Abstract
Purpose
Many reports have described anomalous connections of the superior rectus (SR) with other extraocular rectus muscles, in which additional heads of the other three rectus muscles likely provided the connections. We examined how these connections are established during fetal development.
Methods
We analyzed paraffin-embedded horizontal sections from 25 late-stage fetuses. Horizontal sections are best suited for understanding the mediolateral relationships of muscle origins.
Results
We confirmed a common tendinous origin of the lateral rectus (LR), inferior rectus (IR) and medial rectus (MR) muscles that was separated from the SR origin. Notably, eight fetuses (32%) had tendinous or muscular connections between the SR and other rectus muscles that had one of four morphologies: (a) a thin tendon from the SR to the common tendon of the three rectus muscles (2 fetuses), (b) a thin tendon to the LR (one fetus), (c) a thin tendon to the inferior rectus muscle origin (two fetuses), and (d) SR muscle fibers arising from an additional head of the LR (three fetuses).
Conclusions
The SR seemed to issue a thin tendon that passed along the inferior or lateral side of the oculomotor nerve. Conversely, the LR and inferior rectus muscle were likely to carry a supernumerary bundle that reached the SR. The accessory head of the medial rectus muscle showed a stable morphology in that it seemed to also provide an anomalous double head. However, the presence of an accessory head in the LR was rare. In contrast with our previously published diagram of the orbital apex, the accessory head of the medial rectus muscle passed along the lateral side of the superior oblique.
Keywords: fetal orbit, anomaly, annular tendon, extraocular rectus muscles, superior rectus muscle, human fetus
Many studies have documented variations and anomalies of the human extraocular rectus muscles. Although there are some intermediate morphologies, there seem to be three major types: (a) a supernumerary or additional muscle originating from the annular tendon,1,2 (b) a connecting muscle “slip” or tendon between the rectus muscles,3 and (c) absence of the superior rectus (SR),4 lateral rectus (LR),5 inferior rectus (IR),6 or medial rectus (MR).7 Bergman's textbook summarized these variations.8 These variations tend to involve the upper muscles, such as the SR, the levator palpebrae superioris (LPS), and the superior oblique (SO).1,9 To better understand these variations and abnormalities, it is necessary to further analyze the annular tendon (annulus of Zinn) as a common origin of extraocular muscles, because morphologic variations may involve muscle origins from this tendon. Previous gross observations of this tendon only provided limited information.
Although research on the annular tendon dates back to the eighteenth century,10 there remains gaps in our understanding of this structure. Based on current gross anatomic knowledge, the annular tendon (also known as the common tendinous ring or annulus of Zinn) is divided into two parts. The lower part of the ring, known as the ligament or tendon of Zinn, gives origin to the IR, parts of the MR, and the lower head of the LR. The upper part of the ring, known as the superior tendon of Lockwood, gives origin to the SR, the rest of the MR, and the upper head of the LR.11 However, one of the most recent studies suggested an independent origin of the SR.12 We also described a consistent accessory head of the MR near an origin of the SO or LPS. These interpretations were based on semiserial sagittal sections along the long axis of the orbit in seven late-stage fetuses. However, the section orientation and the 50- to 100-µm intervals between sections likely led to our failure to identify a thin tendinous connection between the rectus muscles. It is therefore necessary to examine horizontal semiserial sections to clarify the suspected tendinous connection and the arrangement of muscle origins from the superior margin of the optic canal opening.
The present study aims to verify previous reports regarding the common origin of the extraocular rectus muscles, with special reference to topographical relations between specific attachments of individual muscles. To establish an embryological basis for anomalous slip and connection of extraocular muscles, we examined 25 late-stage fetuses.
Methods
This study was performed in accordance with the provisions of the fifth revision of the Declaration of Helsinki, as revised in Edinburgh in 2000. We examined histological sections from 25 late-stage fetuses, which included contralateral sides of 7 previously examined fetuses.12 The mean gestational age was 32 weeks (range, 28–40 weeks) and the median crown–rump length (CRL) was 276 mm (range, 217–340 mm). All heads were cut along the midsagittal line, and a left or right half was used for analysis. After routine procedures for paraffin-embedded histology, semiserial 10-µm sections were cut horizontally at 50 µm intervals. Because the orbital content was more than 5 mm along the superoinferior axis, at least 100 sections per orbit were examined, corresponding with almost 3000 sections for 25 fetuses. All sections were stained with hematoxylin and eosin. All fetuses were part of the large collection kept at the Embryology Institute of the Universidad Complutense, Madrid, and were obtained from its Department of Obstetrics. All fetuses were obtained as a result of miscarriages, ectopic pregnancies, and/or abortion. The university ethics committee approved this study (B08/374). Sagittal sections along the long axis of the orbit from another fetus (CRL, 276 mm), which was prepared for our recent study,12 are shown for comparison.
In the present study, the accessory head of the MR was defined as a consistent muscle bundle of arising from the bony optic canal exit in the superomedial side of the optic nerve and merging with the MR main head. The latter head is originated from a common tendon for the LR, IR, and MR in the future superior orbital fissure. Conversely, an accessory head or additional bundle of the SR should originate from a site distant from the optic nerve exit.
