Main Text
Cellular senescence is implicated as a contributing factor in the pathology of several age-related disorders and cancer development. Therefore, targeted removal of senescent cells has emerged as a promising treatment strategy for these conditions. However, current senolytic strategies that selectively eliminate senescent cells are limited by their toxicity and lack of potency. A recent study reported on the use of chimeric antigen receptor (CAR) T cell therapy as a novel senolytic approach and demonstrated improved cancer outcome and reversal of senescence-associated pathologies in animal models. Here, we aim to discuss the opportunities and limitations of translating such a therapeutic strategy into the clinic.
Cellular senescence is an intrinsic stress response commonly characterized by stable cell-cycle arrest and the acquisition of a senescence-associated secretory phenotype (SASP).1,2 Senescence can have both beneficial and detrimental effects. It serves as a cell-intrinsic tumor-suppressive mechanism that prevents the uncontrolled expansion of pre-cancerous cells,3 which are subsequently cleared by the immune system.4 It can, however, promote chronic inflammation and has been shown to contribute to several tissue damage pathologies and chronic disorders.1 Senescent cells have, thus, become therapeutic targets to alleviate age-related diseases and as part of some cancer treatment strategies.5,6 Removal of these cells from damaged tissues in mice has been shown to mitigate symptoms of these diseases and to provide health and lifespan benefits. Previous studies also suggest that the combination of senescence induction and drugs that eliminate senescent cells (“senolytics”) can improve treatment outcomes in certain cancer models.6, 7, 8, 9, 10 Existing senolytic candidates mainly consist of repurposed cancer therapeutics targeting apoptosis resistance pathways that are upregulated in senescent cells such as navitoclax (BCL-XL/BCL-2 inhibitor), or the combination of dasatinib (pan-tyrosine kinase inhibitor), and quercetin (antioxidant flavonoid).5 These drugs have shown evidence of senescent cell clearance in different pathologies. However, these compounds can target a broad spectrum of biological pathways in multiple tissues, thereby increasing the potential for adverse events, such as neutropenia and thrombocytopenia in the case of navitoclax treatment, making it difficult to delineate the underlying mechanism of any potential therapeutic effect.
As such, the need for therapeutic approaches that selectively, efficiently, and safely target senescent cells has advanced the development of new treatment modalities for age-related diseases and cancers. Recently, Amor et al.11 demonstrated that a CAR T cell-based approach could augment immune responses against senescent cells, leading to improvements in treatment outcomes of cancer and senescence-associated pathologies in animal models.
CARs are a class of synthetic receptors that reprogram T cell specificity, function, and metabolism to augment their cytolytic capacity against cancer or other diseases.12 The adoptive transfer of T cells engineered to express CARs has yielded considerable efficacy in patients with hematological B cell malignancies, resulting in the authorization of three CD19-directed CAR therapies: Yescarta and Tecartus from Kite Pharma/Gilead and Kymriah from Novartis.13 Nevertheless, the use of CAR T cells against solid tumors still faces several challenges, such as the risk of on-target off-tumor toxicities owing to the paucity of tumor-specific antigens, antigen heterogeneity, the development of tumor resistance in the form of antigen escape, poor T cell infiltration and persistence, and the immunosuppressive tumor microenvironment.12
Directing CAR T cells against senescent cells unites two emerging research areas: cellular senescence and therapeutic T cell engineering. Therapy-induced senescence and its associated SASP can counteract tumor progression and might favorably alter the tumor microenvironment. This could facilitate trafficking and infiltration of adoptively transferred T cells and enhance CAR T cell activity, resulting in improved clearance of senescent tumor cells (Figure 1). Additionally, the clearance of persistent senescent cancer cells with CAR T cells might prevent SASP-related chronic inflammation and pro-tumorigenic effects that can arise from the accumulation of senescent cells. Amor et al.11 demonstrated the potential of senolytic CAR T cells in mice with lung adenocarcinoma that received a senescence-inducing combination of drugs. CAR T cell administration led to increased infiltration and enhanced activation of transferred T cells and was associated with a reduction of senescent tumor cells and improved overall survival.
