entrapment efficiency of the curcumin-loaded fibers was between 81.13 ± 1.23 for alginate-alone fibers and 99.84 ± 6.98% for A4G6 fibers as shown in (Table 2). Interestingly, the entrapment efficiency was increased with an increase in gelatin content in the fibers which may be ascribed to partial interactions between curcumin and gelatin (Nguyen et al. 2019). Luz stated that the percentage of curcumin per milligram of fiber was 4.0 ± 0.2% for PLA/curcumin fibers and 1.8 ± 0.1% for PLGA/curcumin fibers (Luz et al. 2013).
Table 2.
% Entrapment efficiency of drug-loaded composite microfibers
| Batch code | Entrapment efficiency (%) |
|---|---|
| A10G0 | 81.13 ± 1.23 |
| A9G1 | 87.53 ± 1.58 |
| A8G2 | 88.34 ± 2.10 |
| A7G3 | 90.92 ± 2.98 |
| A6G4 | 94.61 ± 3.47 |
| A5G5 | 94.82 ± 3.78 |
| A4G6 | 99.84 ± 6.98 |
| A3G7 | 98.56 ± 5.99 |
Physicochemical evaluation of curcumin-loaded composite microfibers
FTIR
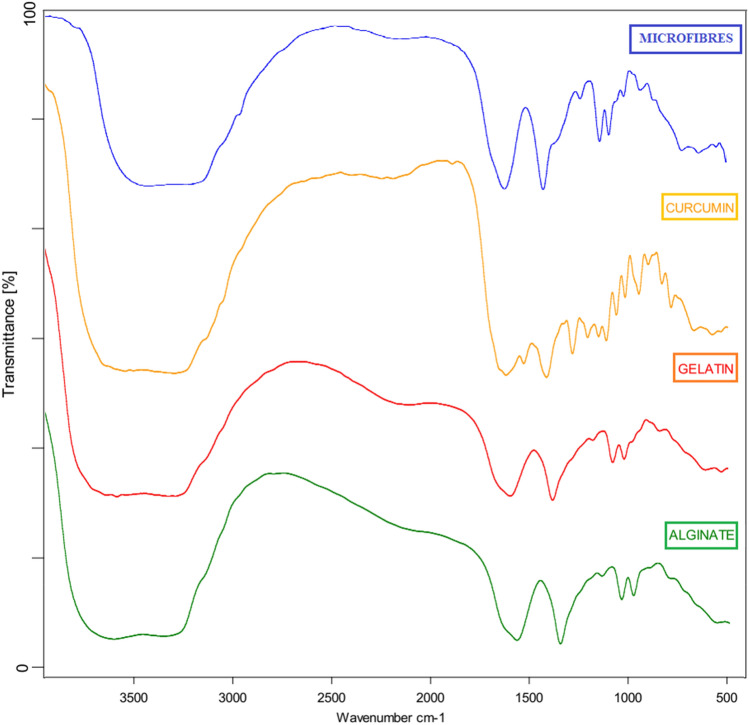
Attenuated total reflectance Fourier transform infrared (FTIR) was employed to provide additional information about molecular interactions within the formulations. Figure 3 shows FTIR spectra of alginate, gelatin, curcumin and curcumin-loaded microfibers. The IR spectrum of alginate showed characteristic absorption bands at 3313 cm−1 (OH stretching), 1608 cm−1 (carboxylic C=O), 1291 cm−1 (C–CH), 1088 cm−1–1051 cm−1 (C–O stretching), 1024 cm−1 (C–C), 949 cm−1 (C–O), 880 cm−1 (CH) and 812 cm−1 (Na–O) (Varaprasad et al. 2020a, b, c). Major peaks of gelatin showed absorption bands at 3411 cm−1 (NH stretching), 1650 cm−1 (amide I, C=O), 1549 cm−1 (amide NH bending) and 1335 cm−1 (for C–N stretching). FTIR of pure curcumin showed peak at 3510 cm−1 which corresponds to phenolic OH vibrations, 1629 cm−1 (C=O) ketonic carbonyl vibration peak at 1597 cm−1 attributed to symmetric aromatic ring stretching vibration (C=C) and C–O attributed to 1279 cm−1. The peaks confirmed the purity of curcumin (Hassani et al. 2020). As characteristic peaks of Curcumin were absent in the FTIR spectra of curcumin-loaded microfibers, it may be postulated that curcumin was well embedded in the fiber matrix.
