ABSTRACT
Passive immunotherapeutics (PITs), including convalescent plasma, serum, or hyperimmune immunoglobulin, have been of clinical importance during sudden outbreaks since the early twentieth century for the treatment of viral diseases such as severe acute respiratory syndrome (SARS), middle east respiratory syndrome (MERS) and swine flu (H1N1). With the recent SARS-CoV-2 pandemic, wherein effective antivirals and vaccines are still lacking, an interest in convalescent plasma therapy as a lifesaving option has resurfaced due to its capacity for antigenic neutralization and reducing viremia. This review summarizes convalescent blood products (CBPs) in terms of current technologies and the shortcomings related to the collection, manufacture, pathogen inactivation, and banking of CBPs, with a specific focus on their plausible applications, benefits, and risks in the COVID-19 pandemic.
KEYWORDS: COVID-19, SARS-CoV-2, immunotherapeutics, convalescent plasma therapy, hyperimmune immunoglobulin, SARS, MERS
Introduction
Viral infections caused by mutant strains of certain viruses with a pandemic potential, such as coronaviruses (SARS-CoV2,1 SARS-CoV,2 MERS-CoV3), influenzaviruses,4-6 flaviviruses (West Nile virus, Dengue virus, Zika virus),7 Chikungunya virus,8 and Ebola virus,9 spread rapidly, without sufficient time to design and develop antiviral drugs or vaccines specific to those mutant strains immediately after the outbreak. To clinically manage such cases, doctors rely exclusively on general supportive care with provisions of critical care including organ support, if necessary.10 In a scenario, where therapeutic drugs or vaccines are not available, one approach that could be used effectively by the medical fraternity, is that of convalescent plasma therapy (CPT) or passive immunotherapy (PIT), which has recently been approved by the FDA to treat critically ill COVID-19 (coronavirus infection disease 2019) patients.11 CPT is a classic adaptive immunotherapy that has been successfully used for decades in the treatment of SARS, MERS, and H1N1 pandemics, with satisfactory efficacy and safety.
SARS-CoV-2 is a novel mutant strain of SARS-CoV12,13 with virological and clinical similarity with no specific antiviral drug or therapeutic vaccine available14 hence, the administration of CPT or hyperimmune immunoglobulin from recovered patients may be a promising approach for the treatment of COVID-19.15 Patients who have recovered from COVID-19 with a high neutralizing antibody titer may be a valuable source of donor convalescent plasma (CP). The efficacy of CPT (Figure 1) has been attributed to active hyperimmune immunoglobulins/antibodies that may directly inactivate the viral load and suppress viremia.16 A meta-analysis from 32 studies of SARS coronavirus infection and influenza A (H1N1) showed a statistically significant reduction in the pooled odds of mortality (odds ratio, 0.25; 95% confidence interval, 0.14–0.45; I2 = 0%) without adverse events in patients administered various doses of CPT compared to placebo or no therapy.6,17-19 Nevertheless, the potential clinical benefits and risks of convalescent blood products (CBPs) in COVID−19 remain uncertain. For example, considering that the virus mutates rapidly, the antibodies generated among one set of infected patients may be different from those of another set of patients infected with a different viral strain. Therefore, there is a theoretical concern that antibodies obtained from a patient recovered from one strain of coronavirus could enhance the clinical risks for a patient infected with another strain of coronavirus, leading to antibody-dependent enhancement (ADE) of infection. Therefore, to avoid ADE, the purified neutralizing antibodies should be collected and administered from donor(s) and recipient(s) respectively, within the same geographic locality.20 On the other hand, if a secondary infection is simultaneously emerging in a particular locality, CBP could be contradicted. Furthermore, CP infusion carries some risks, such as transfusion-related acute lung injury (TRALI).21 There is also a higher risk of active and live antigens being present in CBP which may lead to worsening of clinical symptoms. A minor mistake at any point could worsen the clinical condition of the patient; therefore, CBP source, ADE, time of CP collection, antigenic infectivity index, and transfusion methods should be taken into consideration during the risk-benefit assessment. However, the antigenic infectivity index can be reduced using modern pathogen inactivation (PI) techniques.22
Figure 1.
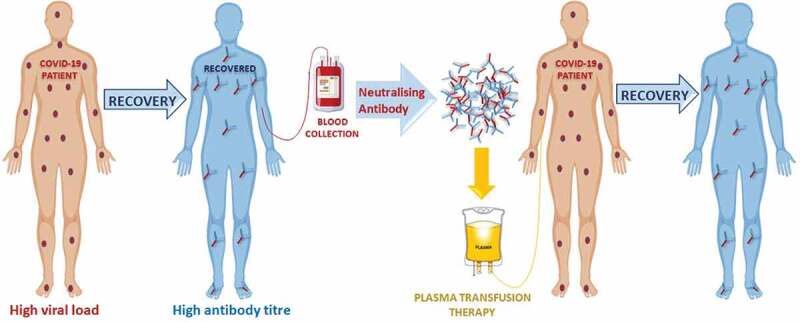
Schematic representation of convalescent plasma therapy
With respect to the COVID-19 pandemic, the present article is dedicated to evaluating the benefits and risks of different CBPs to achieve artificially acquired passive immunity. These CBPs include convalescent whole blood (CWB), CP/convalescent serum (CS), pooled human immunoglobulin (Ig), high titer human Ig, monoclonal antibodies (mAbs), and polyclonal antibodies (pAbs) for intravenous (IV) or intramuscular (IM) administration. This review will also summarize CBPs in terms of current technologies and the shortcomings related to the collection, manufacture, pathogen inactivation, and banking of CBPs with a specific focus on plausible applications in the COVID-19 pandemic.
