Abstract
Background
The clinical efficacy of matrine in treating coronavirus disease (COVID-19) has been confirmed; however, its underlying mechanism of action remains unknown.
Methods
TCMSP, SwissTargetPrediction, SEA, GeneCards, CTD, and TTD were used to identify potential targets for matrine in SARS-CoV-2. Cytoscape software was used to determine the target-pathway network for topographical analysis. The online STRING analysis platform and Cytoscape were together used to generate a PPI network and for GO and KEGG pathway enrichment analysis. Finally, molecular docking simulations were performed to study matrine-Mpro, matrine-ACE2, and matrine-RdRp interactions.
Results
Ten common matrine targets were obtained, particularly including TNF-α, IL-6, and CASP3. GO and KEGG pathway enrichment analysis revealed five significantly enriched signalling pathways involved in cell proliferation, apoptosis, programmed cell death, and immune responses.
Conclusions
During COVID-19 treatment, matrine regulates viral replication, host cell apoptosis, and inflammation by targeting the TNF-α, IL-6, and CASP3 in the TNF signalling pathway.
Keywords: Matrine, COVID-19, network pharmacology, molecular docking, functioning mechanism
Introduction
In late 2019, cases of viral pneumonia of unknown aetiology were reported in China. These were subsequently determined to result from a novel coronavirus, which has now propagated to >100 countries. The WHO named this disease “coronavirus disease” (COVID-19) (Ahmad et al. 2020), and the International Committee on Taxonomy of Viruses named the causative novel coronavirus, a single-strand positive-sense RNA virus, “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2) (Liu et al. 2020). The Center for Systemic Sciences and Engineering of Johns Hopkins University has predicted that >3.6 million individuals worldwide would have COVID-19 as of 6 May 2020. COVID-19 was declared a pandemic on 11 March 2020 by the WHO and is a major public health concern worldwide.
COVID-19 clinically manifests with slight, mild, severe or critically severe symptoms (Tang et al. 2020). The symptoms of slight or mild COVID-19 are fever, fatigue, and dry cough, while those of severe COVID-19 may include shortness of breath, dyspnoea, and reduced blood oxygen saturation at the resting state (Castagnoli et al. 2020; Wang et al. 2020). Critically severe COVID-19 can progress to acute respiratory distress syndrome (ARDS), septic shock, multiple organ failure, and even death (Du et al. 2020). Thus far, however, specific drugs for COVID-19 are unavailable (Zheng et al. 2020). However, traditional Chinese medicine (TCM) has been shown to benefit COVID-19 patients, potentially complementarily improving the treatment efficiency of modern medicine. In China, 91.5% of COVID-19 patients were treated with TCM, indicating a > 90% treatment efficacy.
The TCM Kuh-seng (Latin name: Sophorae Flavescentis Radix) eliminates body heat, eliminates dampness, promotes diuresis and accelerates detoxification (Li et al. 2015). Matrine is an alkaloid present in the root of Sophorae Flavescentis Radix, having natural antiviral, anti-inflammatory and immunoregulatory effects (Fan et al. 2019). Matrine reportedly inactivates H3N2 and Coxsackievirus B3 (Pan et al. 2015, YU et al. 2017). Recent studies on mice have reported that matrine significantly alleviates pathological injury in lung tissues, with a pulmonary index inhibition rate of 86.86% (SUN et al. 2020). A clinical case series of COVID-19 patients reported that the effective COVID-19 treatment rate of matrine-NaCl was 100% (Yang et al. 2020).
