Abstract
Aberrant growth of blood vessels (neovascularization) is a key feature of severe eye diseases that can cause legal blindness, including neovascular age-related macular degeneration (nAMD) and diabetic retinopathy (DR). The development of anti-vascular endothelial growth factor (VEGF) agents has revolutionized the treatment of ocular neovascularization. Novel proangiogenic targets, such as angiopoietin and platelet-derived growth factor (PDGF), are under development for patients who respond poorly to anti-VEGF therapy and to reduce adverse effects from long-term VEGF inhibition. A rapidly advancing area is gene therapy, which may provide significant therapeutic benefits. Viral vector-mediated transgene delivery provides the potential for continuous production of antiangiogenic proteins, which would avoid the need for repeated anti-VEGF injections. Gene silencing with RNA interference to target ocular angiogenesis has been investigated in clinical trials. Proof-of-concept gene therapy studies using gene-editing tools such as CRISPR-Cas have already been shown to be effective in suppressing neovascularization in animal models, highlighting the therapeutic potential of the system for treatment of aberrant ocular angiogenesis. This review provides updates on the development of anti-VEGF agents and novel antiangiogenic targets. We also summarize current gene therapy strategies already in clinical trials and those with the latest approaches utilizing CRISPR-Cas gene editing against aberrant ocular neovascularization.
Keywords: neovascularization, eye, gene therapy, VEGF, age-related macular degeneration, diabetic retinopathy
Graphical Abstract
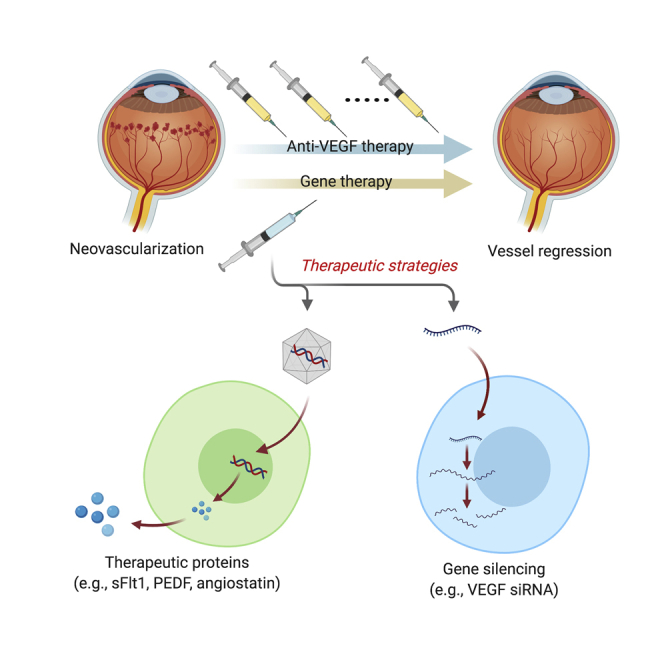
With the clinical success of ocular gene therapy, gene-based therapies might soon expand the therapeutic arsenal for neovascular eye diseases. Lin and colleagues review current gene therapy strategies already in clinical trials and those with the latest approaches utilizing CRISPR-Cas gene editing against aberrant ocular neovascularization.
Main Text
Angiogenesis is the formation of new blood vessels from preexisting vasculature, a process regulated by a dynamic balance between endogenous proangiogenic and antiangiogenic factors. Stimuli, such as biomechanical stress, hypoxia, ischemia, immune or inflammatory responses, and genetic variations, can disturb the balance in favor of angiogenesis.1 Ocular angiogenesis can occur in the retina, choroid, as well as cornea, and unchecked pathological blood vessel formation (neovascularization) can lead to severe visual impairment. These newly formed blood vessels are exudative, resulting in the accumulation of extracellular fluid subsequent to impairment of retinal function. Moreover, the aberrant growth of new vessels interferes with normal tissue structure and corneal transparency.2,3 Neovascularization is associated with a range of ocular disorders, including neovascular age-related macular degeneration (nAMD), diabetic retinopathy (DR), retinopathy of prematurity (ROP), corneal neovascularization, retinal vessel occlusion, and neovascular glaucoma (reviewed in Campochiaro4 and Usui et al.5). Among these neovascularization-related diseases, nAMD and DR are leading causes of visual impairment in the developed world.6
AMD occurs in patients ≥65 years of age. It is predicted that the number of individuals affected globally by AMD will reach 288 million by 2040.7 AMD can be classified as non-neovascular (dry form AMD [dAMD]) or neovascular forms (nAMD). nAMD, an advanced stage of AMD, is characterized by the pathologic growth of blood vessels from the choroid, beneath the macula, which is also termed choroidal neovascularization (CNV). The vessel outgrowth from the choroidal lacks normal vascular structure and function, thus resulting in fluid leakage and ultimately hemorrhagic or exudative retinal detachment. The growth of new vessels is often accompanied by fibrosis, which causes further damage to retina cells, particularly photoreceptors.8,9 Despite a variety of available treatments, nearly half of patients develop retinal pigment epithelium (RPE) atrophy or macular scarring within 2 years, a major cause of permanent vision loss.10 The most susceptible genetic loci relevant to AMD are CFH (complement factor H) and ARMS2 (age-related maculopathy susceptibility 2). Non-genetic risk factors for AMD include smoking and deficiency in dietary intake of antioxidants such as carotenoids and zinc.11,12 During the last two decades, therapeutic intervention for nAMD has shifted from laser photocoagulation to block leaky vessels to pharmacotherapeutic intervention, particularly anti-vascular endothelial growth factor (VEGF) therapy.13 Anti-VEGF therapy has also become a mainstay for the treatment of neovascularization and vascular hyperpermeability in DR.
DR is a leading cause of legal blindness in those of working age (20–65 years).14 According to the International Diabetes Federation (IDF), in 2017 there were an estimated 451 million people with diabetes mellitus (DM), a number projected to increase to 693 million by 2045.15 The prevalence of any form of retinal pathology (retinopathy) in DM patients is approximately 35%, and the number of people with DR is estimated to increase to 191 million in 2030.16,17 Around 7%–10% patients with DM will develop vision-threatening (proliferative) retinopathy, an advanced stage of DR.17,18 Diabetes-associated hyperglycemia damages the retinal vasculature vascular, including endothelial cells, basement membrane, and supporting pericytes.19 Non-proliferative DR (NPDR) is the initial stage of DR characterized by mild changes such as microaneurysms, microhemorrhages, hard exudates, and cotton wool spots, which can be detected clinically using ophthalmoscopy or fundus imaging. Diabetic macular edema (DME) is a frequent cause of vision loss, resulting from the breakdown of the outer blood-retinal barrier due to damaged endothelial tight junctions that allow salts, proteins, and water to accumulate within the retina.20,21 DME can occur at any stage of DR. Proliferative DR (PDR), or advanced DR, is characterized by neovascularization with or without pre-retinal or vitreal hemorrhages. Retinal neovascularization along with fibrosis can promote tractional retinal detachment, a severe complication of PDR that requires surgical interference. Retinal neovascularization can also occur on the iris and fluid drainage angle at the anterior eye, promoting the risk of neovascular glaucoma (reviewed in Rodríguez et al.22). For these reasons, those patients that progress to the PDR stage are at high risk of vision loss. Anti-VEGF therapies, along with laser photocoagulation, are currently the first-line treatment for DR.21,22
VEGF, Vascular Injury, and Current Anti-VEGF Agents
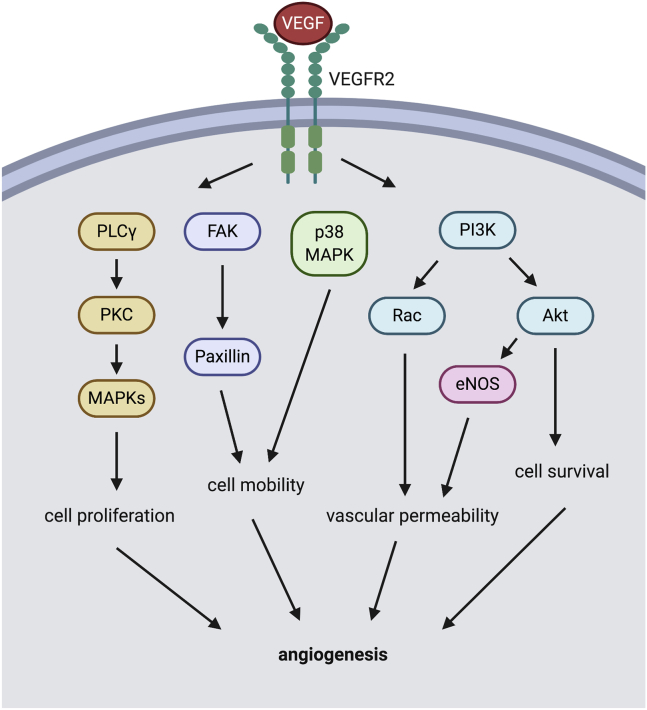
A vasoactive molecule that drives neovascularization common to corneal, retinal, and choroidal vascular beds is VEGF.23,24 VEGF is considered to be the most critical regulator of ocular angiogenesis, where it regulates cellular proliferation, survival, motility, and vascular permeability.25,26 VEGF is released either as soluble or extracellular matrix-bound forms to interact with VEGF receptor 2 (VEGFR2), which are found on vascular endothelial cells.27 Activation of VEGFR2 modulates a number of downstream signaling cascades, one of which is receptor tyrosine kinases. Engagement of VEGFR2 by VEGF activates phosphatidylinositol 3-kinase (PI3K), phospholipase C γ (PLCγ), focal adhesion kinase (FAK), and p38 mitogen-activated protein kinase (p38MAPK) (Figure 1). PI3K, in turn, activates AKT and Rac, resulting in inhibition of apoptotic signaling (promote survival) and decreased cellular adhesion (increase vascular permeability), respectively. PLCγ activates protein kinase C (PKC) and subsequently the MAPK cascade, which promotes cell proliferation and cytoskeletal reorganization (increase motility).26 Both FAK and p38MAPK are known as the key mediators of endothelial cell adhesion and migration (increase motility).28 The combined effects of increased VEGF contribute to several important steps in angiogenesis such as endothelial cell mitogenesis, migration, sprouting, and tube formation.29 Clinical studies have shown that abnormally high VEGF levels are found in several ocular diseases, including nAMD, DR, DME, ROP, and neovascular glaucoma.4 Intravitreal injection of anti-VEGF agents significantly improves visual function in patients with nAMD and DR (Table 1).8,30, 31, 32, 33, 34
Figure 1.
VEGF/VEGFR2 Signaling Pathway in Endothelial Cell Relevant to Angiogenesis
The VEGF-VEGFR2 system engages activation of PI3K, p38MAPK, FAK, and PLCγ. PI3K, in turn, activates AKT and Rac, resulting in cell survival and increased vascular permeability, respectively. PLCγ, in turn, activates PKC with the downstream MAPK cascade facilitating cell proliferation and cell motility. Both p38MAPK and FAK mediate cell mobility. The combined effects of these pathways on the vascular endothelium result in angiogenesis. VEGF, vascular endothelial growth factor; VEGFR2, VEGF receptor 2; PLCγ, phospholipase C gamma; PI3K, phosphatidylinositol 3-kinase; PKC, protein kinase C; MAPK, mitogen-activated protein kinase; FAK, focal adhesion kinase; eNOS, endothelial nitric oxide synthase.
Table 1.
Summary of Available Anti-VEGF Therapies
| Generic Name |
Pegaptanib |
Bevacizumab |
Ranibizumab |
Aflibercept |
Brolucizumab |
|---|---|---|---|---|---|
| Brand name | Macugen | Avastin | Lucentis | Eylea | Beovu |
| Targets | only one VEGF-A isoform (VEGF165) | all VEGF-A isoform | all VEGF-A isoform | all VEGF-A/VEGF-B/PlGF isoforms | all VEGF-A isoform |
| Format | aptamer | full monoclonal antibody | antibody fragment | VEGFR1/2 recombinant fusion protein | single-chain antibody fragment |
| Function | VEGF inhibitor | anti-VEGF antibody | anti-VEGF antibody | VEGF trap | anti-VEGF antibody |
| Molecular mass | 49 kDa | 149 kDa | 48 kDa | 115 kDa | 26 kDa |
| FDA-approved indications | nAMD | no FDA approval for ophthalmic use | nAMD; DR; DME; macular edema after RVO; mCNV | nAMD; DR; DME; macular edema after RVO | nAMD |
| Clinical dosage regimen | 0.3 mg every 6 weeks35 | 1.25 mg every 4 weeks36 | 0.5 mg every 4 weeks for nAMDa or 0.3 mg every 4 weeks for DR or DME37,36 | 2.0 mg every 4 weeks for first three injections, then every 8 weeks for nAMDb or 2.0 mg every 4 weeks for the first five injections, then every 8 weeks for DR or DME36 | 6.0 mg every 4 weeks for the first three injections, then every 8–12 weeks38 |
| Intraocular half-lives | 4 days39 | 6.7 days40 | 7.19 days41 | 11 days42 | 4.3 days43 |
| 4.9 days44 | 9 days45 | ||||
| Molecular structure | 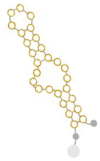 |
 |
 |
 |
 |
VEGF, vascular endothelial growth factor; PlGF, placental growth factor; nAMD, neovascular age-related macular degeneration; DR, diabetic retinopathy; DME, diabetic macular edema; mCNV, myopic choroidal neovascularization; RVO, retinal vein occlusion.
May give every 12 weeks after 3 or 4 monthly injections for nAMD, but is less effective than once monthly dosing.
May give every 12 weeks for selected patients after first year.
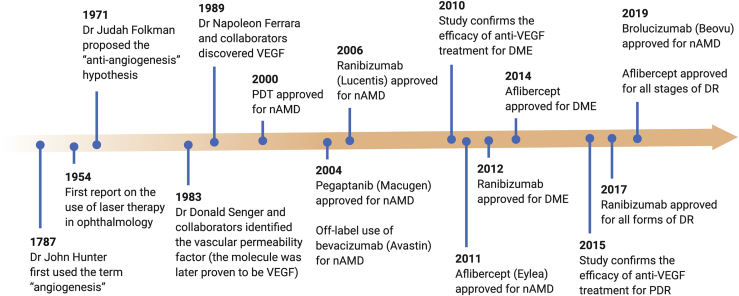
The VEGF aptamer pegaptanib (Macugen; Eyetech Pharmaceuticals/Pfizer) was the first antiangiogenic agent approved at a recommended dosage of 0.3 mg, administered by intravitreal injection every 6 weeks by the US Food and Drug Administration (FDA) for the treatment of nAMD.46,35 Pegaptanib specifically inhibits VEGF165, the dominant isoform of VEGF-A. This selectivity limits its efficacy compared with non-selective VEGF inhibitors (e.g., bevacizumab) for nAMD treatment.8,47 Due to its long-term safety profile, pegaptanib has been suggested for patients who are prone to the development of thromboembolic events (e.g., diabetes, cardiovascular disease) or geographic atrophy.48,49 Other anti-VEGF agents, including ranibizumab, aflibercept, and bevacizumab, are widely used to inhibit ocular neovascularization.50 Bevacizumab (Avastin) and ranibizumab (Lucentis) are immunoglobulin antibodies (monoclonal and fragment, respectively) both targeting VEGF-A. Ranibizumab is 5- to 30-fold more potent at neutralizing VEGF-A than is bevacizumab.51 Ranibizumab was shown to improve vision and was approved at a dose of 0.5 mg every 4 weeks for nAMD treatment in 2006,52,53 and 0.3 mg every 4 weeks for DR and DME in 2012.37 Bevacizumab was originally approved for treatment of metastatic colorectal cancer but has been used since 2005 as an off-label treatment for nAMD at a dose of 1.25 mg every 4 weeks.54 Because of its lower cost and greater availability, bevacizumab has become the first option for many patients.55,56 Aflibercept (Eylea) is a recombinant fusion protein that forms a VEGF trap, which targets VEGF-A, VEGF-B, and placental growth factor (PlGF).57 PlGF is another member of the VEGF family that activates VEGFR1.58 PlGF was reported to potentiate angiogenic signaling by forming heterodimers with VEGF-A, or by displacing VEGF-A from VEGFR1, and contributes to the development of neovascularization.59 Aflibercept is 100-fold higher than both bevacizumab and ranibizumab,60 and as such aflibercept provides comparable effectivity but requires less frequent injection compared with ranibizumab and bevacizumab. Aflibercept was approved for nAMD, DME, and DR treatment in 2011, 2014, and 2019, respectively,55,61 with a recommended dose of 2 mg for three to five initial monthly doses followed by doses every 8 weeks. Ziv-aflibercept (Zaltrap) contains the same aflibercept molecule but with higher osmolarity (1,000 mOsm/kg compared to 300 mOsm/kg for aflibercept), which helps to achieve iso-osmolarity for intravitreal injection. Ziv-aflibercept was approved in 2012 for treatment of metastatic colorectal carcinoma. Several cases have demonstrated that off-label, intravitreal ziv-aflibercept treatment is safe and effective in various chorioretinal vascular diseases.55 As its cost is comparable to bevacizumab (in US dollars, $50 for bevacizumab versus $2,000 for aflibercept), ziv-aflibercept is an attractive treatment option in low- to middle-income countries.62 Similar to aflibercept, conbercept is a recombinant fusion protein that targets VEGF-A, VEGF-B, VEGF-C, and also PlGF.63 In 2013, conbercept was approved for nAMD treatment and had been extensively used in China, and FDA-approved phase III clinical trials are currently well underway in the US (ClinicalTrials.gov: NCT03577899 and NCT03630952). Brolucizumab (Beovu), a humanized single-chain antibody fragment inhibitor of VEGF-A, is the most recent (October 2019) FDA-approved agent for nAMD at a recommended dosage of 6 mg.64 Its small molecular mass (26 kDa) allows for high solubility, extended duration of action, and improved ocular tissue penetration.55 Brolucizumab is the first drug to offer less frequent dosing (3-month dosing interval) in the first year of therapy while maintaining comparable effectiveness. Indeed, brolucizumab-treated patients showed greater fluid reduction, as evidenced by larger decreases in retinal thickness compared with aflibercept.38 A timeline of major advances in understanding ocular angiogenesis and the developments of antiangiogenesis therapy is shown in Figure 2 (reviewed in Yang et al.,65 Ferrara,66 and Shah and Gardner67).