Results
Normal Topographical Anatomy
Figure 1 (sagittal sections) and Figure 2 (horizontal sections) show the normal topographical anatomy used to evaluate advantage and disadvantage in each of sectional planes. The sagittal sections show the main and accessory head of the MR within a single section (Fig. 1D). The inferior end of the SR origin appears as a triangular fibrous mass (Fig. 1C). However, a few semiserial sagittal sections contained the SR origin, identified as a thin muscle bundle along the superior margin of the optic nerve exit (Fig. 1B). In contrast, even when cut at 100-µm intervals, many more horizontal sections show the SR origin extending along the mediolateral axis and surrounding the optic nerve exit (Figs. 2A–D). Our analysis of more than 100 sections revealed that horizontal sections could be used to identify abnormalities at and around the SR origin. In addition, our analysis of the sagittal sections indicated the MR was apparently attached to the IR origin rather than sharing a site on the sphenoid (Fig. 1C), and the IR was apparently attached to the LR (Fig. 1A). Thus, the common origin of these three rectus muscles did not appear in any single section. In contrast, a single horizontal section clearly exhibited a common origin, in which the MR and LR sandwiched the IR (Fig. 2G).
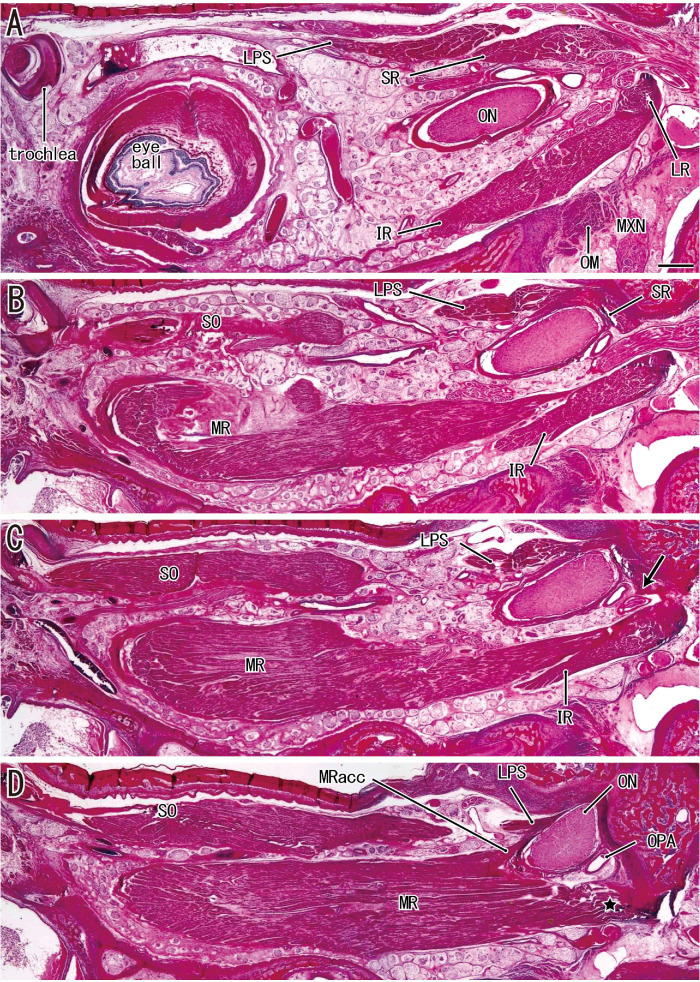
Figure 1.
Sagittal sections along the long axis of the orbit in a fetus with a CRL of 276 mm. (A) The most lateral site and (D) the most medial site. (A) The most posterior part of the LR. (B and C) The SR and LPS originate from the superior margin of the exit of the optic nerve (ON). (C, arrow) The inferomedial end of the SR origin. (D) The major and accessory heads of the MR (MRacc). All images were prepared at the same magnification (scale bar in A, 1 mm; ×1 objective). MXN, maxillary nerve; OM, orbital muscle (smooth muscle); OPA, ophthalmic artery.
