ABSTRACT
Objective
To understand the clinical effectiveness and safety of Shufeng Jiedu Capsules combined with umifenovir (Arbidol) in the treatment of common-type COVID-19.
Methods
A retrospective cohort study was used to analyze the case data of 200 inpatients diagnosed with common-type COVID-19 at Wuhan Hospital. Participants were divided into a control group and an experimental group. The control group was treated with Arbidol hydrochloride capsules while the experimental group was treated with combination Arbidol hydrochloride capsules and Shufeng Jiedu Capsules (SFJDC) for 14 days.
Results
Defervescence was achieved more rapidly in the experimental group (P < 0.05). The white blood cell count and the lymphocyte percentage in the experimental group were higher than that of the control group (P < 0.05). CRP and IL-6 levels in the experimental group were significantly lower than those in the control group (P < 0.05). The proportion of chest CT studies showing resolution of pneumonia in the experimental group was significantly higher than that in the control group (P < 0.05).
Conclusions
A treatment regimen of Shufeng Jiedu Capsules combined with Arbidol to treat common-type COVID-19, combining traditional Chinese and western allopathic medicine, improves time to recovery, has better clinical effectiveness, and is safe.
KEYWORDS: Traditional Chinese medicine (TCM), COVID-19, common type, Arbidol, clinical effectiveness
1. Introduction
Coronaviruses are important human and animal pathogens. In December 2019, a novel coronavirus caused a cluster of pneumonia cases originating in Wuhan, Hubei Province, China. The disease has spread rapidly, causing outbreaks throughout China and beyond, with cases increasing daily. As of 28 August 2020, a total of 85,004 COVID-19 cases have been confirmed in China [1,2] with over 24 million confirmed cases worldwide [3]. In February 2020, the WHO named the disease COVID-19, and its full name as 2019 Coronavirus Disease [4]. Prior to this, the disease was referred to as the 2019 novel coronavirus (2019-nCoV).
Whole-genome sequencing and phylogenetic analysis show that COVID-19 is genetically closer to a novel bat-derived coronavirus and similar to severe acute respiratory syndrome coronavirus (SARS-CoV) [5] and Middle East respiratory syndrome coronavirus (MERS-CoV) [6], both betacoronaviruses. The Coronavirus Study Group of the International Committee on Taxonomy of Viruses proposed to name the virus Severe Acute Respiratory Syndrome Coronavirus Type 2 (SARS-CoV-2) [7]. Since the severe acute respiratory syndrome (SARS) outbreak in 2003 and the Middle East respiratory syndrome coronavirus (MERS) outbreak in 2012, clinicians and virologists have been continuously exploring possible antiviral agents to target these viruses, but to date, there are no specific drugs approved or validated specifically for COVID-19 [8].
At present, the use of Traditional Chinese Medicine (TCM) has gradually shown its unique advantages in the treatment of COVID-19, especially what is referred to in TCM as heat-clearing and detoxifying drugs. Qingrejiedu, as a traditional Chinese medicine is efficacious, has few adverse reactions and is low-cost. Heat-clearing and detoxifying drugs have TCM characteristics of being bitter and cold and have the effects of clearing heat, clearing fire, dampness, cooling blood, and detoxification. Their main function is to clear heat, so they are considered to be antipyretic. A large number of basic and clinical studies have shown that clearing heat and detoxifying drugs can reduce inflammation and effectively supplement Western medicine. Since inflammation is the result of the interaction of multiple inflammatory mediators, heat-clearing and antidote drugs can exert anti-inflammatory effects through different mechanisms, including inhibiting inflammatory cytokines and mediators, blocking inflammatory signals, and interfering with chemokines [9–11]. The use of these drugs to treat COVID-19 has been described in individual case reports [12], but its efficacy is unclear and needs to be evaluated in larger randomized trials. Shufeng Jiedu Capsules are easily administered orally and are low in cost at 28 yuan (approximately 3.95 USD) a box containing 18 capsules. In recent years, Shufeng Jiedu Capsules have been widely used in China to treat diseases such as influenza, upper respiratory tract infections, chronic bronchitis, and community-acquired pneumonia, with good clinical outcomes achieved. This drug is also recommended for COVID-19 in the latest edition of Novel Coronavirus Pneumonia Diagnosis and Treatment Protocol (version 6) [13]. COVID-19 cases have been treated in Wuhan Hospital since January 2020. In this study, 200 inpatients with common-type COVID-19 treated in this hospital from January 20 to 20 February 2020 were selected as the subjects with the purpose of exploring the clinical effectiveness of combination Shufeng Jiedu Capsules and Arbidol therapy in the treatment of common-type COVID-19.
As of 28 August 2020, the cumulative number of people diagnosed with COVID-19 in Mainland China has exceeded 85,000, with a total of 80,064 recovered cases. With the joint efforts of the Chinese government and the people across the country, the current situation has been essentially stable. Although China has achieved a victory in the fight up to this stage against the COVID-19 pandemic, the situation outside of China remains serious, with the number of confirmed cases continuing to rise, and the cumulative number of diagnoses exceeding 24 million. The United States, Brazil, Russia, India, and the United Kingdom have been affected most [3]. At present, it has become clear that COVID-19 is not just a problem for China but has caused a great threat to the health of people around the world. We hope that through this research, we can share our experience in the diagnosis and treatment of COVID-19 to frontline medical workers from all over the world, and strive to win victory in this unprecedented war against an invisible enemy.
