1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in December 2019 and causes coronavirus (CoV) disease 2019 (COVID-19). The virus transmits rapidly in humans and has infected at least 24 million people with >838,000 deaths, leading to a global pandemic with devastating damage and worldwide economic loss. Although several therapeutic agents have shown promise or been approved to treat COVID-19 patients, specific, effective, and safe therapeutics are still needed to effectively control this pandemic.
SARS-CoV-2, together with SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), the other two highly pathogenic human CoVs causing the first human infections in 2002 and 2012, respectively, belong to the beta-CoV genus in the Orthocoronavirinae subfamily of the Coronaviridae family [1]. SARS-CoV-2 is a single-stranded, positive-sense RNA CoV, whose genome encodes four major structural proteins, including spike (S), membrane (M), envelope (E), and nucleocapsid (N) (Figure 1(a)). SARS-CoV-2 S protein plays the most important role in virus infection and pathogenesis. It consists of S1 and S2 subunits: S1 is responsible for virus binding to receptor angiotensin-converting enzyme 2 (ACE2) through receptor-binding domain (RBD), whereas S2 is crucial for mediating virus fusion and entry into target cells (Figures 1(b) and 2(a)) [2,3]. Based on the structural analysis of SARS-CoV-2 RBD/ACE2 complex, the RBD can be further divided into a core structure, which is similar to that of SARS-CoV, and a receptor-binding motif (RBM), which contacts with ACE2 receptor and is slightly different from that of SARS-CoV (Figure 1(c)) [2]. In addition to RBD, the S1 subunit of SARS-CoV-2 also includes an N-terminal domain (NTD) (Figure 1(b)). While specific function of SARS-CoV-2 NTD in viral infection is still under investigation, neutralizing antibodies targeting this region have been identified [4]. The S2 subunit contains heptad repeat region 1 (HR1) and 2 (HR2), both of which interact to form a six-helix bundle (6-HB) fusion core structure, bringing the viral and target cell membranes into close proximity for fusion (Figure 1(b,d) and 2(a) [3]). Therefore, S protein, including its fragments, RBD and NTD in S1 subunit as well as HR1 and HR2 in S2 subunit, can potentially serve as key therapeutic targets against SARS-CoV-2 infection (Figure 2). Here, we summarize recently developed therapeutic antibodies and peptide fusion inhibitors targeting these regions of SARS-CoV-2 S protein (Table 1).
Figure 1.
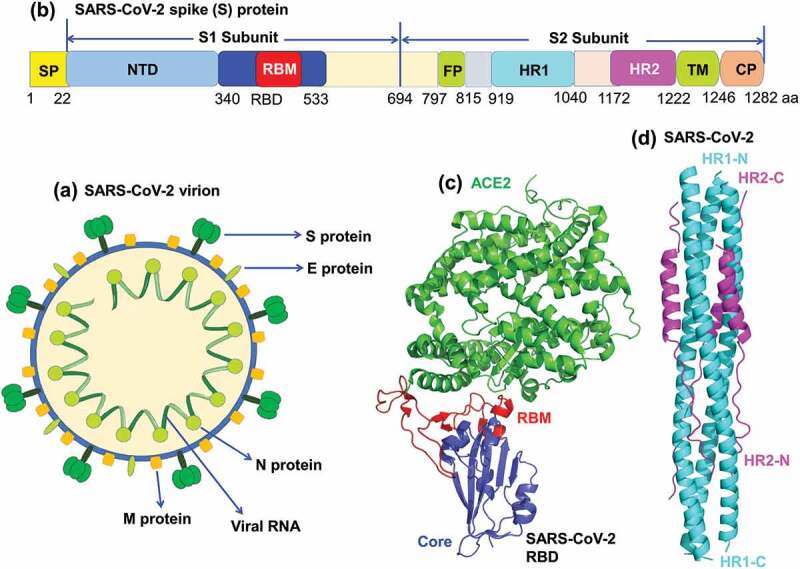
Schematic structures of SARS-CoV-2 virion, spike (S) protein and its functional domains. (a) SARS-CoV-2 virion contains a single-stranded RNA encoding four structural proteins, including S, envelope (E), nucleocapsid (N), and membrane (M) proteins. (b) Schematic structure of SARS-CoV-2 S protein and its functional domains. SP, signal peptide; NTD, N-terminal domain; RBD, receptor-binding domain; RBM, receptor-binding motif; FP, fusion peptide; HR1 and HR2, heptad repeat region 1 and 2; TM, transmembrane; CP, cytoplasmic tail. (c) Crystal structure of SARS-CoV-2 RBD complexed with angiotensin-converting enzyme 2 (ACE2) receptor (PDB ID: 6m0j). SARS-CoV-2 RBD core is colored in blue, RBM in red, and ACE2 in green. (d) Crystal structure of SARS-CoV-2 6-helix-bundle (6-HB) (PDB ID: 6lxt). HR1 is colored in cyan, and HR2 is colored in purple.