Figure 2.
Graphical Timeline of Major Advances in Discovery of VEGF and the Developments of Anti-VEGF Therapy for Neovascular Eye Disease
For each drug, the time indicates the year of FDA approval (except bevacizumab). DR, diabetic retinopathy; DME, diabetic macular edema; nAMD, neovascular age-related macular degeneration; PDT, photodynamic therapy; VEGF, vascular endothelial growth factor.
Novel Ocular Angiogenesis-Related Targets in Clinical Trials
Although anti-VEGF therapy is now the mainstream therapy for ocular neovascularization, its application is not without challenges. First, some patients still show a progression of neovascular pathology despite aggressive anti-VEGF treatment.68 Second, systemic side effects from prolonged use of anti-VEGF associated with thromboembolic complications such as myocardial infarction, stroke, and non-ocular hemorrhage can still occur even though anti-VEGF therapy is locally administered into the eye.69 Finally, as VEGF plays an important role in maintaining healthy choroidal vasculature, corneal nerves, and retinal neurons,70, 71, 72 chronic suppression of VEGF may lead to inhibitory trophic effects. Given these challenges, novel molecular targets and delivery methods are being sought.73 Currently, new treatments targeting angiopoietins (Angs)/Tie-2 or platelet-derived growth factors (PDGFs) are being examined in a number of clinical trials for nAMD and DME.50,73,74 Other novel therapeutic targets are summarized in Table S1.
Angiopoietins/Tie-2
The Tie-2 tyrosine kinase receptor is primarily expressed in vascular endothelial cells and can be found in other cell types, including epithelial cells, smooth muscle cells, fibroblasts, glial cells, neutrophils, and monocytes. The activation of Tie-2 receptors in endothelial cells reinforces junctional proteins and stabilizes the vasculature to limit permeability.75,76 While VEGF promotes sprouting and tube formation from primitive vessels in the early stage of angiogenesis, the angiopoietins/Tie-2 system impacts the late stages of angiogenesis by recruiting mural cells (mainly pericytes), mediating interactions between endothelium cells and pericytes, to stabilize new vessels through the formation of endothelial tight junctions.77 Angiopoietins are ligands that bind to Tie-2, regulating vascular development, maintenance, and permeability. Angiopoietin-1 (Ang-1) has been shown to activate the Tie-2 receptor, thereby reducing vascular leakage and thus inhibiting CNV formation.78 Ang-1 is also shown to be upregulated by VEGF stimulation in RPE cells, indicating that VEGF may selectively regulate the expression of Ang-1 to induce the structural and functional maturation of new blood vessels in the late stage of CNV.79 Angiopoietin-2 (Ang-2) acts as a competitive antagonist to Ang-1, promoting destabilization of vessels and causing vascular leakage.80 Both Ang-2 and VEGF were found to be upregulated in highly vascular areas of surgically excised choroidal neovascular membranes81 and in vitreal fluids from patients with PDR.82 A study has shown that simultaneous VEGF and Ang-2 inhibition reduces vascular lesion size, permeability, retinal edema, and neuron loss in a spontaneous mouse CNV model.83 Faricimab is a bispecific antibody manufactured using CrossMab technology, which is designed to simultaneously neutralize both VEGF-A and Ang-2.84 Two phase II clinical trials (BOULEVARD, ClinicalTrials.gov: NCT02699450 and STAIRWAY, ClinicalTrials.gov: NCT03038880) show that faricimab is safe and effective in treating patients with nAMD and DME, providing evidence that simultaneous inhibition of VEGF and Ang-2 could be a viable therapeutic strategy against ocular angiogenesis.84,85
PDGF
Angiogenesis is associated with the recruitment of pericytes and smooth muscle cells to blood vessels and subsequent extracellular matrix production. Pericytes could directly contact endothelial cells and contribute to vessel maturation through the release of angiogenic growth factors, including VEGF.86,87 PDGF is critical for pericyte recruitment, maturation, and survival;88 thus, a combined therapy with anti-PDGF agents may overcome limitations of anti-VEGF monotherapy. Studies using a range of preclinical ocular neovascularization models have demonstrated that simultaneous inhibition of VEGF and PDGF, especially PDGF-B, results in an enhanced anti-angiogenic effect.89,90 However, clinical studies report that combining pegpleranib (Fovista) or rinucumab (a neutralizing monoclonal antibody to human PDGF-B) with anti-VEGF therapy showed no further benefits in treating nAMD.91 Results from two phase III clinical trials, OPH1002 (ClinicalTrials.gov: NCT01944839) and OPH1003 (ClinicalTrials.gov: NCT01940900), revealed that combined pegpleranib and ranibizumab therapy failed to improve vision at 12 months when compared with ranibizumab monotherapy.92,93 Further studies and subgroup analyses of these data may reveal specific groups of retinal or CNV patients who may benefit from anti-PDGF and anti-VEGF combined therapy.50,91
Drawback of Current Antiangiogenic Therapy
Although intravitreal injections of anti-VEGF agents are effective and safe,25 they do not cure the fundamental problem, and most patients will require repeated intravitreal injections to sustain therapeutic efficacy due to the relatively short half-life of these agents.94,95 Following intravitreal injections (0.5 mg), the half-life of ranibizumab is approximately 9 and 7 days in the vitreous humor and aqueous humor, respectively.41,45 Clinically, a meaningful improvement in visual acuity was only seen in patients receiving monthly ranibizumab injections, rather than those who had received injections every 3 months.96,97 The vitreous half-life of bevacizumab is between 4.9 and 6.7 days after the intravitreal administration (1.25 mg), as reported by two studies in human subjects.40,44 Aflibercept has a longer half-life of 11 days, suggesting that intravitreal aflibercept administration could have a longer dosing interval than either ranibizumab or bevacizumab.42,98 The need for injections every 1–2 months can be a cause of discomfort and anxiety, and this together with the need for repeated office visits with time and cost implications all impact patient compliance with treatment regimens.99 Additionally, frequent intravitreal injections can increase the risk of complications, including submacular hemorrhage, intraocular hypertension, endophthalmitis, and retinal detachment.100, 101, 102 Given this, researchers have sought to develop long-acting anti-VEGF modalities, sustained-release formulation, and device, and they have looked to gene therapies to address the abovementioned issues.73
Gene Therapy for Ocular Disease
Gene therapy designed to directly repair or compensate for defective genes has shown promise for a range of retinal disorders. Clinical success has been realized for Leber’s congenital amaurosis, with voretigene neparvovec-rzyl (Luxturna) becoming the first-ever FDA-approved gene therapy in 2017. The agent is directly administered into the eye to correct a deficiency caused by mutations in the RPE65 gene.103 There are currently more than 60 gene therapy trials ongoing for retinal diseases.104 Hereditary conditions currently being considered for gene therapy include achromatopsia, choroideremia, Leber’s congenital amaurosis, Leber’s hereditary optic neuropathy, retinitis pigmentosa, X-linked retinoschisis, Usher syndrome, and Stargardt’s disease. Gene therapy is also being considered for non-hereditary ocular diseases such as AMD (both dAMD and nAMD), DME, and glaucoma.
Advantage in Using Gene Therapy for Ocular Disease
The eye has advantages as a target organ for gene therapy, including high accessibility, a relative immune-privilege, and relative compartmentalization away from other organs.105 Ease of access and optical clarity facilitate direct visualization of the microsurgical delivery of genetic material to the retina.106,107 These characteristics mean that imaging and functional tools are readily available for quantification of the safety and efficacy of gene therapy. The blood-retinal barrier provides the retina with a relative immune-privilege, thereby blocking the trafficking of immune cells from the systemic circulation to the eye, which dampens inflammatory responses. This functional barrier also prevents the leakage of genetic material into the systemic circulation and thus localizes the expression of the therapeutic gene to the eye.108,109 Given that genetic material can be largely compartmentalized to the eye, smaller doses of genetic material can be delivered.110 Moreover, target cells in the retina such as photoreceptors and RPE do not divide, and as such gene therapy produces prolonged effects.111 Thus, gene therapy provides the possibility for targeted, localized, and sustained delivery of therapeutic genetic material into specific intraocular sites.112
Types of Gene Therapy for Ocular Disease
Gene therapy requires the introduction of exogenous genetic material, such as DNA, RNA, small interfering (siRNA), microRNA (miRNA), and antisense oligonucleotides (synthesized nucleic acid sequence complementary to mRNA), into cells via viral or non-viral vectors to regulate, replace, or modulate specific gene functions.113 Gene therapy approaches are generally categorized into gene augmentation, gene-specific targeting, or genome editing.104 Gene augmentation therapy refers to the introduction of correct copies of genes into the host genome to compensate for the faulty gene, as is the case with Luxturna. Mutations in the RPE65 gene impair the activity of a protein integral to photopigment recycling (visual cycle), leading to the death of photoreceptors and thereby impaired vision. Gene augmentation therapy through the addition of a normal copy of the RPE65 gene delivered by subretinal adeno-associated virus (AAV) vector injection is effective at improving vision.103 AAVs are low in toxicity (minimal pathogenicity, immunogenicity, and capacity for self-replication) and are high-yield single-stranded DNA viruses with a cloning capacity of ∼4.7 kb. AAVs are currently the most commonly used vector for retinal gene transfer in both preclinical studies and clinical trials.114 Although AAV has an excellent safety profile, mild and temporary inflammatory responses have been reported with higher ocular AAV vector doses.115, 116, 117 Recently, Timmers et al.117 studied the contribution of the AAV vector genome and capsid in triggering ocular inflammatory responses. They suggested that lowering the total capsid dose by removing empty AAV capsids could decrease inflammation and improve viral transduction.
Gene augmentation therapy is useful for inherited retinal degenerations where mutations produce loss of function and is a good approach for X-linked or autosomal recessive mutations.118 Gene-specific targeting represents the introduction of genetic materials into a target cell or tissue to specifically alter or turn off a gene responsible for the disease, or the addition of copies of genes for protective or regenerative purposes.104,119 Targeted therapies to modify pathological molecular pathways at the gene level are proving to be beneficial for long-term treatment of both non-genetic diseases and autosomal dominant genetic diseases.104 Genome editing, also known as genome surgery, seeks to excise or correct the faulty gene at a specific genomic location. This approach fundamentally repairs faulty genes rather than just adding normal copies of genes by gene augmentation therapy.120 Genome editing uses programmable site-specific endonucleases to target specific endogenous loci and thus corrects specific pathogenic alleles.121 Endonucleases used for genome editing include meganucleases, zinc finger nucleases, transcription activator-like effector nucleases (TALENs), targetrons, and, most recently, clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated (Cas) systems.122 The CRISPR-Cas system has several potential advantages over other editing tools such as simplicity of target design, ease of generating large-scale libraries, and relatively low cost.122,123 Moreover, genome editing with CRISPR-Cas9 enables multiplex editing through the engagement of multiple guide RNAs (gRNAs).124,125 In addition to gene knockout or transcriptional regulation, base editing of DNA or RNA at specific positions is feasible using the CRISPR-Cas system or Cas variants engineered with cytidine deaminases.126,127 Recently, an upgraded CRISPR-Cas-based technique called prime editing was developed, and it is able to introduce indels and enables all 12 base-to-base conversions (both transitions and transversions) without inducing a double-strand break (DSB).128 Moreover, the action of the CRISPR-Cas system is not restricted to DNA. Recently developed CRISPR-Cas-based RNA editing technology is capable of recognizing and cleaving RNA sequences without altering the sequence or integrity of genomic DNA.129 Compare to other existing RNA interrupting approaches, this system possessed higher efficiency and specificity.130 CRISPR-Cas-based gene therapies have shown promise in a number of animal models of inherited retinal degenerations and non-inherited ocular diseases such as AMD, highlighting their therapeutic potential for ocular diseases.131, 132, 133, 134
Current Gene Therapy Trial for Neovascular Eye Disease
Success in gene therapy has encouraged scientists and clinicians to use this approach for neovascular eye diseases such as nAMD and DR. Gene therapy is particularly attractive, as it has the potential to provide long-term efficacy, thereby eliminating the need for ongoing frequent intraocular injections. Current gene therapy clinical trials for nAMD or DME use two major strategies based on intraocular injection of viral vectors encoding antiangiogenic proteins or non-coding RNA interference molecules that target overexpression of VEGF (Tables 2 and 3). Several antiangiogenic proteins, such as endostatin, angiostatin, pigment epithelium-derived factor (PEDF), and secreted extracellular domain of VEGFR1, soluble fms-like tyrosine kinase-1 (sFLT-1), have been assessed for potential gene therapy. These studies have significantly contributed to our understanding of the safety profile, feasibility (protein expression levels), as well as the optimal evaluation methods for assessing the biological activity of ocular gene therapy.
Table 2.