2. Methods
2.1. Study design and participants
This study is a retrospective cohort study which extracted the electronic medical record data of inpatients of Wuhan Hospital from 20 January 2020 to 20 February 2020. A total of 2583 patients were confirmed to have common-type COVID-19. Those with incomplete medical records and those who did not meet the inclusion criteria were excluded. The remaining 1500 patients were paired 1:1 according to age, gender, and treatment, and the final study included 200 patients. This retrospective cohort study was approved by the Ethics Committee of Wuhan Hospital (HBZY2020-C23-01). Novel coronavirus infection diagnostic standards were in line with the Chinese National Health Commission’s ‘Novel coronavirus pneumonia diagnosis and treatment guidelines (trial version 6)’ [13] and divided into mild, common, severe, and critically severe types. According to the presence or absence of clinical symptoms, pneumonia and its severity, respiratory failure, shock, and presence or absence of other organ failure, the disease is categorized. It is divided into mild type (mild clinical symptoms and no pneumonia manifestations on imaging); common type (fever, respiratory symptoms such as pneumonia can be seen on imaging); severe (respiratory distress, RR ≥30 times/min; at rest, oxygen saturation ≤93%; arterial blood oxygen partial pressure (PaO2)/oxygen concentration (FiO2) ≤300 mmHg) and critically severe (respiratory failure requiring mechanical ventilation; shock occurs; combined with other organ failure requiring ICU admission).
2.2. Inclusion criteria
(1) Diagnosis of common-type COVID-19 (positive on throat swab nucleic acid); (2) Aged above 50; (3) No underlying diagnoses of diabetes, hypertension, or coronary heart disease; (4) No malignant tumor; (5) Compliant with treatment.
2.3. Exclusion criteria
(1) Mild, severe, and critically severe COVID-19 patients; (2) Patients with incomplete medical records.
2.4. Data collection
The research team at Wuhan Hospital collected and summarized the patient’s epidemiology, demographics, clinical symptoms, signs, laboratory test results, imaging characteristics, treatment measures, efficacy, and adverse reactions. The data were collected by a professional medical team who also verified the data. Laboratory confirmation of COVID-19 infection was completed at the Wuhan Center for Disease Control and Prevention. All upper respiratory pharyngeal swab specimens collected at the time of admission from all patients were stored in virus transport medium. COVID-19 was confirmed by real-time RT-PCR [14]. All patients underwent chest CT.
2.5. Treatment method
Participants were divided into control and experimental groups based on the treatment regimen they were on with 100 cases each. The control group was treated with Arbidol Hydrochloride Capsules (Shijiazhuang Fourth Medicine Co., Ltd., Approval Number H20060023) orally, 2 capsules (0.1 g/capsule) 3 times a day for 2 weeks. The experimental group, in addition to what was given to the control group, was given Shufeng Jiedu Capsule (Jiren Pharmaceutical Group, Haozhou, Anhui, China, Approval Number Z20090047) by mouth, 4 capsules (0.52 g/capsule) 3 times a day for 2 weeks.
2.6. Observation of indicators and criteria
Observations were made on the time for improvement in primary clinical symptoms after commencing treatment in the two groups of patients. Venous blood samples were obtained before and after treatment for full blood count, high-sensitivity C-reactive protein (hs-CRP), interleukin 6 (IL-6), and other inflammation markers. The two groups were then compared in the improvement of inflammation markers, resolution of pneumonia in chest CT, clinical effectiveness, and incidence of adverse reactions. Marked resolution on chest CT was defined as more than 50% resolution of infected lesions. Clinical effectiveness was defined as defervescence and maintaining normothermia for more than 3 days, significant improvement of respiratory symptoms, improvement of acute exudative lesions on lung imaging, and negative nucleic acid tests on respiratory specimens for two consecutive times. Treatment was defined as ineffective if the above treatment indicators were not met [13].
2.7. Statistical methods
Categorical variables were represented by frequency and percentage, and continuous variables were represented by mean and standard deviation. When the data were normally distributed, the independent t-test was used to compare the mean of continuous variables. Otherwise, the Mann–Whitney test was used. The χ2 test was used to compare the proportions of categorical variables, and Fisher’s exact test was used when data were limited. All statistical analyses were performed using SPSS version 23.0 software (IBM Corporation, Armonk, NY, USA). P < 0.05 was deemed statistically significant.