Convalescent blood products (CBPs)
In the absence of a specific treatment, CBPs emerge as lifesaving options for the treatment of several infectious diseases such as SARS, MERS, and the ongoing pandemic of SARS-CoV−2. CBPs can be used with or without other drugs and preventive measures. However, considering the health issues of implementing CBP therapy, the WHO has outlined the steps necessary to safely collect CWB or CP from recovered patients. These steps include CP donor recruitment strategies (identification and selection of recovered patients as potential blood donors; donor blood grouping, and screening for plausible transmissible infections, and pathogen inactivation, if any); CP pooling, banking, and transportation (blood collection, labeling, storage, and data collection by blood transfusion services, transportation of CP to the centers where transfusions are to be given); CP recipient recruitment strategies (selection, informed consent, blood grouping, and compatibility testing of patients for clinical transfusion); and clinical data collection and analysis (monitoring treatment response, viral load/clearance after CPT, effectiveness of CPT) at the transfusion center.23
Convalescent whole blood (CWB)/plasma (CP)/serum (CS)
Early studies conducted during the pandemics of 1918 to 1920 suggested that PIT using CWB may be effective.6,24 However, as various clinical risk factors have been taken into consideration,25 the use of CWB is currently limited for the treatment of infectious diseases. Presently, while SARS-CoV−2 infection is increasing rapidly worldwide, Shen et al. recently reported, in an uncontrolled study, that five critically ill patients were successfully treated using CPT. All patients were receiving manual ventilation along with antiviral and steroid therapy, but body temperature normalized 3 days after the administration of CP. The sequential organ failure assessment score decreased and PaO2/FiO2 (ratio of the partial pressure of arterial oxygen to the percentage of inspired oxygen) increased in the 12 days following CP transfusion. The viral load was negative after 12 days and SARS-CoV−2 specific neutralizing antibody titers increased to 80−320 on the seventh day.26 Similarly, another study reported an increase in antibodies in all patients 5 days after medical treatment with an 81% rise in IgM and 100% in IgG. Although narrative analysis of various reports shows consistent evidence of reduction in mortality after early CP transfusion, control groups and quality data are lacking; thus, a well-designed and controlled clinical trial must be conducted.27
Neutralizing antibodies [pooled/high titer human immunoglobulin (Ig)]
Neutralizing antibodies (nAbs), or intravenous immunoglobulins (IVIgs), consist of high titer IgG, isolated and pooled from plasma collected from the blood of recovered patients.28 IVIgs are sterile and contain more than 95% unmodified IgG with two functional fragments; the F(ab)2 fragment for antigen recognition, and the crystallizable fragment (Fc) for the activation of innate immune responses by interacting with Fcγ receptors on B-cells.29 IVIgs are already used to treat patients with viral, bacterial, or fungal infections, along with various autoimmune and chronic inflammatory diseases in clinical and laboratory experimental models.30-35,36 IVIgs do not stimulate the patient’s own immune system to produce more Igs, instead they provide only temporary protection before being metabolized by the body. Therefore, repeated doses are required at regular intervals over the course of treatment until the viral load (in the case of SARS-CoV-2) becomes negative.37 Recently, Cao et al. reported the early recovery of three severely ill COVID-19 patients using high-dose IVIg.38 We recently identified that SARS-CoV-2 has high sequence identity with the spike glycoprotein (S protein) structures of SARS-CoV39; therefore, CS containing nAbs from SARS patients should cross-neutralize SARS-CoV-2 infection by reducing S protein-mediated SARS-CoV-2 entry40 [Figure 2]. Similarly, nAbs targeting the SARS-CoV receptor-binding domain (RBD) could be used for prophylaxis and treatment of SARS-CoV-2 infection.41 However, no cross-neutralization of SARS-CoV-2 is observed for this target.
Figure 2.
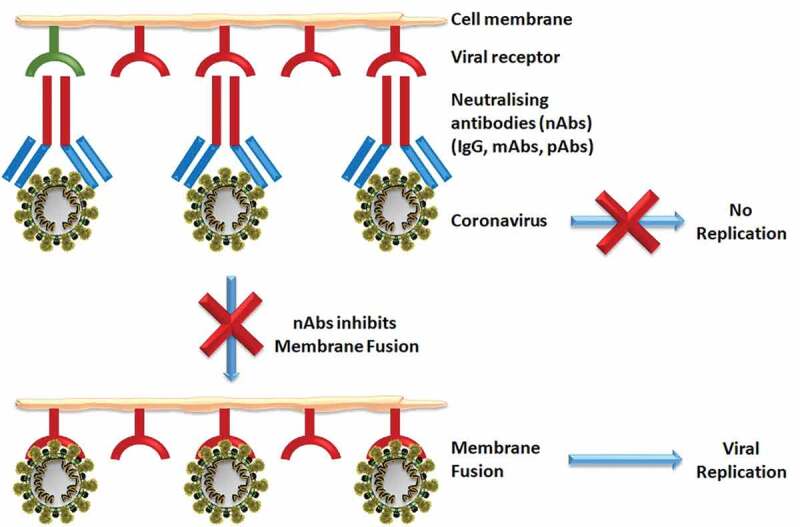
Schematic representation of neutralizing antibodies (nAbs) inhibiting viral membrane fusion and preventing replication
Monoclonal antibodies (mAbs) and polyclonal antibodies (pAbs)
Currently, naturally obtained mAbs specific to SARS-CoV-2 are not available. Work is in progress for the production of prophylactic or therapeutic mAbs to prevent or treat COVID-19. However, a recombinantly expressed human mAb 47D11 has recently been reported which can neutralize both SARS-CoV-2 and SARS-CoV.42 Though the human neutralizing mAb against SARS-CoV RBD, CR3022, has been identified as having high affinity for an RBD epitope but its ability to cross-neutralize SARS-CoV-2 has not been reported. Moreover, pAbs against SARS-CoV RBD have been shown to cross-neutralize SARS-CoV-2 infection in HEK293T cells. The human nAbs against SARS-CoV-2 RBD, SARS-CoV RBD, and MERS-CoV RBD are shown in Table 1.41,42 This research has opened avenues for the potential development of SARS.-CoV-RBD and SARS-CoV-2 RBD-based vaccines for the treatment of COVID-19 using cross-neutralization mAbs and pAbs.42,43
Table 1.