Fig. 3.
FTIR spectra of microfibers, curcumin, gelatin and alginate
Molecular simulations
Model building and energy refinements facilitated by molecular mechanics
The potential energy surfaces were represented by analytical and mathematical techniques, constituting the molecular mechanics energy relationship (MMER), which aided in providing information related to the input of valence terms, non-covalent columbic terms as well as non-covalent Vander Waals forces in molecular interactions. By comparing the total potential energies (isolated and complexed systems), the molecular stability can be determined and it can explain if the total potential energy of complex is lesser than the sum of the potential energies of individual molecules (same conformation), then the complexed form was more stable as depicted in (Table 3).
Table 3.
Different types of energies as depicted through molecular mechanistic study
| Energy type | Gelatin | Alginate | Alg–Gel composite | Alginate–gelatin Ca+2 |
|---|---|---|---|---|
| Total energy | 17.160 | 20.719 | 4.711 | 0.352 |
| Bond energy | 0.769 | 2.689 | 3.253 | 3.216 |
| Angle energy | 17.418 | 21.034 | 36.183 | 37.303 |
| Dihedral energy | 15.777 | 25.651 | 41.573 | 41.456 |
| V dw energy | − 1.503 | 5.393 | − 9.450 | − 13.261 |
| H-bond energy | − 0.429 | − 0.462 | − 2.661 | − 1.623 |
It is evident from the energy minimization data that alginate and gelatin formed a geometrically and energetically stabilized proteo-saccharide complex with an energy stabilization of 33.168 kcal/mol.; interestingly, the final energy of the bimolecular complex (4.711 kcal/mol) was less that of the individual energy values of the individual components (20.719 for alginate and 17.160 for gelatin). The molecular stabilization was well supported by the formation of an H-bond between –COO– of alginate and –NH2 of gelatin as shown in (Fig. 4). The hydrogen bonding may have led to a physical strain in the complex causing a destabilization of the bonding energy values (bond, angle and torsional energies). However, this stabilization was countered by the highly stabilized non-bonding interactions. The electrostatic interactions played a major role in geometrical optimization followed by Vander Waals forces and H-bonding.
Fig. 4.
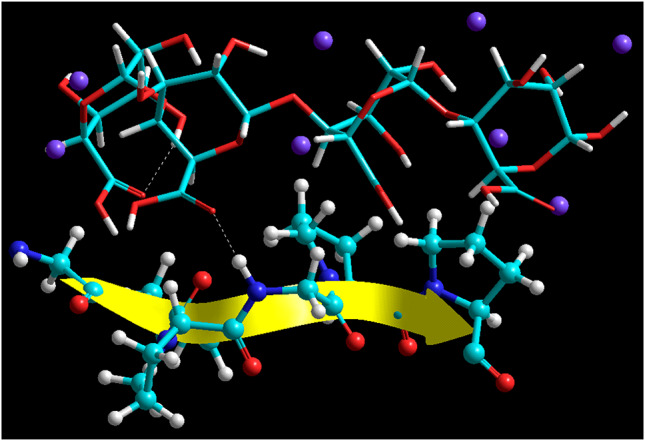
Energy minimized constrained model of the alginate/gelatin proteo-saccharide assemblies derived from molecular mechanics calculations in vacuum. Color codes for elements are as follows: carbon (cyan), hydrogen (white-tubes), oxygen (red) and nitrogen (blue). Molecule rendering: alginate (tubes); gelatin (ball and tube)
To ascertain the role of physical cross linking by Ca++ ions, the molecular Complexation was carried out in the presence of eight Ca++ ions as represented in (Fig. 5). As compared to Alg-Gel molecular complex, Alg-Gel-Ca++ complex was stabilized by minimal energy stabilization of ≈ 4 kcal/mol, whereas in the electrostatic interactions and Vander Waals forces accounted for the molecular stabilization. Although the H-bonding energy term was increased in magnitude, the Alg–Gel H-bonds were intact and confirmed the strong proteo-saccharide complexation between alginate and gelatin.
Fig. 5.

