Highlights
-
•
Diosmetin suppresses the development of DNCB-induced atopic dermatitis.
-
•
Diosmetin inhibits the IL-4 induced activation of JAK/STAT pathway.
-
•
Diosmetin inhibits the LPS induced activation of ERK, p38 and JNK pathway.
-
•
Diosmetin inhibits NO production and iNOS expression by LPS in RAW 264.7 cells.
Keywords: Diosmetin, Atopic dermatitis, Macrophage, Inflammation, Lipopolysaccharide
Abstract
Diosmetin, a citrus flavonoid, has a variety of therapeutic properties such as antibacterial, anti-inflammatory and antioxidant effects. However, the effect of diosmetin on atopic dermatitis (AD) development has not been reported. This study thus aims to investigate whether diosmetin possesses inhibitory effects on AD development. A dinitrochlorobenzene (DNCB)-induced AD mouse model was used to evaluate the effects of diosmetin on AD development. Treatment with diosmetin significantly reduced the dermatitis score, thickness of epidermis and dermis and number of mast cells in comparison with the untreated group. Furthermore, immunohistochemical analysis using an anti-F4/80 antibody demonstrated that diosmetin significantly suppressed macrophage infiltration into the AD lesion. It was observed that the levels of pro-inflammatory cytokines (TNF-α, IL-4 and IL-1β) in skin lesion decreased in response to treatment with diosmetin. In addition, the anti-inflammatory effect of diosmetin was evaluated in LPS- or IL-4-induced a mouse macrophage cell line (raw 264.7). Diosmetin inhibited the production of nitric oxide and decreased the expression of inducible nitric oxide synthase (iNOS). Diosmetin not only suppressed the phosphorylation of MAP kinase (ERK 1/2, p38 and JNK) but the activation of JAK/STAT signaling. The mRNA analysis demonstrated that diosmetin also reduced the level of inflammatory cytokines such as IL-1β and IL-6. Collectively, these results demonstrate that diosmetin exhibits the inhibitory effect on AD, suggesting that diosmetin may be a potential therapeutic agent for this atopic disorder.
1. Introduction
Atopy refers to the predisposition to become sensitized and produce IgE antibodies in response to commonly occurring allergens [1]. Atopic diseases include atopic dermatitis (AD), asthma and allergic rhino-conjunctivitis, and result due to excessive immune responses to usually harmless substances [2]. This hypersensitivity reaction is divided into four types. Among them, type I hypersensitivity includes allergic asthma and rhinitis and involves the IgE-mediated release of histamines and other inflammatory mediators from mast cells and basophils [3]. AD is the most common atopic disease [4]. Symptoms of AD include erythema, diffuse pruritic skin lesions, and occasional flares, often without known causes [5], [6]. An atopic immune response involves T cells and antigen-presenting cells (APCs) such as B cells and neutrophils. The APCs bind and present epitopes of allergens to the T cells to evoke cell-mediated responses and maintain immune responses [7]. AD is a Th2-mediated hyper-immune response [8]. In AD, T cells excessively differentiate to Th2 cells and induce both IgE synthesis and mediate mast cell differentiation through Th2 cytokines such as IL-4, IL-5, and IL-13 [9]. Histamine secreted by mast cells causes itching, a typical symptom of AD [10]. Sustained inflammation and pruritus can result in excessive scratching, damaging the skin barrier, and exposing it to external microbes such as bacteria [11]. Lipopolysaccharide (LPS) present on the outer membrane of Gram-negative bacteria acts as an endotoxin and increases the production of pro-inflammatory cytokines such as TNF-a, IL-1b, and IL-6 in macrophage, which induces the expression of inducible nitric oxide synthase (iNOS). The induction of iNOS produces a large amount of nitric oxide (NO), which promotes tissue damage, edema, and inflammatory responses [12], [13].
Therefore, inhibition of the inflammatory response caused by IL-4 and LPS in AD may be important for the prevention and treatment of atopic diseases. The representative treatment of AD is steroids that have excellent anti-inflammatory effects. However, its use is limited due to various side effects and the development of resistance as a result of long-term usage [14]. The incidence of AD is steadily increasing but no suitable treatment is available. The development of effective therapeutics is thus required.
Globally, multiple groups have explored the use of natural products to inhibit and suppress inflammatory responses to allergens for the treatment of AD [14], [15]. “Flavonoids,” derived from the Latin word “flavus,” means yellow. It is a secondary metabolite of plants and fungi. Diosmetin is a flavonoid that is contained in some medicinal herbs such as oregano and is rich in citrus fruits [16], [17], [18]. Several known therapeutic effects of diosmetin are its antibacterial, anti-inflammatory and antioxidant properties. Previous studies on the effects of diosmetin reported that it also had anti-cancer effects in selected cancer cells and anti-inflammatory effects in hepatocytes [19], [20], [21]. However, its anti-inflammatory and anti-atopic effects are unknown.
In this study, we tested the anti-inflammatory and anti-atopic activity of diosmetin in a mouse macrophage cell line (raw 264.7). Its ability to complement AD treatment using steroids to reduce the side effects and development of resistance to steroids was also tested. In a DNCB-induced AD mouse model, the anti-inflammatory and anti-atopic properties of diosmetin were evaluated to determine its potential as a therapeutic candidate in AD treatment.
2. Materials and methods
2.1. Mice
All animal experiments were performed with approval (CBNU-2019-00254) from the Animal Care Committee of Jeonbuk National University. Specific pathogen-free male 6-week-old male BALB/c mice were purchased from Samtako Bio Korea Co., ltd. (Osan, Korea). All mice were fed with ad libitum access to a standard diet (PMI Lab diet, Richmond, IN, USA) and water.
2.2. Atopic dermatitis model and diosmetin-treatment
Before the experiment, anesthetization the dorsal skin of the mouse was shaved for the first induction of AD in BALB/c mice. Mice were sensitization with 200 μL of 1% DNCB in acetone/olive oil (3:1) for three days (day 1, 3 and 5). After 7 days from first induction, 0.2% DNCB (day 7, 9 and 11) and 0.5% DNCB (day 17, 20, 23, 26 and 30) were painted onto the dorsal skin of the mice for challenge. The 6-week-old male BALB/c mice were divided into four groups (normal group, DNCB-sensitized group, diosmetin-treated group with DNCB-sensitized mice, diosmetin-treated group) of 5 mice per group. On day 31, all mice were sacrificed. Diosmetin (5.0 mg/kg, one time every day, Sigma-Aldrich, St. Louis, MO, USA) was intraperitoneally injected for two weeks from fourteen days after atopic dermatitis challenged. As a control, equal amounts of PBS were injected in the same manner.