Human nAbs against SARS-CoV-2, SARS-CoV, and MERS-CoV
| Human nAb | Neutralizing pathogen | Mechanism |
|---|---|---|
| Recombinant Human 47D11 mAb | SARS-CoV-2 and SARS-CoV | Recognizes conserved epitope on spike RBD independent of receptor binding inhibition. |
| S109.8; S227.14 S230.15; mAbs |
Human SARS-CoV with S mutants | Inhibits the binding of SARS-CoV RBD to ACE2 receptor |
| CR3022; CR3014 scFv; mAb |
Live SARS-CoV, HKU-39849 and CR3014. | Recognizes epitopes on SARS-CoV RBD residues 318–510 with high affinity. |
| 80 R; scFv; mAb | Live SARS-CoV infection | Recognizes epitopes on SARS-CoV S1 and blocks RBD-ACE2 binding. |
| S230.15; m396; mAbs | SARS-CoV strains | Recognizes epitopes on SARS-CoV S1 protein and interferes with RBD–ACE2 receptor interaction. |
| MERS-27; m336 Fab; MCA1; mAbs; |
Strains of pseudo type MERS-CoV. | Recognizes epitopes on MERS-CoV RBD and blocks RBD-DPP4 receptor binding |
Pathogen inactivation (PI) technologies
Pooled plasma from recovered coronavirus patients may be subjected to various methods of PI, to ensure high safety margins. Often, three to seven of the following techniques are used in combination: cryo-precipitation, nano-filtration (15–20 nm), pasteurization, solvent/detergent treatment (0.3% tri-n-butyl phosphate and 1% polysorbate 80 at 37°C), heat treatment, cold ethanolic fractionation, PEG precipitation, ion-exchange chromatography, and low pH incubation [Figure 3]. Once inactivated, the samples are validated and examined for viral infectivity using the tissue culture infectious dose assay, plaque-forming unit assay, or RT-PCR.44-47
Figure 3.
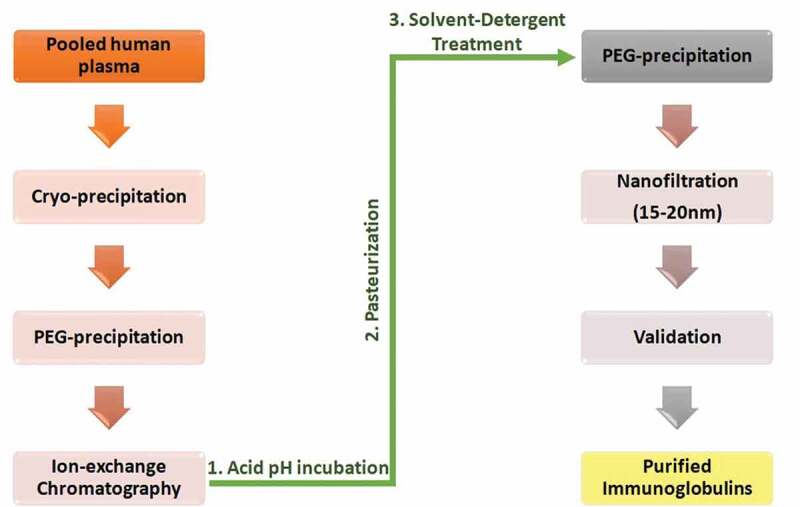
Schematic representation of pathogen inactivation technologies
Lessons from the past (SARS and MERS)
Genome sequencing suggests that SARS-CoV-2 is 82% identical to SARS-CoV and about 50% to MERS-CoV, with a bat origin.48 The clinical presentation and pathology of COVID-19 also greatly resemble the characteristics of SARS and MERS. Therefore, it is essential to learn from our previous experiences with SARS and MERS when treating patients infected with SARS-CoV-2. The primary receptor of MERS-CoV is dipeptidylpeptidase 4 (DPP4)49 whereas SARS-CoV invades the host cells via the binding of S-protein to ACE-2. SARS-CoV-2 has been reported to bind to ACE-2 to gain entry to the host cells in a similar fashion to SARS-CoV,50 as the amino acid sequence of SARS-CoV-2 S-protein is ~76% identical to that of SARS-CoV.51 However, a recent study reported that the RBD-ACE2 binding of SARS-CoV-2 is temperature-sensitive and it is expected that the infectiousness of SARS-CoV-2 would decrease with a rise in temperature.52 Nothing prudent can be taken from this report, but considering the low number of patients in a densely populated tropical country such as India (in comparison to rest of the world), it does appear as though SARS-CoV-2 may be losing its infectivity. An early lockdown in India is a major part of this containment, but another factor which cannot be overlooked is the innate immunity through universal BCG vaccination.53 Therefore, it is proposed that vulnerable population could be immunized with BCG vaccines to attain heterologous nonspecific protection against SARS-CoV-2.
Similar to SARS and MERS,54 elderly people with co-morbidities (mainly hypertension, cardio-cerebrovascular diseases, and diabetes) and males are in general, more clinically susceptible to SARS-CoV-2,55 possibly due to the role of X-chromosomes in innate immunity.56 However, fewer patients with SARS-CoV-2 develop diarrhea in comparison to SARS and MERS.57 During the SARS and MERS outbreaks, 99% of the patients developed high fever,54 whereas in SARS-CoV-2 infection only ~44% of patients develop high fever initially, with the majority being partially symptomatic or asymptomatic58 and inadvertently facilitating a silent spread of the infection. Hospitalized COVID-19 patients also develop pharyngeal pain, dyspnea, dizziness, abdominal pain, and anorexia.59
In pathological studies, it has been demonstrated that a substantial reduction in lymphocytes occurs, leading to lymphopenia, proinflammatory cytokine storm, and higher levels of interleukins and TNFα compared with healthy individuals.57 Under radiological (CT scan) studies, similar to SARS and MERS, COVID-19 patients developed ground-glass opacities with or without vascular enlargement, interlobular septal thickening, and air bronchograms, which adds complexity to the control of the disease.60
For treatment of SARS-CoV infection, chloroquine (an antimalarial drug used in autoimmune diseases) demonstrated remarkable inhibition of the spread of SARS both at entry and during the initial stages of infection.61 Similarly, various observational studies (n = 80), and non-randomized (n = 42) and randomized (n = 62) clinical trials of hydroxychloroquine on COVID-19 patients were carried out along with the standard treatment (oxygen therapy, antivirals and immunoglobulins, with or without corticosteroids). However, it is still not clear whether this drug should be recommended or considered clinically ineffective and stopped.62 Further, since SARS-CoV-2 is associated with an over-activated immune system with severe cytokines storm, similar to SARS and MERS.63 An observational study of corticosteroid treatment in SARS and MERS suggested that steroidal complications resulted in increased mortality, secondary infections, and high viral load. Therefore, corticosteroid treatment is not recommended for COVID-19.64 Finally, CPT has been shown to be very effective for the treatment of SARS and MERS, with reduction in hospital stay and mortality rate upon early administration after onset of symptoms.18 In fact, three infected health-care workers with severely progressed SARS survived only due to CP transfusion, with their viral load dropping to zero within a day of transfusion.65 Similarly, CP containing MERS-CoV-specific antibodies from recovered patients have been suggested as a potential therapy. In murine models, even camel antibodies were observed to be successful for prophylactic and therapeutic use in MERS.66 Therefore, controlled clinical trials are highly recommended for the study of CP in COVID-19 treatment. The FDA has recently issued guidelines on the administration and study of investigational CP from recovered COVID-19 patients during this health emergency.67
Risks and concerns related to convalescent plasma therapy
As mentioned earlier, CPT or PIT has two major risk factors; known risk factors and theoretical risk factors. Known risk factors include the presence of the pathogen against which the plasma is targeted. For example, the presence of SARS-CoV-2 in the plasma of a recovered patient may enhance the clinical symptoms of the recipient rather than act as treatment. The transfusion may also result in an inadvertent infection with another pathogen that was present unknown to the donor. Similarly, another known risk factor is anaphylactic immunological reactions to serum constituents, causing serum sickness. However, with modern blood screening, banking, and PI techniques, the known risks of infection and transfusion reactions may be minimized to negligible levels.
