Main Text
Gene therapy offers much hope for debilitating genetic disorders such as lysosomal storage diseases, which often exhibit a severe phenotype that includes neurological complications. The fact that they tend to be single gene defects and that supply of the missing enzymes through the bloodstream results in cellular uptake and translocation into lysosomes makes them amenable to gene therapy. Sandhoff and Tay-Sachs diseases, however, have additional complications that make treatment particularly challenging. A new study by Lahey et al.,1 published in this issue of Molecular Therapy, constitutes a major advance toward treatment of these devastating GM2 gangliosidoses that cause extensive damage to neurons in the brain.
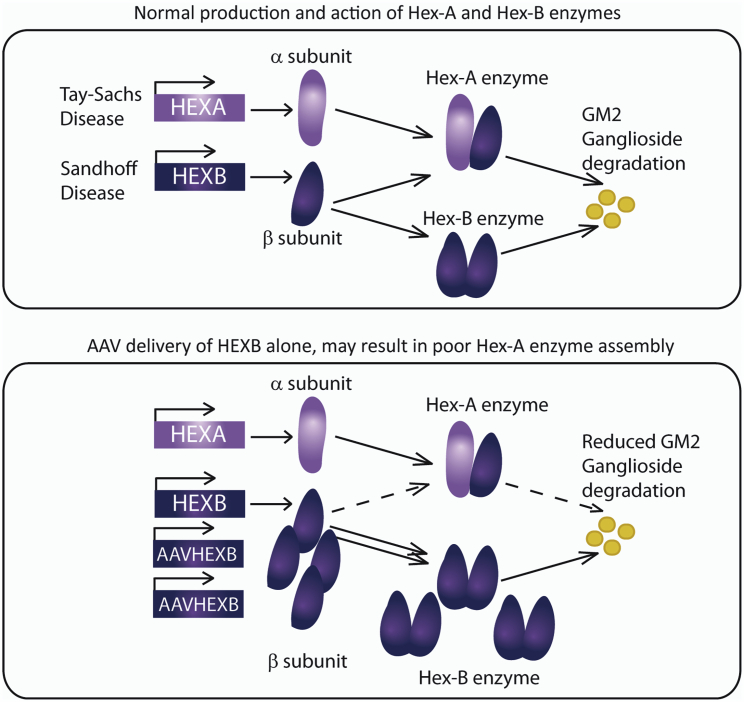
Sandhoff disease is caused by deficiencies in the HEXB gene, leading to accumulation of undegraded GM2 gangliosides, and is clinically indistinguishable from its more well-known counterpart, Tay-Sachs disease, caused by HEXA mutations. HEXA and HEXB genes encode, respectively, α and β subunits of the hexosaminidase enzyme. GM2 ganglioside breakdown requires two isoforms of the hexosaminidase enzyme (confusingly named Hex-A and Hex-B), which are made up of, respectively, (α-β) and (β-β) subunits (Figure 1). Hex-A also requires a co-factor (GM2 activator protein) for activity. Delivery of the β subunit alone as a gene therapy in the Sandhoff mouse model appears to lead to a skewing of enzyme assembly toward Hex-B, with limitation of α subunit availability, perhaps due to steric effects arising from over-production of the Hex-B enzyme2 (Figure 1). Thus, for successful enzyme assembly of both Hex-A and Hex-B enzymes, provision of both HEXA and HEXB genes in roughly equal dosage is critical to successful Sandhoff and Tay-Sachs therapies.
Figure 1.
Constraints on Assembly of Hex-A and Hex-B Isoforms of Hexosaminidase
Tay-Sachs and Sandhoff are caused by deficiencies in the HEXA (α subunit of hexosaminidase) and HEXB (β subunit of hexosaminidase) genes, respectively. These subunits assemble into a heterodimeric (α-β hexosaminidase isoform A; Hex-A) or homodimeric (β-β hexosaminidase isoform B; Hex-B) enzyme. Over-production of one subunit appears to inhibit assembly of the other enzyme isoform, likely due to steric effects. This was demonstrated in 2012 when expression of HEXB in the mouse model of Sandhoff resulted in undetectable Hex-A expression, presumably through reduced α subunit availability or negative regulation.1 Effective gene therapy strategies for Tay-Sachs and Sandhoff, therefore, likely require delivery of both HEXA and HEXB genes at a similar gene dosage.