2.3. Histological analysis
For hematoxylin and eosin (H&E) staining, skin tissues were fixed overnight in 4% paraformaldehyde. After tissue processing using standard procedures, samples were embedded in paraffin, and cut into 5 μm sections followed by H&E staining for measuring the skin thickness. The number of mast cells were counted followed by toluidine blue staining. For immunofluorescence studies, skin tissues were fixed in 4% paraformaldehyde for 4 h, dehydrated in 20% sucrose solution overnight, and embedded in tissue freezing medium (Leica, Wetzlar, Germany). Frozen blocks were cut into 25 μm sections. The sectioned samples were blocked with 5% donkey (or goat) serum in 0.3% Triton X-100 in phosphate-buffered saline (PBS) for 3 h at room temperature (RT) and then incubated overnight at 4 °C with the following primary antibodies: anti-F4/80 (Rat, 14-4801-82, e Bioscience), anti-phospho-STAT6 (Rabbit, 56554, Cell Signaling Technology), anti-iNOS (Rabbit, 13120, Cell Signaling Technology). After several washes, the samples were incubated for 2 h at RT with the following secondary antibodies: Cy3-conjugated donkey anti-Rat IgG, Cy3-conjugated donkey anti-rabbit IgG (all from Jackson ImmunoResearch, West Grove, PA, USA). Nuclei were stained with 4′6-diamidino-2-phenylindole. The samples were then mounted in Fluorescent Mounting Medium (Dako, Carpinteria, CA, USA) and immunofluorescent images were acquired using a laser-scanning confocal microscope (LSM 880; Carl Zeiss, Jena, Germany).
2.4. Cell culture and reagents
The mouse macrophage cell line raw 264.7 was obtained from the Korea Cell Line Bank (KCLB, No.40071, Seoul, Korea). Raw 264.7 cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Atlas Biologicals, For Collins, CO, USA), 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich). Cells were incubated at 37 °C with 5% CO2. Diosmetin was dissolved in dimethyl sulfoxide to obtain a 200 mM stock solution and stored at −20 °C.
2.5. Western blotting
At the indicated time, raw 264.7 cells were homogenized in ice-cold lysis buffer containing a protease inhibitor cocktail (Sigma-aldrich). Each protein was separated with SDS-PAGE and transferred to nitrocellulose membranes. After blocking with 5% skim milk, the membranes were incubated with the following primary antibodies in blocking buffer overnight at 4 °C. The primary antibodies used for immunoblotting were anti-iNOS (13120, Cell Signaling Technology), anti-ERK 1/2 (ab17942, abcam), anti-phospho-ERK 1/2 (ab50011, abcam), anti-p38(9212, Cell Signaling Technology), anti-phospho-p38 (9211S, Cell Signaling Technology), anti-JNK(06-748, Upstate), anti-phospho-JNK (9255S, Cell Signaling Technology), anti-NF-κB p65(3033, Cell Signaling Technology), anti-STAT1 (sc-346, Santa Cruz Biotechnology), anti-phospho-STAT1 (9167, Cell signaling Technology), anti-JAK1 (3344, Cell Signaling Technology), anti-phospho-JAK1 (74129, Cell Signaling Technology), anti-JAK3(8863, Cell Signaling Technology), anti-phospho-JAK3 (5031, Cell Signaling Technology), anti-STAT6(5397, Cell Signaling Technology), anti-phospho-STAT6 (56554, Cell Signaling Technology), and anti-β-actin (mouse monoclonal; Sigma-Aldrich). Membranes were then incubated with HRP-conjugated secondary antibodies for 1 h at Room temperature (RT). Chemiluminescent signals were developed with HRP substrate (Millipore) and detected with a Fusion FX7 acquisition system (Vilbert Lourmat).
2.6. RNA extraction and Reverse-transcription (RT)-PCR
Total RNA was extracted using TRIzol® Reagent (Invitrogen, CA, USA) according to the manufacturer’s instructions. The 2 µg of total RNA was reverse transcribed into cDNA using SuperScript II Reverse Transcriptase (Invitrogen). The cDNA aliquots were amplified on a Mycyler Thermal Cycler (Bio-Rad) using Go Tag DNA polymerase (Promega) and the gene primers listed in Table 1 . Each PCR cycle consisted of 94 °C for 1 min, 58 °C for 1 min, and 72 °C for 1 min, The PCR products were loaded onto a 1.5% agarose gel containing LoadingSTAR nucleic acid dye (6X, Dynebio, Seongnam, Korea), electrophoresed, and photographed using a Fusion FX7 acquisition system (Vilbert Lourmat, Eberhardzell, Germany).
Table 1.
Primer sequence used for RT-PCR.
| Gene | Primer sequence | Size (bp) |
|---|---|---|
| iNOS | Forward: 5′- CCC TTC CGA AGT TTC TGG CAG CAG C-3′ | 496 |
| Reverse: 5′- GGC TGT CAG AGC CTC GTG GCT TTG G-3′ | ||
| TNFα | Forward: 5′- TTG ACC TCA GCG CTG AGT TG-3′ | 364 |
| Reverse: 5′- CCT GTA GCC CAC GTC GTA GC-3′ | ||
| IL-6 | Forward: 5′- GTA CTC CAG AAG ACC AGA GG-3′ | 308 |
| Reverse: 5′- TGC TGG TGA CAA CCA CGG CC-3′ | ||
| IL-1β | Forward: 5′-CAG GAT GAG GAC ATG AGC ACC -3′ | 447 |
| Reverse: 5′-CTC TGC AGA CTC AAA CTC CAC -3′ | ||
| GAPDH | Forward: 5′- ACC ACA GTC CAT GCC ATC AC-3′ | 452 |
| Reverse: 5′- TCC ACC ACC CTG TTG CTG TA-3′ |
2.7. Immunocytochemistry
Immunocytochemical analyses were performed with the following primary antibodies; anti-iNOS (13120, Cell Signaling Technology) and anti-phospho-STAT6 (56554, Cell Signaling Technology) in LPS or IL-4-induced raw 264.7 cells, respectively. The cells were cultured on glass coverslips coated with 0.1% gelatin. Cells were fixed with cold 2% paraformaldehyde and permeabilized with 0.5% Triton X-100 in PBS for 5 min and blocked in 5% donkey (or goat) serum in 0.3% TritonX-100 in PBS for 1 h at RT. Cells were primary-incubated with the antibodies and then secondary-incubated with an Cy3-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch). Nuclei were stained with 4′6-diamidino-2-phenylindole. Then, the cells were mounted in Fluorescent Mounting Medium (Dako) and immunofluorescent images were acquired using a confocal microscope (Carl Zeiss).