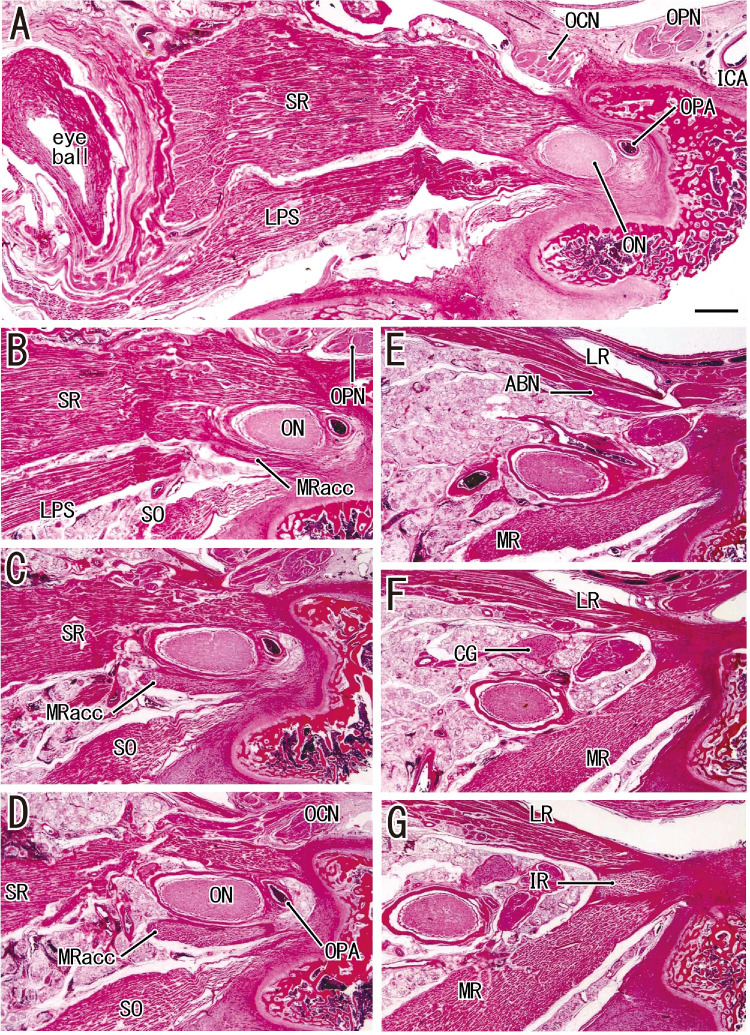
Figure 2.
Horizontal sections in a fetus with a CRL of 272 mm (normal fetus). (A) The most superior site. (G) The most inferior site. (A and B) The origin of the SR surrounds the optic nerve exit. (B) The accessory head of the MR (MRacc) originates from the sphenoid near the SR origin. (D) It provides an independent muscle bundle. (E) The main head of the MR is distant from and inferior to the accessory head. (G) The MR and LR sandwich the IR in the common tendon. ABN, abducent nerve; ICA, internal carotid artery; OCN, oculomotor nerve; ON, optic nerve; OPA, ophthalmic artery; OPN, ophthalmic nerve.
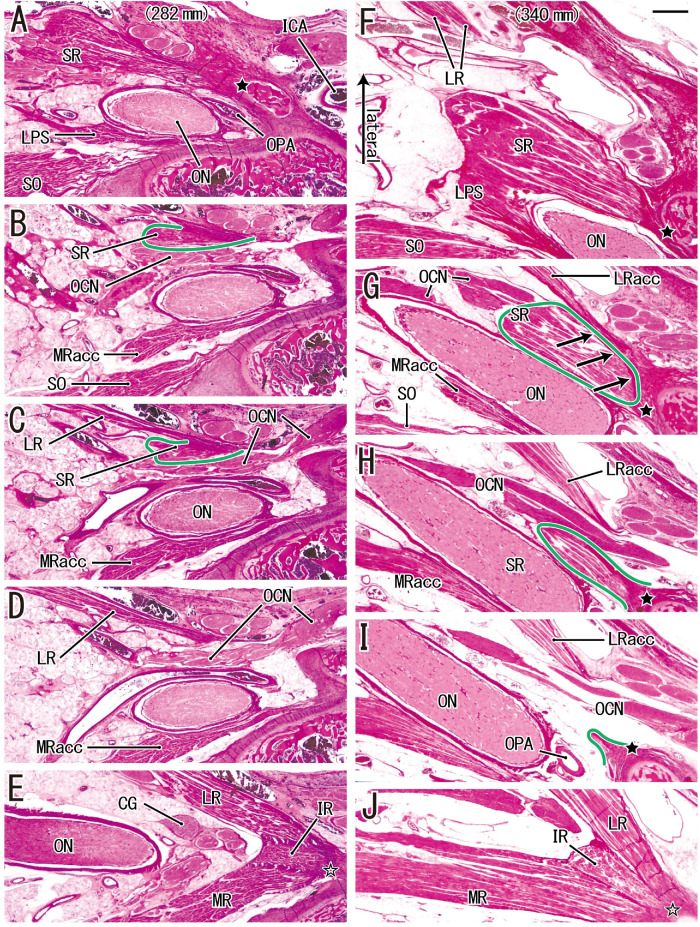
Figures 3, 4, and 5 show fetuses with CRLs of 217 mm, 264 mm, 282 mm, and 340 mm. These four specimens each contain an anomalous tendon or muscle slip (described in detail in the subsection below). A common origin of the three rectus muscles (Figs. 3G; 4H; and 5E, J) were clearly discriminable from the SR origin (Figs. 3B, C; 4A, B; and 5A, F). In this common origin, the IR origin was between the LR origin and the major head of the MR. An accessory head of the MR passed along the lateral side of the SO origin (Figs. 3C and 5B) and attached to a sphenoid on the inferomedial side of the origins of the SR and LPS. The accessory head was more than 1 mm superior to the major head of the MR (Figs. 3G; 4H; and 5E, J). Thus, no single section showed both heads of the MR. Without a lateral bony wall, the orbital fissure opened widely into the middle of the cranial fossa (Fig. 3H). Thus, the superior petrosal sinus attached to the cranial nerves without a bony separation (Fig. 3H). Moreover, the future cavernous portion of the internal carotid artery was very near the orbital apex (2–3 mm posterior; Fig. 5A). Therefore, fetal and adult orbital apexes have different topographical relations.