Figure 2.

Schematic structures of SARS-CoV-2 S protein-mediated receptor-binding and membrane fusion, as well as S-targeting therapeutic antibodies and peptide fusion inhibitors. (a) Schematic structures of SARS-CoV-2 S-mediated receptor-binding and membrane fusion processes. RBD in S1 subunit (S1-RBD) first binds cellular ACE2 receptor to form RBD/ACE2 complex (receptor binding stage), which triggers conformational changes of S protein, resulting in the dissociation of S1 subunit from the S2 subunit and exposure of HR1 and HR2-trimers (intermediate stage) in S2 subunit. This leads to the formation of a 6-helix bundle (6-HB) fusion core and further fusion of virus and cell membranes (membrane fusion). (b-d) Inhibitory mechanisms of SARS-CoV-2 S-targeting therapeutic antibodies and fusion inhibitors against virus infection. (b) Neutralizing antibodies (nAbs) targeting RBD protein bind the RBD, thus blocking RBD-ACE2 binding and subsequent formation of RBD/ACE2 complex. (c) nAbs targeting NTD in S1 subunit block conformational changes of S protein and subsequent processes. (d) S2-HR1-targeting fusion inhibitors bind the HR1, preventing the formation of 6-HB fusion core and membrane fusion.
Table 1.
Selected COVID-19 therapeutic antibodies and peptide fusion inhibitors targeting SARS-CoV-2 S proteina.
| Potential therapeutics | Target | Mechanisms of action | Cryo-EM or crystal structure |
Neutralizing or inhibitory activity against SARS-CoV-2 |
Protective efficacy against SARS-CoV-2 | Ref. |
|---|---|---|---|---|---|---|
| BD-368-2 human mAb |
RBD in S1 | Binding to RBD and blocking RBD-ACE2 interaction | Available | Pseudovirus: IC50: 1.2 ng/ml; Live virus: IC50: 15 ng/ml |
Prophylactically and therapeutically protects hACE2-Tg mice from SARS-CoV-2 infection, with undetectable or reduced viral replication | [5] |
| CB6 CA1 human mAbs |
RBD in S1 | Binding to RBD and blocking RBD-ACE2 interaction | Available | Pseudovirus: CB6’s IC50: 0.036 μg/ml; Live virus: CB6’s IC50: 0.835 μg/ml |
CB6-LALA prophylactically and therapeutically protects rhesus macaques from SARS-CoV-2 infection, with reduced viral load and alleviated infection-related lung damage |
|
| COV2-2196 COV2-2130 COV2-2381 human mAbs |
RBD in S1 | Binding to different epitopes in RBD and blocking RBD-ACE2 interaction. Combining two mAbs exhibits synergistic effect |
Not Available | Pseudovirus: COV2-2196’s IC50: 0.7 ng/ml; Live virus: COV2-2196’s IC50: 15 ng/ml |
Prophylactically protect AdV-hACE2-transduced mice from SARS-CoV-2 infection, prevent wild-type mice from infection with a mouse-adapted SARS-CoV-2, or protect rhesus macaques from SARS-CoV-2 challenge | [10] |
| IgG1 ab1 human mAb |
RBD in S1 | Binding to RBD and blocking RBD-ACE2 interaction | Not available | Pseudovirus: IC50: 10 ng/ml; Live virus: IC50: 200 ng/ml |
Prophylactically protects wild-type mice from mouse-adapted SARS-CoV-2 infection, or hACE2-Tg mice from SARS-CoV-2 infection, preventing weight loss with undetectable viral replication | [8] |