3. Results
3.1. Demographics and baseline characteristics
The study population included 200 hospitalized patients diagnosed with common-type COVID-19, who were allocated into experimental and control groups with 100 patients in each. In the experimental group, there were 66 males and 34 females with a mean age of 60.2 ± 6.6 years (51–75 years). Fifty-eight percent of the patients in the experimental group were between 50 and 60 years old. The time between onset and admission was 4.2 ± 1.4 days (2–7 days). In the control group, there were 64 males and 36 females with a mean age of 60.4 ± 6.6 years (51–75 years), of which 55% were patients aged 50–60. The time between onset and admission was 4.2 ± 1.3 days (2–7 days). All patients were residents of Wuhan. There was no significant difference in demographic data such as age and sex between the two groups (P > 0.05), and thus they were comparable (Table 1).
Table 1.
Demographic and baseline characteristics of 200 common-type COVID-19 patients treated at Wuhan Hospital.
| Variables |
Experimental group n = 100 |
Control group n = 100 |
P value a |
|---|---|---|---|
| Age (years) | |||
| Mean | 60.2 ± 6.6 | 60.4 ± 6.6 | 0.780 |
| Range | 51–75 | 51–75 | |
| Age group [Number (%)] | |||
| 50 to <60 yr | 58 (58.0) | 55 (55.0) | 0.832 |
| 60 to <70 yr | 32 (32.0) | 36 (36.0) | |
| 70 to <80 yr | 10 (10.0) | 9 (9.0) | |
| Sex (%) | |||
| Female | 34 (34.0) | 36 (36.0) | 0.767 |
| Male | 66 (66.0) | 64 (64.0) | |
| Exposure history (%) | |||
| Wuhan resident status | 100 (100.0) | 100 (100.0) | 1.000 |
| Recent travel to Wuhan | 0 | 0 | |
| Time between onset and admission (days) | |||
| Mean | 4.2 ± 1.4 | 4.2 ± 1.3 | 0.877 |
| Range (days) | 2 ~ 7 | 2 ~ 7 |
aP values indicate differences between experimental and control group patients. P < 0.05 was considered statistically significant.
3.2. Clinical characteristics and treatment
The clinical symptoms that were common in both the experimental group as compared to the control group were cough (98% vs 96%), fever (80% vs 78%), fatigue (80% vs 78%), dizziness (40% vs 38%), and rare symptoms of nasal congestion (20% vs 22%), rhinorrhea (20% vs 22%), and constipation (3% vs 2%), with no significant difference between the two groups of patients (P > 0.05). Patients in the experimental and control groups had no significant difference in body temperature (38.1 ± 0.9 vs 38.0 ± 0.9)°C, heart rate (71.2 ± 4.6 vs 71.2 ± 4.7) bpm, respiratory rate (20.0 ± 1.2 vs 20.3 ± 1.1) bpm, systolic blood pressure (131.3 ± 18.7 vs 130.4 ± 22.2) mmHg, diastolic blood pressure (75.1 ± 5.1 vs 75.7 ± 5.3) mmHg, and oxygen saturation (97.9 ± 1.4 vs 98.0 ± 1.4%) (P > 0.05). All patients underwent computed tomography (CT) of the chest upon admission. All lungs were affected simultaneously with patchy, hazily marginated, predominantly peripheral ground-glass shadows (Table 2). Patients in the experimental group and the control group were given corresponding treatment measures according to their clinical situation after admission: oxygen via nasal intubation (30% vs 34%), antibiotics (23% vs 21%), intravenous immunoglobulin (25% vs 28%), and glucocorticoids (36% vs 32%). There was no significant difference in treatment measures given to the two groups (P > 0.05) (Table 2).
Table 2.
Comparison of clinical characteristics and treatment in participants with common-type COVID-19.
| Number (%)/Mean±SD |
Total n = 200 |
Case n = 100 |
Control n = 100 |
t/χ2 value |
P value a |
|---|---|---|---|---|---|
| Clinical symptoms and signs | |||||
| Fever | 158 (79.0) | 80 (80.0) | 78 (78.0) | 0.121 | 0.728 |
| Cough | 194 (97.0) | 98 (98.0) | 96 (96.0) | 0.172 | 0.678 |
| Fatigue | 158 (79.0) | 80 (80.0) | 78 (78.0) | 0.121 | 0.728 |
| Dizziness | 78 (39.0) | 40 (40.0) | 38 (38.0) | 0.084 | 0.772 |
| Nasal congestion | 42 (21.0) | 20 (20.0) | 22 (22.0) | 0.121 | 0.728 |
| Rhinorrhea | 42 (21.0) | 20 (20.0) | 22 (22.0) | 0.121 | 0.728 |
| Constipation | 5 (2.5) | 3 (3.0) | 2 (2.0) | 0.000 | 1.000 |
| Body temperature (°C) | 38.1 ± 0.9 | 38.1 ± 0.9 | 38.0 ± 0.9 | 0.546 | 0.586 |
| Respiratory rate (breaths/min) | 20.2 ± 1.2 | 20.0 ± 1.2 | 20.3 ± 1.1 | −1.457 | 0.147 |
| Pulse (beats/min) | 71.2 ± 4.7 | 71.2 ± 4.6 | 71.2 ± 4.7 | 0.000 | 1.000 |