Energy minimized constrained model of the Ca++ cross linked alginate/gelatin proteo-saccharide assemblies derived from molecular mechanics calculations in vacuum. Color codes for elements are as follows: carbon (cyan), hydrogen (white-tubes), oxygen (red) and nitrogen (blue). Molecule rendering: alginate (tubes); gelatin (ball and tube); Ca++ (purple spheres)
Physical properties of developed fibers
Degradation studies
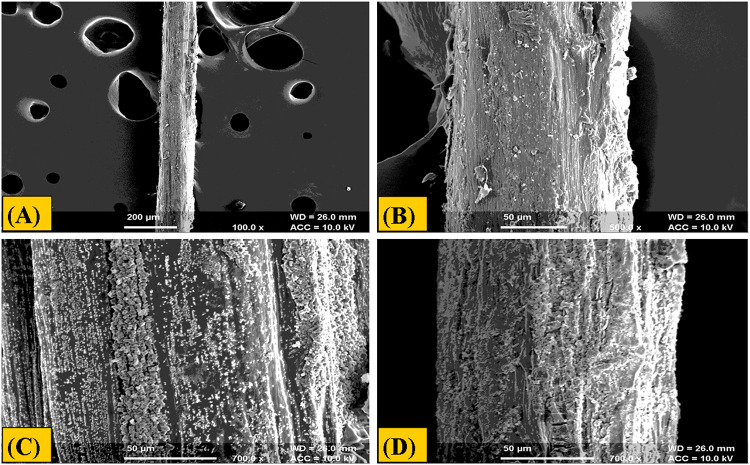
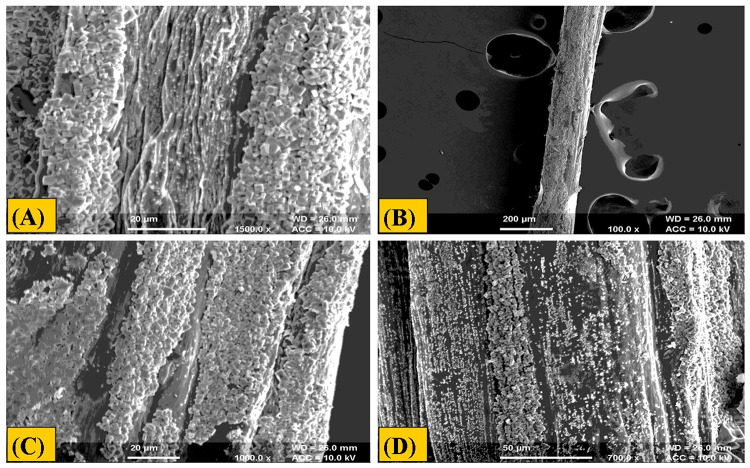
The composite microfibers are relatively used in the field of biomedical, especially in tissue engineering because of their high surface area to volume ratio and high porosity. It is evident that an ideal wound dressing should reveal gradual degradation and reabsorption properties. After 20 days of degradation study the prepared fibers showed no cracks and morphological changes shown in (Figs. 6, 7). Hence, the prepared fibers of alginate-gelatin were found to be stable as exhibited in degradation study.
Fig. 6.
Morphological analysis of batch (A5G5) before degradation at different magnification value (a) at 100 × (b) 500 × (c) 1000 × (d) 700 ×
Fig. 7.
Morphological analysis of batch (A5G5) after degradation at different magnification value (a) at 1500 × (b) 100 × (c) 1000 × (d) 700 ×
(Ranjbar et al. 2018) stated that polymeric electrospun nanofibers are pertained successfully to fabricate scaffolds for biomedical applications (tissue engineering) as they are apparent for the presence of high surface area-to volume ratio and high porosity. The nanofibers manifested randomly oriented porous structure and its interconnected pores. After completing 20 days, PCL/AV nanofibers does not exhibited any significant morphological changes. In PCL/GT/AV nanofibers, the minor cracks were observable but they were evident in maintaining their original structure.