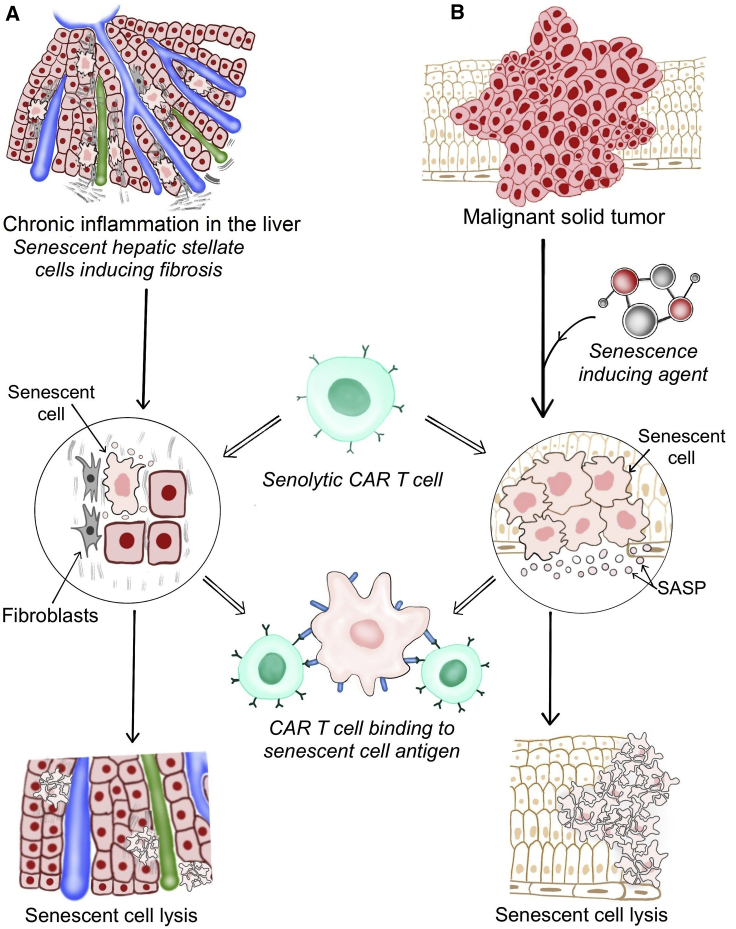
Figure 1.
Senolytic CAR T Cells in Age-Related Pathologies and Solid Tumors
(A) Age-related pathologies and (B) solid malignant tumors. Senolytic CAR T cells migrate to the areas of senescence and infiltrate the senescent tissues. The binding of the CAR to the target antigen expressed on senescent cells (e.g., uPAR) triggers an intracellular signaling cascade that elicits T cell activation, proliferation, and effector functions, resulting in the lysis of senescent cells. Elimination of senescent cells may subsequently restore tissue homeostasis and function.
Beyond cancer, senolytic CAR T cells also have the potential to provide therapeutic benefit in the treatment of diseases related to chronic inflammation, such as liver fibrosis (Figure 1). Amor et al.11 further showed therapeutic benefits in a mouse model of non-alcoholic steatohepatitis (NASH), a condition for which the incidence is increasing and effective therapeutic options are currently lacking. The urokinase-type plasminogen activator receptor (uPAR) was used as the first target for senolytic CAR T cells owing to its broad upregulation in senescent cells in different disease models and its concurrent absence in many vital tissues.14 uPAR regulates extracellular matrix proteolysis by binding its ligand urokinase-type plasminogen activator and activates intracellular signaling pathways through its interaction with other transmembrane receptors. The soluble form of uPAR, which is secreted upon ligand binding, has previously been described as a SASP component and as a serum biomarker for senescence-related pathologies, such as chronic kidney disease and diabetes.15 These findings identify uPAR as a senescence-associated marker and as a promising target for CAR therapy. However, it will require more in-depth analysis to determine if the low expression on the bronchial epithelium and a subset of immune cells may hinder therapeutic efficacy and safety, particularly in human settings.
Existing senolytic approaches have mainly focused on limiting the proinflammatory impact (e.g., through inhibitors of SASP components) or on senolytic drugs that block pro-survival pathways to induce cell death.5 Adapting T cell immunotherapy for senolysis, therefore, represents an attractive addition to the current senolytic treatment landscape. However, the success of this approach in clinical trials will depend on the elucidation of a myriad of factors, such as:
-
(1)
the number of senescent cells amenable to CAR T cell therapy upon senescence induction;
-
(2)
the reduction in senescent cell numbers required to produce therapeutic benefit without interfering with the beneficial aspects of senescence;
-
(3)
the characterization of senescent cell populations and SASP composition in different disease models to further inform target selection and guide improved CAR designs;
-
(4)
differences in CAR designs and their impact on efficacy and safety profiles of senolytic CAR T cells;
-
(5)
the synergistic effects of combined treatment approaches (pro-senescent and anti-senescent);
-
(6)
whether inhibitory SASP factors and recruited myeloid cells could augment immunosuppressive effects against CAR T cells and impair their therapeutic effects; and
-
(7)
whether senescence-associated target antigens are also upregulated on the surface of senescent T cells,16 which could further improve therapeutic efficacy by CAR-mediated elimination of a suppressive and dysfunctional T cell subset with pro-tumorigenic potential.
In terms of potential safety concerns, Amor et al.11 showed that the application of uPAR-specific senolytic CAR T cells at effective doses was safe in different in vivo disease models. However, the administration of supratherapeutic T cell dosages elicited transient toxicities, similar to cytokine release syndrome (CRS). CRS has been observed as a severe side effect of current CAR T cell therapies in cancer, and macrophage involvement plays a crucial role in its pathogenesis.17 uPAR is expressed on a subset of monocytes and macrophages, which may contribute to the observed development of CRS-like syndromes at high CAR T cell doses. Further biological and mechanistic studies will be required to determine the safety profile of senolytic CAR T cells and to investigate potential therapeutic strategies to mitigate toxicities.