| Blood pressure (mmHg) | |||||
| Systolic | 130.9 ± 20.5 | 131.3 ± 18.7 | 130.4 ± 22.2 | 0.310 | 0.757 |
| Diastolic | 75.4 ± 5.2 | 75.1 ± 5.1 | 75.7 ± 5.3 | −0.828 | 0.408 |
| Oxygen saturation (%) | 97.9 ± 1.4 | 97.9 ± 1.4 | 98.0 ± 1.4 | −0.651 | 0.516 |
| Chest CT findings | |||||
| Unilateral pneumonia | 0 | 0 | 0 | 0.000 | 1.000 |
| Bilateral pneumonia | 200 (100.0) | 100 (100.0) | 100 (100.0) | 0.000 | 1.000 |
| Bilateral patchy distribution and ground-glass opacities | 200 (100.0) | 100 (100.0) | 100 (100.0) | 0.000 | 1.000 |
| Treatment | |||||
| Nasal cannula O2 supplementation | 64 (32.0) | 30 (30.0) | 34 (34.0) | 0.368 | 0.544 |
| Mechanical ventilation | 0 | 0 | 0 | - | - |
| Antibiotic treatment | 44 (22.0) | 23 (23.0) | 21 (21.0) | 0.117 | 0.733 |
| Intravenous immunoglobulin therapy | 53 (26.5) | 25 (25.0) | 28 (28.0) | 0.23 | 0.631 |
| Glucocorticoids | 68 (34.0) | 36 (36.0) | 32 (32.0) | 0.357 | 0.550 |
| Other antiviral use | |||||
| Ribavirin | 0 | 0 | 0 | - | - |
| Lopinavir/ritonavir | 0 | 0 | 0 | - | - |
aP values indicate differences between experimental and control patients. P < 0.05 was considered statistically significant.
3.3. Comparison of laboratory-related indicators before and after treatment
Inflammatory indicators such as leukocyte count (WBC), lymphocyte percentage, neutrophil percentage, high-sensitivity C-reactive protein (hs-CRP), and interleukin 6 (IL-6) in the two groups of patients before treatment was made. No statistical significance was found (P > 0.05) (Table 3). The differences in inflammatory markers within the experimental group and the control group before and after the treatment were statistically significant (P < 0.05) (Table 4), but the effect was greater in the experimental group. On day 5 of treatment, the white blood cell count (WBC) of the experimental group (5.5 ± 0.8 vs 4.6 ± 0.5)×109/L and the number of lymphocytes per hundred units of white blood cells (20.6 ± 1.9vs17.0 ± 1.4) were significantly higher than the control group, while the number of neutrophils per hundred units of white blood cells in the experimental group (59.6 ± 1.5 vs 61.5 ± 1.4) was significantly lower than the control group (P < 0.05). For CRP and IL-6, the experimental group was significantly lower than the control group on day 5 [(14.1 ± 1.8 vs 15.6 ± 1.9) mg/L, (8.0 ± 0.6 vs 8.8 ± 0.7) pg/ml], day 10 [(11.2 ± 2.4 vs 13.0 ± 1.8) mg/L; (7.3 ± 0.7 vs 8.2 ± 0.6) pg/ml], and day 14 [(7.2 ± 2.1 vs 10.3 ± 1.7) mg/L; (6.6 ± 0.5 vs 7.2 ± 0.7)] pg/ml, respectively (P < 0.05) (Table 3, Figure 1).
Table 3.
Comparison of laboratory results of common-type COVID-19 patients before and after treatment between experimental and control groups.
| |
Before treatment |
After day 5 of treatment |
||||
|---|---|---|---|---|---|---|
| Case | Control | P value a | Case | Control | P value a | |
| Leucocyte count, (×109 per L; 4–10) |
2.7 ± 0.3 | 2.6 ± 0.2 | 0.067 | 5.5 ± 0.8 | 4.6 ± 0.5 | 0.000 |
| Neutrophil ratio, (%; 50–70) |
65.9 ± 2.5 | 66.3 ± 2.4 | 0.219 | 59.6 ± 1.5 | 61.5 ± 1.4 | 0.000 |
| Lymphocytes ratio, (%;20–40) |
13.0 ± 0.7 | 13.0 ± 0.7 | 0.376 | 20.6 ± 1.9 | 17.0 ± 1.4 | 0.000 |
| Platelet count, (×109 per L; 100–300) |
212.7 ± 46.2 | 211.8 ± 47.9 | 0.892 | 210.9 ± 47.7 | 206.6 ± 44.0 | 0.508 |
aP values indicate differences between experimental and control patients. P < 0.05 was considered statistically significant.
Table 4.
Comparison of laboratory results of common-type COVID-19 patients within the experimental and control groups before and after treatment.
| |
Case |
Control |
||||
|---|---|---|---|---|---|---|
| Before treatment | After day 5 of treatment | P value a | Before treatment | After day 5 of treatment | P value a | |
| Leucocyte count, (×109 per L; 4–10) |
2.7 ± 0.3 | 5.5 ± 0.8 | 0.000 | 2.6 ± 0.2 | 4.6 ± 0.5 | 0.000 |
| Neutrophil ratio, (%; 50–70) |
65.9 ± 2.5 | 59.6 ± 1.5 | 0.000 | 66.3 ± 2.4 | 61.5 ± 1.4 | 0.000 |
| Lymphocytes ratio, (%;20–40) |
13.0 ± 0.7 | 20.6 ± 1.9 | 0.000 | 13.0 ± 0.7 | 17.0 ± 1.4 | 0.000 |
| Platelet count, (×109 per L; 100–300) |
212.7 ± 46.2 | 210.9 ± 47.7 | 0.786 | 211.8 ± 47.9 | 206.6 ± 44.0 | 0.425 |
aP values indicate differences between before treatment and after day 5 of treatment. P < 0.05 was considered statistically significant.