| CC6.29 CC6.30 CC6.33 CC12.1 human mAbs |
RBD in S1 | Binding to RBD and blocking RBD-ACE2 interaction | Not available | Pseudovirus: CC6.29’s IC50: 2 ng/ml; CC6.30’s IC50: 1 ng/ml; CC12.1’s IC50: 19 ng/ml; CC6.33 cross-neutralizes SARS-CoV pseudovirus: IC50: 162 ng/ml |
CC12.1 prophylactically protects hamsters from SARS-CoV-2 infection, preventing weight loss and viral replication | [6] |
| P2B-2F6 P2C-1F11 P2C-1A3 human mAbs |
RBD in S1 | Binding to RBD and blocking RBD-ACE2 interaction | Available | Pseudovirus: P2C-1F11’s IC50: 0.03 μg/ml; Live virus: P2C-1F11’s IC50: 0.03 μg/ml |
Not available | [13] |
| COVA1-18 COVA2-15 COVA2-02 COVA1-16 human mAbs |
RBD in S1 | Binding to RBD and blocking RBD-ACE2 interaction | Not available | Pseudovirus: COVA1-18’s IC50: 0.008 μg/ml; Live virus: COVA1-18’s IC50: 0.007 μg/ml; COVA2-02 cross-neutralizes SARS-CoV pseudovirus: IC50: 0.61 μg/ml |
Not available | [15] |
| n3088 n3130 human scAbs |
RBD in S1 | Binding to different epitopes in RBD and blocking RBD-ACE2 interaction | Not available | Pseudovirus: n3088’s IC50: 3.3 μg/ml; Live virus: n3088’s IC50: 2.6 μg/ml |
Not available | [9] |
| 5F8 3F11 2F2 4D8 1E2 humanized Nbs |
RBD in S1 | Binding to RBD and blocking RBD-ACE2 interaction | Not available | Pseudovirus: 5F8’s IC50: 0.003 μg/ml; Live virus: 5F8’s IC50: 0.238 μg/ml (best) |
Not available | [11] |
| 4A8 human mAb |
NTD in S1 | Binding to S1-NTD, but not RBD, and not blocking RBD-ACE2 interaction | Available | Pseudovirus: IC50: 49 μg/ml; Live virus: IC50: 0.61 μg/ml |
Not available | [4] |
| 2–4 2-15 4–8 2-43 human mAbs |
RBD or/and NTD | 2–4 and 2–15 bind to RBD; 4–8 binds to NTD; 2–43 binds to quaternary epitope, bridging RBD and NTD in S-trimer |
Available | Pseudovirus: 2–4’s IC50: 0.394 μg/ml; 2–15’s IC50: 0.005 μg/ml; 4–8’s IC50: 0.032 μg/ml; 2–43’s IC50: 0.071 μg/ml; Live virus: 2–4’s IC50: 0.057 μg/ml; 2–15’s IC50: 0.0007 μg/ml; 4–8’s IC50: 0.009 μg/ml; 2–43’s IC50: 0.003 μg/ml |
2–15 prophylactically protects hamsters from SARS-CoV-2 infection, preventing viral replication | [12] |
| 414–1 553-15 515–5 human mAbs |
RBD or/and NTD | 414–1 and 553–15 bind to epitopes A and B in RBD; 553–15 could enhance other mAbs’ neutralizing activity; 515–5 does not bind RBD but can cross-neutralize SARS-CoV |
Available | Pseudovirus: 414–1’s IC50: 3.09 nM; 553–15’s IC50: 1.84 nM; 515–5’s IC50: 5.39 nM; Live virus: 414–1’s IC50: 1.75 nM; 553–15’s IC50: ~30 nM; 515–5’s IC50: ~50 nM; 515–5 cross-neutralizes SARS-CoV pseudovirus: IC50: 84 nM |
Not available | [18] |
| EK1 EK1C4 fusion inhibitory peptides |
HR1 in S2 of human CoVs | Binding S2-HR1 to form 6-HB and blocking viral HR1-HR2 interaction, thus inhibiting S2-mediated membrane fusion | Available | Cell-cell fusion: EK1C4’s IC50: 1.3 nM; Pseudovirus: EK1C4’s IC50: 15.8 nM; Live virus: EK1C4’s IC50: 36.5 nM |