Water uptake
The water uptake capacity of alginate–gelatin composite microfibers was found to be increased as the amount of gelatin was increased (Table 4). This could be attributed to enhance network formation as imparted by an increase in the concentration of gelatin and is explained further under FTIR discussion. The % water uptake of the composite fibers decreased with the addition of curcumin with maximum value reaching 56.34 ± 4.68% in case of A3G7. The decrease in water affinity could be attributed to the lipophilic nature of curcumin and the presence of curcumin in the biopolymer matrix. The water-retention properties of the fibers (blended) were utilized as an important function of the weight of gelatin. The water retention of alginate/gelatin fibers effectively increased as the amount of gelatin was gradually increased and resulted as ranging from 108 to 284% which was clearly higher than alginate fiber with lowest value (91%). Alginate’s hydrophilicity was decreased when Ca+2 was added; there was an improvement in water retention because of gelatin hydrophilic nature as that of calcium alginate (Fan et al. 2005).
Table 4.
Water uptake of blank and curcumin-loaded composite microfibers
| Batch code | Blank fibers | Drug loaded fibers |
|---|---|---|
| A10G0 | 30.77 ± 2.17 | 22.34 ± 1.11 |
| A9G1 | 38.22 ± 3.29 | 28.43 ± 2.15 |
| A8G2 | 45.11 ± 3.39 | 33.33 ± 2.28 |
| A7G3 | 56.32 ± 4.49 | 35.32 ± 1.32 |
| A6G4 | 80.13 ± 3.87 | 38.56 ± 2.38 |
| A5G5 | 88.83 ± 4.76 | 43.21 ± 2.64 |
| A4G6 | 95.78 ± 5.65 | 48.32 ± 3.74 |
| A3G7 | 100.00 ± 5.99 | 56.34 ± 4.68 |
Mechanical properties
Mechanical properties of the prepared composite microfibers were assessed in terms of tensile strength and elongation to break (extensibility). Tensile strength and elongation to break were ranging between 1.08 ± 0.01 to 3.53 ± 0.41 N/mm2 and 3.89 ± 0.18 to 0.61 ± 0.03%, respectively. The tensile strength of the fibers was increased with an increase in the gelatin content. Interaction and subsequent bond formation between the two biopolymers could be attributed to increase in the mechanical properties of the composite material as shown in (Table 5). The effect of gelatin on the dry and wet tensile strengths has also been reported by several researchers. The study revealed that the tensile strength of AG10 and AG30 was higher as compared to pure alginate fibers, and the maximum value was obtained at 30% gelatin content. The tensile strength was effectively increased in blended fiber because of the partial interaction between the biomacromolecules as explained under the FTIR discussion and the molecular modeling discussion. However, an inverse relation was observed in case of elongation at break wherein it decreased with an increase in the gelatin content. The variation in this parameter corresponds to tensile strength holding the maximum value (21.3% in the dry state and 70% in the wet state) and was effectuated at the concentration 30% of gelatin. Consequently, by controlling blend conditions, blended fiber can achieve good mechanical property than pure alginate, which is more expensive in contrast (Fan et al. 2005).
Table 5.
Mechanical properties of drug-loaded composite microfibers
| Batch code | Tensile strength (N/mm2) | Elongation to break (%) |
|---|---|---|
| A10G0 | 1.08 ± 0.01 | 3.89 ± 0.18 |
| A9G1 | 1.11 ± 0.21 | 2.47 ± 0.15 |
| A8G2 | 1.80 ± 0.36 | 2.33 ± 0.19 |
| A7G3 | 2.51 ± 0.48 | 1.27 ± 0.13 |
| A6G4 | 2.71 ± 0.34 | 1.03 ± 0.09 |
| A5G5 | 2.92 ± 0.32 | 0.89 ± 0.02 |
| A4G6 | 3.30 ± 0.21 | 0.80 ± 0.01 |
| A3G7 | 3.53 ± 0.41 | 0.61 ± 0.03 |
In vitro drug release study
The results of in vitro dissolution studies are represented in (Fig. 8). There was increase in dissolution rate of A10G0 (46.43 ± 1.12) to A5G5 (12.84 ± 1.74) at 24 h and A10G0 (67.34 ± 2.34) to A5G5 (20.43 ± 2.74) at 48 h because there is formation of matrix due to intermolecular H-Bonding between alginate–gelatin composite microfibers. Despite, A4G6 and A3G7, there was increase in dissolution rate because there was increase in hydrophilicity of composite microfibers.
Fig. 8.