Figure 1.

Changes in CRP (A) and IL-6 (B) in patients with common-type COVID-19 before and after treatment in experimental and control groups. The solid black line indicates the normal upper limit for each indicator.
a When comparing experimental and control groups, P < 0.05.
3.4. Comparison of clinical symptoms before and after treatment
Compared with the control group, defervescence was achieved more rapidly in the experimental group (2.2 ± 1.1) days (P < 0.05). Other clinical symptoms of cough (4.5 ± 2.3 vs 4.2 ± 2.5) days, fatigue (3.2 ± 1.5 vs 3.5 ± 1.7) days, dizziness (3.8 ± 1.8 vs 3.6 ± 1.6) days, nasal congestion (3.4 ± 1.7 vs 3.6 ± 1.4) days, rhinorrhea (3.7 ± 1.5 vs 3.6 ± 1.4) days, and constipation (1.2 ± 0.8 vs 1.3 ± 0.7) days showed no statistically significant difference between the two groups (P > 0.05) (Figure 2).
Figure 2.
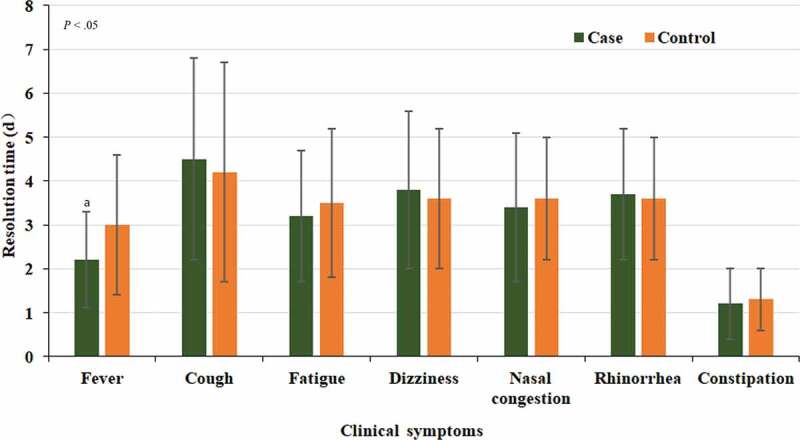
Clinical symptoms resolution time in patients with common-type COVID-19 in the experimental and control groups after treatment.
a When comparing experimental and control groups, P < 0.05.
3.5. Comparison of resolution of pneumonia on chest CT after treatment
On day 7 of treatment, the proportion of those who showed resolution of pneumonia on chest CT in the experimental group was significantly higher than those in the control group (87/100 [87%] vs 72/100 [72%]), with the difference being of statistical significance (P < 0.05) (Table 5). See chest CT images for details (Figure 3).
Table 5.
Comparison of treatment effect between experimental and control group patients with common-type COVID-19.
| Total (%) n = 200 |
Case (%) n = 100 |
Control (%) n = 100 |
χ2 value | P value a | |
|---|---|---|---|---|---|
| Marked resolution of pneumonia on CT (follow-up at day 7) |
159 (79.5) | 87 (87.0) | 72 (72.0) | 6.903 | 0.009 |
| Clinical effectiveness (evaluated at day 14) | |||||
| Effective | 172(86.0) | 92(92.0) | 80(80.0) | 5.980 | 0.014 |
| Ineffective | 28(14.0) | 8(8.0) | 20(20.0) | - | - |
| Adverse effects | 12(6.0) | 6(6.0) | 6(6.0) | 0.000 | 1.000 |
| Nausea | 3(1.5) | 2(2.0) | 1(1.0) | 0.000 | 1.000 |
| Chest tightness | 0 | 0 | 0 | ||
| Allergic reaction | 3(1.5) | 1(1.0) | 2(2.0) | 0.000 | 1.000 |
| Abdominal pain | 4(2.0) | 2(2.0) | 2(2.0) | - | - |
| Diarrhea | 2(1.0) | 1(1.0) | 1(1.0) | - | - |
aP values indicate differences between experimental and control group patients. P < 0.05 was considered statistically significant.
Figure 3.