2.8. NO assay
Effects of diosmetin on NO production in raw 264.7 cells were determined as described previously [22]. Cells were pretreated with diosmetin for 3 h followed by stimulation with 1 μg/mL of lipopolysaccharide (LPS) for 12 h. Griess reagent was used to measure NO level in the supernatant. The results were quantified by measuring the absorbance at 540 nm with a microplate reader (Spectramax M2; Molecular Devices, CA, USA).
2.9. Statistical analysis
Values are presented as the mean ± standard deviation (SD). Significant differences between groups were determined by unpaired Student’s t-tests. For multi group analysis of variances, one-way or two-way ANOVA was performed followed by Bonferroni post-tests. Statistical significance was set at p < 0.1 or p < 0.01 or p < 0.001.
3. Results
3.1. Diosmetin reduced the dermatitis score, epidermis and dermis thickness, and mast cell infiltration in DNCB-induced atopic dermatitis
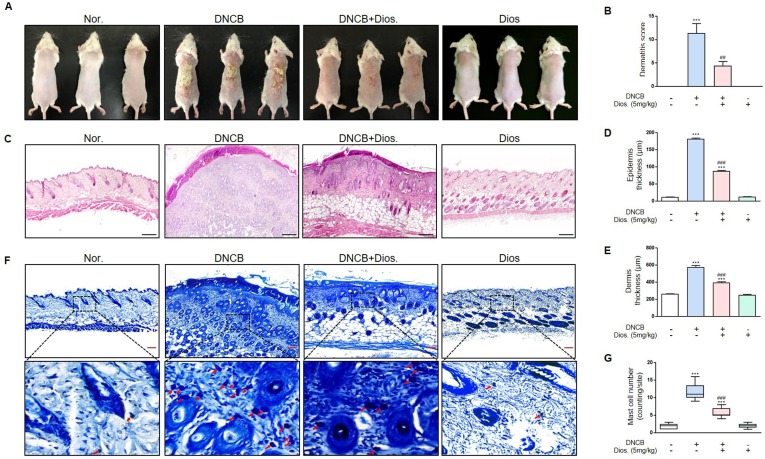
The total clinical severity score for skin lesions was calculated as the sum of the individual scores (graded as 0 (absence), 1 (mild), 2 (moderate), and 3(severe)) for each of the four AD symptoms (erythema/hemorrhage, scaling/dryness, edema, and excoriation/erosion) [23]. Dermatitis scores in each group were calculated. In the normal group and the treated group with only diosmetin, the score was zero in all five mice. In the DNCB treated group, the scores were 7, 15, 14, 6 and 15. In the group treated with both DNCB and diosmetin, the scores were 6, 6, 1, 4 and 5. Thus, diosmetin treatment reduced dermatitis scores (Fig. 1 A and B).
Fig. 1.
Effects of diosmetin in DNCB-induced mouse model. (A) Skin lesions in diosmetin (5 mg/kg) and DNCB-induced AD mice. The image shows the dorsal skin of mice on day 31 after sensitization. (B) Clinical skin severity scores. (C) The images of epidermis and dermis by H&E staining (magnification x100, scale bar: 250 μm). (D) Measurement of the epidermal thickness in five randomized fields. (E) Measurement of the dermal thickness in five randomized fields. (F) Infiltration of mast cells to dermis was examined by toluidine blue staining (magnification x200, scale bar: 500 μm). (G) The number of mast cells were observed in five randomized fields of each groups. Values are mean ± SD from five fields in each group (n = 5). ***p < 0.001 versus normal group, ##p < 0.01, ###p < 0.001 versus DNCB treated group by one-way ANOVA. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In the DNCB-treated group, the thicknesses of the epidermis and dermis were greater compared to the normal group. In the group treated with both DNCB and diosmetin, the thicknesses of epidermis and dermis were significantly decreased compared to the DNCB-treated group (Fig. 1C-E). The group treated with DNCB also had a greater infiltration of mast cells than the normal group. The number of mast cells in the dermis of the group treated with both DNCB and diosmetin was significantly lower compared to the DNCB-treated group (Fig. 1F and G).
