Abstract
The use of field-Effect-Transistor (FET) type biosensing arrangements has been highlighted by researchers in the field of early biomarker detection and drug screening. Their non-metalized gate dielectrics that are exposed to an electrolyte solution cover the semiconductor material and actively transduce the biological changes on the surface. The efficiency of these novel devices in detecting different biomolecular analytes in a real-time, highly precise, specific, and label-free manner has been validated by numerous research studies. Considerable progress has been attained in designing FET devices, especially for biomedical diagnosis and cell-based assays in the past few decades. The exceptional electronic properties, compactness, and scalability of these novel tools are very desirable for designing rapid, label-free, and mass detection of biomolecules. With the incorporation of nanotechnology, the performance of biosensors based on FET boosts significantly, particularly, employment of nanomaterials such as graphene, metal nanoparticles, single and multi-walled carbon nanotubes, nanorods, and nanowires. Besides, their commercial availability, and high-quality production on a large-scale, turn them to be one of the most preferred sensing and screening platforms. This review presents the basic structural setup and working principle of different types of FET devices. We also focused on the latest progression regarding the use of FET biosensors for the recognition of viruses such as, recently emerged COVID-19, Influenza, Hepatitis B Virus, protein biomarkers, nucleic acids, bacteria, cells, and various ions. Additionally, an outline of the development of FET sensors for investigations related to drug development and the cellular investigation is also presented. Some technical strategies for enhancing the sensitivity and selectivity of detection in these devices are addressed as well. However, there are still certain challenges which are remained unaddressed concerning the performance and clinical use of transistor-based point-of-care (POC) instruments; accordingly, expectations about their future improvement for biosensing and cellular studies are argued at the end of this review.
Keywords: Cancer, Biomarkers, Biomedical analysis, Biotechnology, Advanced nanomaterial, Field-effect-transistor, Biosensor
Graphical abstract
Highlights
-
•
Most recent advances and limitations in the field of FET based biosensing systems were discussed.
-
•
The analytical figures of the recently developed methods were also evaluated.
-
•
Recent improvements in the early detection of biomarkers using FET-based biosensors were argued.
1. Introduction
Compact analytical tools that turn a biological response into a measurable electrical signal are called biosensors [1]. These miniaturized devices must be sensitive, selective, rapid, cost-effective, and simple [2]. Besides, they should act independently of external factors such as temperature and pH. Since assembling different parts of a biosensor (biological elements, biocompatible materials, transducing layers, and readout systems) necessitates specialization in various topics, constructing an efficient biosensor requires the active cooperation of scientists from diverse fields, including chemistry, biology, and engineering [3]. There are different types of biosensors that are being studied by researchers from all over the globe, such as, electrical, electrochemical, optical, photonic, magnetic, piezoelectric, etc. [4]. Among a wide range of electrical sensing devices, field-effect transistor (FET) biosensors appeared to be one of the most promising alternatives due to their advantages like being fast, low-cost, and simple [5]. The very first FET biosensor was generated by Bergveld fifty years ago [6]. This novel technology has been evolving since 1970 in different forms and is an ideal approach for swift and precise detection of various analytes and also drug discovery [7]. Although conventional sensing techniques have the capability of specific detection of biomolecules, they entail complicated instrumentations with complex protocols that make them expensive, labor-intensive, and time-consuming [4]. These restrictions can be conquered by fabricating novel and reliable FET-based biosensors which are modified by specific probes on their conducting channel, offering real-time and label-free analyses [8].
Generally, a solid-state device in which the electroconductivity of the semiconductor between the source and drain terminals is regulated by a third gate electrode through an insulator is called a FET [9]. Since constructing most of these devices is done according to standard protocols, they require almost no post-processing, however, to modify their surface and increase their sensitivity, some other materials such as nanostructures are fabricated and added to their structure. Since, they are already commercialized, mass-produced, and widely used, they are very promising in the future of medical diagnostics principally for POC applications [10]. Over the years, there has been an outstanding development in the design and fabrication of FETs employing nanostructures [11]. Their exclusive electronic characteristics, small size, dynamic range, and real-time biological detection with LODs down to zeptomolar levels and even in some cases enabling the evaluation of single molecules or particles, turn them to be one of the most powerful diagnostic platforms [12].
