Abstract
Objectives:
The context of this article is based on two main titles those being Gynecologic Oncology and Minimal invasive surgery. The aim of this study was to report the laparoscopic management of a series of cases of endometrial carcinoma managed by laparoscopic surgical staging in Indian women.
Materials and Methods:
This study was conducted in a private hospital (referral minimally invasive gynecological center).
This was a retrospective study (Canadian Task Force Classification II-3). Eighty-eight cases of clinically early-stage endometrial carcinoma staged by laparoscopic surgery and treated as per final surgicopathological staging. All patients underwent laparoscopic surgical staging of endometrial carcinoma, followed by adjuvant therapy when needed. Data were retrieved regarding surgical and pathological outcomes. Recurrence-free and overall survival durations were measured at follow-up. Survival analysis was calculated using Kaplan–Meier survival analysis.
Results:
The median age of presentation was 56 years, whereas the median body mass index was 28.3 kg/m2. Endometroid variety was the most commonly diagnosed histopathology. There were no intraoperative complications reported. The median blood loss was 100 cc, and the median intraoperative time was 174 min. There were a total of 5 recurrences (5.6%). The outcome of this study was comparable to studies conducted in Caucasian population. The predicted 5-year survival rate according to Kaplan–Meier survival analysis is 95.45%, which is comparable to Caucasian studies.
Conclusion:
Laparoscopic management of early-stage endometrial carcinoma is a standard practice worldwide. However, there is still a paucity of data from the Indian subcontinent regarding the outcomes of laparoscopic surgery in endometrial carcinoma. The Asian perspective has been highlighted by a number of studies from China and Japan. To our knowledge, this study is the first from India to analyze the surgicopathological outcomes following laparoscopic surgery in endometrial carcinoma. The outcome of this study was comparable to studies conducted in Caucasian population.
Keywords: Endometrial carcinoma, laparoscopy, staging
INTRODUCTION
Endometrial carcinoma is the seventh most common malignancy in the world and is one of the leading malignancies in the developed nations.[1] However, Asian countries accounted for a less percentage of the global burden of endometrial carcinoma, but recently, its incidence is on the rise. As per the GLOBOCAN 2018 statistics, India witnessed 13,328 new cases and 5010 deaths due to endometrial carcinoma.[2]
As per the National Comprehensive Cancer Network guidelines, the management of endometrial carcinoma includes a surgical staging comprising hysterectomy, with bilateral salpingo-oophorectomy, with or without pelvic and para-aortic lymphadenectomy, omental biopsy, and peritoneal washings.[3] Traditional route for surgery has been laparotomy. With the introduction of laparoscopy in the surgical staging of endometrial carcinoma by Childersand Surwit, there has been an exponential rise in the adoption of minimally invasive surgical techniques for comprehensive surgical staging.[4] However, in India, there is scant literature regarding adoption of laparoscopy in the management of endometrial carcinoma and laparotomy continues to be the mainstay for comprehensive surgical staging.
The GOG LAP 2 trial firmly consolidated the position and inherent benefits of laparoscopic surgical staging in endometrial carcinoma.[5] Although robotic technology is gaining popularity among gynecologic oncologists, the cost remains a concern for the patients in a developing nation. Majority of the trials regarding application of minimally invasive techniques in gynecologic oncology involve Caucasian population, and there is a need for data to emerge from the Asian subcontinent which has different demographic characteristics when compared with the Caucasian counterparts. As there are scanty data regarding the surgicopathologic outcomes in Indian women undergoing laparoscopic surgical staging, we would like to present our data regarding the same.
MATERIALS AND METHODS
This is a single-institution retrospective chart review of patients with apparently early-stage endometrial carcinoma undergoing laparoscopic comprehensive surgical staging at the author's institute from January 2012 to December 2017. The institutional ethical committee approval was obtained (approval letter obtained on 12 Oct 2018), and necessary consents from patients were taken.