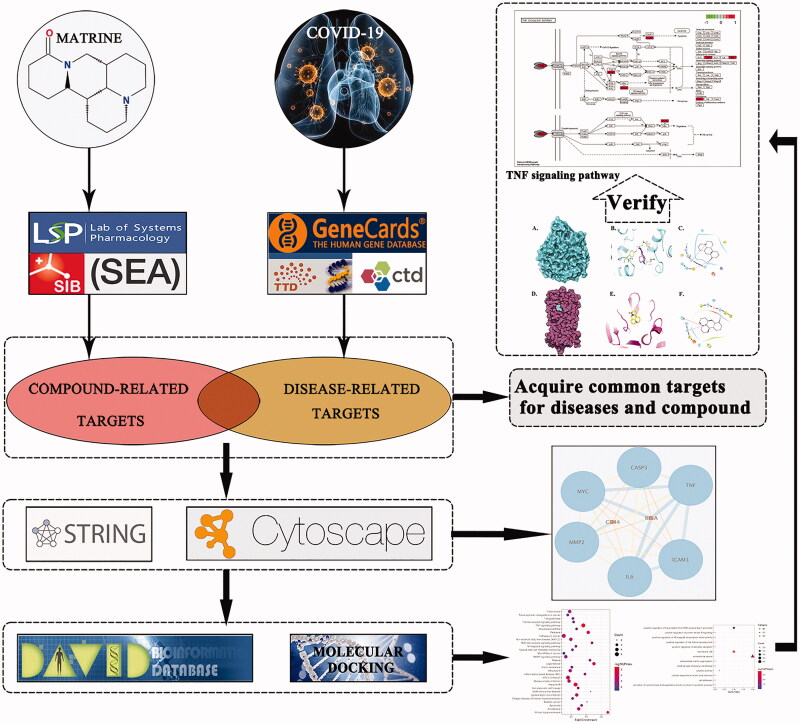
Network pharmacology is an emerging discipline based on the development of systematic biology and polypharmacology, which involves the construction of a complex integrated drug-ingredient-gene-disease network through high-throughput screening and network analysis. This method can systematically elucidate the intervention and influence of drugs on diseases, which meets the development requirements of modernisation of TCM. Molecular docking simulations analyse the geometric structure and spatial interaction between drugs and targets using software specifically developed for drug discovery. SARS-CoV-2 and SARS-CoV bind to angiotensin-converting enzyme II (ACE2) through their S protein to invade host cells (Li et al. 2005, Zhou et al. 2020). SARS-CoV-2-3CL hydrolase (Mpro) is the core component of the proteolytic enzyme precursor of SARS-CoV-2 and contributes to RNA replication and reverse transcription (Zhang et al. 2020b). RNA-dependent RNA polymerase (RdRp) is a necessary enzyme for the replication of RNA viruses with the exception of retroviruses. As these three proteases are crucial for virus replication and control of host cells, they have also become important targets for the development of antiviral drugs (Park et al. 2016, Riccio et al. 2019).This study aimed to assess the mechanism underlying the effects of matrine on COVID-19 through network pharmacology and molecular docking simulations (Figure 1).
Figure 1.
Detailed design and workflow of this study.
Materials and methods
Databases
The databases applied herein included Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, http://tcmspw.com/tcmsp.php), SwissTargetPrediction (http://www.swisstargetprediction.ch), Similarity ensemble approach (SEA, http://sea.bkslab.org), UniProt Universal Protein (http://www.uniprot.org/), STRING (https://string-db.org), The Database for Annotation, Visualization and Integrated Discovery (DAVID, https://david.ncifcrf.gov), GeneCards (http://www.genecards.org), Comparative Toxicogenomics Database (CTD, http://ctd.mdibl.org) and Therapeutic Target Database (TTD, http://bidd.nus.edu.sg/group/ttd/ttd.asp).
Acquisition of potential targets
Potential matrine targets were searched using the online TCMSP, SEA and SwissTargetPrediction database, using “matrine” as the index word. Molecular targets of SARS-CoV-2 were determined using “COVID-19” as the index word, in the CTD, TTD and GeneCards Suite. This search was conducted on 20 April 2020, and the results were pooled, and duplicated data were deleted.
Acquisition of shared pathological and pharmacotherapeutic targets
The pathological and pharmacotherapeutic targets of matrine were intersected using the Venn Diagram package of R software (version 3.6.1) to determine co-targets. These intersecting targets were considered potential targets of matrine to treat COVID-19 patients.
Determination of PPIs
The co-targets thus obtained were imported into the STRING database, with the reference species set as Homo sapiens. Dissociative targets were deleted, and the minimum confidence was set to 0.4. The results were imported into Cytoscape software to determine PPIs.
GO and KEGG pathway enrichment analysis
GO and KEGG pathway enrichment analysis was performed to understand the biological functions of the targets determined herein, with reference to the DAVID database. GO generally has three ontologies: molecular function (MF), cellular component (CC), and biological process (BP). Every gene is subjected to several aspects of definition and description. The potential mechanism underlying the effect of matrine for COVID-19 treatment was determined through KEGG pathway enrichment analysis.