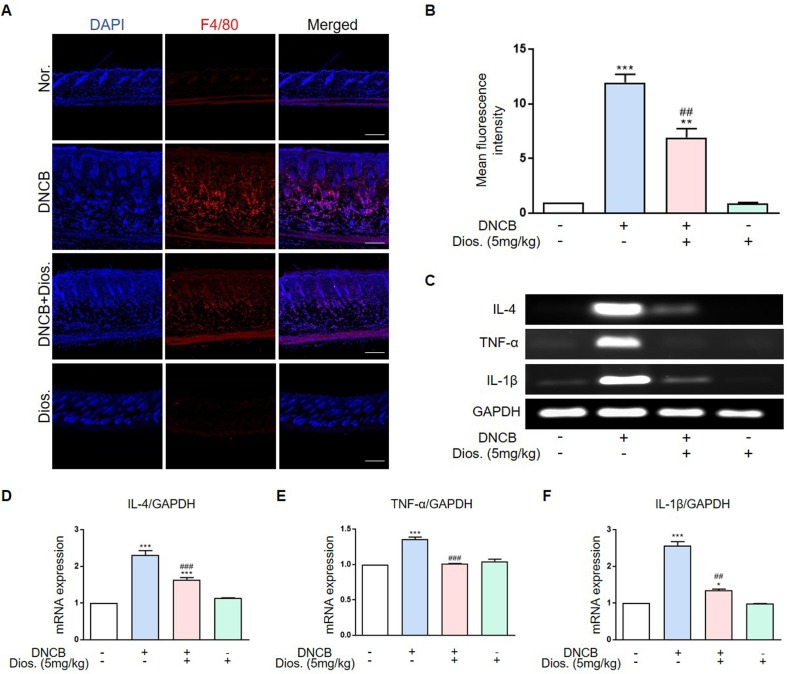
3.2. Diosmetin decreased the infiltration of macrophage in the dermis and decreased cytokines level in DNCB-induced atopic dermatitis
The group treated with DNCB had higher macrophage infiltration than the normal group. Macrophage infiltration was confirmed using immunohistochemistry. The lower red fluorescence intensity in the DNCB and diosmetin treated group suggests a decreased macrophage infiltration compared to the DNCB-treated group (Fig. 2 A and B). The mRNA level of IL-4, an important mediator in AD development, was significantly higher in the DNCB-treated group. The group treated with both DNCB and diosmetin showed a significant decrease in IL-4 mRNA levels. The mRNA levels of inflammatory mediators such as TNF-α IL-1β were also increased in the DNCB group but decreased in the diosmetin-treated group (Fig. 2C-F).
Fig. 2.
Diosmetin decrease infiltration of macrophage to dermis and mRNA expression in DNCB-induced mouse model. (A) Image of infiltration macrophage to dermis was examined in dorsal skin (magnification x200, scale bar: 500 μm). (B) The number of macrophage (Red fluorescence) were observed in three fields. (C) IL-4, TNF-α and IL-1β mRNA were measured by RT-PCR. (D-F) Quantification of mRNA expression (relative to GAPDH). **p < 0.01, ***p < 0.001 versus normal group, ##p < 0.01, ###p < 0.001 versus DNCB treated group by one-way ANOVA. Each group, n = 5. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
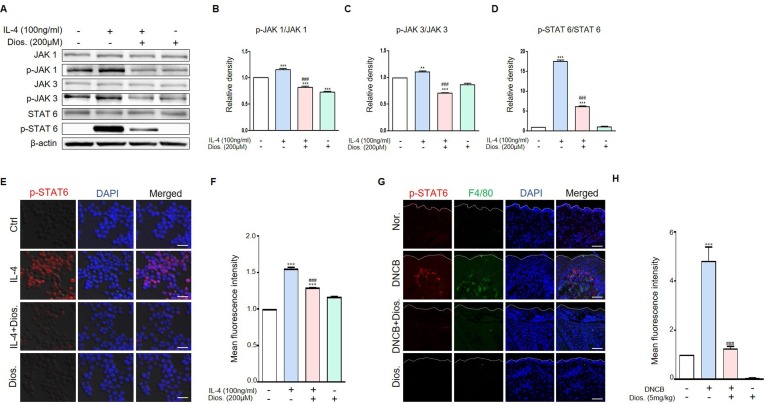
3.3. Diosmetin reduced the IL-4 induced activation of JAK/STAT6 pathway in raw 264.7 cell and DNCB-induced atopic dermatitis
Before the cell experiment, the MTT assay was performed to determine the concentration and cytotoxicity of diosmetin. As a result, diosmetin did not show cytotoxicity under 200 μM concentration treatment in raw 264.7 cells (Data not shown), diosmetin 200 μM concentration was applied to this cell experiment. We investigated the reduction of protein expression caused by IL-4 stimulation in macrophages and DNCB-induced atopic dermatitis. The stimulation of raw 264.7 cells with IL-4 results in the activation of p-JAK1, p-JAK3 and p-STAT6. The phosphorylation of JAk1/3 and STAT6 proteins was higher in the IL-4-treated group than in the control group. Diosmetin treatment of IL-4-stimulated raw 264.7 cells reduced phosphorylation of this proteins. In the diosmetin-treated group, the activation of p-JAK1, p-JAK 3, and p-STAT6 were also lower compared to the untreated IL-4-stimulated group (Fig. 3 A-D). We also examined p-STAT6 expression using immunocytochemistry staining and it was also reduced by diosmetin treatment in raw 264.7 cells (Fig. 3E and F). In animal experiment, the expression of p-STAT6 was higher in the DNCB-treated group than in the control group and this expression was reduced by diosmetin treatment in DNCB induced atopic dermatitis model (Fig. 3G and H).
Fig. 3.