All patients with histologically proven endometrial carcinoma Stage I–II were included in the study. All patients underwent a preoperative imaging which included either a contrast-enhanced computed tomography scan or contrast-enhanced magnetic resonance imaging of the abdomen or pelvis. Preoperative histological confirmation was obtained either through a fractional curettage or hysteroscopy-guided endometrial biopsy. Intraoperative frozen section analysis was performed on the retrieved specimens. Patients underwent a cervical biopsy if imaging was suggestive of gross cervical involvement. Patients referred for completion surgeries after incidental diagnosis of uterine carcinoma were excluded. Sarcomatous lesions and gross abdominal, pelvic, and/or distant metastatic lesions were excluded from the study.
All patients underwent comprehensive surgical staging through laparoscopy. The staging procedure included peritoneal washings, Type A or Type C1 hysterectomy, bilateral salpingo-oophorectomy, omental sampling, and systematic pelvic with or without para-aortic lymphadenectomy as per the International Federation of Gynecology and Obstetrics (FIGO) 2009 surgical staging classification.[6] Systematic para-aortic lymphadenectomy was performed in high-risk cases including Grade 3 endometroid lesions, more than 50% myometrial invasion, cervical involvement, and nonendometroid histology.[3]
The boundaries of pelvic lymphadenectomy were defined from the common iliac vessels superiorly, deep circumflex iliac vein inferiorly, genitofemoral nerve laterally, internal iliac artery medially, and the obturator nerve posteriorly. The para-aortic nodal dissection commenced from the common iliac arteries inferiorly, ureters laterally, left renal vein superiorly, and both the psoas muscles posteriorly. An omental biopsy was obtained from the infracolic portion of the omentum if it grossly appeared normal. Any suspicious area over the omentum was biopsied irrespective of its location.
One supraumbilical 10-mm port was introduced after pneumoperitoneal insufflation with a Veress needle. A patient was placed in Trendelenburg position. Four 5-mm ancillary ports were placed: two on ipsilateral and the remaining two on the contralateral side. Apart from para-aortic lymphadenectomy and omental biopsy, the other components of the surgical staging procedure were performed by the surgeon standing on the left side facing the pelvis. The remainder of the surgery was completed after changing the surgeon position from left to right facing the upper abdomen. Para-aortic lymphadenectomy was approached through a transperitoneal technique, and an additional 10-mm port was introduced in the midline in the suprapubic region for camera insertion. Operative time was measured from port entry to closure of the skin incision. Estimated blood loss was measured by weighing the gauze swabs and also from the amount of blood suctioned during the procedure.
A retrospective chart review was performed, and data were collected from patients who underwent laparoscopic surgical staging for endometrial carcinoma from January 2012 to December 2017. Patient demographic details, operative data, pathological details, and postoperative outcomes were noted. Early postoperative complications were defined as those which occurred within 30 days of surgery, whereas those occurring after 30 days were termed as late postoperative. Postsurgery, all patients were staged as per the FIGO 2009 staging of endometrial carcinoma and appropriate adjuvant therapy was administered. For patients with Stage IA Grade 3, nonendometroid carcinoma, or Stage IB lesions, vaginal brachytherapy was administered. For Stage III tumors, external beam radiations sandwiched between three cycles of platinum-based doublet chemotherapy three cycles each before and after radiation were administered. All patients were followed up every 3 monthly for the first 2 years, 6 monthly for the next 2 years, and annually thereafter. Disease-free survival was calculated from the time of onset of treatment until recurrence or death.
RESULTS
Table 1 summarizes the patient demographics and disease characteristics. The median age at presentation was 56 years (35–77 years), with a median body mass index (BMI) being 28.3kg/m2(20.1–57.9 kg/m2). Preoperative diagnosis of endometrial carcinoma was confirmed using fractional curettage of the endometrium in 71 patients (80.6%), while hysteroscopic-guided endometrial biopsy was used to detect malignancy in 17 patients. Endometroid variant of endometrial carcinoma was the most common histological type accounting for 93.1% of the cases, while four cases were serous and two cases were of malignant mixed Mullerian type of endometrial carcinoma. Among the endometroid type of endometrial carcinoma, 54 (65.8%) comprised Grade 1 lesions, whereas Grade 2 and 3 lesions were detected in 14 cases each. None of the patients had a positive peritoneal cytology or omental metastasis posthisto-cytopathological examination after surgery.
Table 1.