Construction of network diagram
PPIs and the results of KEGG pathway enrichment analysis were inputted in Cytoscape software to generate a PPI network and a Compound-Target-Pathway-Disease network, followed by topology analysis.
Molecular docking
The molecular structure of matrine was downloaded from PubChem; Mpro (PDB ID:6LU7), ACE2 (PDB ID:1R42), and RdRp (Su et al. 2020) PDB database. These molecular structures were processed using Mgltools 1.5.6 and saved in pdbqt format. The self-contained ligands of Mpro, ACE2, and RdRp were used to define the active sites. The coordinates of the Grid Box were defined, and the size for every Box was 30 × 30 × 30 grids. Autodock Vina 1.1.2 was used for the docking between small molecules and proteins. The conformation with the highest score was selected and analysed using Pymol and Maestro 11.9.
Results
Potential targets of matrine
Ten potential matrine targets were identified (OB (%) = 63.7, DL = 0.25) from the TCMSP, SEA, and SwissTargetPrediction database, including RELA, MMP2, TNF, IL6, CASP3, MYC, ICAM1, HPSE, IER3IP1, and CD44 (Table 1).
Table 1.
Potential targets of matrine.
| MolId | Target | Symbol | Relevance score |
|
|---|---|---|---|---|
| GeneCards | CTD | |||
| MOL005944 | Transcription factor p65 | RELA | ns | 25.61 |
| MOL005944 | 72 kDa type IV collagenase | MMP2 | ns | 14.36 |
| MOL005944 | Tumor necrosis factor | TNF | 53.73 | 29.61 |
| MOL005944 | Interleukin-6 | IL6 | 49.73 | 23.63 |
| MOL005944 | Caspase-3 | CASP3 | 27.96 | 25.37 |
| MOL005944 | Myc proto-oncogene protein | MYC | ns | 16.87 |
| MOL005944 | Intercellular adhesion molecule 1 | ICAM1 | ns | 16.65 |
| MOL005944 | Heparanase | HPSE | ns | 2.79 |
| MOL005944 | Immediate early response 3-interacting protein 1 | IER3IP1 | ns | 2.51 |
| MOL005944 | CD44 antigen | CD44 | ns | 1.68 |
The two columns on the right of the table indicate the correlation between the COVID-19 and the targets in different databases. The “ns” means no relevant data. The “Relevance score” indicates how relevant the disease is to the target.
Pathological and pharmacotherapeutic co-targets
In total, 12,677 disease-related targets were determined through GeneCards, CTD, and TTD searches, including 60 targets from the GeneCards database, 12671 from the CTD database, and 0 from the TTD database. All targets were mapped to disease-related targets to determine potential matrine targets included among these disease-related targets.
Target-pathway network diagram
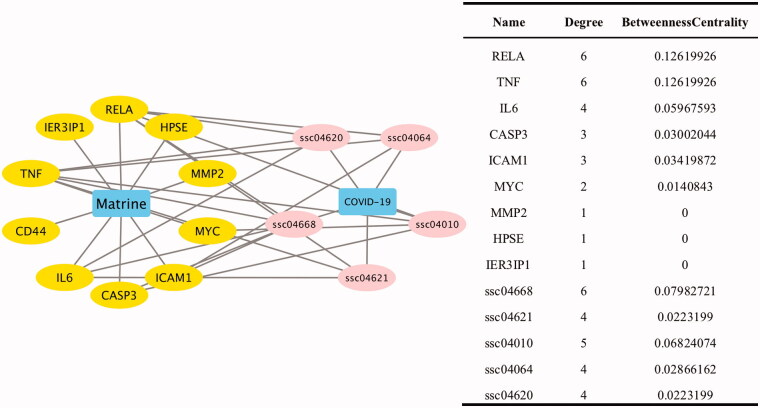
All nodes in the network diagram were subjected to topological analysis. The diagram contained 17 nodes, including 10 targets, 5 pathways, and 25 connecting lines. The network density was 0.243 with a network heterogeneity of 0.598 (Figure 2(A)). ssc04668 represents the TNF signalling pathway; ssc04621, NOD-like receptor signalling pathway; ssc04010, MAPK signalling pathway; ssc04064, NF-κB signalling pathway; and ssc04620, Toll-like receptor signalling pathway. The number of connecting lines represents the degree of nodes. The value of betweenness centrality represents the core value of the node. These two indices are essential indicators to describe nodes. Herein, the TNF signalling pathway had the highest betweenness centrality and the value of the degree of nodes (Figure 2(B)).