In Vitro dissolution profile of curcumin-loaded composite microfibers
Even after 50 h, the pure curcumin dissolution profile depicted negligible results, as depicted by Wang et al. (2015), whereas, a rapid rise in the dissolution rate was observed in case of Curcumin/PVP nanofibers, with the yellow color of the medium in the flask. Fiber mats constituting ibuprofen and PVP K30 were able to dissolve in-vitro previous dissolution tests and that too within 10 s. The dissolution rate of Curcumin/PVP nanofibers was less than 90% within 15 min as observed presently, which gradually decreased to 89% at 48 h. The reduction in dissolution profile of Curcumin over time was mainly due to drug precipitation, as it was present in the buffer in a supersaturated state and was observed visually once the tests were performed. These test results demonstrated the ability of electrospun PVP nanofibers to produce rapidly dissolving drug release systems for drugs which poorly dissolve in water and their derivatives.
The drug release data obtained after in vitro release study wer further analyzed via fitting into various kinetic models, i.e. zero order, first order, Higuchi equation, Hixson-Crowell model, and the power law. The coefficient of determination (r2) was much closer to 1 for first-order equation. All the formulations followed non-Fickian diffusion with n values falling within the 0.5 < n < 1.0 range and depicted anomalous release behavior (Table 6). This value indicates a combination of diffusion- and erosion-controlled mechanisms responsible for the release of drug from the composite fibers.
Table 6.
In vitro drug release (kinetic modeling) data of curcumin-loaded composite microfibers
| Batch | Zero order | First order | Higuchi model | Hixson crowell model | Korsmeyer peppas model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| r2 | k0 | r2 | k1 | r2 | kH | r2 | kHC | r2 | kKP | n | |
| A10G0 | 0.944 | 1.070 | 0.982 | − 0.010 | 0.994 | 9.966 | 0.980 | − 0.024 | 0.991 | 0.958 | 0.520 |
| A3G7 | 0.953 | 0.991 | 0.986 | − 0.008 | 0.992 | 9.184 | 0.984 | − 0.020 | 0.992 | 0.869 | 0.546 |
| A4G6 | 0.956 | 0.831 | 0.976 | − 0.006 | 0.985 | 7.658 | 0.981 | − 0.015 | 0.986 | 0.776 | 0.554 |
| A9G1 | 0.962 | 0.785 | 0.982 | − 0.005 | 0.984 | 6.931 | 0.990 | − 0.013 | 0.971 | 0.560 | 0.652 |
| A8G2 | 0.937 | 0.635 | 0.929 | − 0.003 | 0.943 | 5.784 | 0.965 | − 0.009 | 0.946 | 0.503 | 0.638 |
| A7G3 | 0.958 | 0.496 | 0.982 | − 0.004 | 0.980 | 4.557 | 0.981 | − 0.007 | 0.959 | 0.406 | 0.637 |
| A6G4 | 0.965 | 0.408 | 0.974 | − 0.002 | 0.966 | 3.704 | 0.974 | − 0.006 | 0.938 | 0.295 | 0.644 |
| A5G5 | 0.984 | 0.373 | 0.984 | − 0.003 | 0.976 | 3.376 | 0.996 | − 0.005 | 0.984 | 0.016 | 0.775 |
Wound healing study
The animal study was conducted in accordance with the protocol approved by the Animal Ethics Committee of Chitkara College of Pharmacy, Chitkara University, Patiala, Punjab (1181/PO/ReBi/S/08/CPCSEA). Wound contraction progressed faster in case of treatment group when compared with the control group. Complete healing of wound was observed between 10th and 14th day. Composite microfibers (A5G5) showed 81.90 ± 2.76 degree of contraction, whereas the commercial formulation (Vicco Turmeric®) showed 96.89 ± 3.76 degree of contraction. In case of the control group, only 59.85 ± 3.79 degree of contraction was observed on the 10th day. In terms of period of epithelialization; the commercial sample showed hair growth on the 10th day but it was not observed completely in control until the 14th day (Fig. 9). The accelerated wound contraction and epithelialization period may be due to the wound healing property of curcumin as shown in (Table 7).
Fig. 9.
Wound healing process on 0, 8th and 14th day of treatment with Control Group, Microfibers (curcumin) and marketed formulation (Vicco turmeric)
Table 7.