Patient demographics and tumor characteristics
| Parameter | Median value |
|---|---|
| Age (years) | 56 (35-77) |
| BMI (kg/m2) | 28.3 (20.1-57.9) |
| Method of diagnosis, n (%) | |
| Fractional curettage | 71 (80.6) |
| Hysteroscopic-guided endometrial biopsy | 17 (19.3) |
| FIGO staging 2009, n (%) | |
| IA | 67 (76.1) |
| IB | 10 (11.3) |
| II | 5 ( 5.6) |
| IIIA | 0 |
| IIIB | 0 |
| IIIC1 | 5 (5.6) |
| IIIC2 | 1 (1.1 ) |
| Histology, n (%) | |
| Endometroid | 82 (93.1) |
| Serous papillary | 4 (4.5) |
| Malignant mixed Mullerian tumor | 2 (2.3) |
| Grade (n=82), n (%) | |
| 1 | 54 (65.8) |
| 2 | 14 (17.1) |
| 3 | 14 (17.1) |
| Positive peritoneal cytology in lavage, n (%) | |
| Present | 0 |
| Absent | 88 |
| Omental metastasis, n (%) | |
| Present | 0 |
| Absent | 88 |
MI: Body mass index, FIGO: International Federation of Gynecology and Obstetrics
The median blood loss was 100 cc, and the median total operative time was 174 min (113–268 min), as outlined in Table 2. The average hospital stay was 2 days (2–7 days). All patients underwent systematic pelvic lymphadenectomy, and 33 patients underwent associated para-aortic lymphadenectomy as per indication. The median number of pelvic lymph nodes retrieved was 22 (11 on each side), whereas 9 para-aortic lymph nodes were retrieved (3–22). None of the cases had isolated para-aortic nodal metastasis. Five patients had metastasis in the pelvic lymph nodes, whereas one patient had both pelvic and para-aortic nodal metastasis. The median tumor diameter was 3.5 cm (0.7–7 cm). More than half of the myometrial thickness was invaded by the tumor in 13 cases. Of the five patients that had cervical stromal involvement, all underwent a Type C1 nerve-sparing radical hysterectomy. Parametrial invasion was absent in all the cases of cervical stromal involvement. Lymph-vascular space invasion (LVSI) was detected in 7 patients (7.9%). None of the patients had intraoperative complications, and none received any preoperative or postoperative blood transfusions. Four patients had postoperative pyrexia, and one patient had postoperative ileus. Long-term complications included two patients developing right lower-limb lymphedema.
Table 2.
Intra-operative details
| Parameter | Median value |
|---|---|
| Blood loss (ml) | 100 (60-160) |
| Operative time (min) | 174 (113-268) |
| Maximum tumor diameter (cm) | 3.5 (0.7-7) |
| Pelvic lymph node retrieval | |
| Right | 11 (4-26) |
| Left | 11 (3-25) |
| Para-aortic LN retrieval | 9 (3-22) |
| LVSI | |
| Present | 7 (7.9) |
| Absent | 81 (92.1) |
LVSI: Lymph-vascular space invasion, LN: Lymph node
Posthistological analysis of the surgical specimen, 67 patients were classified as having Stage 1A, 10 having Stage 1B, 5 having Stage 2 and Stage 3C1 disease each, and 1 having Stage 3C2 disease. Twenty-one patients received adjuvant therapy in view of high-risk factors. Ten patients of Stage IB received vaginal brachytherapy. External beam pelvic radiation with concurrent weekly cisplatin was administered in five patients with cervical stromal metastasis. Six patients received external beam pelvic radiation sandwiched between platinum-based 3-weekly systemic chemotherapies. One patient in addition received image-guided radiation to the para-aortic nodal basin.
The median follow-up period was 27 months (9–92 months). At the last follow-up visit, five patients developed recurrence (5.6%). Three patients died of tumor-related deaths. Of the three, two developed malignant ascites and one patient developed right lung metastasis. One patient had port-site metastasis, and another patient developed right supraclavicular node metastasis. Treatment is highlighted in Table 3.
Table 3.