Figure 2.
Target-pathway network diagram. (A) Target-pathway network diagram, yellow nodes represent targets, and pink nodes represent signalling pathways. Edges represent interactions targets and signalling pathways. (B) Topological analysis of target-pathway, the value of degree and betweenness centrality are used to screen the core targets.
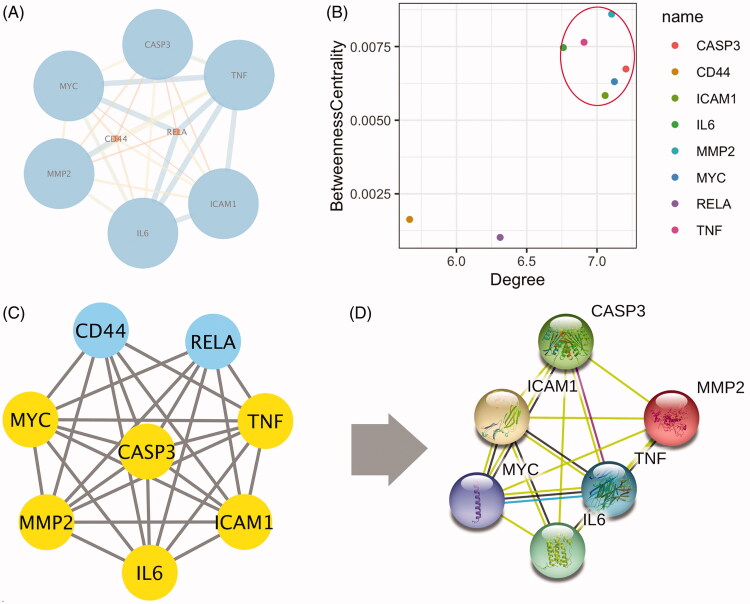
PPI diagram
The 10 targets were introduced into the STRING database. Two free targets were deleted, and the data were introduced in the Cytoscape software to generate the PPI diagram (Figure 3(A)). The topological analysis revealed that IL-6, TNF, CASP3, MMP2, ICAM1, and MYC are nodes with intermediate betweenness centrality and degree higher than the mean value (Figure 3(B–D)).
Figure 3.
PPI diagram and node selection. (A) PPI diagram, the size of nodes represents the value of degree. (B, C, and D) The screening process of core nodes, the nodes whose degree value and centrality are both greater than the mean value are screened.
GO and KEGG pathway enrichment analysis
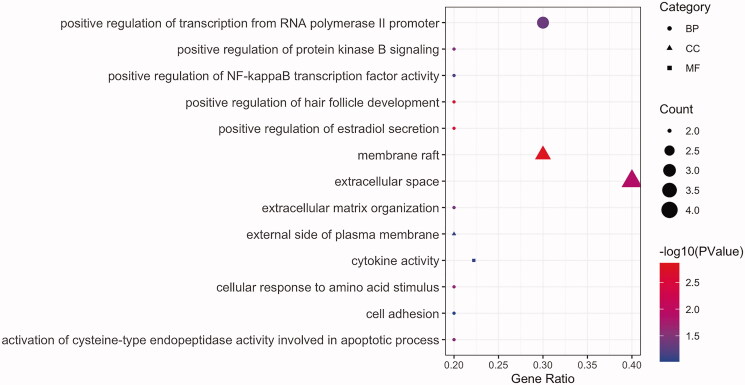
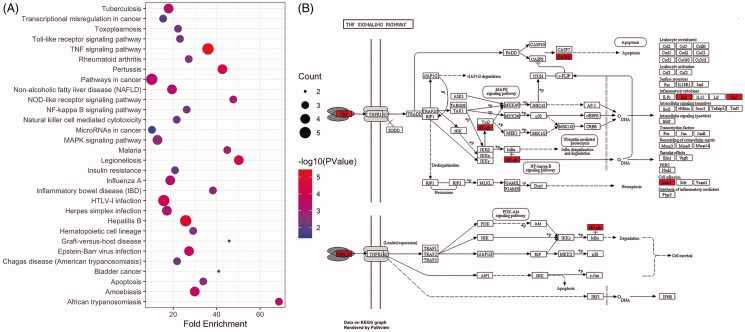
GO enrichment analysis revealed 13 entries with P < .05, including 9 BP, 3 CC, and 1 MF term (Figure 4). These were associated with the regulation of cell proliferation, apoptosis, programmed cell death, intracellular signal cascading responses, immune responses, nucleic acid metabolism, transcription, translation, and angiogenesis. KEGG pathway enrichment analysis revealed five signalling pathways with p < .05 (Figure 5(A)), among which the TNF signalling pathway was most significantly enriched and was most closely associated with viral infection (Figure 5(B)).