Further, TRALI has been the leading cause of transfusion-related mortality. When CP with antibodies is transfused into a patient with the cognate antigen, neutrophils within the pulmonary microvasculature agglutinate, releasing reactive oxygen species and inflammatory mediators that injure the pulmonary endothelium. TRALI is characterized by acute hypoxemia and non-cardiogenic pulmonary edema within 6 hours of transfusion. Some of the risk factors identified in recipients for TRALI are; smoking, alcohol abuse, age, pre-transfusion shock, liver transplant or end-stage liver disease, hematologic malignancy, sepsis, cardio-pulmonary bypass, and massive transfusion.68 Therefore, TRALI should be considered in the risk-benefit assessment before the administration of CPT.
The theoretical risks involve the phenomenon of ADE, which occurs during transfusion therapy in viral diseases due to the natural occurrence of mutant strains. Therefore, the antibodies in the CP of one patient may not be compatible with a patient infected with a different viral variant, leading to enhancement of disease. For coronaviruses, several mechanisms of ADE have been described.69 The available data on the use of CP in patients with SARS and MERS,18 and reports of its use in COVID-19 patients,70 suggest that the use of CP is safe. However, owing to the phylogenetic variants of the virus, precautionary measures are important. Therefore, CP preparation and administration to COVID-19 patients should be carried out in the same geographical locality to avoid ADE. There is another theoretical risk of reduced humoral immunity and subsequent re-infection among exposed individuals undergoing prophylactic CPT to avoid COVID-19.71 Nevertheless, in view of the high mortality rate and risk to elderly people with comorbidities, CPT should be considered based on individual variability and risk-benefit assessment.
CP donor inclusion and exclusion criteria
To define the inclusion and exclusion criteria for CP donors during COVID-19, it is important to learn from previous experiences with SARS and an accumulated knowledge of the present SARS-COV-2 outbreak. In SARS and MERS, viral RNA was detected in respiratory specimens for as long as 4 and 3 weeks, respectively, after the onset of disease. Similarly, SARS-CoV-2 RNA has been observed for up to 20 days after recovery in survivors.72 SARS viral RNA was also detected in the stool and urine samples of three convalescent patients for longer than 4 weeks.73 Similarly, positive RNA signals were detected in the nasopharyngeal swab and stool of SARS-CoV-2 patients.74 As these reports suggest that SARS viral RNA may remain viable in the excretions of convalescent patients, it is vital to perform SARS-CoV-2 RNA screening of both fecal and respiratory specimens of CP donors. Donors should also undergo apheresis therapy.
In a recent investigation of 173 COVID-19 patients, it was observed that the seroconversion rate for Ab, IgM, and IgG production reached 100.0%, 94.3%, and 79.8%, respectively, within 15 days of the onset of symptoms.75 With no data available on the presence or longevity of SARS-CoV-2 IgG and nAbs in convalescent patients, previous reports from SARS need to be reviewed. CP from SARS patients containing IgG and nAbs showed neutralizing activity at a peak of 96% after 3 months, which declined to 48% after 36 months.76 To find a suitable CP donor, various factors such as viral infectivity, gender, antibody titer value, and nAb longevity need to be considered and evaluated. Notably, a total of 1135 clinical trials studying CP and various anti-SARS-CoV-2 polyclonal hyperimmune immunoglobulins for the treatment of COVID-19 patients are listed in WHO’s International Clinical Trial Registry Platform.77
Recently, the FDA has issued guidelines to health-care providers for the collection of COVID-19 CP. As per the guidelines, the plasma must only be collected from documented positive cases, whose symptoms have been completely resolved for at least 14 days prior to donation with negative test results for COVID-19, or who have been symptom-free for 28 days prior to donation. Further, the inclusion criteria allow both male donors and female donors who are not pregnant, or who have had a recent pregnancy but tested negative for HLA antibodies. A SARS-CoV-2 nAb titer of at least 1:160 is recommended. However, a titer of 1:80 may also be accepted if an alternative is not available.78
CP recipient eligibility
The FDA has also issued eligibility criteria for patients to be considered for CPT during the COVID-19 pandemic. These life-threatening parameters include dyspnea with respiratory frequency ≥30/min, blood oxygen saturation ≤93%, PaO2/FiO2 < 300, lung infiltrates >50% within 24 to 48 hours, respiratory failure, septic shock, and multiple organ dysfunction67,78.