Recurrence and treatment
| Age | Stage | Histology | Grade | LVSI | MI (%) | Lymph node metastasis | Adjuvant tx | Local recurrence | Distant recurrence | Time to recur (months) | Tx | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 58 | IIIC1 | Endometroid | 3 | Present | <50 | Present | CCRT | Right port | Nil | 9 | Excision and CT | NED |
| 58 | IA | Endometroid | 3 | Present | <50 | Absent | VBT | Nil | Malignant ascites | 18 | CT | DOD |
| 56 | IIIC2 | Serous | 3 | Present | >50 | Present | CCRT | Nil | Malignant ascites | 15 | CT | DOD |
| 60 | IB | Endometroid | 3 | Absent | >50 | Absent | EBRT | Nil | Right supraclavicular LN | 8 | Excision and CT | NED |
| 62 | IB | Endometroid | 3 | Present | <50 | Absent | EBRT | Nil | Right Lung | 10 | CT | DOD |
LVSI: Lymph-vascular space invasion, MI: Myometrial invasion, LN: Lymph node, tx: Treatment, Tx: Treatment after recurrence, CCRT: Sandwich chemoradiation, CT: Systemic chemotherapy, VBT: Vaginal brachytherapy, EBRT: External beam radiotherapy, NED: No evidence of disease, DOD: Died of disease
DISCUSSION
Laparoscopic management of early-stage endometrial carcinoma is a standard practice worldwide. However, there is still a paucity of data from the Indian subcontinent regarding the outcomes of laparoscopic surgery in endometrial carcinoma. Numerous studies have been published in literature citing the efficacy of laparoscopic surgery, but majority focus on Caucasian population. The Asian perspective has been highlighted by a number of studies from China and Japan. To our knowledge, this study is the first from India to analyze the surgicopathological outcomes following laparoscopic surgery in endometrial carcinoma.
Predominantly a disease of the perimenopausal age group, endometrial carcinoma was most commonly detected in the same, with a median age being 56 years. Three patients presented with endometrial carcinoma at <40 years of age. All patients were thoroughly counseled and opted for comprehensive surgical staging including removal of bilateral ovaries. As noted in literature, Asian women have a higher body fat percentage than Caucasian women when compared to the same BMI.[7,8] However, we had a median BMI of 28.3 kg/m2, which is comparable with Caucasian population studies on endometrial carcinoma.[9,10]
Nearly 20% of the cases were diagnosed preoperatively using hysteroscopic-guided endometrial biopsy. None of the patients had malignant cells in the peritoneal fluid cytological analysis, thereby re-affirming the diagnostic role and safety of hysteroscopy in the preoperative diagnosis of endometrial carcinoma.[11] The median operative time and blood loss are comparable with studies existing in the literature,[12,13] and only a study by Malzoni et al.[9] has operative time and blood loss lesser than the current study. There were no intraoperative complications; however, four patients had postoperative pyrexia treated with antipyretics and antibiotics and one patient had paralytic ileus secondary to possibly prolonged surgical duration. Two patients developed lower-limb lymphedema secondary to extensive pelvic lymphadenectomy, which is reported in literature.[9] The safety with which the procedures were executed highlights the expertise of the operating surgeon, which has reflected in the very minimalistic complication rate. Although there were no wound infections or cases of incisional hernia, there was one case of isolated port-site metastasis. This is in accordance with reported incidence of incisional recurrences postlaparoscopy for endometrial carcinoma being <1%.[14]
With regard to the histopathological outcomes, none of the patients had a positive peritoneal cytology. Although not a part of the staging classification as outlined by FIGO in 2009, it is still recommended to collect peritoneal cytology as it is a significant marker of extrauterine disease.[15,16]. Endometroid type of endometroid carcinoma was the most common, as seen in literature with majority comprising Grade 1 lesions.
Lymphadenectomy in endometrial carcinoma is a controversial aspect of surgical staging, with many prospective studies negating any therapeutic benefits.[16,17] However, we performed systematic pelvic lymphadenectomy in all cases and coupled it with para-aortic lymphadenectomy in high-risk cases. The median number of pelvic and para-aortic lymph nodes was comparable to studies in existing literature.[9,18,19,20,21] Our center did not employ the sentinel node biopsy technique during the period of the current study; hence, data on that front are limited. The wide range in the para-aortic lymph node yield can be attributed to the long learning curve associated with laparoscopic transperitoneal lymphadenectomy. LVSI is another important prognostic marker. LVSI is independently associated with lymph node metastases in women with apparent early-stage endometrial cancer and an independent predictor of survival even after adjustment for the presence of lymph node metastases.[22,23] LVSI is associated with a higher grade of tumor histology, as evident in our study, with all cases being a high grade with metastasis to pelvic lymph node in four out of the seven cases in which lymph vascular space was invaded by tumor emboli.[24]
As is evident earlier and highlighted in Table 3, high grade and LVSI were found to be predictors of recurrence even in early-stage disease.[25] Despite initial adjuvant therapy and a comprehensive surgical staging, one must counsel patients of high grade and LVSI about the importance of posttreatment surveillance as recurrence is common.