To recognize specific analytes, biological receptors, such as, antibodies, nucleic acids, aptamers, enzymes, cells, microorganisms, or artificial biomaterials are immobilized on the sensing channels, which are linked to the source and drain electrodes [13]. After exposing the biosensor to target analytes, and forming specific biological complexes such as antigen-antibody, enzyme-substrate, DNA structures, etc. the transducer system converts biochemical changes into a measurable signal. Attachment of charged biomolecules to the surface of gate dielectric is equivalent to applying a voltage by the use of a gate electrode and results in the threshold voltage variations. Therefore, the strategy underneath the FET biosensors is the reliance of the conductance on the adsorbed species [14].
Two major categories of FETs are n-type and p-type devices in which electrons and holes are the prime charge carriers, respectively [10]. If the target molecule is positively charged, the response of an n-type FET sensor will be an upsurge in the conductance, because of aggregation of electrons. Reversely, if the target is a molecule with a negative charge, the conductance will be decreased. The opposite trend is applicable regarding the p-type FET system [15].
One of the most important factors to take into account is the type of semiconductor in designing a successful biosensor. There is a wide range of materials such as SiO2, Si3N4, and Ta2O5 which are being used in the structure of FET biosensing devices [16]. The integration of nanotechnology paves the way for the use of nano transducers like nanowires, carbon nanotubes, nanoparticles, nanorods, etc. in the fabrication of novel biosensors [17]. Some nanostructures like silicon nanowires, graphene, and carbon nanotubes are among the most popular and functional alternatives when fabricating sensitive, specific, label-free, rapid, and cost-effective devices [11]. Superior mechanical stamina, high surface to volume ratio, extraordinary chemical and thermal firmness, and superior semiconductivity of these materials make them the ideal choice for immobilizing biorecognition elements (BREs) [18]. Numerous pioneering studies have used these strategies to fabricate high-tech devices for POC diagnoses of different diseases which are discussed thoroughly in the following sections.
Another important application of FETs is cell-based screening. To overcome the burden of treating some lethal diseases and address the patients' urgent needs, scientists are trying to find an alternative solution for expensive, time-consuming, and labor-intensive cell-based drug screening studies [19]. High-throughput screening (HTS) systems based on cell and tissue culture are among the most promising methods for both drug discovery and identifying biologically active small molecules or genes rapidly. They reflect the intricacies of actual living systems by imitating their microenvironment [20]. The more advanced the biological assays the more accurate they will perform in assessing the probable biological response of living cells to a controlled stimulus. Characterizing localization, protein expression, cellular morphology, and other phenotypic attributes of cells are necessary for these assays which need sophisticated biochemical and molecular biology tools. FET-based screening systems are one of the most ideal choices for implementing such research works [11].
In this regard, the chief intention of this review is to describe different applications of FET-based devices such as early detection of different diseases, including, recently increasing pandemic coronavirus disease 2019, influenza, hepatitis B virus, Alzheimer's disease, cancer, acute myocardial infarction, malaria, bacterial infection, and cell-based assays for studying dose-dependent cytotoxic effects of drugs, ion efflux, dopamine, and identify specific ligands of cells.
2. Field effect transistor-based biosensors
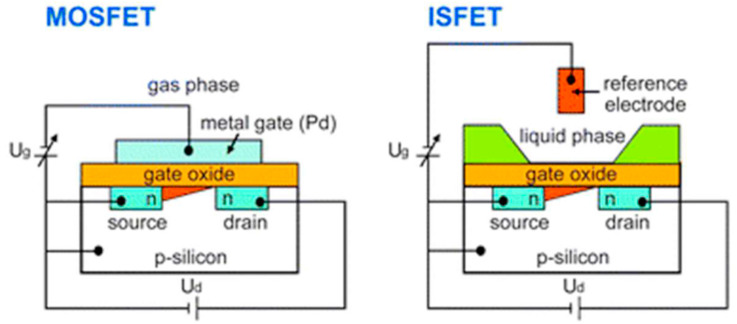
Field-effect transistor biosensors have appeared as the most developed alternatives between various types of biosensors because of several advantages they offer. There are different categories of transistor-based sensing platforms, however, the most used structures for biological applications are ion-sensitive field-effect transistors (ISFETs) and metal-oxide-semiconductor field-effect transistors (MOSFETs), which are separated based on the technique of applying the gate voltage, design, and material of the gate and the channel region. The preliminary basis of regulating the channel conductance is analogous in all FETs, thus, the method of the gate coupling can vary considerably (See Fig. 1 ).
Fig. 1.
Schematic representation of a MOSFET and an ISFET structure. (Ug: gate voltage, Ud: the source-drain voltage) [21].