Clinical Trials Investigating Human Gene Therapy for Ocular Angiogenesis Diseases Based on Viral Vectors Encoding Antiangiogenic Proteins
| Conditions | Identifier: ClinicalTrials.gov | Development Status | Drug | Vector | Mechanism | Route | Company | Study Start Date | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| nAMD | NCT03999801 | prospective, observational study: enrolling by invitation | RGX-314 | AAV8 | encoding for anti-VEGF monoclonal antibody fragment | subretinal | REGENXBIO | May 2019 | a long-term follow-up study for RGX-314; no study results posted. |
| nAMD | NCT03585556 | phase I: active, not recruiting | HMR59 | AAV2 | encoding for sCD59 | IVT | Hemera Biosciences | July 2018 | evaluated the efficacy and safety of HMR59 with anti-VEGF treatment in nAMD; no study results posted |
| nAMD | NCT03748784 | phase I: recruiting | ADVM-022 | AAV2.7m8 | encoding for aflibercept | IVT | Adverum Biotechnologies | November 2018 | 32-week data showed a maintenance of VA with an improvement of retina structural parameters; no rescue injections were needed to control AMD disease activity (S. Kiss, 2019, Amer. Acad. Ophthal., conference; S. Kiss, 2019, Eur. Soc. Gene Cell. Ther., conference) |
| nAMD | NCT03066258 | phase I/IIa: active, not recruiting | RGX-314 | AAV8 | encoding for anti-VEGF monoclonal antibody fragment | subretinal | REGENXBIO | March 2017 | improvement both in VA and retina structural parameters was noted; most patients remained rescue injection-free135 |
| nAMD | NCT01678872 | phase I: active, not recruiting | RetinoStat | lenti-EIAV | encoding for endostatin and angiostatin | subretinal | Oxford Biomedica | August 2012 | a follow-up study to evaluate the safety of RetinoStat; no study results posted |
| nAMD | NCT01494805 | phase I/II: completed | rAAV.sFlt-1 | rAAV | encoding for VEGF-neutralizing protein sFLT-1 | subretinal | Avalanche Biotechnologies | December 2011 | rAAV.sFLT-1 was safe and well tolerated; none of the exploratory endpoints (anti-VEGF retreatment injections, BCVA, and CPT) were statistically significant when rAAV.sFLT-1 was administered alone136,137 |
| nAMD | NCT01301443 | phase I: completed | RetinoStat | lenti-EIAV | encoding for endostatin and angiostatin | subretinal | Oxford Biomedica | February 2011 | EIAV vectors provide a safe platform with robust and sustained transgene expression; no significant change in mean lesion size138 |
| nAMD | NCT01024998 | phase I: completed | AAV2-sFLT01 | AAV2 | encoding for VEGF-neutralizing protein sFLT01 | IVT | Genzyme (Sanofi) | January 2010 | AAV2-sFLT01 was safe and well tolerated; further studies are required to identify sources of variability in expression and anti-permeability activity139 |
| nAMD | NCT00109499 | phase I: completed | AdGVPEDF.11D | adenovirus | encoding for PEDF | IVT | GenVec | April 2005 | no serious adverse events were related to AdGVPEDF.11; a possible dose-escalation response was reported140 |
nAMD, neovascular age-related macular degeneration; AAV, adeno-associated virus; LTFU, long-term follow-up; IVT, intravitreal injection; VA, visual acuity; PEDF, pigment epithelium-derived factor.
Table 3.
Clinical Trials Investigating Human Gene Therapy for Ocular Angiogenesis Diseases Based on Ocular Injection of Non-coding RNA Interference Molecules
| Conditions | Identifier: ClinicalTrials.gov | Development Status | Drug | Vector | Mechanism | Route | Company | Study Start Date | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| DME | NCT01445899 | phase II: completed | PF-04523655 | siRNA | siRNA targeting hypoxia-inducible gene RTP801 | IVT | Quark | February 2012 | evaluated the effect of PF-04523655 with/without ranibizumab in DME; no study results posted |
| nAMD | NCT00713518 | phase II: completed | PF-04523655 | siRNA | siRNA targeting hypoxia-inducible gene RTP801 | IVT | Quark/Pfizer | November 2009 | PF-04523655 monotherapy did not improve AMD as compared with ranibizumab monotherapy; combination of PF-04523655 with ranibizumab provided a synergic therapeutic benefit in visual acuity141 |
| nAMD | NCT00557791 | phase III: withdrawn | bevasiranib (Cand5) | siRNA | siRNA against VEGF | IVT | OPKO Health | November 2009 | study not initiated |
| DME | NCT00701181 | phase II: terminated | PF-04523655 | siRNA | siRNA targeting hypoxia-inducible gene RTP801 | IVT | Quark/Pfizer | June 2008 | a dose-dependent improvement in VA was observed in PF-04523655 therapy compared with laser treatment; the study was terminated due to an unexpectedly high discontinuation rate142 |
| nAMD | NCT00499590 | phase III: terminated | bevasiranib (Cand5) | siRNA | siRNA against VEGF | IVT | OPKO Health | August 2007 | bevasiranib-ranibizumab combination therapy showed a possible benefit for AMD, but the trial was unlikely to meet its primary endpoint and was terminated |
| nAMD | NCT00725686 | phase I: completed | PF-04523655 | siRNA | siRNA targeting hypoxia-inducible gene RTP801 | IVT | Quark/Pfizer | February 2007 | intravitreal injection with PF-04523655 appeared to be tolerable and safe143 |
| nAMD | NCT00395057 | phase II: terminated | AGN211745 (Sirna-027) | siRNA | siRNA targeting VEGFR-1 mRNA | IVT | Allergan | January 2007 | failed to meet therapeutic criteria |
| DME | NCT00306904 | phase II: completed | bevasiranib (Cand5) | siRNA | siRNA against VEGF | IVT | OPKO Health | January 2006 | an improved anatomic outcome and stabilization in VA were observed within 4 months |
| nAMD | NCT00259753 | phase II: completed | bevasiranib (Cand5) | siRNA | siRNA against VEGF | IVT | OPKO Health | July 2005 | bevasiranib-treated patients showed vision loss and increased lesion size, suggesting an insufficient efficacy with bevasiranib monotherapy |
| nAMD | NCT00363714 | phase I/II: completed | AGN211745 (Sirna-027) | siRNA | siRNA targeting VEGFR-1 mRNA | IVT | Allergan | November 2004 | AGN211745 was well tolerated in patients; stabilization or improvement in visual acuity and retinal structure was observed144 |
| nAMD | NCT00722384 | phase I: completed | bevasiranib (Cand5) | siRNA | siRNA against VEGF | IVT | OPKO Health | August 2004 | the safety data of five dosing groups was encouraging |
DME, diabetic macular edema; siRNA, small interfering RNA; IVT, intravitreal injection; nAMD, neovascular age-related macular degeneration; VA, visual acuity.
PEDF
GenVec’s phase I clinical trial (ClinicalTrials.gov: NCT00109499) assessed the safety of AdGVPEDF.11D in patients with advanced nAMD and was one of the earliest human applications of intraocular gene therapy. The replication-deficient adenoviral vector (E1, E3, and E4 deleted) was used to deliver the PEDF gene.140 Adenovirus is a double-stranded DNA virus with the highest loading capacity of up to 37 kb for transgene delivery.145,146 PEDF is a potent endogenous antiangiogenic protein, the levels of which are decreased in patients with nAMD.147,148 Participants received an intravitreal injection of AdGVPEDF.11 ranging in dose from 1E6 to 1E9 particle units (PU). The trial reported that 25% of patients experienced mild, transient intraocular inflammation, but there were no serious adverse events related to AdGVPEDF.11. Although therapeutic efficacy cannot be concluded from this phase I trial, patients receiving 1E8 PU or greater appeared to show stable or a reduction in CNV lesion size compared with those receiving doses lower than 1E8 PU.140
Anti-VEGF
Currently, most clinical trials of gene therapy for AMD predominantly focus on the application of viral vector-delivered FLT-1 (also known as VEGFR-1). The Flt-1 receptor gene is upregulated by hypoxia. As an endogenous inhibitor of VEGF-A, increased sFLT-1 protein neutralizes VEGF-A and prevents heterodimerization with its membrane-bound receptor VEGFR-2, thereby preventing VEGF-A-mediated angiogenesis.149, 150, 151 Several preclinical studies show that increasing levels of sFLT-1 via ocular injection (intravitreal or subretinal) of AAV.sFlt-1 inhibits laser-induced CNV in rodent and non-human primate models.152, 153, 154 These results led to the initiation of a phase I clinical trial (ClinicalTrials.gov: NCT01024998, Sanofi Genzyme) and a phase I/IIa clinical trial (ClinicalTrials.gov: NCT01494805, Avalanche Biotechnologies) in which AAV2-sFLT01 (encodes a fusion protein of the sFLT-01 domain 2 with the Fc domain of immunoglobulin [Ig]G1) and recombinant AAV (rAAV).sFLT-1 (encodes naturally occurring sFlt-1) were intravitreally and subretinally delivered in patients with nAMD, respectively.
Follow-up results released by Sanofi Genzyme in 2017 showed that the AAV2-sFLT01 vector was not detectable systematically and there was no immunogenicity to the vector. sFLT01 was detectable in aqueous humor in 5 out of 10 patients in the highest dose group (2E10 vector genomes [VG]) within 52 weeks. Expression was dose-related but was variable between participants. It was of interest that four of the five non-expressers of sFLT01 had detectable baseline anti-AAV2 antibodies titers of 1:400 or greater, which provides insight into patient-specific factors that can impact efficacy. While the therapy seemed to be safe and well tolerated at all doses, there was no significant improvement in retinal thickness (or central subfield thickness) and best-corrected visual acuity (BCVA).139
In parallel, a clinical trial sought to assess baseline safety and efficacy of rAAV.sFlt-1 in nAMD patients conducted by Avalanche Biotechnologies (ClinicalTrials.gov: NCT01494805). Similar to AAV2-sFLT01, a subretinal injection of the rAAV.sFlt-1 vector was used to deliver naturally occurring sFlt-1. In phase I and phase IIa clinical trials, 40 participants with nAMD were assigned to low-dose (1E10 VG), high-dose (1E11 VG), or control (no vector, ranibizumab only) groups. During the treatment period, rescue treatments with the traditional anti-VEGF drug ranibizumab were given to patients as needed based on vision loss quantified using Early Treatment Diabetic Retinopathy Study (ETDRS) letter scores, increases in intraretinal or subretinal fluid on optical coherence tomography (OCT), and increased leakage on fluorescein angiography. Endpoints including the number of intravitreal anti-VEGF retreatment injections, BCVA, and central point thickness (CPT) were followed during 36 months. The phase I/IIa clinical trial was completed in 2017 with 3-year follow-up results reported in 2019. The authors demonstrated that rAAV.sFLT-1 subretinal gene therapy was safe and tolerable (particularly among the elderly), highly reproducible, and may reduce anti-VEGF drug retreatments for nAMD; however, none of the exploratory endpoints (anti-VEGF retreatment injections, BCVA, and CPT) was statistically significant.136,155,156
Based on encouraging results from studies in the laser-induced CNV model in non-human primates,157 a phase I clinical trial (ClinicalTrials.gov: NCT03748784, Adverum Biotechnologies) assessed the safety and tolerability of ADVM-022 gene therapy for nAMD in 2018. In this study, an AAV2-derived vector, 7m8 (AAV.7m8-aflibercept), was intravitreally injected to produce long-term, robust expression of aflibercept. Other outcome measures included BCVA, anatomic outcomes assessed by OCT, and the needs for rescue aflibercept injections. A novel vector, AAV.7m8, was designed by directed evolution and optimized for intravitreal gene delivery to the outer retina. Eighteen patients with nAMD who were responsive to anti-VEGF treatment were enrolled in this phase I, dose-escalation trial, where three doses of ADVM-022 (2E11, 6E11, and 6E12 VG/eye) were evaluated. The latest data are available on the Adverum Biotechnologies corporate website. No serious adverse events and no drug-related systemic adverse events were noted, and mild inflammatory events responded well to topical steroid drops. The median 44-week data showed general maintenance of BCVA with an improvement in retinal thickness (measured using OCT) in 12 patients injected with 2E11 or 6E11 ADVM-022; most significantly, 10 of 12 (83%) patients did not need rescue injections that help to control AMD disease activity for about 11 months after a single injection of ADVM-022.158
Another encouraging anti-VEGF gene therapy approach is RGX-314 (REGENXBIO). A phase I/IIa clinical trial (ClinicalTrials.gov: NCT03066258, REGENXBIO) was started to evaluate the safety and tolerability of RGX-314 gene therapy in nAMD patients previously treated with VEGF injections in 2017. A novel NAV AAV8 vector was subretinally administered to deliver the gene encoding a monoclonal antibody fragment similar to ranibizumab. Forty-two patients were enrolled and divided into five groups with different viral doses (3E9, 1E10, 6E10, 1.6E11, and 2.5E11 gene copies [GC]). Rescue anti-VEGF treatment was given when patients showed increased, new, or persistent fluid, vision loss of five or more ETDRS letters, or occurrence of new ocular hemorrhages. Results released in October 2019 revealed that RGX-314 was well tolerated in all cohorts with no severe adverse effects. RGX-314 protein levels increased in aqueous humor in a dose-dependent manner across the five cohorts. In comparison to a mean sFLT01 protein level of 73.7 ng/mL at week 26 (peak concentration) after treatment with AAV2-sFLT01,139 RGX-314 protein concentration in cohort 3 (6E10 GC) reached 260.5 ng/mL at 1 year post-injection. The 1.5-year data from cohort 3 showed an improvement both in BCVA (+9 letters) and central retinal thickness (−40 μm thinner) compared with baseline. Approximately 6 months of data from cohorts 4 (1.6E11 GC) and 5 (2.5E11 GC) showed on average a stable improvement in BCVA and central retinal thickness, with 42% and 75% of patients remaining anti-VEGF rescue injection-free, respectively.135 A longer-term follow-up study for RGX-314 (ClinicalTrials.gov: NCT03999801, REGENXBIO) has recently started. Plans are in place to initiate a phase IIb clinical trial for nAMD and also an investigational new drug (IND) phase II clinical trial for DR.
Endogenous Inhibitor of Angiogenesis
Endostatin and angiostatin, cleavage products of collagen VII and plasminogen, respectively, are endogenous inhibitors of angiogenesis.159,160 A number of preclinical studies showed that ocular injection of rAAV-endostatin, rAAV-angiostatin, or lentiviral vector (Lenti)-angiostatin is able to efficiently suppress pathological corneal or retinal angiogenesis.161, 162, 163 Subretinal injection of an EIAV (VSV-G pseudotyped equine infectious anemia virus) lentiviral vector encoding angiostatin and/or endostatin significantly reduced lesion size in a mouse model of laser-induced CNV.164,165 These results led to a phase I clinical study (ClinicalTrials.gov: NCT01301443, Oxford Biomedica) to assess the dose-escalation safety of RetinoStat in patients with advanced AMD, with a long-term follow-up cohort (ClinicalTrials.gov: NCT01678872) initiated in 2012. In these studies, the non-replicating bicistronic EIAV vector encoding both endostatin and angiostatin was subretinally injected to encode both endostatin and angiostatin in 21 participants with advanced nAMD (three cohorts of 2.4E4, 2.4E5, and 8E5 transduction units [TU]). There were no detectable levels of endostatin at baseline in aqueous humor samples from participants. Long-term and stable expression of angiostatin and endostatin were noted for eight subjects after 2.5 years, and two subjects showed stable expression for more than 4 years. At completion, the results suggest that this EIAV lentivector-based gene therapy was safe and well tolerated and that it was clinically feasible to use a cytomegalovirus (CMV) promoter to express two secreted proteins. However, the data failed to show therapeutic benefit for advanced nAMD, although there was a reduction in fluorescein angiographic leakage.138
Targeting the Complement Cascade
Recent evidence implicates an overactivation of the complement cascade in the development of AMD. Activation of the complement cascade leading to membrane attack complex (MAC) accumulation on cell surfaces leads to cell damage and death, causing the clinical features seen in AMD (reviewed in Anderson et al.166). Accumulation of MAC can be found in RPE and choriocapillaris of AMD patients, suggesting a possible role for MAC in the pathogenesis of geographic atrophy or CNV (reviewed in Kumar-Singh167). CD59 is a naturally occurring membrane-bound inhibitor of MAC formation.168 CD59 targets the final step of activation of complement by preventing MAC formation in the cell membrane. In order to enable CD59 to block the formation of MAC at sites distal, a soluble non-membrane binding form of CD59 (sCD59)169,170 has been developed and used for targeted gene therapy. Two phase I clinical trials sponsored by Hemera Biosciences (ClinicalTrials.gov: NCT03144999 and NCT03585556) were initiated in 2017 and 2018 to investigate the efficacy and safety of AAVCAGsCD59 (HMR59) gene therapy for patients with dAMD and nAMD, respectively; however, the results have not been released.
RNA Interference in Ocular Angiogenesis
Synthetic siRNA is usually 19–22 bp long and can selectively complex with complementary mRNA through the RNA-induced silencing complex.171,172 Post-transcriptional gene silencing with siRNAs targeting VEGF and its receptor have demonstrated success in reducing ocular angiogenesis in experimental models of nAMD,173, 174, 175 DR,176 and corneal neovascularization.176,177 These developments have led to clinical trials for bevasiranib (formerly Cand5) (ClinicalTrials.gov: NCT00722384, OPKO Health) and AGN 211745 (formerly Sirna 027) (ClinicalTrials.gov: NCT00363714, Allergan).