Prophylactically and therapeutically protect hDPP4-Tg mice from MERS-CoV infection, or BALB/c mice from hCoV-OC43 infection | [3] |
aACE2, angiotensin-converting enzyme 2; ADV-hACE2-transduced mice, adenovirus-hACE2-transduced mice; hACE2-Tg, human ACE2-transgenic; hDPP4-Tg, human dipeptidyl peptidase 4 (DPP4)-transgenic; HR1, heptad repeat 1; IC50, 50% inhibitory or neutralizing concentration; mAb, monoclonal antibody; Nbs, nanobodies; NTD, N-terminal domain; RBD, receptor-binding domain; 6-HB, six-helix bundle; S, spike; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
2. Antibodies and fusion inhibitors targeting the S protein of SARS-CoV-2
2.1. Therapeutic antibodies targeting RBD in S1 subunit of SARS-CoV-2 S protein
Similar to SARS-CoV and MERS-CoV RBDs, SARS-CoV-2 RBD has been identified as a critical target for the development of effective therapeutic antibodies against SARS-CoV-2. A number of neutralizing antibodies (nAbs) targeting SARS-CoV-2 RBD, including human monoclonal antibodies (mAbs), human single-domain antibodies (scAbs), and humanized nanobodies (Nbs), have been under development (Figure 2(b)) [5–15,6].
Most RBD-targeting antibodies can bind to RBD and block RBD/ACE2 interaction, thus inhibiting attachment of the virion to the host cell and neutralizing SARS-CoV-2 infection (Figure 2(b)). For example, the binding site of human mAb BD-368-2 on RBD overlaps with the ACE2 binding site, neutralizing pseudotyped, and live SARS-CoV-2 infection [5]. Human mAbs CB6 and IgG1 ab1 compete with ACE2 to bind SARS-CoV-2 RBD, and both have potent SARS-CoV-2 neutralizing activity [7,8]. Combination of human mAbs COV2-2196 with COV2-2130, or of human scAbs n3088 with n3130 shows synergistic effect in neutralizing SARS-CoV-2 infection because they target different epitopes in RBD [9,10]. The RBD-specific humanized Nbs, including 1E2, 2F2, 3F11, 4D8, and 5F8, also exhibit potent neutralizing activity against pseudotyped and live SARS-CoV-2 infection [11].
The protective efficacy of SARS-CoV-2 RBD-specific neutralizing mAbs against SARS-CoV-2 infection has been evaluated in available mice, hamster, and non-human primate animal models [5–8,10,12]. It has been shown that human mAbs BD-368-2 and IgG1 ab1 protect transgenic (Tg) mice expressing hACE2 receptor (hACE2-Tg) from SARS-CoV-2 infection [5,8], while IgG1 ab1 also protects wild-type mice from infection of a mouse-adapted SARS-CoV-2 [8]. In addition, human mAbs CC12.1 and 2–15 protect hamsters from viral replication and/or weight loss after SARS-CoV-2 challenge [6,12]. Moreover, human mAbs CB6 containing LALA mutation, COV2-2196, or COV2-2381 protect rhesus macaques against SARS-CoV-2 challenge [7,10]. Particularly, a combinatorial cocktail treatment of human mAbs COV2-2196 and COV2-2130 improves protection against SARS-CoV-2 infection in adenovirus (AdV)-hACE2-transduced wild-type mice or wild-type mice infected with a mouse-adapted SARS-CoV-2 virus [10].