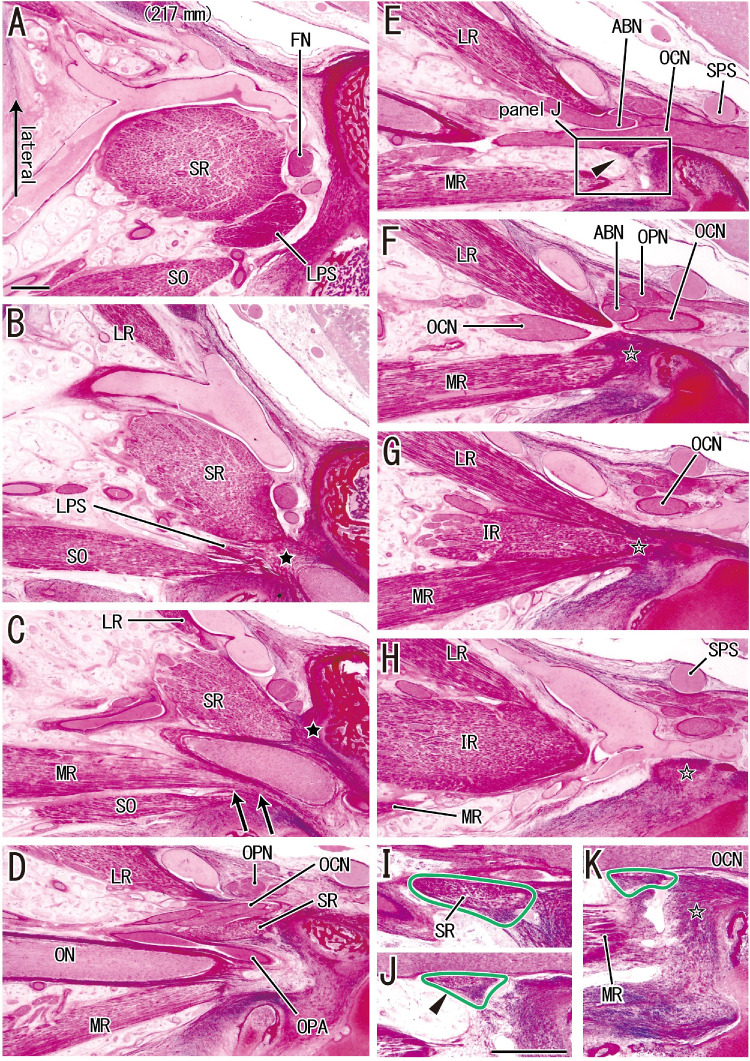
Figure 3.
A thin tendon from the SR origin to the common tendon for the other three rectus muscles in a fetus with a CRL of 217 mm. (A) The most superior site and (H) the most inferior site. (B and C) The SR originates from the sphenoid (black star in each image). (F–H) The LR, IR, and MR have a common origin (open star in K). (G) The common origin. The IR is between the LR and MR. (C) The accessory head of the MR (arrows) is adjacent to the lateral aspect of the origin of the SO. (D and E) The accessory head is detached. (F) An inferior view shows that the major head of the MR originates from the bone. (E) A thin fibrous band (arrowhead) that is shown at a higher magnification in (J). Images (I and K) are near sections of (J). The fibrous band (surrounded by a green line) extends from the inferior end of the SR (I) to the common tendinous origin for three rectus muscles (open star in F–H and K). (A–H) At ×1 objective) and (I–K) at ×2 objective were prepared at the same magnification, respectively (scale bars in A and J = 1 mm). ABN, abducent nerve; FN, frontal nerve; OCN, oculomotor nerve; ON, optic nerve; OPA, ophthalmic artery; OPN, ophthalmic nerve; SPS, superior petrosal sinus.