CP pooling and banking
As mentioned, the nAbs titer values and strength of CP tend to vary based on the severity of disease, gender, comorbidities, time of collection, viral load, pathogenicity, and various other unknown parameters of the donor. Further, serological studies of every donor may not be economically feasible. Therefore, pooling batches of CP is recommended. The CP pools are then subjected to serological studies for nAb titer and used accordingly for eligible patients, depending upon their viral load, infectivity, and disease stage. Lower titer values (≥160) may be used for patients who are asymptomatic or with mild to moderate disease,79 whereas high titer (≥640) doses may be used for severely affected patients with high viral load and life-threatening conditions.26,38 Pooled CP may be stored below −20°C and must be labeled with the date of collection, as an expiry date of 1 year from the collection date is outlined in the FDA guidelines.78
Measurement of anti-SARS-CoV-2 antibodies
During CP collection, potential donors or the pooled plasma undergo serological tests for the presence and strength of anti-SARS-CoV-2 antibodies. Various quantitative immunoassays can be used for the assessment of SARS, MERS, and SARS-CoV-2-infected patients, such as chemiluminescence immunoassay (CLIA), enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA), indirect immunofluorescence test (IIFT), MAb epitope mapping, lateral flow immunoassay (LFIA), and quantitative fluorescence immunoassay.80-83
Clinical data analysis of CPT
Recently, a pilot study of CPT on 10 severely ill COVID-19 patients was reported, where a single transfusion of 200 mL CP (nAb titer ≥ 1:640) was administered to patients along with supportive care and antiviral drugs. This resulted in an undetectable viral load within 3 days of CP transfusion and maintenance of nAb titer at 1:640, along with improved clinical (relief of dyspnea, and normalization of oxygen saturation) and laboratory parameters (increase in lymphocyte count and decrease in C-reactive protein), without any adverse effects. After 7 days, radiological examination also showed absorption of lung lesions to varying degrees. However, the optimal dose of CP and detailed clinical benefits were not ascertained in the study.84 In another study, six critically ill COVID-19 patients with respiratory failure tested negative for SARS-CoV-2 RNA after 3 days of receiving CP transfusion but eventually five patients died.85 Therefore, it was concluded in the study that CPT may reduce viral load and buy some time86 to save lives if treatment is initiated much before the critical stage arrives. However, in a separate study, six confirmed COVID-19 patients who received CP transfusion early recovered with no adverse effect during the treatment. Transfusion of CP led to a resolution of ground-glass opacities and increase in anti-SARS-CoV-2 antibody titer in patients. This study indicates that convalescent plasma therapy is promising and specific for COVID-19.87
Similarly, CPT was conducted on 31 critically ill laboratory-confirmed COVID-19 adult patients ≥18 years of age. Patients on CPT had a lower 14 days (0 versus 35%; p = .033) and 28 days (0 versus 35%; p = .033) all-cause mortality compared to patients not on CPT. However, all-cause mortality was only marginally lower in the CPT group compared to the non-CPT group (9.1% versus 45%; p = .055; power = 66%). Clinical parameters also improved with the CPT showing that the use of CPT in severe COVID-19 patients has been associated with improved outcomes.88 More recently, 20,000 hospitalized patients with COVID-19 were transfused with CP, under US FDA expanded access program for COVID-19, to evaluate the safety issues of CPT in critically ill patients. Interestingly, the incidence of serious adverse events was low which included transfusion reactions (<1%), thromboembolic or thrombotic events (<1%), and cardiac events (~3%) and were judged to be unrelated to the plasma transfusion per se. This data provide robust evidence that CPT is safe in hospitalized patients with COVID-19 and support the notion that earlier administration of plasma within the clinical course of COVID-19 is more likely to reduce mortality.89 Similarly, many reports claimed that CPT has the potential to provide immediate and promising treatment options in early-stage COVID-19 patients.90-92
Conclusions
Amid COVID-19 viral pandemic, where treatment is limited to supportive and critical care as specific drugs and therapeutic vaccines are not currently available, the current evidence suggests that CPT may reduce mortality and result in positive treatment outcomes if commenced early after the onset of symptoms. However, this is based purely on the available data which has come from uncontrolled low-quality studies. Therefore, the present review supports the use of CP in critically ill patients infected with SARS-CoV-2 as part of a well-designed, randomized, controlled clinical trial.
Future perspectives
The understanding of COVID-19 and SARS-CoV-2 is at an early stage. Much research is still needed in various areas such as viral transmission,93 disease progression, differential clinical diagnosis,94 laboratory diagnosis,95 antigenic96 pathogenicity,97 epitopes, immunogenic kinetics,98 drugs, immunotherapeutic products,99 prevention, and control strategies,100,101 cell-based therapy,102 phytotherapy,103 and clinical trials of plasma transfusion. Recently, the potential targets of the immune response to SARS-CoV-2 were predicted by comparison to SARS-CoV. Five antigens that trigger B-cell responses contain epitopes recognized by nAbs in SARS CP. Similarly, predicted antigens of membrane proteins have been shown to elicit marked IgM and IgG responses and reactivity against SARS in mice, monkeys, and humans.104 In spite of these similarities, antigenic analysis performed by our group proved various antigenic differences exist between the two coronavirus strains, including novel glycosylation sites and cytotoxic T-lymphocyte epitopes in SARS-CoV-2.39 In a separate study, cross recognition and reactivity of SARS-CoV mAbs with SARS-CoV-2 was not observed.105 These reports suggest that further studies are required to identify candidate targets for immune responses to SARS-CoV-2. Therefore, epitope mapping needs to be initiated to understand mutational events, epitope escape, and respective immunotherapeutics during viral transmittance within the population. Secondly, there have been many cases of re-infection106 with SARS-CoV-2 after recovery from COVID-19. Therefore, a cocktail antibody approach, where a combination of several nAbs targeting different epitopes on SARS-CoV-2, may be evaluated. This approach may decrease the probability of the virus escaping neutralization. Thirdly, randomized controlled clinical trials with a standardized minimum data set are needed to fully understand nAb production kinetics, their mode of action, the optimal dosage of CP, and effectiveness of repeated CP transfusion for COVID-19 patients with SARS-CoV-2 infection, despite our current recommendation for its early use in critical patients.