Degree of contraction data of control, composite microfibers and marketed drug
| Groups | Day 2 | Day 4 | Day 6 | Day 8 | Day10 | Day12 | Day14 |
|---|---|---|---|---|---|---|---|
| Wound (control group) | 18.54 ± 1.10 | 22.81 ± 2.89 | 35.88± 2.09 | 46.79 ± 1.98 | 59.85 ± 3.79 | 74.57 ± 5.87 | 88.76 ± 2.98 |
| Composite microfibers (A5G5) | 24.35 ± 4.98 | 37.76 ± 3.98 | 57.98 ± 1.98 | 67.87 ± 3.09 | 81.90 ± 2.76 | 96.76 ± 6.53 | 98.75 ± 4.05 |
| Marketed formulation (Vicco turmeric cream) | 34.78 ± 3.98 | 47.98 ± 4.09 | 59.08 ± 5.09 | 69.90 ± 4.07 | 96.89 ± 3.76 | 96.43 ± 2.99 | 97.01 ± 3.51 |
Conclusion
Curcumin-loaded alginate–gelatin based composite microfibers with diameter ranging between 200 and 400 µm were prepared using ionotropic gelation technique. Molecular mechanics based simulation study displayed the formation of energetically stabilized proteo-saccharide complex between alginate and gelatin responsible for adequate mechanical and degradation resistant properties. In vitro drug release study indicated the release of curcumin for extended period of time. In vivo animal study exhibited significant wound healing property of curcumin-loaded composite microfibers when compared with marketed formulation of curcumin. Alginate–gelatin based composite microfibers may be regarded as potential drug delivery system for wound care management.
Acknowledgements
The authors are thankful to Dr. Madhu Chitkara, Vice-Chancellor, Chitkara University, Punjab, India; Dr. Ashok Chitkara, Chancellor, Chitkara University, Punjab, India and Dr. Sandeep Arora, Director, Chitkara University, Punjab, India for constant encouragement and providing necessary facilities.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The animal study was conducted in accordance with the protocol approved by the Animal Ethics Committee of Chitkara College of Pharmacy, Chitkara University, Patiala, Punjab (1181/PO/ReBi/S/08/CPCSEA).
In vivo animal study
In vivo wound healing studies were conducted in a full-thickness wound model and were approved by the Animal Ethics Committee (AEC), Chitkara University, Patiala, Punjab (118/PO/ReBi/S/08/CPCSEA).
References
- Abbas M, Muhammad Arshad HT, et al. Wound healing potential of curcumin cross-linked chitosan/polyvinyl alcohol. Int J BiolMacromol. 2019;140:871–876. doi: 10.1016/j.ijbiomac.2019.08.153. [DOI] [PubMed] [Google Scholar]
- Afjoul H, Shamloo A, Kamali A, et al. Freeze-gelled alginate/gelatin scaffolds for wound healing applications: an in vitro, in vivo study. Mater SciEng. 2020;113:110957. doi: 10.1016/j.msec.2020.110957. [DOI] [PubMed] [Google Scholar]
- Akbik D, Ghadiri M, Chrzanowski W, et al. Curcumin as a wound healing agent. Life Sci. 2014;116(1):1–7. doi: 10.1016/j.lfs.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Bagher Z, Ehterami A, Safdel MH, et al. Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model. J Drug DelivSci Tech. 2020;55:101379. doi: 10.1016/j.jddst.2019.101379. [DOI] [Google Scholar]
- Barchitta M, Maugeri A, Favara G, et al. Nutrition and wound healing: an overview focusing on the beneficial effects of curcumin. Int J MolSci. 2019;20(5):1–14. doi: 10.3390/ijms20051119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayomi SM, El-Kashef HA, El-Ashmawy MB, et al. Synthesis and biological evaluation of new curcumin analogues as antioxidant and antitumor agents: molecular modeling study. Eur J Med Chem. 2015;101:584–594. doi: 10.1016/j.ejmech.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Bediako JK, Lin S, Sarkar AK, Zhao Y, Choi JW, Song MH, Yun YS, et al. Benignly-fabricated crosslinkedpolyethylenimine/calcium-alginate fibers as high-performance adsorbents for effective recovery of gold. J Clean Prod. 2020;252:119389. doi: 10.1016/j.jclepro.2019.119389. [DOI] [Google Scholar]