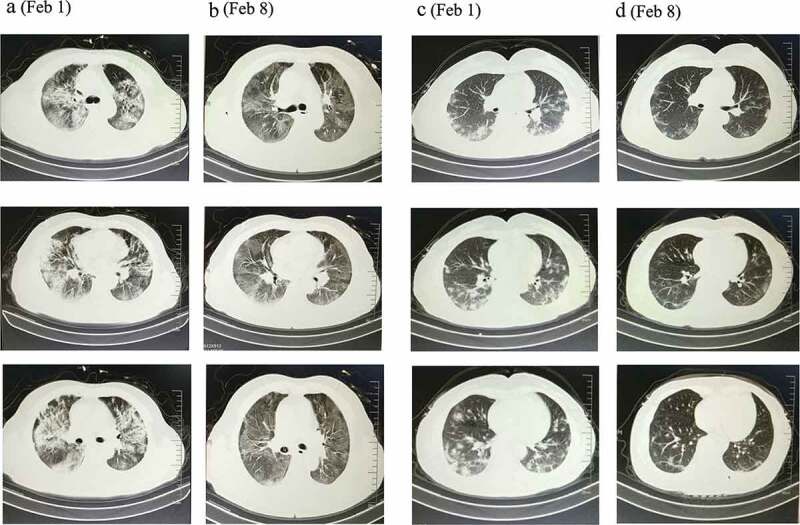
CT images of common-type COVID-19 patients before and after treatment in experimental and control groups.
56-year-old female patient in the control group (a). Before treatment (February 1): Multiple bilateral scattered ground-glass lesions, predominantly peripheral (b). After day 7 of treatment (February 8): Pneumonia is resolved as compared to previous, but a few pulmonary fibrous lesions remain. 62-year-old female patient in the experimental group (c). Before treatment (February 1): Multiple bilateral scattered lesions with predominantly peripheral hazy margins and hilar involvement (d). After day 7 of treatment (February 8): Marked resolution of pneumonia as compared to previous. Peripheral shadows are no longer obvious. Marked improvement as compared to control.
3.6. Comparison of clinical effectiveness between the two groups
On the day 14 of treatment, the rate of recovery in the experimental group was significantly higher than that in the control group (92/100 [92%] vs 80/100 cases [80%]), with the difference being statistically significant (P < 0.05) (Table 5).
3.7. Comparison of the incidence of adverse effects between the two groups
During the course of treatment, a total of 12 patients had adverse drug reactions, of which 6 were in the experimental group. The overall incidence of adverse reactions in the experimental group was 6%. The symptoms included nausea [2 (2%)], allergic reaction [1 (1%)], abdominal pain [2 (2%)], and diarrhea [1 (1%)]. Six patients in the control group experienced adverse drug reaction including nausea [1 (1%)], allergic reaction [2 (2%)], abdominal pain [2 (2%)], and diarrhea [1 (1%)]. All reported adverse reactions were mild and appeared within the first 2 days after commencing treatment. Patients were instructed to take the medication after meals. Side effects such as nausea and vomiting, hypersensitivity, and abdominal pain were treated symptomatically. Adverse effects resolved 1–2 days after symptomatic treatment was implemented. The total incidence of adverse reactions in the control group was 6%, and as such there was no significant difference in the incidence of adverse reactions between the two groups (P > 0.05) (Table 5).
4. Discussion
Current studies have confirmed that COVID-19 is the cause of the unexplained cases of pneumonia in Wuhan. The apparent structure of the COVID-19 receptor-binding gene region is very similar to that of the SARS coronavirus, and it has been speculated that both viruses use the same receptor to enter cells. The RNA sequence of COVID-19 is most similar to two bat coronaviruses, so bats may be its main source thought it is not yet clear whether COVID-19 was transmitted directly from bats to humans or through some other mechanism, such as via an intermediate host [15]. Pneumonia is the most common manifestation of the virus and is mainly characterized by fever, cough, and dyspnea, with chest imaging showing bilateral lung infiltration. Although many of the reported cases are not critical, about 20% of patients diagnosed are classified as severe, which present with respiratory failure, septic shock, or other organ failure requiring intensive care [14,16]. The overall fatality rate is uncertain but is estimated to be approximately 3% [17]. At present, commonly used antiviral drugs, including neuraminidase inhibitors (i.e. oseltamivir, peramivir, and zanamivir) are not effective against COVID-19 because neuraminidase is not produced by coronaviruses. Drugs such as ganciclovir, acyclovir, and ribavirin have little effect and are not recommended for clinical use. Antiviral drugs targeting COVID-19 are currently being studied. Drugs that have been shown to be effective by current studies include radsivir, lopinavir/ritonavir, lopinavir/ritonavir combined with interferon-β, convalescent plasma, and monoclonal antibodies [18,19]. However, the efficacy and safety of these drugs in COVID-19 need to be confirmed by further clinical trials.