Crystal or Cryo-EM structures of several RBD-targeting neutralizing mAbs (BD-368-2, CB6, P2B-2F6, 2–4, REGN10933, and REGN10987) complexed with SARS-CoV-2 S-trimer or RBD have been solved [5,7,12–14]. Negative-stain EM structures are available for neutralizing mAbs, such as COV-2196 and COV2-2130, complexed with SARS-CoV-2 S-trimer [10]. The structural information of the complexes formed by these mAbs and SARS-CoV-2 S protein provide a molecular basis to elucidate the mechanisms of mAbs in inhibiting SARS-CoV-2 infection and rationally designing S-RBD-based COVID-19 vaccines.
Notably, several SARS-CoV-2 RBD-targeting antibodies, such as P2C-1F11, P2B-2F6, P2C-1A3, and IgG1 ab1, have no cross-reactivity with SARS-CoV and MERS-CoV RBDs, S1, or S proteins, and do not cross-neutralize SARS-CoV or MERS-CoV infection [8,13]. Although a number of SARS-CoV-2 RBD-specific nAbs cross-react with SARS-CoV RBD, they do not neutralize SARS-CoV infection, and only a few, including CC6.33, COVA2-02, and COVA1-16, cross-neutralize pseudotyped or live SARS-CoV infection in vitro [6,15]. Generally, while SARS-CoV-2 RBD-targeting nAbs (such as CC6.33, COVA2-02, COV2-2678, and COV2-2514) potently neutralize SARS-CoV-2 infection, they have weak neutralizing activity against SARS-CoV infection [6,15,16].
2.2. Therapeutic antibodies targeting NTD in S1 subunit of SARS-CoV-2 S protein
The NTD is another target for the development of therapeutic antibodies against SARS-CoV-2 (Figure 2(c)). Several NTD-specific human mAbs, 4A8, 4–8, 2–17, 5–24, and COVA1-22, neutralize pseudotyped and live SARS-CoV-2 infection [4,12,15]. These NTD-targeting nAbs generally do not bind SARS-CoV-2 RBD, thus not directly blocking the RBD-ACE2 binding, but rather potentially restrain S conformational changes from the pre-fusion to post-fusion stages (Figure 2(c)) [4,15]. Previous studies on MERS-CoV also indicate that the NTD-targeting mAbs might inhibit viral entry and block receptor engagement at the cell membrane [17]. Interestingly, some mAbs with potent SARS-CoV-2 neutralizing activity, such as 2–43, can bind both RBD and NTD, and block RBD-ACE2 interaction [12]. It is worthy of noting that SARS-CoV-2 NTD-targeting nAbs usually have less neutralizing activity than RBD-specific nAbs against SARS-CoV-2 infection [4]. A few SARS-CoV-2 NTD-targeting nAbs can cross-neutralize SARS-CoV infection, but with relatively lower neutralizing potency [18]. Cryo-EM structures of complexes of 4A8/S and 4–8/S-trimer of SARS-CoV-2 are available [4,12], providing a structural information for understanding the binding site(s) of these mAbs in the S protein and their mechanisms of action against SARS-CoV-2 infection.
2.3. Peptide fusion inhibitors targeting HR1 domain in S2 subunit of SARS-CoV-2 S protein
The HR1 domain in S2 of SARS-CoV-2 is one of the most conserved regions in S protein, thus being considered as a key target for developing broad-spectrum viral fusion inhibitors for inhibiting 6-HB formation and blocking virus and cell membrane fusion (Figure 2(d)). A pan-CoV fusion inhibitor, EK1, binds to HR1 domains of many human CoVs, including SARS-CoV, MERS-CoV, SARS-CoV-2, and inhibits fusion of these CoVs with target cells [3]. Recently, the cholesterol has been conjugated to the C-terminus of EK1 and the resultant lipopeptide EK1C4 exhibits improved inhibitory activity against infection of SARS-CoV-2, SARS-CoV, MERS-CoV, and SARS-related CoVs from bats (bat SARSr-CoVs) [3]. These studies suggest the potential for further development of the aforementioned fusion inhibitors as pan-CoV prophylactics and therapeutics to prevent and treat current COVID-19 and future emerging CoV diseases.