The predicted 5-year survival rate as per the Kaplan–Meier survival rate curve is 95.45%, which is comparable with Caucasian studies.[9] The survival curve is depicted in Figure 1.
Figure 1.
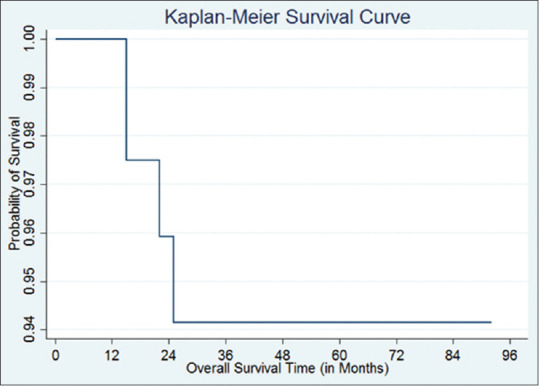
Overall survival curve
The limitations of this study are the retrospective nature and its inherent flaws. Furthermore, the median follow-up period of 27 months is inadequate and cannot be labeled as a long-term follow-up to assess survival. However, this being the first study to encompass laparoscopic surgical management of endometrial carcinoma from India, where the application of laparoscopy itself is limited, deserves credit. Furthermore, the minimalistic incidence of surgical complications is promising of the surgical skill. More data need to emerge on the role of minimal invasive management of endometrial carcinoma from India.
CONCLUSION
Laparoscopic management of early-stage endometrial carcinoma is a standard practice worldwide. However, there is still a paucity of data from the Indian subcontinent regarding the outcomes of laparoscopic surgery in endometrial carcinoma. The Asian perspective has been highlighted by a number of studies from China and Japan. To our knowledge, this study is the first from India to analyze the surgicopathological outcomes following laparoscopic surgery in endometrial carcinoma. The outcome of this study was comparable to studies conducted in Caucasian population.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Boyle P, Levin B. World Cancer Report 2008. Lyon: WHO; 2008. p. 428. [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Olawaiye AB, Mutch DG. Lymphnode staging update in the American Joint Committee on Cancer 8th Edition cancer staging manual. Gynecol Oncol. 2018;150:7–8. doi: 10.1016/j.ygyno.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Childers JM, Surwit EA. Combined laparoscopic and vaginal surgery for the management of two cases of Stage I endometrial cancer. Gynecol Oncol. 1992;45:46–51. doi: 10.1016/0090-8258(92)90489-6. [DOI] [PubMed] [Google Scholar]
- 5.Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30:695–700. doi: 10.1200/JCO.2011.38.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FIGO Committee on Gynecologic Oncology. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int J Gynaecol Obstet. 2014;125:97–8. doi: 10.1016/j.ijgo.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Pan WH, Flegal KM, Chang HY, Yeh WT, Yeh CJ, Lee WC. Body mass index and obesity-related metabolic disorders in Taiwanese and US whites and blacks: Implications for definitions of overweight and obesity for Asians. Am J Clin Nutr. 2004;79:31. doi: 10.1093/ajcn/79.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Deurenberg-Yap M, Schmidt G, van Staveren WA, Deurenberg P. The paradox of low body mass index and high body fat percentage among Chinese, Malays and Indians in Singapore. Int J Obes Relat Metab Disord. 2000;24:1011. doi: 10.1038/sj.ijo.0801353. [DOI] [PubMed] [Google Scholar]
- 9.Malzoni M, Tinelli R, Cosentino F, Perone C, Rasile M, Iuzzolino D, et al. Total laparoscopic hysterectomy versus abdominal hysterectomy with lymphadenectomy for early-stage endometrial cancer: A prospective randomized study. Gynecol Oncol. 2009;112:126. doi: 10.1016/j.ygyno.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Nezhat F, Yadav J, Rahaman J, Gretz H, Cohen C. Analysis of survival after laparoscopic management of endometrial cancer. J Minim Invasive Gynecol. 2008;15:181. doi: 10.1016/j.jmig.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Kudela M, Pilka R. Is there a real risk in patients with endometrial carcinoma undergoing diagnostic hysteroscopy (HSC)? Eur J Gynaecol Oncol. 2001;22:342. [PubMed] [Google Scholar]