Diosmetin decrease protein levels in IL-4-induced raw 264.7 cells. (A) Raw 264.7 cells were pre-treated with 200 μM diosmetin for 3 h and then exposed to 100 ng/ml IL-4 for 30 m. The treated cells were assessed for p-JAK1, p-JAK3 and p-STAT6 expression by western blot analysis. (B-D) Quantification of protein levels (relative to total protein). (E) Immunocytochemistry for the p-STAT6 was analyzed from raw 264.7 cells. Images of p-STAT6. Scale bar, 20 μm. (F) Quantification of p-STAT6 protein levels (Red fluorescence). (G) Immunohistochemistry for the p-STAT6 was analyzed from DNCB treated mouse model. Images of p-STAT6. Scale bar, 20 μm. (H) Quantification of p-STAT6 protein levels (Red fluorescence). Values are mean ± SD from four independent experiments. **p < 0.01, ***p < 0.001 versus control group or normal group, ###p < 0.001 versus IL-4-induced group or DNCB-induced group by one-way ANOVA. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
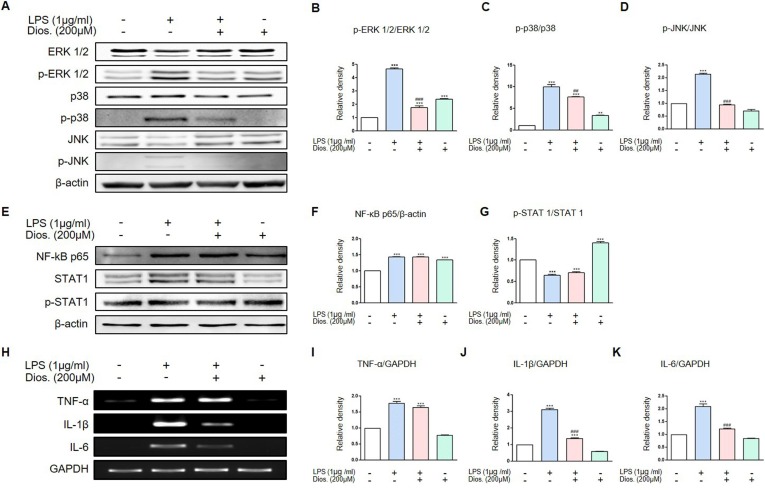
3.4. Diosmetin inhibited the LPS induced activation of MAP kinase pathway in raw 264.7 cell
In macrophage, MAP kinase pathway mainly activated through three signaling pathways induced by LPS. We investigated the phosphorylation of MAP kinase proteins as ERK1/2, p38 and JNK by diosmetin treatment after LPS stimulation in raw 264.7 cells. As a result, LPS induced the phosphorylation of ERK1/2, p38 and JNK and this phosphorylation inhibited by diosmetin treatment (Fig. 4 A-D). The other two pathway stimulated by LPS are NF-κB p65 and STAT1 pathway. LPS treatment increased NF-κB p65 expression but the diosmetin treatment did not decrease its expression. In the case of STAT1, total STAT1 increased during LPS treatment and LPS treatment after diosmetin pretreatment in raw 264.7 cells, but there was no difference between the two groups. Phosphorylation of STAT1 also showed no difference between the two groups (Fig. 4E-G). Therefore, it was found that diosmetin affected MAP kinase pathway but not that of NF-κB p65 and p-STAT1 pathway. Representative inflammatory mediators studied here are TNF-α, IL-1β and IL-6. Raw 264.7 cells were treated with LPS and diosmetin to investigate the mRNA expression of these mediators. Diosmetin treatment was performed to determine the degree of inhibition of the expression of these inflammatory mediators. LPS treatment of raw 264.7 cells showed increased expressions of TNF-α, IL-1β, IL-6 mRNA than in the control group. In the group treated with LPS and diosmetin, IL-1β and IL-6 mRNA was lower than in the group treated with LPS only. However, TNF-α mRNA was not decrease by diosmetin treatment (Fig. 4H-K).
Fig. 4.
Inhibitory effects or diosmetin on the protein levels and mRNA expression in LPS-induced raw 264.7 cells. (A) Raw 264.7 cells were pre-treated with 200 μM diosmetin for 3 h and then exposed to 1 μg/ml LPS for 12 h. The treated cells were assessed for p-ERK 1/2, p-p38 and p-JNK expression by western blot analysis. (B-D) Quantification of protein levels (relative to total protein) in (A). (E) Raw 264.7 cells were pre-treated with 200 μM diosmetin for 3 h and then exposed to 1 μg/ml LPS for 12 h. The treated cells were assessed for NF-κB p65 and p-STAT1 expression by western blot analysis. (F-G) Quantification of protein levels (relative to total protein) in (E). (H) Raw 264.7 cells were pre-treated with 200 μM diosmetin for 3 h and then exposed to 1 μg/ml LPS for 12 h. The treated cells were assessed for TNF-α, IL-1β, IL-6 expression by RT-PCR. (I-K) Quantification of mRNA expression (relative to GAPDH) in (H). Values are mean ± SD from four independent experiments. **p < 0.01, ***p < 0.001 versus control group, ##p < 0.01, ###p < 0.001 versus LPS-induced group by one-way ANOVA.
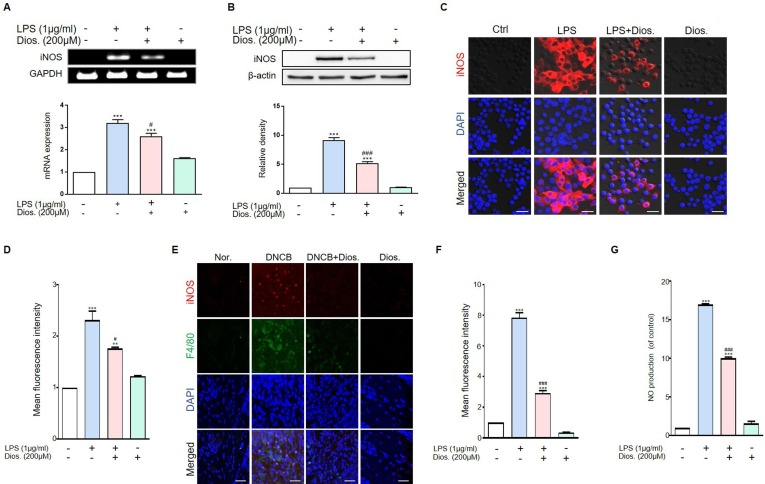
3.5. Diosmetin reduced iNOS expression and NO production in LPS-treated raw 264.7 cell and DNCB-induced atopic dermatitis
LPS stimulates raw 264.7 cells and induces iNOS that are not normally present in cells. Because of the inflammatory signals, iNOS mRNA is expressed in the nucleus upon LPS stimulation. In this studies, iNOS mRNA was significantly increased in the LPS-treated group. In LPS and diosmetin-treated groups, the expression of iNOS mRNA was significantly reduced (Fig. 5 A). Increased protein expression of iNOS was observed in the LPS-treated group. In the LPS and diosmetin treated groups, iNOS protein expression was also significantly reduced (Fig. 5B). In this studies, iNOS expression in raw 264.7 cells was further confirmed using immunocytochemistry staining. As the result, the iNOS protein expression stimulated by LPS was significantly decreased by diosmetin treatment (Fig. 5C and D). In animal experiment, iNOS protein expression was significantly increased in the DNCB induced atopic dermatitis group and it was significantly decreased by diosmetin treatment (Fig. 5E and F). The activation of iNOS in macrophagy produce large quantities of NO in response to inflammatory stimuli such as LPS. We investigated the change of NO production by diosmetin in raw 264.7 cells. NO production increased in the LPS-treated group, but it was reduced when diosmetin was pretreated before LPS treatment in raw 264.7 cells (Fig. 5G).
Fig. 5.