Bevasiranib, a complex of two 21-nt RNA molecules that selectively silence mRNA encoding VEGF, was the first siRNA protocol granted IND status and tested in a clinical trial for treating nAMD via intravenous administration.178 The safety data for five dosing groups (0.1, 0.33, 1, 1.5, and 3 mg) in a phase I clinical trial (ClinicalTrials.gov: NCT00722384) was encouraging.179 However, in the phase II study (ClinicalTrials.gov: NCT00259753) comparing three doses (0.2, 1.5, or 3.0 mg) in patients with CNV secondary to AMD, efficacy could not be shown with bevasiranib monotherapy treatment. A phase II study (ClinicalTrials.gov: NCT00306904) evaluating bevasiranib’s safety and efficacy in patients with DME reported improved anatomic outcomes between weeks 8 and 12, with 91% of patients showing stabilization of BCVA through 6–12 weeks. The detailed results of these three clinical trials have not yet been published.180, 181, 182, 183 As bevasiranib may only inhibit new VEGF synthesis, without impacting existing VEGF levels, a phase III trial (ClinicalTrials.gov: NCT00499590) was performed to assess the efficacy of combining bevasiranib and ranibizumab therapy for nAMD treatment. Although bevasiranib-ranibizumab combination therapy showed a possible benefit in AMD treatment, the trial was unlikely to meet its primary endpoint and thus was terminated.
A subsequent phase III trial (ClinicalTrials.gov: NCT00557791) evaluating the efficacy of combination bevasiranib and ranibizumab treatment in nAMD was withdrawn prior to enrolment, following the discovery that angiogenesis suppression by siRNA agents such as bevasiranib could be acting through non-specific activation of Toll-like receptor (TLR)3 rather than the presumed sequence-dependent property of the siRNA.184,185 Specifically, Kleinman et al.184 demonstrated that suppression of CNV was only observed in Tlr3+/+ but not Tlr3−/− mice after intravitreal injection of anti-VEGF siRNA, suggesting the involvement of TLR3 in CNV. Subsequent studies also confirmed that regardless of their targeting sequences, 21-nt siRNAs can inhibit experimental CNV.186,187 Retinal toxicity remains a concern, as 21-nt siRNAs were found to induce apoptosis in mouse retinal pigmented epithelial cells through TLR3 and interferon regulatory factor 3.188
AGN211745 is a 21-nt RNA duplex designed to target FLT1 (VEGFR1) mRNA. Despite initial positive reports from a phase I trial (ClinicalTrials.gov: NCT00363714),144 the phase II trial (ClinicalTrials.gov: NCT00395057) for treatment of CNV associated with AMD was halted due to failure to meet therapeutic criteria and uncertainty around possible widespread involvement of TLR3. To avoid TLR3 activation and thus enhance therapeutic specificity,189 PF-04523655 (developed by Quark Pharmaceuticals, licensed to Pfizer) targeting the hypoxia-inducible RTP801 gene (also known as DDIT4) was designed as a 19-nt siRNA with 2′-O-methylation in every pair of oligonucleotides and was successfully tested clinically in CNV or DME with/without ranibizumab. The phase I trial (ClinicalTrials.gov: NCT00725686) reported that intravitreal injection with PF-04523655 (≤3,000 μg) was tolerable and safe in patients with nAMD.143 The phase II MONET study (ClinicalTrials.gov: NCT00713518) demonstrated that PF-04523655 monotherapy did not improve visual acuity as compared with ranibizumab monotherapy, but there was the suggestion of a synergetic therapeutic benefit for visual acuity when PF-04523655 was combined with ranibizumab.141 Concurrently, the phase II DEGAS study compared PF-04523655 with laser photocoagulation therapy in patients with DME (ClinicalTrials.gov: NCT00701181). Improvement in visual acuity was dose related and was more evident in PF-04523655 than in the laser therapy group; however, the study was terminated due to an unexpected high discontinuation rate.142 The latest MATISSE study (ClinicalTrials.gov: NCT01445899) assesses the effect of PF-04523655 with/without ranibizumab in DME, with detailed results not yet made public.
Although siRNA-based therapies showed some benefits for patients with nAMD and DME, this approach has no advantage over conventional anti-VEGF treatments since repeated injections are still needed to deliver the siRNA. Furthermore, the gene-silencing effect of siRNAs is transient, typically effective only for 3–7 days, as siRNAs are degraded by tissue nucleases. Nevertheless, prolonged effects could be achieved by chemical modifications or using viral vectors to sustain the effectiveness of RNA interference-based therapies.182 Short hairpin RNAs (shRNAs), structurally similar to cellular miRNAs, are processed to generate siRNAs.190 A number of studies have demonstrated that subretinal administration of viral vectors encoding VEGF-targeting shRNAs can reduce CNV lesions in VEGF-induced or laser-injured CNV mouse models.191,192 Recently, Kaadt et al.193 demonstrated a potentially safer and more efficient strategy for gene knockdown by Dicer-independent shRNAs expressed from miRNA scaffolds, which produce no passenger strand activity and remain active in Dicer-knockout cells. Given encouraging results from clinical studies of dual therapy using siRNA and ranibizumab,24,183,141 there has been interest in investigating the efficacy of combination therapy. Askou et al.194,195 have developed multigenic lentiviral vectors, enabling the cell- or tissue-specific simultaneously expression of anti-VEGF miRNAs and antiangiogenic factors. Later, the authors proved that dual-acting therapy using AAV encoded for anti-VEGF miRNAs and secreted PEDF protects against CNV in vivo.196 As multiple dysregulated pathways are involved in ocular angiogenesis and pathogenesis,24,183 an ideal drug candidate might combine antiangiogenic, anti-inflammatory, or neuroprotective genes. Given the feasibility of loading multiple transgenes into one vector, such a combined approach may be an important future advance.
Preclinical Studies of CRISPR-Cas Gene Editing to Treat Ocular Angiogenesis
Currently, more than 20 clinical trials are underway to test the therapeutic effects of CRISPR-Cas systems in diseases, including cancers and thalassemia, or to develop this approach as a diagnostic tool for tuberculosis and sepsis (reviewed in ClinicalTrials.gov). Of particular note in the field of genome editing for eye diseases, a phase I/II clinical trial has been approved by the FDA (ClinicalTrials.gov: NCT03872479, Allergan/Editas Medicine) to assess AGN-151587 (EDIT-101), a CRISPR-Cas-based genome editing approach for the treatment of Leber’s congenital amaurosis type 10 (the most common form of inherited childhood blindness).197 In addition to those inherited retinal diseases caused by specific gene mutations,132,198 numerous preclinical studies had investigated CRISPR-Cas applications for complex retinal diseases with multiple risk factors such as AMD (Table 4).
Table 4.
Preclinical Genome Surgery with CRISPR-Cas to Treat Ocular Angiogenesis
| Target Gene | Experiment |
Specific Nuclease | Delivery Method | Outcome | References | |
|---|---|---|---|---|---|---|
| In Vivo | In Vitro | |||||
| VEGF-A | human ARPE-19 cell line | SpCas9 | lentivirus (single vector) | CRISPR-Cas9 reduced VEGF-A secretion from human RPE cells and suppressed angiogenesis | 199 | |
| VEGFR2 | primary human retinal microvascular endothelial cell (HREC) | SpCas9 | rAAV5 (dual vector) | CRISPR-Cas9-mediated depletion of VEGFR2 prevented VEGF-induced Akt activation, as well as proliferation, migration, and tube formation in HRECs | 131 | |
| VEGFR2 | primary human retinal microvascular endothelial cell (HREC) | SpCas9 | lentivirus (single vector) | CRISPR-Cas9 decreased VEGF-induced Akt activation, as well as proliferation, tube formation, and migration in VEGFR2-depleted HRECs, and did so to a greater extent than ranibizumab and aflibercept | 200 | |
| VEGFR2 | oxygen-induced retinopathy and laser-induced CNV | C57BL/6 mouse primary brain microvascular endothelial cell (MVEC) | SpCas9 | rAAV1 (dual vector) | CRISPR-Cas9-edited MVECs significantly reduced VEGFR2 production in vitro; intravitreal administration of CRISPR-Cas9 targeting VEGFR2 reduced CNV area in both animal models | 201 |
| VEGF-A | non-disease mouse | SpCas9 | lentivirus (single vector) | in vivo subretinal administration of CRISPR-Cas9 disrupted VEGF-A gene specifically in mouse RPE | 202 | |
| VEGF-A and HIF-1α | mouse mode of laser-induced CNV | CjCas9 | AAV9 (single vector) | intravitreal injection of CRISPR-Cas9 targeting either VEGF-A or HIF-1α suppressed CNV, particular in the RPE; cone dysfunction was observed in the VEGF-A-edited but not in the HIF-1α-edited group; no detectable off-target indels were noted 6 weeks after AAV9-CjCas9 injection | 203 | |
| HIF-1α | mouse mode of laser-induced CNV | CjCas9 | AAV9 (single vector) | intravitreal injection of AAV9-CjCas9 targeting HIF-1α induced long-term gene disruption by constitutive expression of CjCas9 nuclease without detectable off-target indels 14 months after injection; the treatment did not affect retina function or histology | 204 | |
| VEGF-A and HIF-1α | mouse mode of laser-induced CNV | LbCpf1 | AAV2/9 (single vector) | intravitreal injection of AAV2/9-LbCpf1 targeting either VEGF-A or HIF-1α reduced CNV area and did not affect cone function; the antiangiogenic effects of CRISPR-LbCpf1 were comparable to the aflibercept group | 134 | |
| VEGF-A | mouse mode of laser-induced CNV | human ARPE-19 cell line | SpCas9 | Cas9 ribonucleoproteins (RNPs) | Cas9 RNP reduced the VEGFA mRNA and protein levels in ARPE-19 in vitro; subretinal injection of Cas9 RNPs induces indels specific in RPE cells, resulting in the reduction of VEGF-A protein and CNV; Cas9 protein was completely degraded 3 days post-injection, and no cone dysfunction was observed 7 days post-injection | 205 |
CRISPR, clustered regularly interspaced short palindromic repeats; Cas, CRISPR-associated; VEGF, vascular endothelial growth factor; rAAV, recombinant adeno-associated virus; CNV, choroidal neovascularization; NV, neovascularization; RPE, retinal pigment epithelium; HIF, hypoxia-induced factor.
CRISPR-SpCas9 System
Streptococcus pyogenes Cas9 (SpCas9) was first applied in human cells and has become one of the most commonly used Cas endonuclease variants.124 Huang et al.201 adapted CRISPR-Cas9-mediated disruption of VEGFR2 in both the mouse model of oxygen-induced retinopathy (OIR) mice and laser-induced CNV. The authors first tested the rAAV1-CRISPR-SpCas9 systems to target human VEGFR2 in C57BL/6 mouse primary brain microvascular endothelial cells (MVECs) using a dual AAV vector system. An 80% decrease in VEGFR2 production in CRISPR-SpCas9-edited MVECs was detected by western blot. Equal amounts of rAAV1-SpCas9 and rAAV1-single guide RNA (sgRNA) targeting VEGFR2 were intravitreally administered in OIR or laser-induced CNV mice. In CRISPR-edited OIR mice retina, there was approximately a 30% reduction in VEGFR2 production with significant inhibition of retinal neovascularization. Similarly, CRISPR-SpCas9-mediated disruption of VEGFR2 reduced CNV size compared with vehicle control laser-induced CNV mice. The authors speculate that rAAV1 preferentially transduces pathological vascular endothelial cells over healthy cells, as a way to account for the limited efficacy of in vivo VEGFR2 disruption compared with its effect in vitro. Holmgaard et al.202 examined in vivo knockout of the VEGF gene by lentiviral delivery of SpCas9 with sgRNA targeting exon 3 of murine VEGF-A. Five weeks after subretinal administration of the lentivirus in mouse eyes, robust expression of sgRNA with SpCas9 and successful indel formation in the VEGF-A gene were found specifically in the RPE cell layer.
Smaller CRISPR-Cas System
AAV-mediated delivery of CRISPR-Cas appears to be a safer, more efficient, and precise tool for retinal genome editing.206 However, the relatively small packaging capacity (<4.7 kb) of AAVs means that the most frequently used SpCas9 gene (∼4.10 kb)207 and sgRNA sequences cannot be packaged into the same AAV vector.208 As compared with AAV, lentiviral vectors have larger payload capacity (8–10 kb) but limited ability to transduce outer retina,209 whereas EIAV-based lentiviral vector was found to efficiently transduce both RPE and photoreceptors in the macaque.210 In addition, dual-vector AAV systems,201,211 AAV-split-Cas9 systems,212,213 smaller Cas9 orthologs (such as Staphylococcus aureus [SaCas9; 3.16 kb],214 Campylobacter jejuni [CjCas9; 2.95 kb],203 Neisseria meningitidis [NmCas9; 3.25 kb],203 and Streptococcus thermophilus [StCas9]215), or other class 2, type V nucleases such as Cpf1 (also known as Cas12a) from Acidaminococcus sp. (AsCpf1) or Lachnospiraceae bacterium (LbCpf1)134,216 have been developed. Despite successful gene knockout using dual-vector AAV systems, the need for co-transduction could be a drawback for their application.202 For example, the co-delivery of two AAV vectors has been reported to be less efficient than delivery by a single AAV vector in vivo.203,217 Alternatively, a split SpCas9 approach is less active compared with the delivery of intact SpCas9.212,218 Other Cas endonucleases, such as NmCas9 and StCas9, have received less attention, as their longer protospacer-adjacent motif (PAM) regions (5′-NNNNGATT-3′ and 5′-NNAGAAW-3′, respectively) limit sequences available for targeting.208
Kim et al.203 employed intravitreal injections of AAV9 vector encoding for CjCas9 and sgRNA targeting VEGF-A or HIF-1a genes in a laser-induced CNV mouse model. They show that partial knockdown of either VEGF-A or HIF-1α in the mouse retina, particularly in RPE cells, suppressed CNV by approximately 20% compared with the control group. Of note, cone dysfunction was observed in the VEGF-A-edited group but not in the HIF-1α-edited group. This might suggest that HIF-1α is a safer therapeutic target for CNV. The authors reported that there were no detectable off-target indels 6 weeks after AAV9-CjCas9 injection.203 Later, the authors reported that 14 months after gene editing with AAV9-CjCas9, HIF-1α indels reached frequencies of 79% ± 2%. Importantly, after 14 months, there were no indels at potential off-target sites, and the treatment did not affect retinal function or histology. These results indicated that a single intravitreal injection of AAV9-CjCas9 could induce long-term gene editing without significant genotoxic risk, supporting the contention that AAV-CRISPR-CjCas9-mediated gene therapy is long-lasting, effective, and safe in eyes.204 The same team also considered whether LbCpf1-based gene surgery could be used in the treatment of CNV. Following laser-induced CNV in the mouse retina, intravitreal injection of AAV2/9-expressing VEGF-A or HIF-1a-specific LbCpf1 reduced CNV area by 42% ± 4% and 34% ± 5%, respectively. The antiangiogenic effects of CRISPR-LbCpf1 were comparable to an aflibercept-injected group and more effective than the aforementioned CjCas9-based gene therapy.134,203 In addition, no deleterious effects on cones were observed either with VEGF-A- or HIF-1a-specific LbCpf1-edited groups, providing further support for CRISPR-LbCpf1 as a safer therapeutic approach. Cpf1 has the advantage of higher genome-wide specificity in human cells with minimum off-target potential compared with SpCas9, as well as the capacity for streamlined multiplex genome editing.219,220 These results support the potential application of LbCpf1-based gene editing for pathologic angiogenesis diseases in the eye.
CRISPR-Cas Ribonucleoprotein
Significant challenges for the field remain, namely viral vector-evoked host immune responses and constitutively expressed Cas9-related off-target events.208,221, 222, 223 Kim et al.205 thus tested a non-viral approach by delivering preassembled VEGF-A-specific Cas9 ribonucleoproteins (RNPs) in human RPE cells and a mouse model of CNV. The recombinant Cas9 protein complexed with sgRNA targeting VEGF-A was delivered via transfection using cationic lipids. The subretinal injection of RNPs induced indels specifically in RPE cells near the injection site, resulting in a significant reduction of VEGF-A protein levels and CNV area. The Cas9 protein was completely degraded 3 days after subretinal injection, and no cone dysfunction was observed 7 days post-injection. The rapid turnover of Cas9 RNP is less likely to elicit host immune responses and to produce off-target effects. Also, note that large amounts of recombinant Cas9 (8 μg) and sgRNA (4.5 μg) were used in the RNPs, but the authors had previously shown that RNP delivery was safer than plasmid delivery in human embryonic cells.224 These results highlight the possibilities of locally modifying VEGF-A genes using Cas9 RNPs.