- 12.Chiou HY, Chiu LH, Chen CH, Yen YK, Chang CW, Liu WM. Comparing robotic surgery with laparoscopy and laparotomy for endometrial cancer management: A cohort study. Int J Surg (London, England) 2015;13:17–22. doi: 10.1016/j.ijsu.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Ind TE, Marshall C, Hacking M, Harris M, Bishop L, Barton D, et al. Introducing robotic surgery into an endometrial cancer service – A prospective evaluation of clinical and economic outcomes in a UK institution. Int J Med Rob. 2016;12:137–44. doi: 10.1002/rcs.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogani G, Dowdy SC, Cliby WA, Gostout BS, Kumar S, Ghezzi F, et al. Incisional recurrences after endometrial cancer surgery. Anticancer Res. 2015;35:6097–104. [PubMed] [Google Scholar]
- 15.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynecol Obstet. 2009;105:103–4. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Frost JA, Webster KE, Bryant A, Morrison J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst Rev. 2017;10:CD007585. doi: 10.1002/14651858.CD007585.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Candido EC, Rangel Neto OF, Toledo MC, Torres JC, Cairo AA, Braganca JF, et al. Systematic lymphadenectomy for intermediate risk endometrial carcinoma treatment does not improve the oncological outcome. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100020. doi: 10.1016/j.eurox.2019.100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho YH, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Laparoscopic management of early uterine cancer: 10-year experience in Asan Medical Center. Gynecol Oncol. 2007;106:585. doi: 10.1016/j.ygyno.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Tozzi R, Malur S, Koehler C, Schneider A. Laparoscopy versus laparotomy in endometrial cancer:First analysis of survival of a randomized prospective study. J Minim Invasive Gynecol. 2005;12:130. doi: 10.1016/j.jmig.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Fram KM. Laparoscopically assisted vaginal hysterectomy versus abdominal hysterectomy in Stage I endometrial cancer. Int J Gynecol Cancer. 2002;12:57. doi: 10.1046/j.1525-1438.2002.01038.x. [DOI] [PubMed] [Google Scholar]
- 21.Palomba S, Falbo A, Mocciaro R, Russo T, Zullo F. Laparoscopic treatment for endometrial cancer: A meta-analysis of randomized controlled trials (RCTs) Gynecol Oncol. 2009;112:415. doi: 10.1016/j.ygyno.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Jorge S, Hou JY, Tergas AI, Burke WM, Huang Y, Hu JC, et al. Magnitude of risk for nodal metastasis associated with lymphvascular space invasion for endometrial cancer. Gynecol Oncol. 2016;140:387–93. doi: 10.1016/j.ygyno.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayhan A, Şahin H, Sari ME, Yalçin I, Haberal A, Meydanli MM. Prognostic significance of lymphovascular space invasion in low-risk endometrial cancer. Int J Gynecol Cancer. 2019;29:505–12. doi: 10.1136/ijgc-2018-000069. [DOI] [PubMed] [Google Scholar]
- 24.Sahin H, Meydanli MM, Sari ME, Kocaman E, Cuylan ZF, Yalcin I, et al. Recurrence patterns and prognostic factors in lymphovascular space invasion-positive endometrioid endometrial cancer surgically confined to the uterus. Taiwan J Obstet Gynecol. 2019;58:82–9. doi: 10.1016/j.tjog.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien DJ, Mooney EE, Foley M. Lymphovascular space involvement in early stage well-differentiated endometrial cancer is associated with increased mortality. BJOG. 2009;116:991. doi: 10.1111/j.1471-0528.2009.02162.x. [DOI] [PubMed] [Google Scholar]