Diosmetin has inhibitory effects iNOS mRNA expression, protein level and NO in LPS-induced raw 264.7 cells. (A) Raw 264.7 cells were pre-treated with 200 μM diosmetin for 3 h and then exposed to 1 μg/ml LPS for 12 h. The treated cells were assessed for iNOS expression by RT-PCR. Quantification of iNOS mRNA expression (relative to GAPDH). (B) Raw 264.7 cells were pre-treated with 200 μM diosmetin for 3 h and then exposed to 1 μg/ml LPS for 12 h. The treated cells were assessed for iNOS expression by western blot. Quantification of iNOS protein levels (relative to β-actin). (C) Immunocytochemistry for the iNOS was analyzed from raw 264.7 cells. Images of iNOS. Scale bar, 20 μm. (D) Quantification of iNOS protein levels (Red fluorescence). (E) Immunohistochemistry for the iNOS was analyzed from DNCB treated mouse model. Images of iNOS. Scale bar, 20 μm. (F) Quantification of iNOS protein levels (Red fluorescence). (G) NO production in LPS-induced raw 264.7 cells. RAW 264.7 cells were pretreated with diosmetin for 3 h followed by stimulation with LPS for 12 h. Values are mean ± SD from four independent experiments. **p < 0.01, ***p < 0.001 versus control group, #p < 0.1, ###p < 0.001 versus LPS-induced group by one-way ANOVA. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Atopic dermatitis (AD) is caused by many factors; recent studies suggest that a major cause is an allergen-induced immune response [24], [25]. In allergies, the excessive differentiation of T cells into Th2 cells results in the release of a large number of inflammatory cytokines such as IL-4 and IL-13 [26]. As a result, the release of histamine from mast cells causes itching, the main symptom of AD [27]. Excessive scratching due to the itch damages the skin barrier and leads to various skin infections, causing secondary infections [28]. The result is an excessive inflammatory response that eventually leads to AD, a chronic skin condition [29].
In this study, we investigated the inhibitory effect of diosmetin, a flavonoid rich in tangerines and herbs, in AD. DNCB was used to induce an AD-like disorder in vivo. The DNCB treated group showed an observable worsening of symptoms. Histologically, there were increases in epidermis and dermis thicknesses, mast cell and macrophage invasion [30]. These are the typical symptoms of AD. However, treatment with diosmetin was found to suppress these symptoms. In addition, the expression of pro-inflammatory cytokines such as IL-4, TNF- α and IL-1β in the tissues was reduced (Fig. 6 ). Two phases of AD, the primary response and secondary infection were investigated in vitro. In both situations, murine macrophages (raw 264.7 cells) were used. After treatment with IL-4, an important cytokine in the primary response [31], it was confirmed that the activation of downstream signaling proteins such as p-JAK1, p-JAK3, and p-STAT6 were increased. In addition, it was confirmed that the activation was significantly inhibited by diosmetin treatment. In secondary infection, inflammation was induced by LPS. There were thus resultant increases in the expression of both LPS-related proteins and cytokines. In previous studies on AD, several flavonoids showed anti-inflammatory effects on LPS-treated raw 264.7 cells [30], [32]. In this study, we examined the inhibitory effect of diosmetin on MAP kinase pathway and NO production activated by LPS in raw 264.7 cells, and as a result, diosmetin inhibits the LPS induced activation of ERK, p38 and JNK pathway and it also inhibits iNOS expression and NO production (Fig. 6). However, unlike what we expected, diosmetin did not have inhibitory effects on proteins such as NF-kb and STAT1 induced by LPS as well as cytokine such as TNF-α. Further research will be needed to confirm these results.
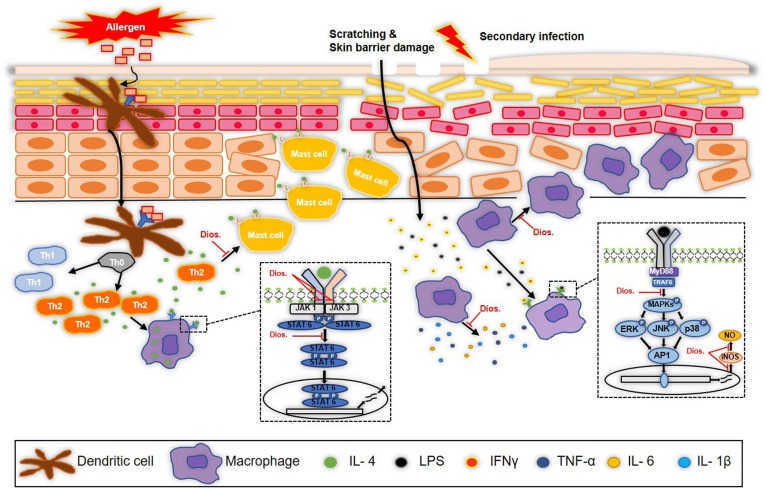
Fig. 6.
Schematic diagram of anti-inflammatory effects of diosmetin in atopic dermatitis. Diosmetin decrease the number of mast cells, the infiltration of macrophage and the cytokines activation in DNCB-induced atopic dermatitis. In activated macrophagy by IL-4 and LPS, diosmetin inhibits the activation of JAK/STAT and MAP kinase pathway and it also inhibits NO production and iNOS expression.
In order to apply the diosmetin applied to humans, it is expected to be administered at a concentration of 0.04 mg/kg according to conversion of animal doses to human equivalent doses based on body surface area [33]. According to a recent paper, atopic dermatitis has been reported to adversely affect children's learning and nerve development [34]. Therefore, natural flavonoids such as diosmetin on atopic dermatitis is thought to help improve the learning and neurodevelopment of young children, and further research is needed. Also, a paper has been reported that human coronavirus, which has recently become an issue, can be improved by inhibiting IL-1 family and IL-6 and suppressing mast cells [35], [36]. According to our result, it has been shown that diosmetin inhibits IL-1b and IL-4/6 and decreases mast cells. Therefore, natural flavonoids such as diosmetin may also be consider a therapeutic strategy of human coronavirus-infected patients. In order to determine the exact inhibitory effect of diosmetin on AD, it was necessary to investigate whether there is an inhibitory effect on other cell types such as Th2 cells and B cells [37]. Further studies on keratinocytes will also be needed to identify the inhibitory effect of diosmetin on the thickening of the skin barrier in AD patients [38]. In addition, it is necessary to study the inhibitory effect of diosmetin by inducing AD using house dust mites, a cause of AD gaining prominence in recent years [39].