Figure 4.
GO functional enrichment analysis. Each node signals a GO term, and its size represents the gene number. The colour indicates the –log10(p values). The shape means the category.
Figure 5.
KEGG enrichment analysis and pathway mapping. (A) Representative bubble plots of the pathway enrichment analysis of the core targets. (B) Reprinted of TNF signalling pathway with permission from Kyoto Encyclopaedia of Genes and Genome.
Molecular docking
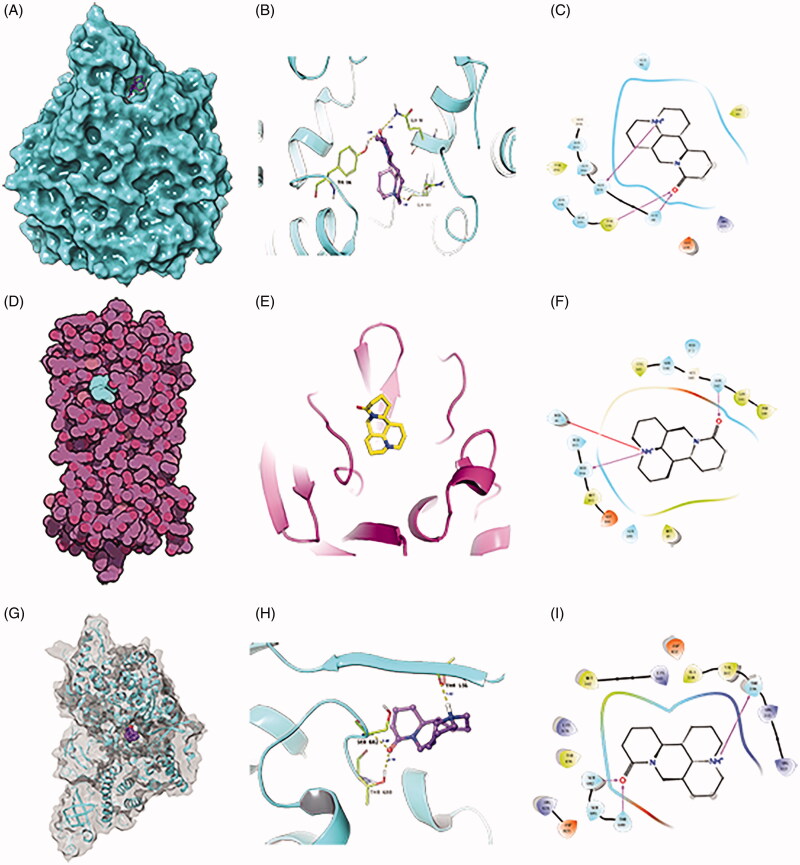
Figure 6A–C represents the mode of molecular docking between matrine and ACE2, Figure 6D–F shows the molecular docking between matrine and Mpro, and Figure 6G–I reveals the molecular docking between matrine and RdRp. The binding sites of ACE2 are GLN101, TYR196, and GLN98; Mpro, ASN142, HID41, and HID164; RdRp, THR556, SER682, and THR680. Combined with the binding energy of ribavirin and lopinavir in Table 2, it suggests the high binding potential of matrine to three key target proteins ACE2, Mpro, and RdRp.
Figure 6.