2.4. Therapeutic antibodies targeting other regions of CoV-2 S protein
Some nAbs, such as 2–43, target the region in S1 of SARS-CoV-2 S protein, other than RBD, NTD, or S2. Cryo-EM structural analysis of 2–43-Fab/S-trimer complex indicates that the Fab of 2–43 recognizes a quaternary epitope bridging the RBD and NTD in the S-trimer [12]. This mAb also exhibits potent neutralizing activity against SARS-CoV-2 infection, although its mechanism of action is still elusive.
3. Conclusions
In this editorial, we briefly summarize currently developed therapeutic antibodies and peptide fusion inhibitors targeting RBD, NTD, HR1, and some other regions in the SARS-CoV-2 S protein. These therapeutics have capacity to bind the target site(s), to neutralize/inhibit SARS-CoV-2 infection or cross-neutralize/inhibit SARS-CoV infection, and/or to protect animals against SARS-CoV-2 challenge. Overall, it provides the updated information for the development of therapeutics and/or prophylactics against infection of SARS-CoV-2, and possibly SARS-CoV.
4. Expert opinion
Since the pandemic of COVID-19 began, anti-SARS-CoV-2 nAbs have been developed more rapidly than previously seen for any other virus. Notably, most potent nAbs are those specific to the RBD in SARS-CoV-2 S1 subunit [6,19], suggesting that this region is a critical target for developing highly effective therapeutics against SARS-CoV-2. In general, RBD-specific antibodies often have strong potency against the same CoV species, but less efficacy in neutralizing different CoV species.
The S2 protein of human CoVs has been identified as a target to develop broad-spectrum prophylactic and therapeutic antibodies for preventing and treating virus infection. Several SARS-CoV or MERS-CoV S2-specific nAbs have shown neutralizing activity against SARS-CoV or MERS-CoV infection. Different from RBD-specific nAbs, S2-targeting nAbs generally have broad-spectrum neutralizing activity owing to the relatively conserved sequences in S2 region among different CoVs in the same groups. Indeed, some SARS-CoV S2-targeting mAbs do cross-react with SARS-CoV-2 [20]. It appears that no SARS-CoV-2 S2-specific antibodies have been reported so far. Thus, efforts are necessary to develop such mAbs with potent and broad-spectrum neutralizing ability. Except for its role as a neutralizing target, SARS-CoV-2 S2 subunit could also serve as a key target to develop fusion inhibitors to block virus and cell membrane fusion. Such inhibitors have been identified, showing potent inhibitory activity.
Combinatorial treatments by combining nAbs targeting different epitopes on S protein or combining a nAb with a peptide-based fusion inhibitor are expected to present synergistic antiviral effects [14,21]. Such combinatorial or antibody cocktail therapy is anticipated to improve the overall efficacy of each individual therapeutic in neutralization, inhibitory activity, and/or protection against infection of SARS-CoV-2 with or without mutations, as well as SARS-CoV and bat-SARSr-CoVs.
Antibody-dependent enhancement (ADE) has been a key issue in CoV vaccines and therapeutic antibodies, in which antibodies with no, or low-titer, neutralizing activity enhance virus infection. ADE can be caused by SARS-CoV full-length S protein-targeting antibodies in an Fc receptor-dependent manner [22]. Although the ADE phenomenon has not been clearly observed in SARS-CoV-2 S-targeting antibodies, it is possible that ADE may occur when the antibody has no neutralizing activity, or the titer is too low to neutralize virus infection. Notably, the Fc portion of mAbs, or the Fc fusion tag of Nbs, can be substituted with LALA mutation to remove potential ADE [7]. It is expected that more potent S protein-targeting neutralizing therapeutics with strong safety can be developed and progressed to clinical trials to control the COVID-19 pandemic.
Funding Statement
The research of the authors is supported by National Institutes of Health (NIH) grants (R01AI137472, R01AI139092, and R01AI157975).