Improving the Safety of the CRISPR-Cas System
It is clear that therapeutic applications of CRISPR-Cas in ocular angiogenesis are an active area of research, particularly to limit off-target effects, which is a significant concern to scientists and clinicians. Higher-fidelity CRISPR-Cas9 nucleases such as SpCas9-HF1, HypaCas9, and eSpCas9 have been developed to address these issues.225, 226, 227 Moreover, AAV-based CRISPR-Cas delivery system raises concern around the effect of persistent in vivo expression of the bacterially derived Cas enzyme. Systems including anti-CRISPR proteins, inducible promoters, and split-intein Cas9 are being developed to modulate Cas9 cleavage activity (not remove the Cas9 protein).212,228,229 Other approaches involving lentiviral vector-based KamiCas9 and lentiSLiCES, as well as a self-deleting AAV-CRISPR-Cas system, have been exploited to remove the Cas protein to circumvent problems related to long-term Cas expression.230, 231, 232
Conclusion
There has been a rapid expansion of efforts to explore the potential benefits of ocular gene therapy. Current clinical results suggest that viral vector-mediated gene augmentation holds the potential to provide long-lasting therapeutic benefits for patients with nAMD or DME. This stands to diminish the a need for repeated intraocular injections. Although post-transcriptional gene silencing with RNA interference has also shown some promise as an adjunct to anti-VEGF therapies, there is need for further study. CRISPR-Cas-based gene editing may be on the cusp of clinical application for ocular neovascularization. Further developments in vector design, genetic material modification, and delivery strategies are needed to improve the safety, specificity, and therapeutic efficacy of gene transfer. Reliable tools for assessment of in vivo genotoxicity risks such as insertional mutagenesis or off-target activity are also needed to monitor the biosafety of ocular gene therapy. Furthermore, the measurement of treatment outcomes should also be a key consideration to assess and monitor the efficacy and safety of gene therapy in clinical trials. With continued development in both preclinical and clinical settings, gene therapy may be a viable alternative approach for the treatment of ocular neovascularization.
Author Contributions
Conceptualization: F.-L.L. and G.-S.L. Writing – Original Draft: F.-L.L. and G.-S.L. Writing – Review & Editing: P.-Y.W., J.-H.W., B.V.B., Y.-F.C., and V.H.Y.W. Funding Acquisition: P.-Y.W. and G.-S.L.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council of Australia (GNT1185600), the Ophthalmic Research Institute of Australia, and the Shenzhen Key Laboratory of Biomimetic Materials and Cellular Immunomodulation (ZDSYS20190902093409851). The Centre for Eye Research Australia receives Operational Infrastructure Support from the Victorian Government.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2020.06.029.
Contributor Information
Peng-Yuan Wang, Email: py.wang@siat.ac.cn.
Guei-Sheung Liu, Email: rickliu0817@gmail.com.
Supplemental Information
References
- 1.Carmeliet P., Jain R.K. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 2.Sharif Z., Sharif W. Corneal neovascularization: updates on pathophysiology, investigations & management. Rom. J. Ophthalmol. 2019;63:15–22. [PMC free article] [PubMed] [Google Scholar]
- 3.Witmer A.N., Vrensen G.F., Van Noorden C.J., Schlingemann R.O. Vascular endothelial growth factors and angiogenesis in eye disease. Prog. Retin. Eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 4.Campochiaro P.A. Ocular neovascularization. J. Mol. Med. (Berl.) 2013;91:311–321. doi: 10.1007/s00109-013-0993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Usui Y., Westenskow P.D., Murinello S., Dorrell M.I., Scheppke L., Bucher F., Sakimoto S., Paris L.P., Aguilar E., Friedlander M. Angiogenesis and eye disease. Annu. Rev. Vis. Sci. 2015;1:155–184. doi: 10.1146/annurev-vision-082114-035439. [DOI] [PubMed] [Google Scholar]
- 6.Flaxman S.R., Bourne R.R.A., Resnikoff S., Ackland P., Braithwaite T., Cicinelli M.V., Das A., Jonas J.B., Keeffe J., Kempen J.H., Vision Loss Expert Group of the Global Burden of Disease Study Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob. Health. 2017;5:e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 7.Wong W.L., Su X., Li X., Cheung C.M., Klein R., Cheng C.Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob. Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 8.Solomon S.D., Lindsley K., Vedula S.S., Krzystolik M.G., Hawkins B.S. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst. Rev. 2019;3:CD005139. doi: 10.1002/14651858.CD005139.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green W.R., Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100:1519–1535. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- 10.Martin D.F., Maguire M.G., Fine S.L., Ying G.S., Jaffe G.J., Grunwald J.E., Toth C., Redford M., Ferris F.L., 3rd, Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–1398. doi: 10.1016/j.ophtha.2020.01.029. [DOI] [PubMed] [Google Scholar]
- 11.Vavvas D.G., Small K.W., Awh C.C., Zanke B.W., Tibshirani R.J., Kustra R. CFH and ARMS2 genetic risk determines progression to neovascular age-related macular degeneration after antioxidant and zinc supplementation. Proc. Natl. Acad. Sci. USA. 2018;115:E696–E704. doi: 10.1073/pnas.1718059115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awh C.C., Lane A.M., Hawken S., Zanke B., Kim I.K. CFH and ARMS2 genetic polymorphisms predict response to antioxidants and zinc in patients with age-related macular degeneration. Ophthalmology. 2013;120:2317–2323. doi: 10.1016/j.ophtha.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 13.Emerson M.V., Lauer A.K. Current and emerging therapies for the treatment of age-related macular degeneration. Clin. Ophthalmol. 2008;2:377–388. doi: 10.2147/opth.s1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simó-Servat O., Hernández C., Simó R. Diabetic retinopathy in the context of patients with diabetes. Ophthalmic Res. 2019;62:211–217. doi: 10.1159/000499541. [DOI] [PubMed] [Google Scholar]
- 15.Cho N.H., Shaw J.E., Karuranga S., Huang Y., da Rocha Fernandes J.D., Ohlrogge A.W., Malanda B. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y., He M., Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J. Ophthalmol. 2012;60:428–431. doi: 10.4103/0301-4738.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yau J.W., Rogers S.L., Kawasaki R., Lamoureux E.L., Kowalski J.W., Bek T., Chen S.J., Dekker J.M., Fletcher A., Grauslund J., Meta-Analysis for Eye Disease (META-EYE) Study Group Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maniadakis N., Konstantakopoulou E. Cost effectiveness of treatments for diabetic retinopathy: a systematic literature review. Pharmacoeconomics. 2019;37:995–1010. doi: 10.1007/s40273-019-00800-w. [DOI] [PubMed] [Google Scholar]
- 19.Abcouwer S.F., Gardner T.W. Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment. Ann. N Y Acad. Sci. 2014;1311:174–190. doi: 10.1111/nyas.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stitt A.W., Lois N., Medina R.J., Adamson P., Curtis T.M. Advances in our understanding of diabetic retinopathy. Clin. Sci. (Lond.) 2013;125:1–17. doi: 10.1042/CS20120588. [DOI] [PubMed] [Google Scholar]
- 21.Sacconi R., Giuffrè C., Corbelli E., Borrelli E., Querques G., Bandello F. Emerging therapies in the management of macular edema: a review. F1000Res. 2019;8:F1000. doi: 10.12688/f1000research.19198.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodríguez M.L., Pérez S., Mena-Mollá S., Desco M.C., Ortega A.L. Oxidative stress and microvascular alterations in diabetic retinopathy: future therapies. Oxid. Med. Cell. Longev. 2019;2019:4940825. doi: 10.1155/2019/4940825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kvanta A. Ocular angiogenesis: the role of growth factors. Acta Ophthalmol. Scand. 2006;84:282–288. doi: 10.1111/j.1600-0420.2006.00659.x. [DOI] [PubMed] [Google Scholar]
- 24.Rubio R.G., Adamis A.P. Ocular angiogenesis: vascular endothelial growth factor and other factors. Dev. Ophthalmol. 2016;55:28–37. doi: 10.1159/000431129. [DOI] [PubMed] [Google Scholar]
- 25.Campochiaro P.A., Aiello L.P., Rosenfeld P.J. Anti-vascular endothelial growth factor agents in the treatment of retinal disease: from bench to bedside. Ophthalmology. 2016;123(10S):S78–S88. doi: 10.1016/j.ophtha.2016.04.056. [DOI] [PubMed] [Google Scholar]
- 26.Cross M.J., Dixelius J., Matsumoto T., Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem. Sci. 2003;28:488–494. doi: 10.1016/S0968-0004(03)00193-2. [DOI] [PubMed] [Google Scholar]
- 27.Chen T.T., Luque A., Lee S., Anderson S.M., Segura T., Iruela-Arispe M.L. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J. Cell Biol. 2010;188:595–609. doi: 10.1083/jcb.200906044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshizuka N., Chen R.M., Xu Z., Liao R., Hong L., Hu W.Y., Yu G., Han J., Chen L., Sun P. A novel function of p38-regulated/activated kinase in endothelial cell migration and tumor angiogenesis. Mol. Cell. Biol. 2012;32:606–618. doi: 10.1128/MCB.06301-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ucuzian A.A., Gassman A.A., East A.T., Greisler H.P. Molecular mediators of angiogenesis. J. Burn Care Res. 2010;31:158–175. doi: 10.1097/BCR.0b013e3181c7ed82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solomon S.D., Chew E., Duh E.J., Sobrin L., Sun J.K., VanderBeek B.L., Wykoff C.C., Gardner T.W. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:412–418. doi: 10.2337/dc16-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spooner K., Fraser-Bell S., Hong T., Chang A.A. Five-year outcomes of retinal vein occlusion treated with vascular endothelial growth factor inhibitors. BMJ Open Ophthalmol. 2019;4:e000249. doi: 10.1136/bmjophth-2018-000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang H.G., Choi E.Y., Byeon S.H., Kim S.S., Koh H.J., Lee S.C., Kim M. Anti-vascular endothelial growth factor treatment of retinopathy of prematurity: efficacy, safety, and anatomical outcomes. Korean J. Ophthalmol. 2018;32:451–458. doi: 10.3341/kjo.2018.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petsoglou C., Balaggan K.S., Dart J.K., Bunce C., Xing W., Ali R.R., Tuft S.J. Subconjunctival bevacizumab induces regression of corneal neovascularisation: a pilot randomised placebo-controlled double-masked trial. Br. J. Ophthalmol. 2013;97:28–32. doi: 10.1136/bjophthalmol-2012-302137. [DOI] [PubMed] [Google Scholar]
- 34.Puliafito C.A., Wykoff C.C. Looking ahead in retinal disease management: highlights of the 2019 angiogenesis, exudation and degeneration symposium. Int. J. Retina Vitreous. 2019;5:22. doi: 10.1186/s40942-019-0174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosina C., Bottoni F., Staurenghi G. Clinical experience with pegaptanib sodium. Clin. Ophthalmol. 2008;2:485–488. doi: 10.2147/opth.s3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holekamp N.M. Review of neovascular age-related macular degeneration treatment options. Am. J. Manag. Care. 2019;25(10, Suppl):S172–S181. [PubMed] [Google Scholar]
- 37.Stewart M.W. A review of ranibizumab for the treatment of diabetic retinopathy. Ophthalmol. Ther. 2017;6:33–47. doi: 10.1007/s40123-017-0083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yannuzzi N.A., Freund K.B. Brolucizumab: evidence to date in the treatment of neovascular age-related macular degeneration. Clin. Ophthalmol. 2019;13:1323–1329. doi: 10.2147/OPTH.S184706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drolet D.W., Nelson J., Tucker C.E., Zack P.M., Nixon K., Bolin R., Judkins M.B., Farmer J.A., Wolf J.L., Gill S.C., Bendele R.A. Pharmacokinetics and safety of an anti-vascular endothelial growth factor aptamer (NX1838) following injection into the vitreous humor of rhesus monkeys. Pharm. Res. 2000;17:1503–1510. doi: 10.1023/a:1007657109012. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Q., Ziemssen F., Henke-Fahle S., Tatar O., Szurman P., Aisenbrey S., Schneiderhan-Marra N., Xu X., Grisanti S.;, Tübingen Bevacizumab Study Group Vitreous levels of bevacizumab and vascular endothelial growth factor-A in patients with choroidal neovascularization. Ophthalmology. 2008;115:1750–1755.e1. doi: 10.1016/j.ophtha.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 41.Krohne T.U., Liu Z., Holz F.G., Meyer C.H. Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. Am. J. Ophthalmol. 2012;154:682–686.e2. doi: 10.1016/j.ajo.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 42.Do D.V., Rhoades W., Nguyen Q.D. Pharmacokinetic study of intravitreal aflibercept in humans with neovascular age-related macular degeneration. Retina. 2020;40:643–647. doi: 10.1097/IAE.0000000000002566. [DOI] [PubMed] [Google Scholar]
- 43.Caruso A., Füth M., Alvarez-Sánchez R., Belli S., Diack C., Maass K.F., Schwab D., Kettenberger H., Mazer N.A. Ocular half-life of intravitreal biologics in humans and other species: meta-analysis and model-based prediction. Mol. Pharm. 2020;17:695–709. doi: 10.1021/acs.molpharmaceut.9b01191. [DOI] [PubMed] [Google Scholar]
- 44.Moisseiev E., Waisbourd M., Ben-Artsi E., Levinger E., Barak A., Daniels T., Csaky K., Loewenstein A., Barequet I.S. Pharmacokinetics of bevacizumab after topical and intravitreal administration in human eyes. Graefes Arch. Clin. Exp. Ophthalmol. 2014;252:331–337. doi: 10.1007/s00417-013-2495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu L., Lu T., Tuomi L., Jumbe N., Lu J., Eppler S., Kuebler P., Damico-Beyer L.A., Joshi A. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: a population approach. Invest. Ophthalmol. Vis. Sci. 2013;54:1616–1624. doi: 10.1167/iovs.12-10260. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt-Erfurth U., Pollreisz A., Mitsch C., Bolz M. Antivascular endothelial growth factors in age-related macular degeneration. Dev. Ophthalmol. 2010;46:21–38. doi: 10.1159/000320007. [DOI] [PubMed] [Google Scholar]
- 47.Ng E.W., Shima D.T., Calias P., Cunningham E.T., Jr., Guyer D.R., Adamis A.P. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug Discov. 2006;5:123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 48.Grunwald J.E., Pistilli M., Daniel E., Ying G.S., Pan W., Jaffe G.J., Toth C.A., Hagstrom S.A., Maguire M.G., Martin D.F., Comparison of Age-Related Macular Degeneration Treatments Trials Research Group Incidence and growth of geographic atrophy during 5 years of comparison of age-related macular degeneration treatments trials. Ophthalmology. 2017;124:97–104. doi: 10.1016/j.ophtha.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dombi T., Kwok K.K., Sultan M.B. A retrospective, pooled data analysis of the safety of pegaptanib sodium in the treatment of age-related macular degeneration in subjects with or without diabetes mellitus. BMC Ophthalmol. 2012;12:37. doi: 10.1186/1471-2415-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cabral T., Mello L.G.M., Lima L.H., Polido J., Regatieri C.V., Belfort R., Jr., Mahajan V.B. Retinal and choroidal angiogenesis: a review of new targets. Int. J. Retina Vitreous. 2017;3:31. doi: 10.1186/s40942-017-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y., Wiesmann C., Fuh G., Li B., Christinger H.W., McKay P., de Vos A.M., Lowman H.B. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J. Mol. Biol. 1999;293:865–881. doi: 10.1006/jmbi.1999.3192. [DOI] [PubMed] [Google Scholar]