Molecular docking mode of matrine with ACE2, Mpro, and RdRp molecules. (A–C) Pattern diagram of molecular docking of matrine with ACE2. (D–F) Pattern diagram of molecular docking of matrine with Mpro. (G–I) Pattern diagram of molecular docking of matrine with RdRp.
Table 2.
Results of molecular docking efficiency.
| Drugs | Mpro(kcal/ml–1) | ACE2(kcal/ml–1) | RdRp(kcal/ml–1) |
|---|---|---|---|
| Matrine | –5.8 | –6.1 | –6.3 |
| Lopinavir | –6.6 | –6.5 | –6.2 |
| Ribavirin | –7.7 | –7.4 | –7.6 |
Ribavirin and Lopinavir are used as a positive control.
Discussion
TCM helps prevent and control infectious diseases and enhances immunity and homeostasis, serving as an effective control strategy for epidemic diseases with unknown pathogenesis, especially in the absence of vaccines or specific drugs(Zhang et al. 2020a). COVID-19 has high infectivity and epidemicity, being within the treatment scope of the plague according to TCM theories (Zhang et al. 2020). COVID-19 is primarily characterised by fever, fatigue, and dry cough. Sometimes, nasal obstruction, a running nose, pharyngalgia, and diarrhoea may occur (Tay et al. 2020). TCM is effective against COVID-19 and is widely favoured worldwide (Zhang et al. 2020c). As an aspect of COVID-19 treatment strategies in China, TCM potentially provides potential insights into the prevention and control of this disease worldwide.
Recent animal and clinical studies reported significant therapeutic effects of matrine on COVID-19 patients (Yang et al. 2020, SUN et al. 2020). Matrine can alleviate pathological injuries in lung tissue in model mice, reduce the lung index, decrease IL-16, IL-10, and TNF-α levels and the viral load in lung tissue, and increase the percentage of peripheral lymphocytes. Furthermore, matrine can significantly shorten the time to viral clearance in COVID-19 patients, leading to the patients testing negative for SARS-CoV-2 sooner. Moreover, lung-computed tomographic imaging has suggested favourable lung focal absorption, especially for a marked improvement in networked and fibrotic foci. Furthermore, no adverse reactions were noted among all age groups.
Based on the aforementioned findings that matrine can defend against SARS-CoV-2 and help alleviate COVID-19, our data further report that matrine probably targets TNF, IL-6, and CASP3 via the TNF signalling pathway. TNF is secreted by activated mononuclear macrophages and converted to TNF-α after maturation. TNF-α exerts antiviral effects similar to interferons and can inhibit viral replication and block viral protein synthesis and the assembly of new virus particles (Wong and Goeddel 1986).
In a previous study, upon stimulation with purified recombinant S protein, macrophagocytes were activated to produce pro-inflammatory factors IL-6 and TNF-α in a nucleic factor-κB (NF-κB)-dependent manner (Wang et al. 2007). As shown in Figure 5B, the higher the NF-κB activation, the greater the influence on downstream IL-6 and TNF-α signalling, thus regulating intracellular replication and inflammatory responses to the virus. When SARS-CoV-2 infects the respiratory tract, it causes mild or severe ARS, subsequently releasing pro-inflammatory cytokines including IL-1β and IL-6 (Conti et al. 2020). Clinical experts primarily attributed ARDS in severe COVID-19 patients to cytokine storms, which involves rapid T cell activation and the production of various cytokines including granulocyte-macrophagocyte community stimulation factor (GM-CSF), interleukins, and chemokines (Chen et al. 2020). The cytokine storm potentially induces a supersensitive inflammatory reaction and blocks pulmonary gas exchange, subsequently progressing to ARDS and multiple organ failure (Merad and Martin 2020). Studies have confirmed that matrine can inhibit pig alveolar macrophages by inducing IL-1β secretion via the MyD88/NF-κB pathway and the NLRP3 inflammasome, thus alleviating PRRSV infection-induced respiratory tract inflammation (Sun et al. 2019). The dominant substrate of CASP3 is poly (ADP-ribose) polymerase (PARP), which is associated with DNA repair and facilitates monitoring of genome integrity (Hsu et al. 2018). Upon initiation of the cell response, PARP is cleaved to two fragments at Asp216-Gly217, thus separating two DNA-binding zinc finger motifs from the catalytic domain of the hydroxyl terminal in PARP, probably causing PARP malfunction and enhancing endonuclease activity, thus fragmenting internucleosomal DNA and ultimately leading to apoptosis (Affar et al. 2001, Riccio et al. 2016). Matrine reportedly downregulates N protein in Marc-145 in vitro and reduces porcine reproductive and respiratory syndrome virus (PRRS)-induced apoptosis by inhibiting CASP3 activation. Herein, molecular docking simulation revealed that matrine had a high potential to interact with ACE2, Mpro, and RdRp, the key proteins during the SARS-CoV-2 infection. Overall, the present results suggest that matrine helps treat COVID-19 by inhibiting the synthesis of key SARS-CoV-2 proteins and blocking their replication, primarily through the TNF signalling pathway (Sun et al. 2014).