Short-lived effector CAR T cells, such as CD28z-based CARs, are known to induce high efficacy but relatively short persistence compared to CARs incorporating BBz signaling domains. Hence, they are likely to provide potent senolytic activity while reducing interference with beneficial functions of senescence. Nevertheless, favorable CAR designs and beneficial T cell properties might differ between age-related pathologies and cancer settings, and further efforts should be directed at developing CAR constructs tailored to senescence and treatment specific conditions. Systemic surface profiling of senescent cells might indicate alternative or additional targets for senolytic CAR T cells that could be exploited for combinatorial targeting approaches to minimize toxicities and improve efficacy. These efforts would also benefit from the design of suitable preclinical models to test the toxicity of senolytic CAR T cells.
In summary, the observed efficacy of senolytic CAR T cells in different disease models suggests a therapeutic potential that could overcome obstacles of current senolytic agents and may open new treatment avenues for a variety of chronic diseases and cancers. However, a lot of work remains to be done to translate these prospects into clinical therapy. Clinical studies should further address differences between young and old patients, as senolytics are likely to have different effects on the aging immune system and organism. Most importantly, assessment of the crucial role of cellular senescence in the pathophysiology of disease as well as a better understanding of short- and long-term consequences of senescent cell elimination will guide the selection of suitable disease and treatment settings, time points, dosing, and frequency of CAR T cell administration. Continuing progress in T cell engineering and synthetic biology will provide options to optimize CAR T cell designs for senescence-specific conditions, which could advance cancer and aging research and offer more effective therapies for these disorders
Conflicts of Interest
J.F. is listed as an inventor on a patent application (PCT/US2020/016290) that has been filed on 02/01/2020 and is based in part on information presented in this manuscript on the use of CAR T cells that target uPAR as senolytic agents. J.F. holds other unrelated patents on CAR technologies. M.A. declares no competing interests.
References
- 1.He S., Sharpless N.E. Senescence in Health and Disease. Cell. 2017;169:1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coppe J.P., Desprez P.Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serrano M., Lin A.W., McCurrach M.E., Beach D., Lowe S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 4.Ruscetti M., Leibold J., Bott M.J., Fennell M., Kulick A., Salgado N.R., Chen C.-C., Ho Y., Sanchez-Rivera F.J., Feucht J. NK cell–mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science. 2018;362:1416–1422. doi: 10.1126/science.aas9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ovadya Y., Krizhanovsky V. Strategies targeting cellular senescence. J. Clin. Invest. 2018;128:1247–1254. doi: 10.1172/JCI95149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sieben C.J., Sturmlechner I., van de Sluis B., van Deursen J.M. Two-Step Senescence-Focused Cancer Therapies. Trends Cell Biol. 2018;28:723–737. doi: 10.1016/j.tcb.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu M., Pirtskhalava T., Farr J.N., Weigand B.M., Palmer A.K., Weivoda M.M., Inman C.L., Ogrodnik M.B., Hachfeld C.M., Fraser D.G. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker D.J., Wijshake T., Tchkonia T., LeBrasseur N.K., Childs B.G., van de Sluis B., Kirkland J.L., van Deursen J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L., Leite de Oliveira R., Wang C., Fernandes Neto J.M., Mainardi S., Evers B., Lieftink C., Morris B., Jochems F., Willemsen L. High-Throughput Functional Genetic and Compound Screens Identify Targets for Senescence Induction in Cancer. Cell Rep. 2017;21:773–783. doi: 10.1016/j.celrep.2017.09.085. [DOI] [PubMed] [Google Scholar]
- 10.Wang C., Vegna S., Jin H., Benedict B., Lieftink C., Ramirez C. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature. 2019;574:268–272. doi: 10.1038/s41586-019-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amor C., Feucht J., Leibold J., Ho Y.-J., Zhu C., Alonso-Curbelo D., Mansilla-Soto J., Boyer J.A., Li X., Giavridis T. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020;583:127–132. doi: 10.1038/s41586-020-2403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadelain M., Rivière I., Riddell S. Therapeutic T cell engineering. Nature. 2017;545:423–431. doi: 10.1038/nature22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elsallab M., Levine B.L., Wayne A.S., Abou-El-Enein M. CAR T-cell product performance in haematological malignancies before and after marketing authorisation. Lancet Oncol. 2020;21:e104–e116. doi: 10.1016/S1470-2045(19)30729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perna F., Berman S.H., Soni R.K., Mansilla-Soto J., Eyquem J., Hamieh M. Integrating Proteomics and Transcriptomics for Systematic Combinatorial Chimeric Antigen Receptor Therapy of AML. Cancer Cell. 2017;32:506–519.e505. doi: 10.1016/j.ccell.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorgoulis V., Adams P.D., Alimonti A., Bennett D.C., Bischof O., Bishop C. Cellular Senescence: Defining a Path Forward. Cell. 2019;179:813–827. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Liu X., Hoft D.F., Peng G. Senescent T cells within suppressive tumor microenvironments: emerging target for tumor immunotherapy. J Clin Invest. 2020;130:1073–1083. doi: 10.1172/JCI133679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giavridis T., van der Stegen S.J.C., Eyquem J., Hamieh M., Piersigilli A., Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 2018;24:731–738. doi: 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]