- 52.Heier J.S., Antoszyk A.N., Pavan P.R., Leff S.R., Rosenfeld P.J., Ciulla T.A., Dreyer R.F., Gentile R.C., Sy J.P., Hantsbarger G., Shams N. Ranibizumab for treatment of neovascular age-related macular degeneration: a phase I/II multicenter, controlled, multidose study. Ophthalmology. 2006;113:633.e1-4. doi: 10.1016/j.ophtha.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 53.Frennesson C., Nilsson U.L., Peebo B.B., Nilsson S.E. Significant improvements in near vision, reading speed, central visual field and related quality of life after ranibizumab treatment of wet age-related macular degeneration. Acta Ophthalmol. 2010;88:420–425. doi: 10.1111/j.1755-3768.2009.01576.x. [DOI] [PubMed] [Google Scholar]
- 54.Stewart M.W. Off-label drug use: the bevacizumab story. Mayo Clin. Proc. 2013;88:305. doi: 10.1016/j.mayocp.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 55.Hussain R.M., Ciulla T.A. Emerging vascular endothelial growth factor antagonists to treat neovascular age-related macular degeneration. Expert Opin. Emerg. Drugs. 2017;22:235–246. doi: 10.1080/14728214.2017.1362390. [DOI] [PubMed] [Google Scholar]
- 56.Rosenfeld P.J., Moshfeghi A.A., Puliafito C.A. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg. Lasers Imaging. 2005;36:331–335. [PubMed] [Google Scholar]
- 57.Malik D., Tarek M., Caceres del Carpio J., Ramirez C., Boyer D., Kenney M.C., Kuppermann B.D. Safety profiles of anti-VEGF drugs: bevacizumab, ranibizumab, aflibercept and ziv-aflibercept on human retinal pigment epithelium cells in culture. Br. J. Ophthalmol. 2014;98(Suppl 1):i11–i16. doi: 10.1136/bjophthalmol-2014-305302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park J.E., Chen H.H., Winer J., Houck K.A., Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J. Biol. Chem. 1994;269:25646–25654. [PubMed] [Google Scholar]
- 59.Rakic J.M., Lambert V., Devy L., Luttun A., Carmeliet P., Claes C., Nguyen L., Foidart J.M., Noël A., Munaut C. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2003;44:3186–3193. doi: 10.1167/iovs.02-1092. [DOI] [PubMed] [Google Scholar]
- 60.Papadopoulos N., Martin J., Ruan Q., Rafique A., Rosconi M.P., Shi E., Pyles E.A., Yancopoulos G.D., Stahl N., Wiegand S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171–185. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heier J.S., Brown D.M., Chong V., Korobelnik J.F., Kaiser P.K., Nguyen Q.D., Kirchhof B., Ho A., Ogura Y., Yancopoulos G.D., VIEW 1 and VIEW 2 Study Groups Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Mansour A.M., Stewart M.W., Farah M.E., Mansour H.A., Chhablani J. Ziv-aflibercept: a cost-effective, off-label, highly potent antagonist of vascular endothelial growth factor. Acta Ophthalmol. 2019 doi: 10.1111/aos.14328. Published online December 20, 2019. [DOI] [PubMed] [Google Scholar]
- 63.de Oliveira Dias J.R., de Andrade G.C., Novais E.A., Farah M.E., Rodrigues E.B. Fusion proteins for treatment of retinal diseases: aflibercept, ziv-aflibercept, and conbercept. Int. J. Retina Vitreous. 2016;2:3. doi: 10.1186/s40942-016-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Markham A. Brolucizumab: first approval. Drugs. 2019;79:1997–2000. doi: 10.1007/s40265-019-01231-9. [DOI] [PubMed] [Google Scholar]
- 65.Yang W.H., Xu J., Mu J.B., Xie J. Revision of the concept of anti-angiogenesis and its applications in tumor treatment. Chronic Dis. Transl. Med. 2017;3:33–40. doi: 10.1016/j.cdtm.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 67.Shah A.R., Gardner T.W. Diabetic retinopathy: research to clinical practice. Clin. Diabetes Endocrinol. 2017;3:9. doi: 10.1186/s40842-017-0047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang S., Zhao J., Sun X. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: a comprehensive review. Drug Des. Devel. Ther. 2016;10:1857–1867. doi: 10.2147/DDDT.S97653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Costagliola C., Morescalchi F., Duse S., Romano D., Mazza G., Parmeggiani F., Bartollino S., Semeraro F. Systemic thromboembolic adverse events in patients treated with intravitreal anti-VEGF drugs for neovascular age-related macular degeneration: an update. Expert Opin. Drug Saf. 2019;18:803–815. doi: 10.1080/14740338.2019.1643838. [DOI] [PubMed] [Google Scholar]
- 70.Brar V.S., Sharma R.K., Murthy R.K., Chalam K.V. Bevacizumab neutralizes the protective effect of vascular endothelial growth factor on retinal ganglion cells. Mol. Vis. 2010;16:1848–1853. [PMC free article] [PubMed] [Google Scholar]
- 71.Kurihara T., Westenskow P.D., Bravo S., Aguilar E., Friedlander M. Targeted deletion of Vegfa in adult mice induces vision loss. J. Clin. Invest. 2012;122:4213–4217. doi: 10.1172/JCI65157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu C.Q., Zhang M., Matis K.I., Kim C., Rosenblatt M.I. Vascular endothelial growth factor mediates corneal nerve repair. Invest. Ophthalmol. Vis. Sci. 2008;49:3870–3878. doi: 10.1167/iovs.07-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Al-Khersan H., Hussain R.M., Ciulla T.A., Dugel P.U. Innovative therapies for neovascular age-related macular degeneration. Expert Opin. Pharmacother. 2019;20:1879–1891. doi: 10.1080/14656566.2019.1636031. [DOI] [PubMed] [Google Scholar]
- 74.Whitehead M., Wickremasinghe S., Osborne A., Van Wijngaarden P., Martin K.R. Diabetic retinopathy: a complex pathophysiology requiring novel therapeutic strategies. Expert Opin. Biol. Ther. 2018;18:1257–1270. doi: 10.1080/14712598.2018.1545836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Makinde T., Agrawal D.K. Intra and extravascular transmembrane signalling of angiopoietin-1-Tie2 receptor in health and disease. J. Cell. Mol. Med. 2008;12:810–828. doi: 10.1111/j.1582-4934.2008.00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sato T.N., Tozawa Y., Deutsch U., Wolburg-Buchholz K., Fujiwara Y., Gendron-Maguire M., Gridley T., Wolburg H., Risau W., Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 77.Augustin H.G., Koh G.Y., Thurston G., Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 78.Lee J., Park D.Y., Park D.Y., Park I., Chang W., Nakaoka Y., Komuro I., Yoo O.J., Koh G.Y. Angiopoietin-1 suppresses choroidal neovascularization and vascular leakage. Invest. Ophthalmol. Vis. Sci. 2014;55:2191–2199. doi: 10.1167/iovs.14-13897. [DOI] [PubMed] [Google Scholar]
- 79.Hangai M., Murata T., Miyawaki N., Spee C., Lim J.I., He S., Hinton D.R., Ryan S.J. Angiopoietin-1 upregulation by vascular endothelial growth factor in human retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 2001;42:1617–1625. [PubMed] [Google Scholar]
- 80.Maisonpierre P.C., Suri C., Jones P.F., Bartunkova S., Wiegand S.J., Radziejewski C., Compton D., McClain J., Aldrich T.H., Papadopoulos N. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 81.Otani A., Takagi H., Oh H., Koyama S., Matsumura M., Honda Y. Expressions of angiopoietins and Tie2 in human choroidal neovascular membranes. Invest. Ophthalmol. Vis. Sci. 1999;40:1912–1920. [PubMed] [Google Scholar]
- 82.Watanabe D., Suzuma K., Suzuma I., Ohashi H., Ojima T., Kurimoto M., Murakami T., Kimura T., Takagi H. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am. J. Ophthalmol. 2005;139:476–481. doi: 10.1016/j.ajo.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 83.Regula J.T., Lundh von Leithner P., Foxton R., Barathi V.A., Cheung C.M., Bo Tun S.B., Wey Y.S., Iwata D., Dostalek M., Moelleken J. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol. Med. 2016;8:1265–1288. doi: 10.15252/emmm.201505889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharma A., Kumar N., Kuppermann B.D., Bandello F., Loewenstein A. Faricimab: expanding horizon beyond VEGF. Eye (Lond) 2020;34:802–804. doi: 10.1038/s41433-019-0670-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sahni J., Patel S.S., Dugel P.U., Khanani A.M., Jhaveri C.D., Wykoff C.C., Hershberger V.S., Pauly-Evers M., Sadikhov S., Szczesny P. Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth factor-A with faricimab in diabetic macular edema: BOULEVARD phase 2 randomized trial. Ophthalmology. 2019;126:1155–1170. doi: 10.1016/j.ophtha.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 86.Armulik A., Abramsson A., Betsholtz C. Endothelial/pericyte interactions. Circ. Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 87.Eilken H.M., Diéguez-Hurtado R., Schmidt I., Nakayama M., Jeong H.W., Arf H., Adams S., Ferrara N., Adams R.H. Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1. Nat. Commun. 2017;8:1574. doi: 10.1038/s41467-017-01738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Andrae J., Gallini R., Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dong A., Seidel C., Snell D., Ekawardhani S., Ahlskog J.K., Baumann M., Shen J., Iwase T., Tian J., Stevens R. Antagonism of PDGF-BB suppresses subretinal neovascularization and enhances the effects of blocking VEGF-A. Angiogenesis. 2014;17:553–562. doi: 10.1007/s10456-013-9402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jo N., Mailhos C., Ju M., Cheung E., Bradley J., Nishijima K., Robinson G.S., Adamis A.P., Shima D.T. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am. J. Pathol. 2006;168:2036–2053. doi: 10.2353/ajpath.2006.050588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dunn E.N., Hariprasad S.M., Sheth V.S. An Overview of the Fovista and Rinucumab Trials and the Fate of Anti-PDGF Medications. Ophthalmic Surg. Lasers Imaging Retina. 2017;48:100–104. doi: 10.3928/23258160-20170130-02. [DOI] [PubMed] [Google Scholar]
- 92.Ophthotech Corporation A phase 3 safety and efficacy study of Fovista® (E10030) intravitreous administration in combination with Lucentis® compared to Lucentis® monotherapy [ NCT01944839] 2013. https://ClinicalTrials.gov/show/NCT01944839
- 93.Ophthotech Corporation A phase 3 safety and efficacy study of Fovista® (E10030) intravitreous administration in combination with Lucentis® compared to Lucentis® monotherapy [NCT09140900] 2013. https://ClinicalTrials.gov/show/NCT01940900
- 94.García-Quintanilla L., Luaces-Rodríguez A., Gil-Martínez M., Mondelo-García C., Maroñas O., Mangas-Sanjuan V., González-Barcia M., Zarra-Ferro I., Aguiar P., Otero-Espinar F.J., Fernández-Ferreiro A. Pharmacokinetics of Intravitreal anti-VEGF drugs in age-related macular degeneration. Pharmaceutics. 2019;11:365. doi: 10.3390/pharmaceutics11080365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yorston D. Anti-VEGF drugs in the prevention of blindness. Community Eye Health. 2014;27:44–46. [PMC free article] [PubMed] [Google Scholar]
- 96.Rosenfeld P.J., Brown D.M., Heier J.S., Boyer D.S., Kaiser P.K., Chung C.Y., Kim R.Y., MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 97.Singer M.A., Awh C.C., Sadda S., Freeman W.R., Antoszyk A.N., Wong P., Tuomi L. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119:1175–1183. doi: 10.1016/j.ophtha.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 98.Stewart M.W., Rosenfeld P.J. Predicted biological activity of intravitreal VEGF Trap. Br. J. Ophthalmol. 2008;92:667–668. doi: 10.1136/bjo.2007.134874. [DOI] [PubMed] [Google Scholar]
- 99.Smith A.G., Kaiser P.K. Emerging treatments for wet age-related macular degeneration. Expert Opin. Emerg. Drugs. 2014;19:157–164. doi: 10.1517/14728214.2014.884559. [DOI] [PubMed] [Google Scholar]
- 100.Jager R.D., Aiello L.P., Patel S.C., Cunningham E.T., Jr. Risks of intravitreous injection: a comprehensive review. Retina. 2004;24:676–698. doi: 10.1097/00006982-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 101.Krishnan R., Goverdhan S., Lochhead J. Submacular haemorrhage after intravitreal bevacizumab compared with intravitreal ranibizumab in large occult choroidal neovascularization. Clin. Exp. Ophthalmol. 2009;37:384–388. doi: 10.1111/j.1442-9071.2009.02043.x. [DOI] [PubMed] [Google Scholar]
- 102.Adelman R.A., Zheng Q., Mayer H.R. Persistent ocular hypertension following intravitreal bevacizumab and ranibizumab injections. J. Ocul. Pharmacol. Ther. 2010;26:105–110. doi: 10.1089/jop.2009.0076. [DOI] [PubMed] [Google Scholar]
- 103.Smalley E. First AAV gene therapy poised for landmark approval. Nat. Biotechnol. 2017;35:998–999. doi: 10.1038/nbt1117-998. [DOI] [PubMed] [Google Scholar]
- 104.Lee J.H., Wang J.H., Chen J., Li F., Edwards T.L., Hewitt A.W., Liu G.S. Gene therapy for visual loss: opportunities and concerns. Prog. Retin. Eye Res. 2019;68:31–53. doi: 10.1016/j.preteyeres.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 105.Boye S.E., Boye S.L., Lewin A.S., Hauswirth W.W. A comprehensive review of retinal gene therapy. Mol. Ther. 2013;21:509–519. doi: 10.1038/mt.2012.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boon C.J., Jeroen Klevering B., Keunen J.E., Hoyng C.B., Theelen T. Fundus autofluorescence imaging of retinal dystrophies. Vision Res. 2008;48:2569–2577. doi: 10.1016/j.visres.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 107.Zysk A.M., Nguyen F.T., Oldenburg A.L., Marks D.L., Boppart S.A. Optical coherence tomography: a review of clinical development from bench to bedside. J. Biomed. Opt. 2007;12:051403. doi: 10.1117/1.2793736. [DOI] [PubMed] [Google Scholar]
- 108.Kumaran N., Michaelides M., Smith A.J., Ali R.R., Bainbridge J.W.B. Retinal gene therapy. Br. Med. Bull. 2018;126:13–25. doi: 10.1093/bmb/ldy005. [DOI] [PubMed] [Google Scholar]
- 109.Caspi R.R. A look at autoimmunity and inflammation in the eye. J. Clin. Invest. 2010;120:3073–3083. doi: 10.1172/JCI42440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.DiCarlo J.E., Mahajan V.B., Tsang S.H. Gene therapy and genome surgery in the retina. J. Clin. Invest. 2018;128:2177–2188. doi: 10.1172/JCI120429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jolly J.K., Bridge H., MacLaren R.E. Outcome measures used in ocular gene therapy trials: a scoping review of current practice. Front. Pharmacol. 2019;10:1076. doi: 10.3389/fphar.2019.01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gupta K., Zhang J. Angiogenesis: a curse or cure? Postgrad. Med. J. 2005;81:236–242. doi: 10.1136/pgmj.2004.023309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Campbell J.P., McFarland T.J., Stout J.T. Ocular gene therapy. Dev. Ophthalmol. 2016;55:317–321. doi: 10.1159/000434698. [DOI] [PubMed] [Google Scholar]
- 114.Dismuke D.J., Tenenbaum L., Samulski R.J. Biosafety of recombinant adeno-associated virus vectors. Curr. Gene Ther. 2013;13:434–452. doi: 10.2174/15665232113136660007. [DOI] [PubMed] [Google Scholar]