Acknowledgments
The authors are grateful to the Vice Chancellor, King George’s Medical University (KGMU), Lucknow, India for the encouragement for this work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, Chan P, Wong KC, Leung CB, Cheng G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, Walmsley SL, Mazzulli T, Avendano M, Derkach P, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–09. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 4.Bermingham A, Chand MA, Brown CS, Aarons E, Tong C, Langrish C, Hoschler K, Brown K, Galiano M, Myers R, et al. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro Surveill. 2012;17:20290. [PubMed] [Google Scholar]
- 5.Vogel OA, Manicassamy B.. Broadly protective strategies against influenza viruses: universal vaccines and therapeutics. Front Microbiol. 2020;11:135. doi: 10.3389/fmicb.2020.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 7.Guarner J, Hale GL. Four human diseases with significant public health impact caused by mosquito-borne flaviviruses: west Nile, Zika, dengue and yellow fever. Semin Diagn Pathol. 2019;36:170–76. doi: 10.1053/j.semdp.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Vairo F, Haider N, Kock R, Ntoumi F, Ippolito G, Zumla A. Chikungunya: epidemiology, pathogenesis, clinical features, management, and prevention. Infect Dis Clin North Am. 2019;33:1003–25. doi: 10.1016/j.idc.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Hoenen T, Groseth A, Feldmann H. Therapeutic strategies to target the Ebola virus life cycle. Nat Rev Microbiol. 2019;17:593–606. doi: 10.1038/s41579-019-0233-2. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . Clinical management of severe acute respiratory infections when novel coronavirus is suspected: what to do and what not to do. [cited 2020 May 7]. https://www.who.int/csr/disease/coronavirus_infections/InterimGuidance_ClinicalManagement_NovelCoronavirus_11Feb13u.pdf
- 11.Tanne JH. Covid-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ. 2020;368:m1256. doi: 10.1136/bmj.m1256. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization . [cited 2020 May 7]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 13.Wang C, Liu Z, Chen Z, Huang X, Xu M, He T, Zhang Z. The establishment of reference sequence for SARS‐CoV‐2 and variation analysis. J Med Virol. 2020. doi: 10.1002/jmv.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck BR, Shin B, Choi Y, Park S, Kang K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput Struct Biotechnol J. 2020;18:784–90. doi: 10.1016/j.csbj.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. The Lancet. 2020;20(4):398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forthal DN. Functions of antibodies. Microbiol Spectr. 2014;2:1–17. [PMC free article] [PubMed] [Google Scholar]
- 17.Liu JP, Manheimer E, Shi Y. Systematic review and meta-analysis on the integrative traditional Chinese and Western medicine in treating SARS. Chin J Integr Med. 2005;25:1082–88. [PubMed] [Google Scholar]
- 18.Jenkins MJ, Campos SM, Baillie JK, Cleary P, Khaw FM, Lim WS, Makki S, Rooney KD, Nguyen-Van-Tam JS, Beck CR. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manzoli L, Vito CD, Salanti G, D’Addario M, Villari P, Ioannidis JPA. Meta-analysis of the immunogenicity and tolerability of pandemic influenza A 2009 (H1N1) vaccines. PlosOne. 2011;6(9):e24384. doi: 10.1371/journal.pone.0024384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jawhara S. Could intravenous immunoglobulin collected from recovered coronavirus patients protect against COVID-19 and strengthen the immune system of new patients? Int J Mol Sci. 2020;21(7):2272. doi: 10.3390/ijms21072272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, O’Byrne MM, Evenson LK, Malinchoc M, DeGoey SR, et al. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med. 2007;176(9):886–91. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seltsam A. Pathogen inactivation of cellular blood products - an additional safety layer in transfusion medicine. Front Med (Lausanne). 2017;4:219. doi: 10.3389/fmed.2017.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an empirical treatment during outbreaks. [cited 2020 May 7]. https://apps.who.int/iris/bitstream/handle/10665/135591/WHO_HIS_SDS_2014.8_eng.pdf?sequence=1
- 24.Sahr F, Ansumana R, Massaquoi TA, Idriss BR, Sesay FR, Lamin JM, Baker S, Nicol S, Conton B, Johnson W, et al. Evaluation of convalescent whole blood for treating Ebola virus disease in Freetown, Sierra Leone. J Infect. 2017;74(3):302–09. doi: 10.1016/j.jinf.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medscape: Drugs & Diseases Whole Blood (Blood component) . [cited 2020 May 7]. https://reference.medscape.com/drug/whole-blood-999509#4
- 26.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L, et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA. 2020. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, Wang YY, Xiao GF, Yan B, Shi ZL, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386–89. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz J, Parikh K. Intravenous Immunoglobulin. Medscape: drugs & diseases clinical procedures. [cited 2020 May 7]. https://emedicine.medscape.com/article/210367-overview
- 29.Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29:491–98. doi: 10.1093/intimm/dxx039. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Nathan D, Lustig S, Tam G, Robinzon S, Segal S, Rager-Zisman B. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating West Nile virus infection in mice. J Infect Dis. 2003;188(1):5–12. doi: 10.1086/376870. [DOI] [PubMed] [Google Scholar]
- 31.Shopsin B, Kaveri SV, Bayry J. Tackling difficult Staphylococcus aureus infections: antibodies show the way. Cell Host Microbe. 2016;20:555–57. doi: 10.1016/j.chom.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Jolles S, Sewell WA, Misbah SA. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol. 2005;142:1–11. doi: 10.1111/j.1365-2249.2005.02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samson M, Fraser W, Lebowitz D. Treatments for primary immune thrombocytopenia: a review. Cureus. 2019;11:e5849. doi: 10.7759/cureus.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diep BA, Le VT, Badiou C, Le HN, Pinheiro MG, Duong AH, Wang X, Dip EC, Aguiar-Alves F, Basuino L, et al. IVIG-mediated protection against necrotizing pneumonia caused by MRSA. Sci Transl Med. 2016;8(357):357ra124. doi: 10.1126/scitranslmed.aag1153. [DOI] [PubMed] [Google Scholar]
- 35.Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Intravenous immunoglobulin for infectious diseases: back to the pre-antibiotic and passive prophylaxis era? Trends Pharmacol Sci. 2004;25(6):306–10. doi: 10.1016/j.tips.2004.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaveri SV, Maddur MS, Hegde P, Lacroix-Desmazes S, Bayry J. Intravenous immunoglobulins in immunodeficiencies: more than mere replacement therapy. Clin Exp Immunol. 2011;164:2–5. doi: 10.1111/j.1365-2249.2011.04387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Immunoglobulin therapy and other medical therapies for antibody deficiencies . [cited 2020 May 7]. https://primaryimmune.org/treatment-information/immunoglobulin-therapy