2.1. Ion-sensitive field-effect transistors (ISFETs)
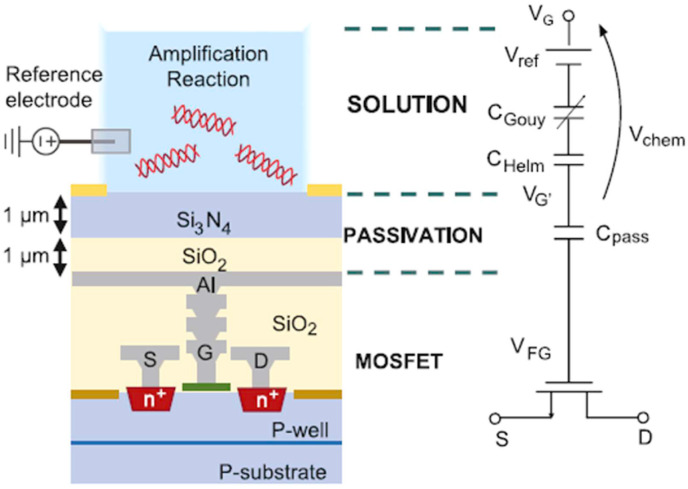
ISFETs are the most common types of integrated devices for micro electrochemical LOC arrangements [14]. Their use as a transducer signifies a favorable tool for biological applications. Their configuration is very similar to the normal MOSFETs. The only difference is their sensitive area or transistor gate that transduces ion concentration to a measurable voltage. In other words, a sample solution with an immersed reference electrode and an insulating layer which is sensitive to ions are representing the metal gate and gate oxide of a MOSFET [22]. The sensitivity and capability of an ISFET sensor are highly dependable on the nature of the insulating layer [23]. It isolates the channel of the FET from the liquid and couples the surface layer charge into the channel electrostatically [16]. This dielectric layer can be made of different materials such as Si3N4, Al2O3, or Ta2O5 on SiO2, which act selectively particularly for H+ ions owing to their active groups on the oxide surface [24]. These types of ISFETs are pH-ISFET that is the most common category [25]. To enhance the specificity of the device and enable identifying other analytes, ion-sensing or ion-blocking membranes such as ionophores can be employed [26]. By regulating the current flowing between the two semiconductors (S and D), the measurement is done. Source and drain are linked together by a third electrode (gate terminal) that is directly contacted to the sample to be measured [27]. To make them sensitive towards biomolecules, a bio-recognition layer is introduced at their surface. In this case, the conventional ISFET is called a biologically-sensitive field-effect transistor (BioFET) [10]. This layer which provides selective interaction with analytes decreases non-specific binding and, enables a charge transfer, binds to the transducer surface by either chemical or electrostatic interactions [28]. If an attachment takes place between targets and BREs, a variation will be seen in the surface charge. Accordingly, the potential in the semiconductor and the conductivity of the channel will change. By measuring the change in conductance, the binding of the analytes can be detected [29]. For instance, Synhaivska et al. used silicon nanoribbon ISFET structures amended with a Gly-Gly-His peptide for the identification of copper ions in a wide range of concentrations. The attachment of copper ions results in a structural alteration of the ligand, and also a loss of proton in the secondary amine assemblies [30]. These devices can be developed through complementary metal oxide semiconductor technology (CMOS). CMOS is a commonly used technology for producing integrated circuits that are being exploited in a wide range of electronic apparatuses such as, digital cameras, batteries, and microprocessors [31]. The “MOS” in CMOS denotes the MOSFET transistors in a CMOS. Although recently developed MOSFETs usually employ polysilicon as a substitute for aluminum as the conductive material, we still call them metal oxide semiconductor transistors. Whereas, the N-doped or P-doped semiconductors represent the “complementary” part in CMOS [32]. These devices are well-known for their efficient consumption of electrical power [33]. No electrical current is needed excluding when they are shifting from one state to another. The fact that complementary semiconductors work together results in the control of output voltage and the design of low-power devices that emit negligible heat [34]. Consequently, these transistors substituted other former structures and been utilized in most up-to-date processors [35]. This unique technology facilitates the development of inexpensive, extensive, and mass-produced devices capable of detecting tiny amounts of analytes on miniaturized chips [36]. For example, Malpartida-Cardenas and coworkers used an ISFET, manufactures in standard CMOS technology for instantaneous DNA sensing. This fully-electronic Lab-on-Chip platform was designed to detect plasmodium falciparum [37].