Conclusions
In this study, we utilised network pharmacology and molecular docking simulation to investigate the potential mechanism underlying the effects of matrine during COVID-19 treatment. The present results suggest that matrine helps treat COVID-19 by regulating viral replication, cell apoptosis, and inflammatory responses by targeting TNF-α, IL-6, and CASP3 via the TNF signalling pathway. Furthermore, matrine has high binding potential to Mpro, ACE2, and RdRp. Together with existing evidence from animal studies and clinical trials, our results suggest that matrine may serve as a promising drug for treating COVID-19. A randomised and double-blind clinical trial with a large patient cohort was required to further extrapolate the present results among the population for COVID-19 treatment.
Acknowledgements
We pay tribute all the people who are fighting the COVID-19 epidemic around the world. We would like to thank Editage (www.editage.cn) for English language editing.
Funding Statement
This research was funded by grants from the National Natural Science Foundation of China [81673936].
Authors’ contributions
Z.X.M and W.Q. conceptualised this study; P.W.P. and X.Y. contributed to methodology of this study; X.Y. provided software; H.D. and F.F.C. carried out formal analysis; Z.Z.C. and G.C. carried out the investigation; P.W.P. and X.Y. provided resources; P.W.P. contributed to data curation; P.W.P and X.Y. carried out writing-original draft preparation; Z.X.M. contributed to writing-review and editing; W.Q. supervised this study; P.W.P. looked after project administration; Z.X.M. looked after funding acquisition. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Affar, E.B., et al. , 2001. Caspase-3-mediated processing of poly (ADP-ribose) glycohydrolase during apoptosis. The journal of biological chemistry, 276 (4), 2935–2942. [DOI] [PubMed] [Google Scholar]
- Ahmad, A., Rehman, M.U., and Alkharfy, K.M., 2020. An alternative approach to minimize the risk of coronavirus (Covid-19) and similar infections. European review for medical and pharmacological sciences, 24 (7), 4030–4034. [DOI] [PubMed] [Google Scholar]
- Castagnoli, R., et al. , 2020. Severe acute respiratory syndrome coronavirus 2 (sars-cov-2) infection in children and adolescents: a systematic review. JAMA pediatrics. doi: 10.1001/jamapediatrics.2020.1467 [DOI] [PubMed] [Google Scholar]
- Yang, M.W., et al. , 2020. [Clinical efficacy of Matrine and Sodium Chloride Injection in treatment of 40 cases of COVID-19]. Zhongguo Zhong Yao za Zhi = Zhongguo Zhongyao Zazhi = China journal of Chinese materia medica, 45 (10), 2221–2231. [DOI] [PubMed] [Google Scholar]
- Chen, N., et al. , 2020. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet, 395 (10223), 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti, P., et al. , 2020. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. Journal of biological regulators & homeostatic agents, 34 (2), 327–331. [DOI] [PubMed] [Google Scholar]
- Du, Y., et al. , 2020. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. American journal of respiratory and critical care medicine, 201 (11), 1372–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, C.L., et al. , 2019. Alopecuroides A-E, matrine-type alkaloid dimers from the aerial parts of Sophora alopecuroides. Journal of natural products, 82 (12), 3227–3232. [DOI] [PubMed] [Google Scholar]
- Hsu, H.Y., et al. , 2018. Fucoidan upregulates TLR4/CHOP-mediated caspase-3 and PARP activation to enhance cisplatin-induced cytotoxicity in human lung cancer cells. Cancer letters, 432, 112–120. [DOI] [PubMed] [Google Scholar]
- Li, F., et al. , 2005. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science, 309 (5742), 1864–1868. [DOI] [PubMed] [Google Scholar]
- Li, G., et al. , 2015. Matrine regulates expression of Caspase-3, Smac and XIAP protein to induce the apoposis of tumor cell. Journal of Hebei medical university, 36, 800–806. [Google Scholar]