- 38.Cao W, Liu X, Bai T, Fan H, Hong K, Song H, Han Y, Lin L, Ruan L, Li T. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease. Open Forum Infect Dis. 2020;7(3):ofaa102. doi: 10.1093/ofid/ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S, Maurya VK, Prasad AK, Bhatt MLB, Saxena SK. Structural, glycosylation and antigenic variation between 2019 novel coronavirus (2019-nCoV) and SARS coronavirus (SARS-CoV). Virusdisease. 2020;31(1):13–21. doi: 10.1007/s13337-020-00571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41(5):355–59. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang C, Li W, Drabek D, Okba NMA, van Haperen R, Osterhaus ADME, van Kuppeveld FJM, Haagmans BL, Grosveld F, Bosch BJ. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun. 2020;11(1):2251. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, Zhou Y, Du L. Characterization of the receptor binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17(6):613-620. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terpstra FG, Parkkinen J, Tolo H, Koenderman AH, Ter Hart HG, von Bonsdorff L, Torma E, van Engelenburg FA. Viral safety of Nanogam, a new 15 nm-filtered liquid immunoglobulin product. Vox Sang. 2006;90:21–32. doi: 10.1111/j.1423-0410.2005.00710.x. [DOI] [PubMed] [Google Scholar]
- 45.Caballero S, Nieto S, Gajardo R, Jorquera JI. Viral safety characteristics of Flebogamma DIF, a new pasteurized, solvent-detergent treated and Planova 20 nm nano filtered intravenous immunoglobulin. Biologicals. 2010;38(4):486–93. doi: 10.1016/j.biologicals.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Poelsler G, Berting A, Kindermann J, Spruth M, Hammerle T, Teschner W, Schwarz HP, Kreil TR. A new liquid intravenous immunoglobulin with three dedicated virus reduction steps: virus and prion reduction capacity. Vox Sang. 2008;94(3):184–92. doi: 10.1111/j.1423-0410.2007.01016.x. [DOI] [PubMed] [Google Scholar]
- 47.Roberts PL, Dunkerley C, Walker C. Virus reduction in an intravenous immunoglobulin by solvent/detergent treatment, ion-exchange chromatography and terminal low pH incubation. Biologicals. 2012;40(5):345–52. doi: 10.1016/j.biologicals.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 48.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang N, Shi X, Jiang L, Zhang S, Wang D, Tong P, Guo D, Fu L, Cui Y, Liu X, et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23:986–93. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiu Y, BoZhao Y, Wang Q, YanLi J, JianZhou Z, Ce-Heng L, YiGe X. Predicting the angiotensin converting enzyme 2 (ACE2) utilizing capability as the receptor of SARS-CoV-2. Microbes Infect. 2020. doi: 10.1016/j.micinf.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W, Hao P. Evolution of the novel corona virus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63(3):457–60. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He J, Tao H, Yan Y, Huang SY, Xiao Y. Molecular mechanism of evolution and human infection with SARS-CoV-2. Viruses. 2020;12:428. doi: 10.3390/v12040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gursel M, Gursel I. Is global BCG vaccination-induced trained immunity relevant to the progression of SARS-CoV-2 pandemic? Allergy. 2020. doi: 10.1111/all.14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–33. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China. Eur Respir J. 2020;2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jaillon S, Berthenet K, Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol. 2019;56(3):308–21. doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- 57.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–69. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. Am J Roentgenol. 2020;214:1–7. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 61.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taccone FS, Gorham J, Vincent JL. Hydroxychloroquine in the management of critically ill patients with COVID-19: the need for an evidence base. Lancet Respir Med. 2020. doi: 10.1016/S2213-2600(20)30172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130(5):2202–05. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clark DR, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–75. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yeh K, Chiueh T, Siu L. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56(5):919–22. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao J, Perera RAPM, Kayali G, Meyerholz D, Perlman S, Peiris M. Passive immunotherapy with dromedary immune serum in an experimental animal model for Middle East respiratory syndrome coronavirus infection. J Vir. 2015;89(11):6117–20. doi: 10.1128/JVI.00446-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.U.S. FDA . Recommendations for investigational COVID-19 convalescent plasma. [cited 2020 May 7]. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma
- 68.Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012;52(1):65S–79S. doi: 10.1111/j.1537-2995.2012.03663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wan Y, Shang J, Sun S, Tai W, Chen J, Geng Q, He L, Chen Y, Wu J, Shi Z, et al. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J Virol. 2020;94(5):e02015–19. doi: 10.1128/JVI.02015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roback JD, Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. 2020. doi: 10.1001/jama.2020.4940. [DOI] [PubMed] [Google Scholar]
- 71.Crowe JE, Firestone CY, Murphy BR. Passively acquired antibodies suppress humoral but not cell-mediated immunity in mice immunized with live attenuated respiratory syncytial virus vaccines. J Immunol. 2001;167(7):3910–18. doi: 10.4049/jimmunol.167.7.3910. [DOI] [PubMed] [Google Scholar]
- 72.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. Clinical course and risk factors for mortality of adult inpatients withCOVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu D, Zhang Z, Jin L, Chu F, Mao Y, Wand H, Liu M, Wang M, Zhang L, Gao GF, et al. Persistent shedding of viable SARS-CoV in urine and stool of SARS patients during the convalescent phase. Eur J Clin Microbiol Infect Dis. 2005;24(3):165–71. doi: 10.1007/s10096-005-1299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lo IL, Lio CF, Cheong HH, Lei CI, Cheong TH, Zhong X, Tian Y, Sin NN. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int J Biol Sci. 2020;16(10):1698–707. doi: 10.7150/ijbs.45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu L, Xie J, Sun J, Han Y, Zhang C, Fan H, Liu Z, Qiu Z, He Y, Li T. Longitudinal profiles of immunoglobulin G antibodies against severe acute respiratory syndrome coronavirus components and neutralizing activities in recovered patients. Scand J Infect Dis. 2011;43(6–7):515–21. doi: 10.3109/00365548.2011.560184. [DOI] [PubMed] [Google Scholar]