2.2. Metal-oxide field-effect transistors (MOSFETs)
MOSFETs are the most frequent form of insulated gate FETs (IGFET) which are exploited in various types of electronic circuits for switching and amplifying electronic signals. They are the core of integrated circuits and are capable of being used in a single chip because of their small sizes [38]. The key characteristic of MOSFETs is their metal oxide gate electrode. An ultra-thin layer of insulating material (usually SiO2) insulates this controlling gate from the main channel which is located in the middle of the drain and source, making the MOSFET' input resistance exceptionally high. Therefore, no current flows into the gate and the device acts like a voltage-controlled resistor [39]. MOSFETs can easily become damaged due to the accumulation of large amounts of static charge resulting from high input resistance. Thus, they need to be used carefully [40]. For example, a commercial biosensing device based on MOSFET for the recognition of C-reactive protein (CRP) was created by Lyu and colleagues. The binding of the CRP to its specific antibody was sensed by quantifying the drain current of the system. The manufactured MOSFET biosensor paved the way for the emergence of cheap and simple FET-based biosensors [41]. Or in another research work done by Mostafa et al., MOSFET-fixed cantilevers were designed to spot Bacillus thuringiensis. Attachment of this target to the cantilever which is modified by gold caused variations in the current of MOSFET drain as a result of the flexing of the cantilever. It is important to mention that the electronic attributes of the target were also accountable for the alteration in the current of the drain. Their reported limit of detection was 10 μL [42].
3. Selective adhesion on FET for biosensing applications
One of the most common uses of FET-based biosensors is the early detection of diseases rapidly and reliably. Since the majority of biomolecules transmit electrical charges. The FET biosensors can act as capable candidates intended for utilizations necessitating high preciseness and speed. Also, novel CMOS manufacturing strategies offer the advantages of miniaturization and concurrent sensing. To demonstrate competencies of FET biosensing systems, a wide range of biological analytes were studied during the last few years. To illustrate, they were exploited to sense different viruses such as recently emerged COVID-19, influenza, hepatitis B virus (HBV), or biomarkers of diseases such as Alzheimer's, cancer, acute myocardial infarction (AMI), bacteria-related disorders, etc. Besides, they have been utilized for designing nucleic acid sensors that are dependent on DNA hybridization. Considering these improvements, FET biosensing platforms have been highlighted as a perfect choice for the pioneering class of point-of-care diagnostics.
3.1. Coronavirus disease 2019 (COVID-19)
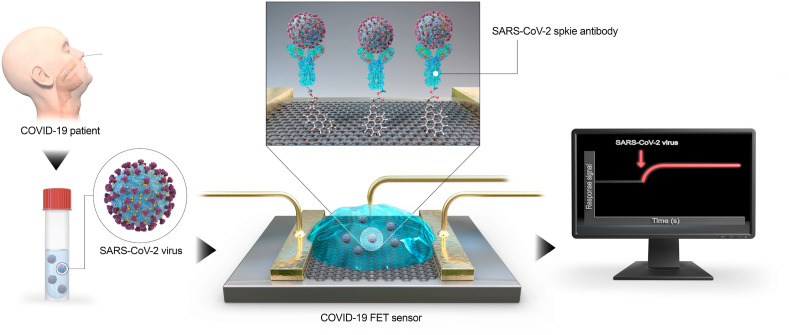
The COVID-19 that has made more than a billion people stay in quarantine and has brought much of global economic activity to a halt, is a recently emerged contagious malady instigated by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [43]. In the last months of 2019, several unknown pneumonia cases occurred in China. As person-to-person transfer augmented promptly, the outbreak was acknowledged by the World Health Organization (WHO) to be a global health hazard [44]. There is not a reliable and accurate source of information regarding the actual morbidity and mortality rates that have given rise to vagueness in assessing and envisaging the degree of this pandemic [45]. Thus, to effectively monitor patients and control new infections, it is vital to design accurate COVID-19 recognition assays [46]. There is a wide range of available diagnostic tests, however, FET biosensors offer many benefits, in particular, high speed, sensitivity, and specificity which are required in COVID-19 detection. As it is demonstrated in Fig. 2 , Seo and colleagues presented a FET-based biosensor for identifying SARS-CoV-2 in biological fluids. The device was fabricated by modifying sheets of graphene as sensing materials with a monoclonal antibody specific for the SARS-CoV-2 spike protein. This approach was successful to sense SARS-CoV-2 in culture medium and clinical samples with LODs of 1.6 × 101 pfu/ml and 2.42 × 102 copies/ml, respectively. Therefore, this device with an off-chip readout system is a promising immunosensing strategy for COVID-19 that necessitates no sample pre-processing [47]. Besides, designing customized biosensing tools for controlling the transmission rate of this pandemic-causing virus is possible through using the already developed immuno-, or genosensors which are proposed for detecting other proteins or nucleic acids. In addition, utilizing nanomaterials in the structure of these sensors will further improve their efficiency and sensitivity.