For the treatment of COVID-19 patients, effective control of fever, duration of acute infection and hospitalization, and minimization of serious complications are issues that clinicians have been working to address. Effective antiviral treatment thus becomes a very important part of overall treatment. Umifenovir (Arbidol) was developed by the Russian Research Chemical‐Pharmaceutical Institute. It is a synthetic broad-spectrum antiviral drug that was previously used to prevent and treat human influenza A and B infections and influenza complications. It has been used as an anti-flu drug in some countries with a history of use spanning decades [20]. Umifenovir is active against many DNA/RNA and enveloped/non-enveloped viruses. It inhibits membrane fusion between virus particles and the plasma membrane and between virus particles and endosome membranes by embedding in membrane lipids. Umifenovir has been reported to be effective against COVID-19 in a concentration range of 10–30 μM in vitro [21]. At present, umifenovir has been widely used in Wuhan, other urban areas in Hubei, and for patients with COVID-19 elsewhere in China [22] with long-term treatment effects requiring further verification. Shufeng Jiedu Capsule (SFJDC) is a TCM drug that was approved by China’s State Food and Drug Administration in 2009. It belongs to a class of heat-clearing and detoxifying drugs with anti-infective and anti-inflammatory effects [23] according to the TCM treatment modality and theories which differ in paradigm from Western allopathic medicine. Shufeng Jiedu Capsules are mainly composed of eight components: Polygonum cuspidatum, Forsythia, Radix, Banlangen, Bupleurum, Verbena, Verbena, Reed Root, and Licorice, each of which produces its own therapeutic effect and has a synergistic effect. Modern research shows that the Forsythia suspensa in Shufeng Jiedu Capsules has the functions such as clearing away heat, detoxification, and alleviation of swelling. Banlangen has the effect of clearing away heat and of detoxification. Reed root can clear the lungs, calm the stomach, and alleviate thirst. Bupleurum plays the role of reconciliation; Polygonum cuspidatum has the effect of removing wind, dehumidification, and detoxification; the sapodilla has the functions of clearing away heat and detoxification, removing carbuncles and heat. Licorice has the effect of nourishing the stomach and has a regulating effect. Verbenaside can effectively regulate G protein-coupled receptor 18 (GPR18) protein, improve p21 protein-activated kinase 1 (PAK)-induced lung injury in mice, and has a significant clinical effect on the repair of viral lung injury. Shufeng Jiedu Capsules can produce good antibacterial and antiviral effects after a reasonable combination of drugs, to achieve the purpose of reducing fever, relieving pain, and eliminating cough [24–26].
This study provides a treatment plan of Shufeng Jiedu Capsules combined with Arbidol for inpatients diagnosed with common-type COVID-19 from a study population at Wuhan Hospital. The results show that pulmonary inflammation lesions of the experimental group were significantly resolved on the day 7 of treatment, with treatment effectiveness higher than that of the control group on day 14 of treatment (P < 0.05), suggesting that the combination of Arbidol and Shufeng Jiedu Capsules for the treatment of common-type COVID-19 has a degree of feasibility and effectiveness. On day 5 of treatment, leukocyte count and lymphocyte counts of the experimental group increased significantly (P < 0.05). On days 5, 10, and 14 of treatment, CRP and IL-6 were significantly reduced as compared with the control group (P < 0.05), and time to defervescence was significantly shorter than the control group (P < 0.05), suggesting that the combined drug regimen may reduce the expression of tumor necrosis factor-ɑ (TNF-ɑ), interleukin-lβ (IL-lβ), and other factors. This indicates alleviation of the inflammatory state and the reduction in the degree of tissue damage, thus leading to an improvement of signs and symptoms. According to the findings of this study, the advantages of Arbidol combined with Shufeng Jiedu Capsules in the treatment of common-type COVID-19 patients include improvement of immunity and reducing viral load in the lung tissue, maximizing anti-viral and anti-inflammatory effects, inhibiting the inflammatory response, and thus achieving the reduction of endotoxins and alleviating lung injury, and achieving effective and appropriate defervescence.
5. Conclusion
In conclusion, Shufeng Jiedu Capsules are cost-effective, have few side effects, and have good anti-inflammatory effects. In the absence of specific antiviral drugs, combination Arbidol and Shufeng Jiedu Capsules to treat common-type COVID-19 reduces the duration of symptoms and increases achievement of clinical effectiveness with no significant adverse reactions observed. However, this study also has certain limitations. Due to the limited sample size of this study and that it was a single-center study, there may be a certain selection bias, and the lack of an empty control group does not rule out the placebo effect. Patients with underlying diseases were excluded, and it is these patients who are more likely to develop severe or even critical illness because of their increased likelihood to be immunocompromised. If in the unfortunate situation where there is be a future outbreak in China, we will continue to conduct large-scale, prospective, multi-center randomized controlled clinical studies for further verification.
Acknowledgments
We thank all our colleagues who helped us during the current study. We are grateful to all the participants of this study. We also thank all the investigators for relentless efforts in collecting clinical and imaging data. We are also grateful to the many front-line medical staff for their dedication in the face of this outbreak, despite the potential threat to their own lives and the lives of their families.
Funding Statement
This study was supported by a grant from the Guiding project of the Natural Science Foundation of Fujian (2019D010), Fujian Young/Middle-aged Talent Cultivation Project (2019-ZQNB-31), and Xiamen Science and Technology Commission (3502Z20164029).
Article highlights
Shufeng Jiedu Capsules are cost-effective.
Shufeng Jiedu Capsules have few side effects and good anti-inflammatory effects.
Shufeng Jiedu Capsules combined with Arbidol in the treatment of common-type COVID-19 can effectively promote the improvement of patients’ clinical symptoms.