- Blanco PA, López RA, Loarca PG, et al. Characterization, release and antioxidant activity of curcumin-loaded amaranth-pullulanelectrospun fibers. LWT-Food SciTechnol. 2015;63(2):1137–1144. doi: 10.1016/j.lwt.2015.03.081. [DOI] [Google Scholar]
- Fan L, Du Y, Huang R, et al. Preparation and characterization of alginate/gelatin blend fibers. J ApplPolymSci. 2005;96(5):1625–1629. doi: 10.1002/app.21610. [DOI] [Google Scholar]
- Fereydouni N, Darroudi M, Movaffagh J, et al. Curcuminnanofibers for the purpose of wound healing. J Cell Physiol. 2019;234(5):5537–5554. doi: 10.1002/jcp.27362. [DOI] [PubMed] [Google Scholar]
- Golchin A, Hosseinzadeh S, Staji M, et al. Biological behavior of the curcumin incorporated chitosan/poly (vinyl alcohol) nanofibers for biomedical applications. J Cell Biochem. 2019;120(9):15410–15421. doi: 10.1002/jcb.28808. [DOI] [PubMed] [Google Scholar]
- Hassani A, Mahmood S, Enezei HH, et al. Formulation, characterization and biological activity screening of sodium alginate-gum arabic nanoparticles loaded with curcumin. Molecules. 2020;25(9):1–21. doi: 10.3390/molecules25092244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlings SJ, Kalman DS. Curcumin: a review of it’s effects on human health. Foods. 2017;6(10):1–11. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R, Sharma A, Puri,, et al. Preparation and characterization of biocomposite films of carrageenan/locust bean gum/montmorrillonite for transdermal delivery of curcumin. BI. 2019;9(1):37–43. doi: 10.15171/bi.2019.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AS, Gurav DD, Khan AA, Shinde VS, et al. Curcumin loaded nanofibrous mats for wound healing application. Colloid Surface B. 2020;189:110885–110885. doi: 10.1016/j.colsurfb.2020.110885. [DOI] [PubMed] [Google Scholar]
- Kumar P, Choonara YE, Pillay V, et al. In silicoanalytico-mathematical interpretation of biopolymeric assemblies: quantification of energy surfaces and molecular attributes via atomistic simulations. BioengTransla Med. 2018;3(3):222–231. doi: 10.1002/btm2.10105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz PP, Silva ML, Hinestroza JP, et al. Curcumin-loaded biodegradable electrospun fibers: preparation, characterization, and differences in fiber morphology. Int J Polym Anal Ch. 2013;18(7):534–544. doi: 10.1080/1023666X.2013.816207. [DOI] [Google Scholar]
- Mahmud MM, Zaman S, Perveen A, et al. Controlled release of curcumin from electrospun fiber mats with antibacterial activity. J Drug DelivSci Tech. 2020;55:101386. doi: 10.1016/j.jddst.2019.101386. [DOI] [Google Scholar]
- Mutlu G, Calamak S, Ulubayram K, et al. Curcumin-loaded electrospun PHBV nanofibers as potential wound-dressing material. J Drug DelivSci Tech. 2018;43:185–193. doi: 10.1016/j.jddst.2017.09.017. [DOI] [Google Scholar]
- Nguyen DT, Dinh VT, Dang LH. Dual interactions of amphiphilic gelatin copolymer and nanocurcumin improving the delivery efficiency of the nanogels. Polymers. 2019;11(5):1–15. doi: 10.3390/polym11050814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjan R, Kaushik M, Prakash J. Enhanced wound healing by PVA/chitosan/curcumin patches: in vitro and in vivo study. Colloid Surf B. 2019;182:1–8. doi: 10.1016/j.colsurfb.2019.06.068. [DOI] [PubMed] [Google Scholar]
- Pankongadisak P, Sangklin S, Chuysinuan P, et al. The use of electrospuncurcumin-loaded poly (l-lactic acid) fiber mats as wound dressing materials. J Drug DelivSci Tech. 2019;53:1–9. doi: 10.1016/j.jddst.2019.06.018. [DOI] [Google Scholar]