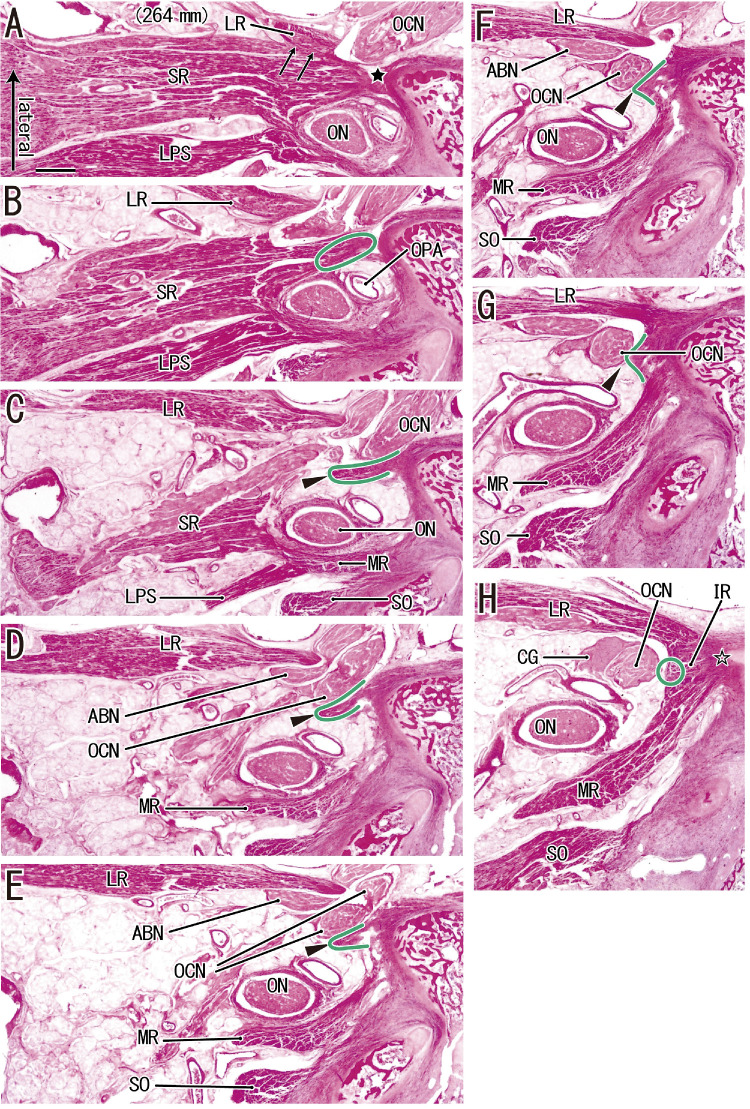
Figure 4.
SR–LR connection consists of a thin tendon connecting the SR with the common tendon of the three rectus muscles in a fetus with a CRL of 264 mm. (A) The most superior site and (H) the most inferior site. (A) The SR originates from the sphenoid (black star). (H) The LR, IR, and MR have a common origin (open star). (A) An additional head of the LR originates from the lateral margin of the SR (arrows). (C, D, and E) The abducent and oculomotor nerves (ABN, OCN) pass between the major and additional heads of the LR. (C, D, and G) A thin tendon (arrowheads; surrounded by green line) extends from the inferolateral end of the SR origin (C), via the medial side of the OCN (D), to a superior margin of the common tendon (G). All images were prepared at the same magnification (scale bar in A, 1 mm; ×1 objective). CG, ciliary ganglion; ON, optic nerve; OPA, ophthalmic artery.
Figure 5.
An additional origin of the SR from a part of the LR in a fetus with a CRL of 282 mm (A–E) and a fetus with a CRL of 340 mm (F–J). (A and F) The most superior site of each fetus. (A, F, and I) The SR originates from the sphenoid (black star in each image). (E and J) The LR, IR, and MR have a common origin (open star in each image). (B and C) An inferolateral marginal part of the SR (surrounded by a green line) originates from the superior margin of the LR. Much inferiorly (1.0 mm), (E) shows that major parts of the LR and MR sandwich the IR origin. In contrast, (G) shows that an inferior part of the SR (surrounded by a green line) originates from an additional head of the LR (arrows). (H and I) The accessory head (LRacc) is detached from the bone. Much inferiorly (1.3 mm), (J) shows a major head of the LR. All images were prepared at the same magnification (scale bar in F, 1 mm; ×1 objective). ICA, internal carotid artery; MRacc, accessory head of the MR; OCN, oculomotor nerve; ON, optic nerve; OPA, ophthalmic artery.
Abnormal Connection of the SR
Notably, we found muscular or tendinous connections between the SR and another rectus near the origin in 8 of the 25 fetuses (CRLs of 217, 230, 256, 264, 282, 296, 326, and 340 mm). We classified each connection or bridge as having one of four morphologies: (a) a thin tendon from the SR to the common tendon of the three rectus muscles (two fetuses; Fig. 3E), (b) a thin tendon to the SR that was connected with the LR (one fetus; Fig. 5C), (c) a thin tendon from the SR to the IR origin (two fetuses; Figs. 4C–G), or (d) an additional head of the LR that connected with the SR (three fetuses; Fig. 5G). These connecting tendons from the SR usually rode over the oculomotor and/or abducent nerves, but often passed along the medial side (Figs. 4F, G). Figure 6 provides a schematic summary of these connections.
Figure 6.