Shufeng Jiedu Capsule combined with Arbidol in the treatment of common-type COVID-19 is safe.
The combination of traditional Chinese and Western allopathic medicine is worth promoting in the clinical treatment of COVID-19.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
JC and QX conceived and designed the study. JC, QX, and SL contributed to the literature search. QX contributed to data collection. JC and SL contributed to data analysis. SL contributed to data interpretation. JC, QX, and SL contributed to the figures. JC, QX, and CN contributed to writing of the report. All authors have read and approved the final manuscript.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020. February;382(8):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Notification of COVID-19 infection. National Health Commission of the People’s Republic of China. [cited 2020 August28]. http://www.nhc.gov.cn/xcs/yqtb/202002/17a03704a99646ffad6807bc806f37a4.shtml (in Chinese). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Coronavirus disease 2019 (COVID-19) situation report-146[EB/OL]. [cited 2020 Aug 28]. https://www.who.int/docs/default-source/coronaviruse/situationreports/20200321-sitrep-61-covid-19.pdf?sfvrsn=f201f85c_2
- 4.World Health Organization . Director-general’s remarks at the media briefing on 2019-nCoV on 11 February 2020. [cited 2020. February 12]. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020
- 5.Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003. May;348(20):1953–1966. [DOI] [PubMed] [Google Scholar]
- 6.Zaki AM, van Boheemen S, Bestebroer TM, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012. November;367(19):1814–1820. [DOI] [PubMed] [Google Scholar]
- 7.https://www.biorxiv.org/content/10.1101/2020.02.07.937862v1 [cited 2020. February 12].
- 8.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends. 2020. March;14(1):69–71. [DOI] [PubMed] [Google Scholar]
- 9.Tang Decai XJ. Science of Chinese materia medica. Shanghai: Shanghai University of Traditional Chinese Medicine; 2006. [Google Scholar]
- 10.Pan MH, Chiou YS, Tsai ML, et al. Anti-inflammatory activity of traditional Chinese medicinal herbs. J Tradit Complement Med. 2011. October;1(1):8–24. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Article reviewing the anti-inflammatory constituents of traditional Chinese medicine (TCM) and their molecular targets.
- 11.Wang Q, Kuang H, Su Y, et al. Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J Ethnopharmacol. 2013. March 7;146(1):9–39. [DOI] [PubMed] [Google Scholar]; •• Article which highlights Chinese Material Medica (CMM) remains a promising source of new therapeutic agents based on their multi-active ingredient characteristics.
- 12.Wang Z, Chen X, Lu Y, et al. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020. March;14(1):64–68. [DOI] [PubMed] [Google Scholar]; • Article may provide clues for combining Chinese and Western medicine treatment ofCOVID-19.
- 13.Diagnosis and treatment of pneumonia caused by 2019-nCoV (version 6). [cited 2020. February 5]. http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440.shtml (in Chinese)
- 14.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020. February;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perlman S. Another decade, another coronavirus. N Engl J Med. 2020. February;382(8):760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020. February;395(10223):514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet. 2020. February;395(10223):470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020. March;382(10):929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim J, Jeon S, Shin HY, et al. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020. February;35(6):e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Ding Y, Yang C, et al. Inhibition of the infectivity and inflammatory response of influenza virus by arbidol hydrochloride in vitro and in vivo (mice and ferret). Biomed Pharmacother. 2017. July;91:393–401. [DOI] [PubMed] [Google Scholar]; • Article highlighting the activity of Arbidol hydrochloride in both suppressing virus propagation and modulating the expression of inflammatory cytokines in vitro and in vivo.
- 21.News. [cited 2020 February5]. http://www.sd.chinanews.com/2/2020/0205/70145.html (in Chinese)
- 22.Chinese clinical trial registry. [cited 2019 February5] http://www.chictr.org.cn/showproj.aspx?proj =49065
- 23.Tao Z, Yang Y, Shi W, et al. Complementary and alternative medicine is expected to make greater contribution in controlling the prevalence of influenza. Biosci Trends. 2013. October;7(5):253–256. [PubMed] [Google Scholar]
- 24.Ainsworth DM, Reyner CL. Effects of in vitro exposure to autologous blood and serum on expression of interleukin-8, interleukin-1β, and chemokine (C-X-C motif) ligand 2 in equine primary bronchial epithelial cell cultures. Am J Vet Res. 2012. February;73(2):296–301. [DOI] [PubMed] [Google Scholar]
- 25.Silva MA, Bercik P. Macrophages are related to goblet cell hyperplasia and induce MUC5B but not MUC5AC in human bronchus epithelial cells. Lab Invest. 2012. June;92(6):937–948. [DOI] [PubMed] [Google Scholar]
- 26.Yuan Y, Liao Q, Xue M, et al. Shufeng jiedu capsules alleviate lipopolysaccharide- induced acute lung inflammatory injury via activation of GPR18 by verbenalin. Cell Physiol Biochem. 2018;50(2):629–639. [DOI] [PubMed] [Google Scholar]; •• Article demonstrating that verbenalin was a significant anti-inflammatory compound, which may function through GPR18.