Fig. 2.
Outline of COVID-19 FET sensor functioning method [47].
3.2. Influenza
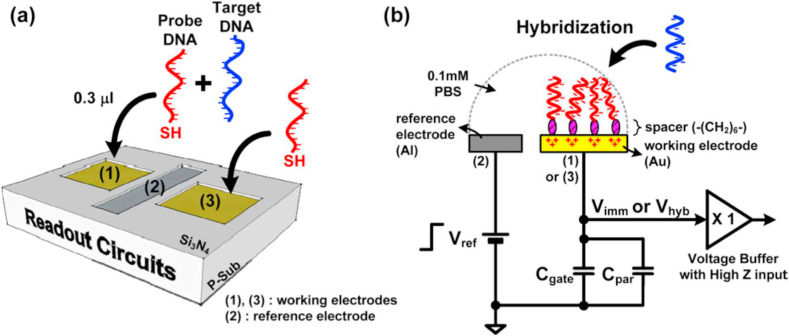
Influenza is a contagious viral respiratory sickness caused by the influenza virus that is responsible for up to 1 billion contagions, around 4 million serious cases [48]. The main reason for this high morbidity and mortality rate of pandemic disease is the alterations in the surface antigens that happen occasionally [49]. Everyone is indeed in danger of being affected by influenza, but, the major proportion of influenza-related deaths are related to the elderly and especially to those with cardiovascular and respiratory disorders [50]. Influenza viruses are separated into different subtypes based on surface viral glycoproteins [51]. The infection initiates after the virus links to the sialic acid receptors on host respiratory epithelial cells via H proteins [52]. Despite the significant number of researchers working on influenza prevention, the death rate is still high. Thus rapid diagnostic approaches may be the solution [53]. Up until now, a wide range of tests with different sensitivity, selectivity, cost, and ease of use have been designed. To reduce the number of patients suffering from adverse effects of inappropriate drugs and attain accurate results in a short time, it is necessary to use ultra-sensitive and selective methods [54]. The novel technology of FET-based biosensors is highly capable of detecting any nano-sized object, like an antibody, biomolecules, pathogens, and even viruses [55]. Owing to a large amount of hemagglutinin (HA) and neuraminidase (NA) on a viral particle, these glycoproteins are an ideal target for virus detection [56]. Additionally, the nucleoprotein (NP) of influenza types A and B are different, so, it can be a perfect target in antigen-detection type assays [57]. Table 1 indicates various characteristics of lately projected biosensors for identifying of influenza virus. The most efficient device is an immunosensor fabricated by Chiang and coworkers, which had a LOD of 10−17 M. Whereas, the least sensitivity which is 100 pM is reported by Lee and colleagues. The first system was a reusable SiNW-FET as an ultrasensitive biosensor to detect avian influenza virus (AIV) in buffer solution. The employment of nanowires on the surface of this device boosted the available surface area for immobilizing the receptors. They used a disulfide linker to functionalize the surface of SiNW-FET. After the immobilization of BREs, the final immunosensor was successful to identify 10−17 M of H5N2 AIVs [58]. As it is demonstrated in Fig. 3 , the latter device was a genosensor for measuring the electrical potential on electrodes amended by an oligonucleotide. It was designed to repeatedly measure the charges provoked in the working electrode. The reference electrode, working electrodes, and readout circuits were arranged in one package via CMOS technology. It was acknowledged that the system was able to recognize down to 100 pM of oligonucleotide sequences derived from the H5N1 avian influenza virus [59].
Table 1.