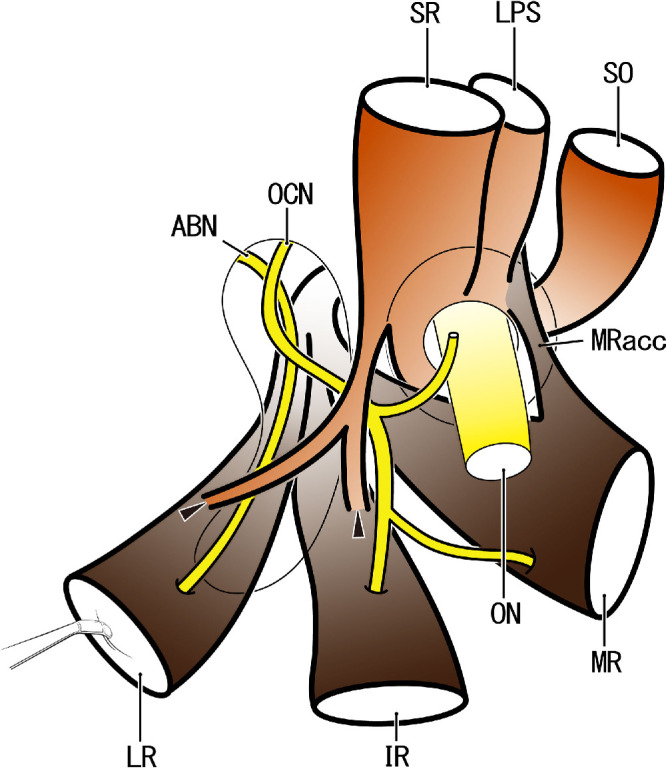
Extraocular muscle origins and their anomalous connections: a revision of our previous diagram (IOVS 2019; 60:4573). Anterior view of the right orbital apex. The SR originates from the lateral half of the optic canal opening, and the LR, IR, and MR muscles provide a common tendon originating from the medial wall of the orbital fissure. The MR has an accessory head (MRacc) that originates from the superomedial margin of the optic canal opening. In contrast to our previous diagram, the MRacc passes along the lateral side of the origin of the SO. Thin tendons likely connect the SR and the LR and/or IR (arrowheads). The connection is usually over the abducent and oculomotor nerve (ABN, OCN), but likely passes along the inferior side of the nerve. ON, optic nerve.
Discussion
Gray's Anatomy 11 described that the annular tendon is divided into two parts: (1) the lower part for origins of the IR, parts of the MR and the lower head of the LR and (2) the upper part for the SR, the rest of the MR, and the upper head of the LR. In near-term fetuses, we had also demonstrated a similar morphology12: (1) the lower part for the IR, LR, and major parts of the MR and (2) the upper part for the SR, the rest of the MR and the upper head of the LR (an additional head of the MR). However, we had not found the upper head of the LR originating near the SR origin.12 The present observations ensured the consistent presence of a common tendinous origin for the three rectus muscles (LR, MR, and IR) that was independent of the origin of the SR. Because the accessory head of the MR was more than 1 mm superior to the major head of the MR, no single horizontal section showed both heads together, in contrast with sagittal sections; this factor is a limitation of total reliance on horizontal sections. However, the present horizontal sections revealed details of the topographical relationships of muscle origins along the optic nerve exit. Thus, the present results indicate a need to revise the location of the accessory head of the MR reported in our previous schematic diagram.12 In fact, the accessory head of the MR passed along the lateral side (not the medial side) of the SO. Our revised diagram (Fig. 6) emphasizes that the muscular origin of the IR was sandwiched by the tendinous origins of the LR and MR. Although it was consistently seen, the MR accessory head seemed to only rarely grow an independent and thick muscle slip that is evident in radiology or dissection.13 Previous examinations might have interpreted the accessory head as a part of the annular tendon, a connection between the MR and LPS, because of its proximity.
As a basis of extraocular muscle anomalies, we found that 8 of 25 fetuses had a tendinous or muscular connection or bridge between the SR and the other rectus muscles. There was a thin tendon from the SR to the common tendon of the three rectus muscles or one of the rectus muscles in five fetuses and the SR muscle fibers arose from an additional head of the LR in three fetuses. The former anomaly contained a tendinous connection between the SR and LR in one fetus and between the SR and IR in two fetuses. Although we did not find the coexistence of these two types of connections, Kakizaki et al.3 described a 45-year-old female cadaver who had a tendon from the SR muscle belly and another tendon from the LR origin that joined to provide a tendinous band inserted in the IR muscle belly. This female had no reported ocular movement disorders during her lifetime. Therefore, the coexistence and/or fusion between tendinous connections may be likely and is apparently asymptomatic. Rather than a tendinous connection, several reports identified muscular connections between the SR and IR.2,9,14 These courses of the muscular slip appeared similar to the tendinous connection identified here.
We also identified an anomaly in three fetuses in which SR muscle fibers arose from an additional head of the LR. This might grow into the accessory LR or SR of adults, as shown by Liao et al.,15 Park and Oh,16 and Nayak et al.17 However, in contrast with Schaeffer18 and Tawfik and Dutton,19 the additional head of the LR in fetuses was not usual. If present in adults, the additional head of the LR could be misinterpreted as being a part of the annular tendon, that is, a connection from the SR, via the LR and IR and to the MR. Conversely, a classical concept of the annular tendon might mislead an imagination of the usual existence of the LR additional head. Likewise, the present tendinous connections between the SR and other rectus muscles (see previous paragraph) may also be interpreted as being a part of the annular tendon because they were near the muscle origins from the sphenoid.