- 77.WHO . International clinical trials registry platform (ICTRP). [cited 2020 May 7]. https://www.who.int/ictrp/en/
- 78.U.S. FDA . Recommendations for investigational COVID-19 convalescent plasma. [cited 2020 May 7]. https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/investigational-new-drug-ind-or-device-exemption-ide-process-cber
- 79.Arabi Y, Balkhy H, Hajeer AH, Bouchama A, Hayden FG, Al-Omari A, Al-Hameed FM, Taha Y, Shindo N, Whitehead J, et al. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. SpringerPlus. 2015;4:709. doi: 10.1186/s40064-015-1490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jin Y, Wang M, Zuo Z, Fan C, Ye F, Cai Z, Wang Y, Cui H, Pan K, Xu A. Diagnostic value and dynamic variance of serum antibody in corona virus disease 2019. Int J Infect Dis. 2020;94:49–52. doi: 10.1016/j.ijid.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vashist SK. Invitro diagnostic assays for Covid-19: recent advances and emerging trends. Diagnostics. 2020;10(4):202. doi: 10.3390/diagnostics10040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tripp RA, Haynes LM, Moore D, Anderson B, Tamin A, Harcourt BH, Jones LP, Yilla M, Babcock GJ, Greenough T, et al. Monoclonal antibodies to SARS-associated coronavirus (SARS-CoV): identification of neutralizing and antibodies reactive to S, N, M and E viral proteins. J Vir Methods. 2005;128:21–28. doi: 10.1016/j.jviromet.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu HS, Chiu SC, Tseng TC, Lin SF, Lin JH, Hsu YF, Wang MC, Lin TL, Yang WZ, Ferng TL. Serologic and molecular biologic methods for SARS-associated coronavirus infection, Taiwan. Emerg Infect Dis. 2004;10(2):305–10. doi: 10.3201/eid1002.030731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, Zhou M, Chen L, Meng S, Hu Y. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;117(17):9490–96. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeng QL, Yu ZJ, Gou JJ, Li GM, Ma SH, Zhang GF, Xu JH, Lin WB, Cui GL, Zhang MM, et al. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J Infect Dis. 2020. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tamburello A, Marando M. Immunoglobulins or convalescent plasma to tackle COVID-19: buying time to save lives - current situation and perspectives. Swiss Med Wkly. 2020;150:w20264. doi: 10.4414/smw.2020.20264. [DOI] [PubMed] [Google Scholar]
- 87.Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, Xia X, Lv T. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020. doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khamis F, Al-Zakwani I, Al-Hashmi S, Al-Dowaiki S, Al-Bahrani M, Pandak N, Al-Khalili H, Memish Z. Therapeutic Plasma Exchange in Adults with Severe COVID-19 Infection. Int J Infect Dis. 2020. doi: 10.1016/j.ijid.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, Lesser ER, Wiggins CC, Senefeld JW, Klompas AM, Hodge DO, et al. Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients. Mayo Clinic Proc. 2020. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Erkurt MA, Sarici A, Berber I, Kuku I, Kaya E, Ozgul M. Life-saving effect of convalescent plasma treatment in covid-19 disease: clinical trial from eastern Anatolia. Transfus Apher Sci. 2020. doi: 10.1016/j.transci.2020.102867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abdullah HM, Hama-Ali HH, Ahmed SN, Ali KM, Karadakhy KA, Mahmood SO, Mahmood ZH, Amin KQH, Atta PM, Nuradeen BE, et al. Severe refractory COVID-19 patients responding to convalescent plasma; A case series. Ann Med Surg. 2020;56:125–27. doi: 10.1016/j.amsu.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abolghasemi H, Eshghi P, Cheraghali AM, Fooladi AAI, Moghaddam FB, Imanizadeh S, Maleki MM, Ranjkesh M, Rezapour M, Bahramifar A, et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transf Apher Sci. 2020;102875. doi: 10.1016/j.transci.2020.102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yadav T, Saxena SK. Transmission cycle of SARS-CoV and SARS-CoV-2. Coronavirus Disease 2019 (COVID-19). 2020;33–42. doi: 10.1007/978-981-15-4814-7_4. [DOI] [Google Scholar]
- 94.Sharma R, Agarwal M, Gupta M, Somendra S, Saxena SK. Clinical characteristics and differential clinical diagnosis of novel coronavirus disease 2019 (COVID-19). Coronavirus Disease 2019 (COVID-19). 2020:55–70. doi: 10.1007/978-981-15-4814-7_6. [DOI] [Google Scholar]
- 95.Padhi A, Kumar S, Gupta E, Saxena SK. Laboratory diagnosis of novel coronavirus disease 2019 (COVID-19) infection. Coronavirus Disease 2019 (COVID-19) 2020;95–107. doi: 10.1007/978-981-15-4814-7_9. [DOI] [Google Scholar]
- 96.Baxi P, Saxena SK. Emergence and reemergence of severe acute respiratory syndrome (SARS) coronaviruses. Coronavirus Disease 2019 (COVID-19) 2020:151–163. doi: 10.1007/978-981-15-4814-7_13. [DOI] [Google Scholar]
- 97.Kumar S, Nyodu R, Maurya VK, Saxena SK. Morphology, genome organization, replication, and pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Coronavirus Disease 2019 (COVID-19) 2020:23–31. doi: 10.1007/978-981-15-4814-7_3. [DOI] [Google Scholar]
- 98.Kumar S, Nyodu R, Maurya VK, Saxena SK. Host immune response and immunobiology of human SARS-CoV-2 infection. Coronavirus Disease 2019 (COVID-19) 2020:43–53. doi: 10.1007/978-981-15-4814-7_5. [DOI] [Google Scholar]
- 99.Maurya VK, Kumar S, Bhatt MLB, Saxena SK. Therapeutic development and drugs for the treatment of COVID-19. Coronavirus Disease 2019 (COVID-19) 2020:109–126. doi: 10.1007/978-981-15-4814-7_10. [DOI] [Google Scholar]
- 100.Srivastava N, Saxena SK. Prevention and control strategies for SARS-CoV-2 infection. Coronavirus Disease 2019 (COVID-19) 2020:127–140. doi: 10.1007/978-981-15-4814-7_11. [DOI] [Google Scholar]
- 101.Chawla S, Saxena SK. Preparing for the perpetual challenges of pandemics of coronavirus infections with special focus on SARS-CoV-2. Coronavirus Disease 2019 (COVID-19) 2020:165–186. doi: 10.1007/978-981-15-4814-7_14. [DOI] [Google Scholar]
- 102.Khoury M, Cuenca J, Cruz FF, Figueroa FE, Rocco PRM, Weiss DJ. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J. 2020:2000858. doi: 10.1183/13993003.00858-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mani JS, Johnson JB, Steel JC, Broszczak DA, Neilsen PM, Walsh KB, Naiker M. Natural product-derived phytochemicals as potential agents against coronaviruses: a review. Virus Res. 2020:197989. doi: 10.1016/j.virusres.2020.197989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grifoni A, Sidney J, Zhang Y, Scheuermann RH, Peters B, Sette A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe. 2020;27(4):671–680.e2. doi: 10.1016/j.chom.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Sci. 2020;367(6483):1260–63. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ota M. Will we see protection or reinfection in COVID-19? Nat Rev Immunol. 2020. doi: 10.1038/s41577-020-0316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