Previous efforts to correct Sandhoff or Tay-Sachs have attempted to deliver two adeno-associated viral (AAV) vectors expressing HEXA and HEXB, respectively,2,3 which leads to multiple problems. These include reliance on efficient co-delivery of both vectors to the same cell, which is particularly difficult when targeting the brain by direct injection and even more challenging with an intravenous delivery approach. An alternative strategy was to develop a HEXA modified gene, incorporating sections of the similar HEXB, that could form a monomeric dimer and bind GM2 activator protein (dubbed Hex-M).4 While this may prove quite successful for Tay-Sachs, it still does not solve the problem of Sandhoff.
Lahey et al.1 have neatly solved this old problem by developing a single AAV vector expressing both HEXA and HEXB genes from a bidirectional promoter. They demonstrate that these constructs are able to achieve long-term expression, reduce GM2 ganglioside accumulation, and improve behavioral outcomes and survival in Sandhoff disease mice, with the AAV9-Bic construct (a vector containing a bidirectional CBA promoter—a cytomegalovirus [CMV] enhancer, flanked by two β-actin promoters) demonstrating the highest level of correction. This result is truly impressive, given that each gene is approximately 1.6 Kb in size, leaving precious little effective packaging capacity to work with when incorporated into an AAV vector. Both transcripts were able to effectively deliver each Hex subunit and correct disease in the mouse model. Importantly, gene expression was sustained for up to 2 years in some cases, suggesting that promoter methylation, typically a feature of unmodified CMV promoters, may not be a significant factor in this design.
Innovative promoter design in gene therapy vectors has perhaps not received as much attention in recent years as it deserves. In order to improve the effectiveness, specificity, and safety of vectors, optimal design of promoters remains critical. Bidirectional promoters, such as the one described here, also open up the prospect for designing effective and controlled regulated gene expression. Many such systems require dual gene expression, although few are sufficiently tightly regulated to be suitable for clinical use. Lahey et al.1 have elegantly addressed an old problem in the lysosomal disease community with a vector that should correct both Sandhoff and Tay-Sachs disease, and its design likely has even broader applicability. Going forward, the same research group is in the unique position to test the concept in a sheep model of Tay-Sachs disease (because mice possess an alternative pathway for GM2 ganglioside catabolism, murine models of Tay-Sachs disease are not suitable for development of therapies). Experiments in these large animals should prove informative if this vector does indeed serve the dual purpose of treating both Sandhoff and Tay-Sachs.
References
- 1.Lahey H.G., Hwang M. Pronounced therapeutic benefit of a single bidirectional AAV vector administered systemically in Sandhoff mice. Mol Ther. 2020;28:2150–2160. doi: 10.1016/j.ymthe.2020.06.021. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cachón-González M.B., Wang S.Z., McNair R., Bradley J., Lunn D., Ziegler R., Cheng S.H., Cox T.M. Vol. 20. 2012. Gene transfer corrects acute GM2 gangliosidosis--potential therapeutic contribution of perivascular enzyme flow; pp. 1489–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sargent T.J., Wang S., Bradley J., Smith N.J.C., Raha A.A., McNair R., Ziegler R.J., Cheng S.H., Cox T.M., Cachón-González M.B. Vol. 20. 2011. Adeno-associated virus-mediated expression of β-hexosaminidase prevents neuronal loss in the Sandhoff mouse brain; pp. 4371–4380. [DOI] [PubMed] [Google Scholar]
- 4.Karumuthil-Melethil, S., Kalburgi, S.N., Thompson, P., Tropak, M., Kaytor, M.D., Keimel, J.G., Mark, B.L., Mahuran, D., Walia, J.S., and Gray, S.J. (2016). 27, 509–521. [DOI] [PMC free article] [PubMed]