There are many reports of aplasia or dysplasia of the extraocular rectus muscles4–7,20–26 and of anomalous insertions.1,16,27–31 However, none of our fetuses had these anomalies, probably owing to the limited numbers of specimens we examined. Likewise, we did not find the well-known insertion anomaly of the LR, (i.e., a retractor bulbi that is an additional slip of the LR and provides a muscular funnel around the optic nerve's exit from the eye ball).8 This aplasia or dysplasia is considered to be caused by a failure of innervation during early development (reviewed by Tawfik and Dutton19). Determination of the normal muscle insertions of a fetus seems to depend on the timing and sequence of developing muscles and the rotating eye ball.32 A muscle insertion can change significantly owing to changing topographical relationships during growth.33 Indeed, the initial extraocular muscles can have transient origins from the optic or oculomotor nerve because of the delayed development of cartilage.12 Tawfik and Dutton19 reviewed such a muscle insertion to a nerve.
Study Limitations
The major limitation of this study was that we had no observations of complete serial sections. However, compared with semiserial sagittal sections, the semiserial horizontal sections presented here successfully demonstrated tendinous connections between the rectus muscles. Our examination of a relatively small number of fetuses was another limitation, although the collection at the Embryology Institute of the Universidad Complutense is extensive and might have the greatest number of such samples in the world. Although the fetuses we examined were the products of miscarriages or ectopic pregnancies, we found no common abnormalities of the head, such as cleft palate or underdeveloped auricles.
Conclusions
The SR likely issued a thin tendon that passed along the inferior or lateral side of the oculomotor nerve. Conversely, the LR and IR likely carried a supernumerary bundle that reached the SR. The accessory head of the MR, which is a stable morphology, seemed to also provide an anomalous double head. However, an accessory head of the LR was rare. An accessory head of the MR passed along the lateral side (not the medial side) of the SO.
Acknowledgments
Disclosure: J.H. Kim, None; S. Hayashi, None; M. Yamamoto, None; G. Murakami, None; J. Wilting, None; J.F. Rodríguez-Vázquez, None
References
- 1. Haladaj R, Wysiadecki G, Tubbs RS, Topol M. Anatomical variations of the levator palpebrae supeioris, including observations on its innervation and intramuscular nerves’ distribution pattern. Ann Anat. 2020; 228: 151439. [DOI] [PubMed] [Google Scholar]
- 2. von Lüdinghausen M. Bilateral supernumerary rectus muscles of the orbit. Clin Anat. 1998; 11: 271–277. [DOI] [PubMed] [Google Scholar]
- 3. Kakizaki H, Zako M, Nakano T, Asamoto K, Miyaishi O, Iwaki M. An anomalous muscle linking superior and inferior rectus muscles in the orbit. Anat Sci Int. 2006; 81: 197–199. [DOI] [PubMed] [Google Scholar]
- 4. Mather TR, Saunders RA.. Congenital absence of the superior rectus muscle: a case report. J Pediatr Ophthalmol Strabismus. 1987; 24: 291–295. [DOI] [PubMed] [Google Scholar]
- 5. Zőller CC, Gräf M, Kaufmann H. Unilateral aplasia of a lateral rectus muscle. Lin Monbl Augenheikd. 2001; 218: 55–60. [DOI] [PubMed] [Google Scholar]
- 6. Pimenides D, Young S, Minty I, Spratt J, Tiffin PA. Familial aplasia of the inferior rectus muscles. J Pediatr Ophthalmol Strabismus. 2005; 42: 222–227. [DOI] [PubMed] [Google Scholar]
- 7. Lee DW, Lee S, Ahn M. Congenital bilateral medial rectus muscle aplasia. J Pediatr Ophthalmol Strabismus. 2013; 50: 134–135. [DOI] [PubMed] [Google Scholar]
- 8. Kacabiyik N. Orbital muscles. In: Tubbs RS, Shoja MM, Loukas M. Bergman's Comprehensive Encyclopedia of Human Anatomic Variation. 1st ed. Hoboken, NJ: Wiley-Blackwell; 2016: 207–211. [Google Scholar]
- 9. Haladaj R, Wysiadecki G, Polguj M, Topol M. Bilateral muscular slips between superior and inferior rectus muscles: a case report with discussion on classification of accessory rectus muscles within the orbit. Surg Radiol Anat. 2018; 40: 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zampieri F, Marrone D, Zanatta A. Should the annular tendon of the eye be named ‘annulus of Zinn’ or ‘of Valsalva’? Acta Ophthalmol. 2015; 93: 97–99. [DOI] [PubMed] [Google Scholar]