In conclusion, the flavonoid diosmetin inhibited IL-4 and LPS signaling pathways in vitro and improved clinical lesions in the DNCB-induced AD model. It was also effective in inhibiting Th2-associated mast cells and in suppressing the expression of inflammatory cytokines, such as IL-4, associated with secondary infection. Diosmetin is thus potentially an effective therapeutic agent in AD treatment.
CRediT authorship contribution statement
Dae-hyo Lee: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing - original draft. Jeong-ki Park: Investigation, Methodology, Validation, Visualization. Jawun Choi: Investigation, Methodology, Validation, Visualization. Hyuk Jang: Investigation, Methodology, Validation, Visualization. Jae-won Seol: Conceptualization, Project administration, Funding acquisition, Supervision, Validation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Agriculture, Food and Rural Affairs Convergence Technologies Program for Educating Creative Global Leader, and this research was supported by ‘Research Base Construction Fund Support Program” funded by Jeonbuk National University in 2020.
References
- 1.Novak N., Bieber T. Allergic and nonallergic forms of atopic diseases. J. Allergy Clin. Immunol. 2003;112(2):252–262. doi: 10.1067/mai.2003.1595. [DOI] [PubMed] [Google Scholar]
- 2.W. Miller, C. Griffin, K. Campbell, Muller & Kirk's Small Animal Dermatology 7th Edition. St. Louis, United States: Elsevier.[Google Scholar], 2013.
- 3.Akdis M., Akdis C.A. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J. Allergy Clin. Immunol. 2014;133(3):621–631. doi: 10.1016/j.jaci.2013.12.1088. [DOI] [PubMed] [Google Scholar]
- 4.Darlenski R., Kazandjieva J., Hristakieva E., Fluhr J.W. Atopic dermatitis as a systemic disease. Clin. Dermatol. 2014;32(3):409–413. doi: 10.1016/j.clindermatol.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Park Y.M. Advances in the pathophysiology of atopic dermatitis. Pediatr. Allergy Respir. Dis. 2006;16(3):189. [Google Scholar]
- 6.Hanifin J.M. Atopic dermatitis: broadening the perspective. J. Am. Acad. Dermatol. 2004;51(1 Suppl):S23. doi: 10.1016/j.jaad.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Kim M.-A., Son H.-U., Nam D.-Y., Cha Y.-S., Shin Y.-K., Choi Y.-H., Lee S.-H. Inhibitory effect of Angelica keiskei extract in an atopic dermatitis animal model. Korean J. Food Preservation. 2012;19(5):792–798. [Google Scholar]
- 8.Jartti T., Burmeister K.A., Seroogy C.M., Jennens-Clough M.L., Tisler C.J., Salazar L.P., DaSilva D.F., Evans M.D., Vrtis R.F., Wallace P.K. Association between CD4+ CD25high T cells and atopy in children. J. Allergy Clin. Immunology. 2007;120(1):177–183. doi: 10.1016/j.jaci.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Leung D.Y., Soter N.A. Cellular and immunologic mechanisms in atopic dermatitis. J. Am. Acad. Dermatol. 2001;44(1):S1–S12. doi: 10.1067/mjd.2001.109815. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto Y., Takano N., Nakamura A., Nakaike S., Yu Z., Endo Y., Arai I. Scratching behavior in NC/Nga mice with dermatitis: Involvement of histamine-induced itching. Allergology Int. 2004;53(4):349–358. [Google Scholar]
- 11.Lübbe J. Secondary infections in patients with atopic dermatitis. Am. J. Clin. Dermatol. 2003;4(9):641–654. doi: 10.2165/00128071-200304090-00006. [DOI] [PubMed] [Google Scholar]
- 12.An S., Pae H., Oh G., Choi B., Jeong S., Jang S., Oh H., Kwon T., Song C., Chung H. Inhibition of TNF-α, IL-1β, and IL-6 productions and NF-κB activation in lipopolysaccharide-activated RAW 264.7 macrophages by catalposide, an iridoid glycoside isolated from Catalpa ovata G. Don (Bignoniaceae) Int. Immunopharmacol. 2002;2(8):1173–1181. doi: 10.1016/s1567-5769(02)00085-1. [DOI] [PubMed] [Google Scholar]
- 13.Lee H.-J., Jeong Y.-S., Ryu S.-Y., Ryu J.-H. Inhibition of nitric oxide synthesis by 8-epi-xanthatin in activated RAW 264.7 cells. Yakhak Hoeji. 1998;42(5):540–543. [Google Scholar]
- 14.Zuberbier T., Orlow S.J., Paller A.S., Taïeb A., Allen R., Hernanz-Hermosa J.M., Ocampo-Candiani J., Cox M., Langeraar J., Simon J.C. Patient perspectives on the management of atopic dermatitis. J. Allergy Clin. Immunol. 2006;118(1):226–232. doi: 10.1016/j.jaci.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 15.Lee G.-S., Pena I.D., Choi J.-Y., Yoon S.-Y., Choi J.-H., Kang T.-J., Oh S.-K., Cheong J.-H. Effect of SPZZC, a composition of herb extracts, on atopic dermatitis in BALB/c and NC/Nga mouse. Yakhak Hoeji. 2008;52(3):232–239. [Google Scholar]
- 16.Roowi S., Crozier A. Flavonoids in tropical citrus species. J. Agric. Food Chem. 2011;59(22):12217–12225. doi: 10.1021/jf203022f. [DOI] [PubMed] [Google Scholar]
- 17.Mueller M., Lukas B., Novak J., Simoncini T., Genazzani A.R., Jungbauer A. Oregano: a source for peroxisome proliferator-activated receptor γ antagonists. J. Agric. Food Chem. 2008;56(24):11621–11630. doi: 10.1021/jf802298w. [DOI] [PubMed] [Google Scholar]
- 18.Bıtıs L., Kultur S., Melıkoglu G., Ozsoy N., Can A. Flavonoids and antioxidant activity of Rosa agrestis leaves. Nat. Prod. Res. 2010;24(6):580–589. doi: 10.1080/14786410903075507. [DOI] [PubMed] [Google Scholar]
- 19.AlGamdi N., Mullen W., Crozier A. Tea prepared from Anastatica hirerochuntica seeds contains a diversity of antioxidant flavonoids, chlorogenic acids and phenolic compounds. Phytochemistry. 2011;72(2–3):248–254. doi: 10.1016/j.phytochem.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Chan B.C., Ip M., Gong H., Lui S., See R.H., Jolivalt C., Fung K., Leung P., Reiner N.E., Lau C.B. Synergistic effects of diosmetin with erythromycin against ABC transporter over-expressed methicillin-resistant Staphylococcus aureus (MRSA) RN4220/pUL5054 and inhibition of MRSA pyruvate kinase. Phytomedicine. 2013;20(7):611–614. doi: 10.1016/j.phymed.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Chandler D., Woldu A., Rahmadi A., Shanmugam K., Steiner N., Wright E., Benavente-García O., Schulz O., Castillo J., Münch G. Effects of plant-derived polyphenols on TNF-α and nitric oxide production induced by advanced glycation endproducts. Mol. Nutr. Food Res. 2010;54(S2):S141–S150. doi: 10.1002/mnfr.200900504. [DOI] [PubMed] [Google Scholar]
- 22.M. Dominguez, E.H. Jeffery, C.L. Cespedes, Effects of extracts, flavonoids and iridoids from Penstemon gentianoides (Plantaginaceae) on inhibition of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2) in LPS-Activated RAW 264.7 macrophage cells and their antioxidant activity, Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 9(5) (2010) 397–413.