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Wang N, Shang J, Jiang S, et al. Subunit vaccines against emerging pathogenic human coronaviruses. Front Microbiol. 2020;11:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lan J, Ge J, Yu J.. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. [DOI] [PubMed] [Google Scholar]
- 3.Xia S, Liu M, Wang C, et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30(4):343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper identifies a S2-targeting pan-coronavirus peptide fusion inhibitor that potently inhibits infection of SARS-CoV-2 and other coronaviruses.
- 4.Chi X, Yan R, Zhang J, et al. A neutralizing human antibody binds to the N-terminal domain of the spike protein of SARS-CoV-2. Science. 2020;369(6504):650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper identifies a SARS-CoV-2 S1-NTD-specific human mAb with neutralizing activity agaisnt SARS-CoV-2 infection.
- 5.Cao Y, Su B, Guo X, et al. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell. 2020;182(1):73–84.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers TF, Zhao F, Huang D, et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020;369(6506):956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper identifies a SARS-CoV-2 RBD-targeting human neutralizing mAb in protection of hamsters from SARS-CoV-2 infection.
- 7.Shi R, Shan C, Duan X, et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020;584(7819):120–124. [DOI] [PubMed] [Google Scholar]
- 8.Li W, Drelich A, Martinez DR, et al. Rapid selection of a human monoclonal antibody that potently neutralizes SARS-CoV-2 in two animal models. bioRxiv. 2020. DOI: 10.1101/2020.05.13.093088 [DOI] [Google Scholar]; • This paper describes a SARS-CoV-2 RBD-targeting human neutralizing mAb in protection of transgenic mice from SARS-CoV-2 challenge, as well as wild-type mice from mouse-adapted SARS-CoV-2 infection.
- 9.Wu Y, Li C, Xia S, et al. Identification of human single-domain antibodies against SARS-CoV-2. Cell Host Microbe. 2020;27(6):891–898.e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zost SJ, Gilchuk P, Case JB, et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020;584(7821):443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper describes several SARS-CoV-2 RBD-targeting human neutralizing mAbs in protection of mice and non-human primates from SARS-CoV-2 infection.
- 11.Chi X, Liu X, Wang C, et al. Humanized single domain antibodies neutralize SARS-CoV-2 by targeting spike receptor binding domain. bioRxiv. 2020. DOI: 10.1101/2020.04.14.042010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Wang P, Nair MS, et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584(7821):450–456. [DOI] [PubMed] [Google Scholar]
- 13.Ju B, Zhang Q, Ge J, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584(7819):115–119. [DOI] [PubMed] [Google Scholar]
- 14.Hansen J, Baum A, Pascal KE, et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369(6506):1010–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brouwer PJM, Caniels TG, Straten KVD, et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020;369(6504):643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper identifies several SARS-CoV-2 BBD-targeting human neutralizing mAbs with neutralizing activity agaisnt SARS-CoV-2 infectioin.
- 16.Zost SJ, Gilchuk P, Chen RE, et al. Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein. Nature Med. 2020. DOI: 10.1038/s41591-020-0998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou H, Chen Y, Zhang S, et al. Structural definition of a neutralization epitope on the N-terminal domain of MERS-CoV spike glycoprotein. Nat Commun. 2019;10(1):3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan J, Xing S, Ding L, et al. Human-IgG-neutralizing monoclonal antibodies block the SARS-CoV-2 infection. Cell Rep. 2020;32(3):107918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robbiani DF, Gaebler C, Muecksch F. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Z, Monteil VM, Maurer-Stroh S, et al. Monoclonal antibodies for the S2 subunit of spike of SARS-CoV-1 cross-react with the newly-emerged SARS-CoV-2. Euro Surveill. 2020;25(28):2000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baum A, Fulton BO, Wloga E, et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369(6506):1014–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper describes antibody cocktail treatment in preventing SARS-CoV-2 escape mutants.
- 22.Jaume M, Yip MS, Kam YW, et al. SARS CoV subunit vaccine: antibody-mediated neutralisation and enhancement. Hong Kong Med J. 2012;18(2):31–36. [PubMed] [Google Scholar]