- Liu, Y.C., Kuo, R.L., and Shih, S.R., 2020. COVID-19: the first documented coronavirus pandemic in history. Biomedical journal, 4170 (20), 30044–30045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad, M., and Martin, J.C., 2020. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nature reviews: immunology, 20 (6), 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Q.M., et al. , 2015. Antiviral matrine-type alkaloids from the rhizomes of Sophora tonkinensis. Journal of natural products, 78 (7), 1683–1688. [DOI] [PubMed] [Google Scholar]
- Park, J.-Y., et al. , 2016. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. Journal of enzyme inhibition and medicinal chemistry, 31 (1), 23–30. [DOI] [PubMed] [Google Scholar]
- Riccio, A.A., Cingolani, G., and Pascal, J.M., 2016. PARP-2 domain requirements for DNA damage-dependent activation and localization to sites of DNA damage. Nucleic acids research, 44 (4), 1691–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio, F., et al. , 2019. Development and validation of RdRp Screen, a crystallization screen for viral. Biology open, 8 (1), bio037663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, Y., et al. , 2020. Screening of anti-2019-nCoV inhibitors by taking RNA-dependent RNA polymerase as target. Chinese archives of traditional Chinese medicine, 38 (05), 7–13+259–260. [Google Scholar]
- Sun, J., et al. , 2020. Effect of matrine sodium chloride injection on a mouse model combining disease with syndrome of human coronavirus pneumonia with cold-dampness pestilence attacking the lung. Acta pharmaceutica sinica, 55, 366–373. [Google Scholar]
- Sun, P., et al. , 2019. Matrine inhibits IL-1β secretion in primary porcine alveolar macrophages through the MyD88/NF-κB pathway and NLRP3 inflammasome. Veterinary research, 50 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, N., et al. , 2014. Antiviral activity and underlying molecular mechanisms of Matrine against porcine reproductive and respiratory syndrome virus in vitro. Research in veterinary science, 96 (2), 323–327. [DOI] [PubMed] [Google Scholar]
- Tang, W., et al. , 2020. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ (Clinical Research ed.), 369, m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay, M.Z., et al. , 2020. The trinity of COVID-19: immunity, inflammation and intervention. Nature reviews: immunology, 20 (6), 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D., et al. , 2020. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. JAMA, 323 (11), 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W., et al. , 2007. Up-regulation of IL-6 and TNF-alpha induced by SARS-coronavirus spike protein in murine macrophages via NF-kappaB pathway. Virus research, 128 (1–2), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, G.H., and Goeddel, D.V., 1986. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. Nature, 323 (6091), 819–822. [DOI] [PubMed] [Google Scholar]
- Yu, Y., Yang, L., and Liu, Q., 2017. Protective effects of matrine on cardiac microvascular endothelial cells infected by coxsackievirus B3. Chinese archives of traditional Chinese medicine, 35, 1506–1509. [Google Scholar]
- Zhang, D., et al. , 2020. a. The clinical benefits of Chinese patent medicines against COVID-19 based on current evidence. Pharmacological research, 157, 104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L., et al. , 2020. b. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science, 368, 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., et al. , 2020. c. Current targeted therapeutics against COVID-19: based on first-line experience in China. Pharmacological research, 157, 104854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., et al. , 2020. The academic insight of SARS-CoV-2 in traditional Chinese medicine from treatise on warm pestilence. Journal of Nanjing university of traditional Chinese medicine, 36, 290–294. [Google Scholar]
- Zheng, Y., Li, R., and Liu, S., 2020. Immunoregulation with mTOR inhibitors to prevent COVID-19 severity: a novel intervention strategy beyond vaccines and specific antiviral medicines. Journal of medical virology, 92 (9), 1495–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P., et al. , 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579 (7798), 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]