- Rajkumar SRJ, Gnanavel G, Nadar MM, et al. Wound healing activity of MorindatinctoriaRoxb aqueous leaf extract. 3 Biotech. 2018;8(8):343. doi: 10.1007/s13205-018-1361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbar-Mohammadi M. Characteristics of aloe vera incorporated poly (ε-caprolactone)/gum tragacanthnanofibers as dressings for wound care. J Ind Text. 2018;47(7):1464–1477. doi: 10.1177/1528083717692595. [DOI] [Google Scholar]
- Saraswathy N, Rohit R, Shanmugam K. A preliminary investigation of turmeric-agar composite film as bioactive wound dressing material on excision wound in rat model. Indian J Nat Prod Resour. 2012;3(2):237–241. [Google Scholar]
- Shababdoust A, Ehsani M, Shokrollahi P, et al. Fabrication of curcumin-loaded electrospunnanofiberous polyurethanes with anti-bacterial activity. ProgrBiomat. 2018;7(1):23–33. doi: 10.1007/s40204-017-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifah IS, Qairol AA, Azlina HN, et al. Thermal, structural and mechanical properties of melt drawn cur-loaded poly (lactic acid) fibers. ProcediaEng. 2017;184:544–551. doi: 10.1016/j.proeng.2017.04.129. [DOI] [Google Scholar]
- Shrivastav A, Mishra AK, Ali SS, et al. In vivo models for assesment of wound healing potential: a systematic review. Wound Med. 2018;20:43–53. doi: 10.1016/j.wndm.2018.01.003. [DOI] [Google Scholar]
- Si J, Yang Y, Xing X, et al. Controlled degradable chitosan/collagen composite scaffolds for application in nerve tissue regeneration. PolymDegradStabil. 2019;166:73–85. doi: 10.1016/j.polymdegradstab.2019.05.023. [DOI] [Google Scholar]
- Sorg H, Tilkorn DJ, Hager S, et al. Skin wound healing: an update on the current knowledge and concepts. EurSurg Res. 2017;58(1–2):81–94. doi: 10.1159/000454919. [DOI] [PubMed] [Google Scholar]
- Sun XZ, Williams GR, Hou XX, et al. Electrospuncurcumin-loaded fibers with potential biomedical applications. CarbohydrPolym. 2013;94(1):147–153. doi: 10.1016/j.carbpol.2012.12.064. [DOI] [PubMed] [Google Scholar]
- Varaprasad K, Jayaramudu T, Kanikireddy V, Toro C, Sadiku ER, et al. Alginate-based composite materials for wound dressing application: a mini review. CarbohyrPolym. 2020;236:116025. doi: 10.1016/j.carbpol.2020.116025. [DOI] [PubMed] [Google Scholar]
- Varaprasad K, Lopez M, Nunez D, Jayaramudu T, Sadiku ER, Karthikeyan C, Oyarzúnc P, et al. Antibiotic copper oxide-curcuminnanomaterials for antibacterial applications. J MolLiq. 2020;300:112353. doi: 10.1016/j.molliq.2019.112353. [DOI] [Google Scholar]
- Varaprasad K, Nunez D, Ide W, Jayaramudu T, Sadiku ER, et al. Development of high alginate comprised hydrogels for removal of Pb(II) ions. J MolLiq. 2020;298:112087. doi: 10.1016/j.molliq.2019.112087. [DOI] [Google Scholar]
- Wang C, Ma C, Wu Z, et al. Enhanced bioavailability and anticancer effect of curcumin-loaded electrospunnanofiber: in vitro and in vivo study. Nanoscale Res Lett. 2015;10(1):1–10. doi: 10.1186/s11671-015-1146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Hao L, Wang P, et al. Release kinetics and antibacterial activity of curcumin loaded zein fibers. Food Hydrocoll. 2017;63:437–446. doi: 10.1016/j.foodhyd.2016.09.028. [DOI] [Google Scholar]
- Zahiri M, Khanmohammadi M, Goodarzi A, et al. Encapsulation of curcumin loaded chitosan nanoparticle within poly (ε-caprolactone) and gelatin fiber mat for wound healing and layered dermal reconstitution. Int J BiolMacromol. 2020;153:1241–1250. doi: 10.1016/j.ijbiomac.2019.10.255. [DOI] [PubMed] [Google Scholar]