- 23.H. Matsuda, N. Watanabe, G.P. Geba, J. Sperl, M. Tsudzuki, J. Hiroi, M. Matsumoto, H. Ushio, S. Saito, P.W.J.I.i. Askenase, Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice, 9(3) (1997) 461–466. [DOI] [PubMed]
- 24.H. Kim, Relation between atopic dermatitis and residential environment, Dept. of Housing & Interior Design of Yonsei Uni. Master's thesis (2005).
- 25.Park J.M., Chae J.W. Effects of Aurantii Immaturus Fructus (AI) on atopic dermatitis (AD) induced by DNCB in mice. J. Pediatr. Korean Med. 2015;29(1):27–43. [Google Scholar]
- 26.Morren M.-A., Przybilla B., Bamelis M., Heykants B., Reynaers A., Degreef H. Atopic dermatitis: triggering factors. J. Am. Acad. Dermatol. 1994;31(3):467–473. doi: 10.1016/s0190-9622(94)70213-6. [DOI] [PubMed] [Google Scholar]
- 27.Rukwied R., Lischetzki G., McGlone F., Heyer G., Schmelz M. Mast cell mediators other than histamine induce pruritus in atopic dermatitis patients: a dermal microdialysis study. Br. J. Dermatol. 2000;142(6):1114–1120. doi: 10.1046/j.1365-2133.2000.03535.x. [DOI] [PubMed] [Google Scholar]
- 28.S.J.A.o.n. Nutten, metabolism, Atopic dermatitis: global epidemiology and risk factors, 66(Suppl. 1) (2015) 8–16. [DOI] [PubMed]
- 29.Buske-Kirschbaum A., Ebrecht M., Kern S., Hellhammer D. Endocrine stress responses in TH1-mediated chronic inflammatory skin disease (psoriasis vulgaris)—do they parallel stress-induced endocrine changes in TH2-mediated inflammatory dermatoses (atopic dermatitis)? Psychoneuroendocrinology. 2006;31(4):439–446. doi: 10.1016/j.psyneuen.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 30.H.N. Lee, S.A. Shin, G.S. Choo, H.J. Kim, Y.S. Park, B.S. Kim, S.K. Kim, S.D. Cho, J.S. Nam, C.S.J.I.j.o.m.m. Choi, Anti‑inflammatory effect of quercetin and galangin in LPS‑stimulated RAW264. 7 macrophages and DNCB‑induced atopic dermatitis animal models, 41(2) (2018) 888–898. [DOI] [PMC free article] [PubMed]
- 31.L. Horsmanhetmo, I. Harvima, A. Järvikallio, R. Harvima, A. Naukkarinen, M.J.B.J.o.D. Horsmanheimo, Mast cells are one major source of interleukin‐4 in atopic dermatitis, 131(3) (1994) 348–353. [DOI] [PubMed]
- 32.M. Wang, J.J.O.M. Ma, Effect of NGR1 on the atopic dermatitis model and its mechanisms, 14(1) (2019) 847–853. [DOI] [PMC free article] [PubMed]
- 33.U. Food, D. Administration, Guidance for industry: Estimating the maximum safe starting dose in adult healthy volunteer, (2005).
- 34.T. Theoharides, M. Kavalioti, R.J.J.B.R.H.A. Martinotti, Factors adversely influencing neurodevelopment, 33(6) (2019) 1663–1667. [DOI] [PubMed]
- 35.S. Kritas, G. Ronconi, A. Caraffa, C. Gallenga, R. Ross, P.J.J.B.R.H.A. Conti, Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy, 34(1) (2020) 10.23812. [DOI] [PubMed]
- 36.P. Conti, G. Ronconi, A. Caraffa, C. Gallenga, R. Ross, I. Frydas, S.J.J.B.R.H.A. Kritas, Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies, 34(2) (2020) 1. [DOI] [PubMed]
- 37.T.R. Mosmann, R.J.A.r.o.i. Coffman, TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties, 7(1) (1989) 145-173. [DOI] [PubMed]
- 38.C. Vestergaard, K. Bang, B. Gesser, H. Yoneyama, K. Matsushima, C.G.J.J.o.I.D. Larsen, A Th2 chemokine, TARC, produced by keratinocytes may recruit CLA+ CCR4+ lymphocytes into lesional atopic dermatitis skin, 115(4) (2000) 640–646. [DOI] [PubMed]
- 39.T.A. Platts-Mills, M.D.J.J.o.A. Chapman, C. Immunology, Dust mites: immunology, allergic disease, and environmental control, 80(6) (1987) 755–775. [DOI] [PubMed]