- 115.Maclachlan T.K., Lukason M., Collins M., Munger R., Isenberger E., Rogers C., Malatos S., Dufresne E., Morris J., Calcedo R. Preclinical safety evaluation of AAV2-sFLT01—a gene therapy for age-related macular degeneration. Mol. Ther. 2011;19:326–334. doi: 10.1038/mt.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marangoni D., Wu Z., Wiley H.E., Zeiss C.J., Vijayasarathy C., Zeng Y., Hiriyanna S., Bush R.A., Wei L.L., Colosi P., Sieving P.A. Preclinical safety evaluation of a recombinant AAV8 vector for X-linked retinoschisis after intravitreal administration in rabbits. Hum. Gene Ther. Clin. Dev. 2014;25:202–211. doi: 10.1089/humc.2014.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Timmers A.M., Newmark J.A., Turunen H.T., Farivar T., Liu J., Song C., Ye G.J., Pennock S., Gaskin C., Knop D.R., Shearman M.S. Ocular inflammatory response to intravitreal injection of adeno-associated virus vector: relative contribution of genome and capsid. Hum. Gene Ther. 2020;31:80–89. doi: 10.1089/hum.2019.144. [DOI] [PubMed] [Google Scholar]
- 118.Lipinski D.M., Thake M., MacLaren R.E. Clinical applications of retinal gene therapy. Prog. Retin. Eye Res. 2013;32:22–47. doi: 10.1016/j.preteyeres.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 119.Goswami R., Subramanian G., Silayeva L., Newkirk I., Doctor D., Chawla K., Chattopadhyay S., Chandra D., Chilukuri N., Betapudi V. Gene therapy leaves a vicious cycle. Front. Oncol. 2019;9:297. doi: 10.3389/fonc.2019.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cox D.B., Platt R.J., Zhang F. Therapeutic genome editing: prospects and challenges. Nat. Med. 2015;21:121–131. doi: 10.1038/nm.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.DiCarlo J.E., Deeconda A., Tsang S.H. Viral vectors, engineered cells and the CRISPR revolution. Adv. Exp. Med. Biol. 2017;1016:3–27. doi: 10.1007/978-3-319-63904-8_1. [DOI] [PubMed] [Google Scholar]
- 122.Guha T.K., Wai A., Hausner G. Programmable genome editing tools and their regulation for efficient genome engineering. Comput. Struct. Biotechnol. J. 2017;15:146–160. doi: 10.1016/j.csbj.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sander J.D., Joung J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A., Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sakuma T., Nishikawa A., Kume S., Chayama K., Yamamoto T. Multiplex genome engineering in human cells using all-in-one CRISPR/Cas9 vector system. Sci. Rep. 2014;4:5400. doi: 10.1038/srep05400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rees H.A., Liu D.R. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018;19:770–788. doi: 10.1038/s41576-018-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marx V. Base editing a CRISPR way. Nat. Methods. 2018;15:767–770. doi: 10.1038/s41592-018-0146-4. [DOI] [PubMed] [Google Scholar]
- 128.Anzalone A.V., Randolph P.B., Davis J.R., Sousa A.A., Koblan L.W., Levy J.M., Chen P.J., Wilson C., Newby G.A., Raguram A., Liu D.R. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576:149–157. doi: 10.1038/s41586-019-1711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abudayyeh O.O., Gootenberg J.S., Essletzbichler P., Han S., Joung J., Belanto J.J., Verdine V., Cox D.B.T., Kellner M.J., Regev A. RNA targeting with CRISPR-Cas13. Nature. 2017;550:280–284. doi: 10.1038/nature24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Konermann S., Lotfy P., Brideau N.J., Oki J., Shokhirev M.N., Hsu P.D. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell. 2018;173:665–676.e14. doi: 10.1016/j.cell.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yu W., Mookherjee S., Chaitankar V., Hiriyanna S., Kim J.W., Brooks M., Ataeijannati Y., Sun X., Dong L., Li T. Nrl knockdown by AAV-delivered CRISPR/Cas9 prevents retinal degeneration in mice. Nat. Commun. 2017;8:14716. doi: 10.1038/ncomms14716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bakondi B., Lv W., Lu B., Jones M.K., Tsai Y., Kim K.J., Levy R., Akhtar A.A., Breunig J.J., Svendsen C.N., Wang S. In vivo CRISPR/Cas9 gene editing corrects retinal dystrophy in the S334ter-3 rat model of autosomal dominant retinitis pigmentosa. Mol. Ther. 2016;24:556–563. doi: 10.1038/mt.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu W., Duan Y., Ma G., Zhou G., Park-Windhol C., D’Amore P.A., Lei H. AAV-CRISPR/Cas9-mediated depletion of VEGFR2 blocks angiogenesis in vitro. Invest. Ophthalmol. Vis. Sci. 2017;58:6082–6090. doi: 10.1167/iovs.17-21902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koo T., Park S.W., Jo D.H., Kim D., Kim J.H., Cho H.Y., Kim J., Kim J.H., Kim J.S. CRISPR-LbCpf1 prevents choroidal neovascularization in a mouse model of age-related macular degeneration. Nat. Commun. 2018;9:1855. doi: 10.1038/s41467-018-04175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.REGENXBIO Inc. Key takeaways from the RGX-314 phase I/IIa clinical trial for wet AMD (cohorts 1–5) 2019. https://regenxbio.com/wp-content/uploads/2019/10/Key-Takeaways-From-The-RGX-314-Phase-I-IIa-Clinical-Trial-For-Wet-AMD-Cohorts-1-5.pdf
- 136.Rakoczy E.P., Lai C.M., Magno A.L., Wikstrom M.E., French M.A., Pierce C.M., Schwartz S.D., Blumenkranz M.S., Chalberg T.W., Degli-Esposti M.A., Constable I.J. Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: 1 year follow-up of a phase 1 randomised clinical trial. Lancet. 2015;386:2395–2403. doi: 10.1016/S0140-6736(15)00345-1. [DOI] [PubMed] [Google Scholar]
- 137.Constable I.J., Pierce C.M., Lai C.M., Magno A.L., Degli-Esposti M.A., French M.A., McAllister I.L., Butler S., Barone S.B., Schwartz S.D. Phase 2a randomized clinical trial: safety and post hoc analysis of subretinal rAAV.sFLT-1 for wet age-related macular degeneration. EBioMedicine. 2016;14:168–175. doi: 10.1016/j.ebiom.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Campochiaro P.A., Lauer A.K., Sohn E.H., Mir T.A., Naylor S., Anderton M.C., Kelleher M., Harrop R., Ellis S., Mitrophanous K.A. Lentiviral vector gene transfer of endostatin/angiostatin for macular degeneration (GEM) study. Hum. Gene Ther. 2017;28:99–111. doi: 10.1089/hum.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Heier J.S., Kherani S., Desai S., Dugel P., Kaushal S., Cheng S.H., Delacono C., Purvis A., Richards S., Le-Halpere A. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: a phase 1, open-label trial. Lancet. 2017;390:50–61. doi: 10.1016/S0140-6736(17)30979-0. [DOI] [PubMed] [Google Scholar]
- 140.Campochiaro P.A., Nguyen Q.D., Shah S.M., Klein M.L., Holz E., Frank R.N., Saperstein D.A., Gupta A., Stout J.T., Macko J. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Hum. Gene Ther. 2006;17:167–176. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- 141.Nguyen Q.D., Schachar R.A., Nduaka C.I., Sperling M., Klamerus K.J., Chi-Burris K., Yan E., Paggiarino D.A., Rosenblatt I., Aitchison R., Erlich S.S., MONET Clinical Study Group Evaluation of the siRNA PF-04523655 versus ranibizumab for the treatment of neovascular age-related macular degeneration (MONET Study) Ophthalmology. 2012;119:1867–1873. doi: 10.1016/j.ophtha.2012.03.043. [DOI] [PubMed] [Google Scholar]
- 142.Nguyen Q.D., Schachar R.A., Nduaka C.I., Sperling M., Basile A.S., Klamerus K.J., Chi-Burris K., Yan E., Paggiarino D.A., Rosenblatt I., DEGAS Clinical Study Group Dose-ranging evaluation of intravitreal siRNA PF-04523655 for diabetic macular edema (the DEGAS study) Invest. Ophthalmol. Vis. Sci. 2012;53:7666–7674. doi: 10.1167/iovs.12-9961. [DOI] [PubMed] [Google Scholar]
- 143.Nguyen Q.D., Schachar R.A., Nduaka C.I., Sperling M., Basile A.S., Klamerus K.J., Chi-Burris K., Yan E., Paggiarino D.A., Rosenblatt I., PF-04523655 Study Group Phase 1 dose-escalation study of a siRNA targeting the RTP801 gene in age-related macular degeneration patients. Eye (Lond.) 2012;26:1099–1105. doi: 10.1038/eye.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kaiser P.K., Symons R.C., Shah S.M., Quinlan E.J., Tabandeh H., Do D.V., Reisen G., Lockridge J.A., Short B., Guerciolini R., Nguyen Q.D., Sirna-027 Study Investigators RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. Am. J. Ophthalmol. 2010;150:33–39.e2. doi: 10.1016/j.ajo.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 145.Bennett J., Wilson J., Sun D., Forbes B., Maguire A. Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Invest. Ophthalmol. Vis. Sci. 1994;35:2535–2542. [PubMed] [Google Scholar]
- 146.Parks R.J., Chen L., Anton M., Sankar U., Rudnicki M.A., Graham F.L. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dawson D.W., Volpert O.V., Gillis P., Crawford S.E., Xu H., Benedict W., Bouck N.P. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 148.Holekamp N.M., Bouck N., Volpert O. Pigment epithelium-derived factor is deficient in the vitreous of patients with choroidal neovascularization due to age-related macular degeneration. Am. J. Ophthalmol. 2002;134:220–227. doi: 10.1016/s0002-9394(02)01549-0. [DOI] [PubMed] [Google Scholar]
- 149.Gerber H.P., Condorelli F., Park J., Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J. Biol. Chem. 1997;272:23659–23667. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- 150.Kendall R.L., Thomas K.A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kendall R.L., Wang G., Thomas K.A. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem. Biophys. Res. Commun. 1996;226:324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- 152.Lai C.M., Shen W.Y., Brankov M., Lai Y.K., Barnett N.L., Lee S.Y., Yeo I.Y., Mathur R., Ho J.E., Pineda P. Long-term evaluation of AAV-mediated sFlt-1 gene therapy for ocular neovascularization in mice and monkeys. Mol. Ther. 2005;12:659–668. doi: 10.1016/j.ymthe.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 153.Lukason M., DuFresne E., Rubin H., Pechan P., Li Q., Kim I., Kiss S., Flaxel C., Collins M., Miller J. Inhibition of choroidal neovascularization in a nonhuman primate model by intravitreal administration of an AAV2 vector expressing a novel anti-VEGF molecule. Mol. Ther. 2011;19:260–265. doi: 10.1038/mt.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lai C.M., Estcourt M.J., Himbeck R.P., Lee S.Y., Yew-San Yeo I., Luu C., Loh B.K., Lee M.W., Barathi A., Villano J. Preclinical safety evaluation of subretinal AAV2.sFlt-1 in non-human primates. Gene Ther. 2012;19:999–1009. doi: 10.1038/gt.2011.169. [DOI] [PubMed] [Google Scholar]
- 155.Rakoczy E.P., Magno A.L., Lai C.M., Pierce C.M., Degli-Esposti M.A., Blumenkranz M.S., Constable I.J. Three-year follow-up of phase 1 and 2a rAAV.sFLT-1 subretinal gene therapy trials for exudative age-related macular degeneration. Am. J. Ophthalmol. 2019;204:113–123. doi: 10.1016/j.ajo.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 156.Constable I.J., Lai C.M., Magno A.L., French M.A., Barone S.B., Schwartz S.D., Blumenkranz M.S., Degli-Esposti M.A., Rakoczy E.P. Gene therapy in neovascular age-related macular degeneration: three-year follow-up of a phase 1 randomized dose escalation trial. Am. J. Ophthalmol. 2017;177:150–158. doi: 10.1016/j.ajo.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 157.Grishanin R., Vuillemenot B., Sharma P., Keravala A., Greengard J., Gelfman C., Blumenkrantz M., Lawrence M., Hu W., Kiss S., Gasmi M. Preclinical evaluation of ADVM-022, a novel gene therapy approach to treating wet age-related macular degeneration. Mol. Ther. 2019;27:118–129. doi: 10.1016/j.ymthe.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Adverum Biotechnologies Transforming gene therapy. 2020. http://investors.adverum.com/static-files/c8256955-641c-45a3-bdda-5b99c2336a14
- 159.O’Reilly M.S., Boehm T., Shing Y., Fukai N., Vasios G., Lane W.S., Flynn E., Birkhead J.R., Olsen B.R., Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 160.O’Reilly M.S., Holmgren L., Shing Y., Chen C., Rosenthal R.A., Moses M., Lane W.S., Cao Y., Sage E.H., Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 161.Cheng H.C., Yeh S.I., Tsao Y.P., Kuo P.C. Subconjunctival injection of recombinant AAV-angiostatin ameliorates alkali burn induced corneal angiogenesis. Mol. Vis. 2007;13:2344–2352. [PubMed] [Google Scholar]
- 162.Igarashi T., Miyake K., Kato K., Watanabe A., Ishizaki M., Ohara K., Shimada T. Lentivirus-mediated expression of angiostatin efficiently inhibits neovascularization in a murine proliferative retinopathy model. Gene Ther. 2003;10:219–226. doi: 10.1038/sj.gt.3301878. [DOI] [PubMed] [Google Scholar]
- 163.Lai L.J., Xiao X., Wu J.H. Inhibition of corneal neovascularization with endostatin delivered by adeno-associated viral (AAV) vector in a mouse corneal injury model. J. Biomed. Sci. 2007;14:313–322. doi: 10.1007/s11373-007-9153-7. [DOI] [PubMed] [Google Scholar]
- 164.Kachi S., Binley K., Yokoi K., Umeda N., Akiyama H., Muramatu D., Iqball S., Kan O., Naylor S., Campochiaro P.A. Equine infectious anemia viral vector-mediated codelivery of endostatin and angiostatin driven by retinal pigmented epithelium-specific VMD2 promoter inhibits choroidal neovascularization. Hum. Gene Ther. 2009;20:31–39. doi: 10.1089/hum.2008.046. [DOI] [PubMed] [Google Scholar]
- 165.Balaggan K.S., Binley K., Esapa M., MacLaren R.E., Iqball S., Duran Y., Pearson R.A., Kan O., Barker S.E., Smith A.J. EIAV vector-mediated delivery of endostatin or angiostatin inhibits angiogenesis and vascular hyperpermeability in experimental CNV. Gene Ther. 2006;13:1153–1165. doi: 10.1038/sj.gt.3302769. [DOI] [PubMed] [Google Scholar]
- 166.Anderson D.H., Radeke M.J., Gallo N.B., Chapin E.A., Johnson P.T., Curletti C.R., Hancox L.S., Hu J., Ebright J.N., Malek G. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog. Retin. Eye Res. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kumar-Singh R. The role of complement membrane attack complex in dry and wet AMD—from hypothesis to clinical trials. Exp. Eye Res. 2019;184:266–277. doi: 10.1016/j.exer.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 168.Rollins S.A., Sims P.J. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J. Immunol. 1990;144:3478–3483. [PubMed] [Google Scholar]
- 169.Sugita Y., Ito K., Shiozuka K., Suzuki H., Gushima H., Tomita M., Masuho Y. Recombinant soluble CD59 inhibits reactive haemolysis with complement. Immunology. 1994;82:34–41. [PMC free article] [PubMed] [Google Scholar]
- 170.Fraser D.A., Harris C.L., Williams A.S., Mizuno M., Gallagher S., Smith R.A., Morgan B.P. Generation of a recombinant, membrane-targeted form of the complement regulator CD59: activity in vitro and in vivo. J. Biol. Chem. 2003;278:48921–48927. doi: 10.1074/jbc.M302598200. [DOI] [PubMed] [Google Scholar]
- 171.McManus M.T., Sharp P.A. Gene silencing in mammals by small interfering RNAs. Nat. Rev. Genet. 2002;3:737–747. doi: 10.1038/nrg908. [DOI] [PubMed] [Google Scholar]
- 172.Kubo T., Yanagihara K., Takei Y., Mihara K., Sato Y., Seyama T. siRNAs conjugated with aromatic compounds induce RISC-mediated antisense strand selection and strong gene-silencing activity. Biochem. Biophys. Res. Commun. 2012;426:571–577. doi: 10.1016/j.bbrc.2012.08.128. [DOI] [PubMed] [Google Scholar]
- 173.Shen J., Samul R., Silva R.L., Akiyama H., Liu H., Saishin Y., Hackett S.F., Zinnen S., Kossen K., Fosnaugh K. Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther. 2006;13:225–234. doi: 10.1038/sj.gt.3302641. [DOI] [PubMed] [Google Scholar]
- 174.Reich S.J., Fosnot J., Kuroki A., Tang W., Yang X., Maguire A.M., Bennett J., Tolentino M.J. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol. Vis. 2003;9:210–216. [PubMed] [Google Scholar]