- 11. Standring S. Gray's Anatomy: The Anatomical Basis of Clinical Practice. 41st ed. Edinburgh, London: Churchill Livingstone; 2015: 670–671. [Google Scholar]
- 12. Naito T, Cho KH, Yamamoto M, Hirouchi H, Murakami G, Hayashi S, Abe SI. Examination of the topographical anatomy and fetal development of the tendinous annulus of Zinn for a common origin of the extraocular recti. Invest Ophthal Vis Sci. 2019; 60: 4564–4573. [DOI] [PubMed] [Google Scholar]
- 13. Fernández-de-Luna ML, Rodríguez-Martínez AC, Mohamed-Noriega J, Fernández-de-Luna CA, Mohamed-Hamsho J. Double-bellied medial rectus muscle in a patient with Down syndrome and congenital esotropia. Surg Radiol Anat. 2020; 42: 859–861. [DOI] [PubMed] [Google Scholar]
- 14. Fichter N, von Arx G, Kirsch EC. Accessory lateral rectus muscle graves’ orbitopathy: a case report. Clin Neuroradiol. 2014; 24: 277–279. [DOI] [PubMed] [Google Scholar]
- 15. Liao YJ, Hwang JJ.. Accessory lateral rectus in a patient with normal ocular motor control. J Neuroophthalmol. 2014; 34: 153–154. [DOI] [PubMed] [Google Scholar]
- 16. Park CY, Oh SY.. Accessory lateral rectus muscle in a patient with congenital third-nerve palsy. Am J Ophthalmol. 2003; 136: 355–356. [DOI] [PubMed] [Google Scholar]
- 17. Nayak SB, Shetty SD, Kumar N, Aithal AP. Double-bellied superior rectus muscle. Surg Radiol Anat. 2019; 41: 713–715. [DOI] [PubMed] [Google Scholar]
- 18. Schaeffer JP. Morris’ Human Anatomy. 11th ed. New York: Blakiston; 1953: 1248. [Google Scholar]
- 19. Tawfik HA, Dutton JJ.. Embryologic and fetal development of the human orbit. Ophthalmic Plast. Reconstr Surg. 2018; 34: 405–421. [DOI] [PubMed] [Google Scholar]
- 20. Drummond GT, Keech RV. Absent and anomalous superior oblique and superior rectus muscles. Can J Ophthalmol. 1989; 24: 275–279. [PubMed] [Google Scholar]
- 21. Matsuo T, Watanabe T, Furuse T, Hasebe S, Ohtsuki H. Case report and literature review of inferior rectus muscle aplasia in 16 Japanese patients. Strabismus. 2009; 17: 66–74. [DOI] [PubMed] [Google Scholar]
- 22. Cuttone JM, Brazis PT, Miller MT, Folk ER. Absence of the superior rectus muscle in Apert's syndrome. J Pediatr Ophthalmol Strabismus. 1979; 16: 349–354. [DOI] [PubMed] [Google Scholar]
- 23. Murthy R. Congenital dystrophic medial rectus muscle. Indian J Ophthalmol. 2017; 65: 62–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Almahmoudi F, Khan AO.. Inferior oblique anterior transposition for the unilateral hypertropia associated with bilateral inferior rectus muscle aplasia. J AAPOS. 2014; 18: 301–303. [DOI] [PubMed] [Google Scholar]
- 25. Astle WF, Hill VE, Ells AL, Chi NT. Martinovic E. Congenital absence of the inferior rectus muscle-diagnosis and management. J AAPOS. 2003; 7: 339–344. [DOI] [PubMed] [Google Scholar]
- 26. Muňoz M. Congenital absence of the inferior rectus muscle. Am J Ophthalmol. 1996; 121: 327–329. [DOI] [PubMed] [Google Scholar]
- 27. Rosenbaum AL, Jampolsky A.. Pseudoparalysis caused by anomalous insertion of superior rectus muscle. Arch Ophthalmol. 1975; 93: 535–537. [DOI] [PubMed] [Google Scholar]
- 28. Ozkan SB, Cakmak H, Dayanir V. Fibrotic superior oblique and rectus rectus muscles with an accessory tissue band. J AAPOS. 2007; 11: 491–494. [DOI] [PubMed] [Google Scholar]
- 29. Verma R, Hertle RW.. An interesting case of bilateral bifid insertion of superior rectus muscle as an intra-operative finding in a patient with oculocutaneous albinism. Surg Radiol Anat. 2014; 36: 605–606. [DOI] [PubMed] [Google Scholar]
- 30. Coats DK, Ou R.. Anomalous medial rectus muscle insertion in a child with craniosynostosis. Binocul Vis Strabismus Q. 2001; 16: 119–120. [PubMed] [Google Scholar]
- 31. Okano M, Matsuo T, Konishi H, Hasebe S, Tadokoro Y, Ohtsuki H. Anomalous posterior insertion of medial rectus muscle simulating congenital oculomotor palsy. Jpn J Ophthalmol. 1990; 34: 275–279. [PubMed] [Google Scholar]
- 32. Katori Y, Rodríguez-Vázquez JF, Kawase T, Murakami G, Cho BH, Abe S. Early fetal development of hard tissue pulleys for the human superior oblique and tensor veli palatini muscles. Ann Anat. 2011; 193: 127–133. [DOI] [PubMed] [Google Scholar]
- 33. Naito M, Suzuki R, Abe H, Rodríguez-Vázquez JF, Murakami G, Aizawa S. Fetal development of the human obturator internus muscle with special reference to the tendon and pulley. Anat Rec. 2015; 298: 1282–1293. [DOI] [PubMed] [Google Scholar]