- 175.Tolentino M.J., Brucker A.J., Fosnot J., Ying G.S., Wu I.H., Malik G., Wan S., Reich S.J. Intravitreal injection of vascular endothelial growth factor small interfering RNA inhibits growth and leakage in a nonhuman primate, laser-induced model of choroidal neovascularization. Retina. 2004;24:660. doi: 10.1097/00006982-200408000-00039. [DOI] [PubMed] [Google Scholar]
- 176.Zuo L., Fan Y., Wang F., Gu Q., Xu X. A siRNA targeting vascular endothelial growth factor-A inhibiting experimental corneal neovascularization. Curr. Eye Res. 2010;35:375–384. doi: 10.3109/02713681003597230. [DOI] [PubMed] [Google Scholar]
- 177.Singh N., Higgins E., Amin S., Jani P., Richter E., Patel A., Kaur R., Wang J., Ambati J., Dong Z., Ambati B.K. Unique homologous siRNA blocks hypoxia-induced VEGF upregulation in human corneal cells and inhibits and regresses murine corneal neovascularization. Cornea. 2007;26:65–72. doi: 10.1097/ICO.0b013e31802b4201. [DOI] [PubMed] [Google Scholar]
- 178.Castanotto D., Rossi J.J. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457:426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Garba A.O., Mousa S.A. Bevasiranib for the treatment of wet, age-related macular degeneration. Ophthalmol. Eye Dis. 2010;2:75–83. doi: 10.4137/OED.S4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Iacono P., Battaglia Parodi M., Bandello F. Antivascular endothelial growth factor in diabetic retinopathy. Dev. Ophthalmol. 2010;46:39–53. doi: 10.1159/000320008. [DOI] [PubMed] [Google Scholar]
- 181.Silva P.S., Haddad Z.A., Sun J.K., Cavallerano J.D., Aiello L.P. Anti-VEGF therapies in diabetic macular edema. In: Das A., Friedberg T., editors. Therapy for Ocular Angiogenesis: Principles and Practice. Lippincott Williams & Wilkins; 2011. pp. 154–169. [Google Scholar]
- 182.Guzman-Aranguez A., Loma P., Pintor J. Small-interfering RNAs (siRNAs) as a promising tool for ocular therapy. Br. J. Pharmacol. 2013;170:730–747. doi: 10.1111/bph.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Corydon T.J. Antiangiogenic eye gene therapy. Hum. Gene Ther. 2015;26:525–537. doi: 10.1089/hum.2015.064. [DOI] [PubMed] [Google Scholar]
- 184.Kleinman M.E., Yamada K., Takeda A., Chandrasekaran V., Nozaki M., Baffi J.Z., Albuquerque R.J., Yamasaki S., Itaya M., Pan Y. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cho W.G., Albuquerque R.J., Kleinman M.E., Tarallo V., Greco A., Nozaki M., Green M.G., Baffi J.Z., Ambati B.K., De Falco M. Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth. Proc. Natl. Acad. Sci. USA. 2009;106:7137–7142. doi: 10.1073/pnas.0812317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ashikari M., Tokoro M., Itaya M., Nozaki M., Ogura Y. Suppression of laser-induced choroidal neovascularization by nontargeted siRNA. Invest. Ophthalmol. Vis. Sci. 2010;51:3820–3824. doi: 10.1167/iovs.09-5121. [DOI] [PubMed] [Google Scholar]
- 187.Gu L., Chen H., Tuo J., Gao X., Chen L. Inhibition of experimental choroidal neovascularization in mice by anti-VEGFA/VEGFR2 or non-specific siRNA. Exp. Eye Res. 2010;91:433–439. doi: 10.1016/j.exer.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 188.Kleinman M.E., Kaneko H., Cho W.G., Dridi S., Fowler B.J., Blandford A.D., Albuquerque R.J., Hirano Y., Terasaki H., Kondo M. Short-interfering RNAs induce retinal degeneration via TLR3 and IRF3. Mol. Ther. 2012;20:101–108. doi: 10.1038/mt.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Karikó K., Buckstein M., Ni H., Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 190.Hutvágner G., Zamore P.D. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–2060. doi: 10.1126/science.1073827. [DOI] [PubMed] [Google Scholar]
- 191.Cashman S.M., Bowman L., Christofferson J., Kumar-Singh R. Inhibition of choroidal neovascularization by adenovirus-mediated delivery of short hairpin RNAs targeting VEGF as a potential therapy for AMD. Invest. Ophthalmol. Vis. Sci. 2006;47:3496–3504. doi: 10.1167/iovs.05-1610. [DOI] [PubMed] [Google Scholar]
- 192.Askou A.L., Pournaras J.A., Pihlmann M., Svalgaard J.D., Arsenijevic Y., Kostic C., Bek T., Dagnaes-Hansen F., Mikkelsen J.G., Jensen T.G., Corydon T.J. Reduction of choroidal neovascularization in mice by adeno-associated virus-delivered anti-vascular endothelial growth factor short hairpin RNA. J. Gene Med. 2012;14:632–641. doi: 10.1002/jgm.2678. [DOI] [PubMed] [Google Scholar]
- 193.Kaadt E., Alsing S., Cecchi C.R., Damgaard C.K., Corydon T.J., Aagaard L. Efficient knockdown and lack of passenger strand activity by Dicer-independent shRNAs expressed from Pol II-driven microRNA scaffolds. Mol. Ther. Nucleic Acids. 2019;14:318–328. doi: 10.1016/j.omtn.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Askou A.L., Corydon T.J. Development of multigenic lentiviral vectors for cell-specific expression of antiangiogenic miRNAs and protein factors. Methods Mol. Biol. 2018;1715:47–60. doi: 10.1007/978-1-4939-7522-8_4. [DOI] [PubMed] [Google Scholar]
- 195.Askou A.L., Aagaard L., Kostic C., Arsenijevic Y., Hollensen A.K., Bek T., Jensen T.G., Mikkelsen J.G., Corydon T.J. Multigenic lentiviral vectors for combined and tissue-specific expression of miRNA- and protein-based antiangiogenic factors. Mol. Ther. Methods Clin. Dev. 2015;2:14064. doi: 10.1038/mtm.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Askou A.L., Alsing S., Benckendorff J.N.E., Holmgaard A., Mikkelsen J.G., Aagaard L., Bek T., Corydon T.J. Suppression of choroidal neovascularization by AAV-based dual-acting antiangiogenic gene therapy. Mol. Ther. Nucleic Acids. 2019;16:38–50. doi: 10.1016/j.omtn.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Maeder M.L., Stefanidakis M., Wilson C.J., Baral R., Barrera L.A., Bounoutas G.S., Bumcrot D., Chao H., Ciulla D.M., DaSilva J.A. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat. Med. 2019;25:229–233. doi: 10.1038/s41591-018-0327-9. [DOI] [PubMed] [Google Scholar]
- 198.Zhong H., Chen Y., Li Y., Chen R., Mardon G. CRISPR-engineered mosaicism rapidly reveals that loss of Kcnj13 function in mice mimics human disease phenotypes. Sci. Rep. 2015;5:8366. doi: 10.1038/srep08366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yiu G., Tieu E., Nguyen A.T., Wong B., Smit-McBride Z. Genomic disruption of VEGF-A expression in human retinal pigment epithelial cells using CRISPR-Cas9 endonuclease. Invest. Ophthalmol. Vis. Sci. 2016;57:5490–5497. doi: 10.1167/iovs.16-20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Huang X., Zhou G., Wu W., Ma G., D’Amore P.A., Mukai S., Lei H. Editing VEGFR2 blocks VEGF-induced activation of Akt and tube formation. Invest. Ophthalmol. Vis. Sci. 2017;58:1228–1236. doi: 10.1167/iovs.16-20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Huang X., Zhou G., Wu W., Duan Y., Ma G., Song J., Xiao R., Vandenberghe L., Zhang F., D’Amore P.A., Lei H. Genome editing abrogates angiogenesis in vivo. Nat. Commun. 2017;8:112. doi: 10.1038/s41467-017-00140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Holmgaard A., Askou A.L., Benckendorff J.N.E., Thomsen E.A., Cai Y., Bek T., Mikkelsen J.G., Corydon T.J. In vivo knockout of the Vegfa gene by lentiviral delivery of CRISPR/Cas9 in mouse retinal pigment epithelium cells. Mol. Ther. Nucleic Acids. 2017;9:89–99. doi: 10.1016/j.omtn.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kim E., Koo T., Park S.W., Kim D., Kim K., Cho H.Y., Song D.W., Lee K.J., Jung M.H., Kim S. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat. Commun. 2017;8:14500. doi: 10.1038/ncomms14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jo D.H., Koo T., Cho C.S., Kim J.H., Kim J.S., Kim J.H. Long-term effects of in vivo genome editing in the mouse retina using Campylobacter jejuni Cas9 expressed via adeno-associated virus. Mol. Ther. 2019;27:130–136. doi: 10.1016/j.ymthe.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kim K., Park S.W., Kim J.H., Lee S.H., Kim D., Koo T., Kim K.E., Kim J.H., Kim J.S. Genome surgery using Cas9 ribonucleoproteins for the treatment of age-related macular degeneration. Genome Res. 2017;27:419–426. doi: 10.1101/gr.219089.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lau C.H., Suh Y. In vivo genome editing in animals using AAV-CRISPR system: applications to translational research of human disease. F1000Res. 2017;6:2153. doi: 10.12688/f1000research.11243.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Anders C., Niewoehner O., Duerst A., Jinek M. Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature. 2014;513:569–573. doi: 10.1038/nature13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Recchia A. AAV-CRISPR persistence in the eye of the beholder. Mol. Ther. 2019;27:12–14. doi: 10.1016/j.ymthe.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Grüter O., Kostic C., Crippa S.V., Perez M.T., Zografos L., Schorderet D.F., Munier F.L., Arsenijevic Y. Lentiviral vector-mediated gene transfer in adult mouse photoreceptors is impaired by the presence of a physical barrier. Gene Ther. 2005;12:942–947. doi: 10.1038/sj.gt.3302485. [DOI] [PubMed] [Google Scholar]
- 210.Binley K., Widdowson P., Loader J., Kelleher M., Iqball S., Ferrige G., de Belin J., Carlucci M., Angell-Manning D., Hurst F. Transduction of photoreceptors with equine infectious anemia virus lentiviral vectors: safety and biodistribution of StarGen for Stargardt disease. Invest. Ophthalmol. Vis. Sci. 2013;54:4061–4071. doi: 10.1167/iovs.13-11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yang Y., Wang L., Bell P., McMenamin D., He Z., White J., Yu H., Xu C., Morizono H., Musunuru K. A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat. Biotechnol. 2016;34:334–338. doi: 10.1038/nbt.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zetsche B., Volz S.E., Zhang F. A split-Cas9 architecture for inducible genome editing and transcription modulation. Nat. Biotechnol. 2015;33:139–142. doi: 10.1038/nbt.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Truong D.J., Kühner K., Kühn R., Werfel S., Engelhardt S., Wurst W., Ortiz O. Development of an intein-mediated split-Cas9 system for gene therapy. Nucleic Acids Res. 2015;43:6450–6458. doi: 10.1093/nar/gkv601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ran F.A., Cong L., Yan W.X., Scott D.A., Gootenberg J.S., Kriz A.J., Zetsche B., Shalem O., Wu X., Makarova K.S. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–191. doi: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Müller M., Lee C.M., Gasiunas G., Davis T.H., Cradick T.J., Siksnys V., Bao G., Cathomen T., Mussolino C. Streptococcus thermophilus CRISPR-Cas9 systems enable specific editing of the human genome. Mol. Ther. 2016;24:636–644. doi: 10.1038/mt.2015.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zetsche B., Gootenberg J.S., Abudayyeh O.O., Slaymaker I.M., Makarova K.S., Essletzbichler P., Volz S.E., Joung J., van der Oost J., Regev A. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163:759–771. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Trapani I., Colella P., Sommella A., Iodice C., Cesi G., de Simone S., Marrocco E., Rossi S., Giunti M., Palfi A. Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol. Med. 2014;6:194–211. doi: 10.1002/emmm.201302948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wright A.V., Sternberg S.H., Taylor D.W., Staahl B.T., Bardales J.A., Kornfeld J.E., Doudna J.A. Rational design of a split-Cas9 enzyme complex. Proc. Natl. Acad. Sci. USA. 2015;112:2984–2989. doi: 10.1073/pnas.1501698112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kim D., Kim J., Hur J.K., Been K.W., Yoon S.H., Kim J.S. Genome-wide analysis reveals specificities of Cpf1 endonucleases in human cells. Nat. Biotechnol. 2016;34:863–868. doi: 10.1038/nbt.3609. [DOI] [PubMed] [Google Scholar]
- 220.Kleinstiver B.P., Tsai S.Q., Prew M.S., Nguyen N.T., Welch M.M., Lopez J.M., McCaw Z.R., Aryee M.J., Joung J.K. Genome-wide specificities of CRISPR-Cas Cpf1 nucleases in human cells. Nat. Biotechnol. 2016;34:869–874. doi: 10.1038/nbt.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fu Y., Foden J.A., Khayter C., Maeder M.L., Reyon D., Joung J.K., Sander J.D. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 2013;31:822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pattanayak V., Lin S., Guilinger J.P., Ma E., Doudna J.A., Liu D.R. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat. Biotechnol. 2013;31:839–843. doi: 10.1038/nbt.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chew W.L., Tabebordbar M., Cheng J.K., Mali P., Wu E.Y., Ng A.H., Zhu K., Wagers A.J., Church G.M. A multifunctional AAV-CRISPR-Cas9 and its host response. Nat. Methods. 2016;13:868–874. doi: 10.1038/nmeth.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kim S., Kim D., Cho S.W., Kim J., Kim J.S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014;24:1012–1019. doi: 10.1101/gr.171322.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kleinstiver B.P., Pattanayak V., Prew M.S., Tsai S.Q., Nguyen N.T., Zheng Z., Joung J.K. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016;529:490–495. doi: 10.1038/nature16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Slaymaker I.M., Gao L., Zetsche B., Scott D.A., Yan W.X., Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2016;351:84–88. doi: 10.1126/science.aad5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chen J.S., Dagdas Y.S., Kleinstiver B.P., Welch M.M., Sousa A.A., Harrington L.B., Sternberg S.H., Joung J.K., Yildiz A., Doudna J.A. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature. 2017;550:407–410. doi: 10.1038/nature24268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Shin J., Jiang F., Liu J.J., Bray N.L., Rauch B.J., Baik S.H., Nogales E., Bondy-Denomy J., Corn J.E., Doudna J.A. Disabling Cas9 by an anti-CRISPR DNA mimic. Sci. Adv. 2017;3:e1701620. doi: 10.1126/sciadv.1701620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Dow L.E., Fisher J., O’Rourke K.P., Muley A., Kastenhuber E.R., Livshits G., Tschaharganeh D.F., Socci N.D., Lowe S.W. Inducible in vivo genome editing with CRISPR-Cas9. Nat. Biotechnol. 2015;33:390–394. doi: 10.1038/nbt.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Merienne N., Vachey G., de Longprez L., Meunier C., Zimmer V., Perriard G., Canales M., Mathias A., Herrgott L., Beltraminelli T. The self-inactivating KamiCas9 system for the editing of CNS disease genes. Cell Rep. 2017;20:2980–2991. doi: 10.1016/j.celrep.2017.08.075. [DOI] [PubMed] [Google Scholar]
- 231.Petris G., Casini A., Montagna C., Lorenzin F., Prandi D., Romanel A., Zasso J., Conti L., Demichelis F., Cereseto A. Hit and go CAS9 delivered through a lentiviral based self-limiting circuit. Nat. Commun. 2017;8:15334. doi: 10.1038/ncomms15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Li A., Lee C.M., Hurley A.E., Jarrett K.E., De Giorgi M., Lu W., Balderrama K.S., Doerfler A.M., Deshmukh H., Ray A. A self-deleting AAV-CRISPR ysstem for in vivo genome editing. Mol. Ther. Methods Clin. Dev. 2018;12:111–122. doi: 10.1016/j.omtm.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.