FET-base biosensors for early detection of influenza.
| Application | Target | RE | Linker | Surface | Sensor | Readout | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| IV diagnosis | GST-tagged-HA | CMP-NANA/Biotin-tagged GST antibody | APTES GA Gold-labeled streptavidin |
SiO2/SiNW | CMOS-compatible FET | Off-chip | 1 fM | Buffer solution (PBS) | [60] |
| AIV diagnosis | AIa | antibody | SBP | SiO2/CYTOPTM (hydrophobic) and Si3N4 (hydrophilic) | FET | Off-chip | 1.9 fM 0.19 pM |
Buffer solution (PBS) | [61] |
| AIV detection | AIV | mAbH5 | MPTMS DTT biotin-HPDP |
SiNW | SiNW-FET | Off-chip | 10−17 M | Buffer solution (PBS) | [58] |
| Detection of DNA Hybridization | oligonucleotide sequences derived from the H5N1AIV | 18-mer ssDNA | Thiol chain mercaptohexanol | Al/Au | FET | On-chip | 100 pM | Buffer solution (PBS) | [59] |
APTES: (3-Aminopropyl) triethoxysilane, GST: glutathione S-transferase, CMP-NANA: Cytidine-50-monophosphate-N-acetylneuraminic acid, AIa: avian influenza antigen, SBP: silica binding protein, MPTMS: 3-mercaptopropyltrimethoxysilane, Biotin-HPDP: N-(6-(biotinamido)hexyl)-3’-(2′-pyridyldithio)-propionamide, DTT: dithiothreitol.
Fig. 3.
(a) A conceptual view of the proposed device. (b) The operation procedure with a top-notch model [59].
3.3. Hepatitis B virus (HBV)
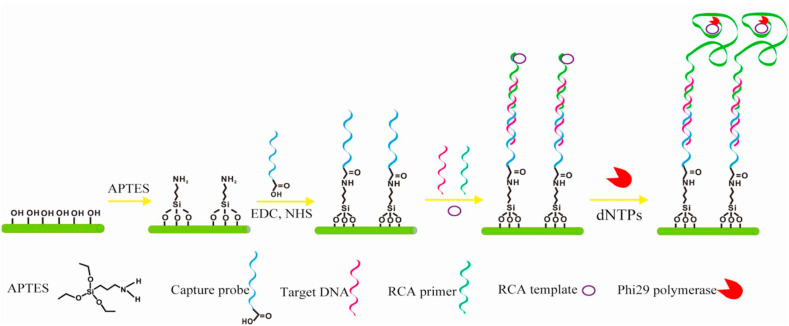
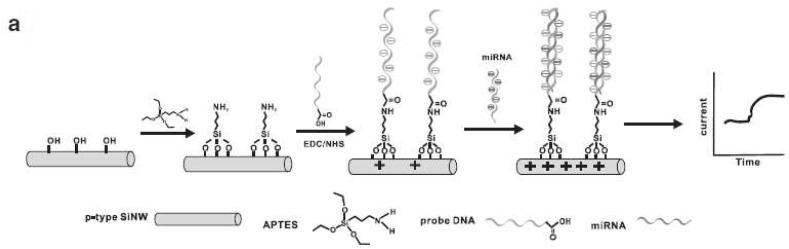
HBV infection is one of the most critical universal public health burdens [62]. According to global estimates over one-third of the World's population have been diseased with HBV, and about 5% of this population are infected recurrently and around 25% of these chronic carriers have severe liver diseases like cirrhosis, hepatic carcinoma, and chronic hepatitis [63]. There is a high risk of emerging chronic infection and following complications if the infection happens before birth [64]. Perinatal transmission at birth, horizontal transmission between youngsters, sexual interaction, and injecting drug use are among the most prevalent infections in most of the countries [65]. For that reason, it is of high importance to prevent and control this viral disease by implementing strategies such as promoting consciousness via public education, vaccination, blood transfusion safety policies, early identification, and effectual medical care [66]. Lately, there is an extensive focus on the HBV early detection and evaluation systems improvement [67]. The nanoscience-based approaches have been very successful in HBV detection biosensor structures [68]. Amongst the recently developed FET-based biosensors for detecting HBV, Shariati designed a FET genosensor for sensing HBV founded on indium tin oxide nanowires (ITO NWs) modified by gold. Owing to ITONWs extraordinary conductivity, the immobilization of BREs (SS-DNA) and target attachment were enhanced intensely. The reported LOD of the genosensor was about 1 fM [69]. In another work done by Gao and coworkers, a novel biosensor was fabricated for DNA detection based on silicon nanowire (SiNW) FET. According to Fig. 4 , the surface of SiNW was modified by probe DNA, succeeded by hybridization with the complementary target DNA and rolling circle amplification (RCA) primer. A limit of detection of 1 fM was achieved [70]. Using nanostructures like nanowires on the topmost layer of the FET devices increased the surface to volume ratio significantly which paved the way for enhancing the number of immobilized bioreceptor. Accordingly, the number of interactions between BREs and the target analytes augmented considerably which further amplified the signal.
Fig. 4.
Schema of SiNW detection of DNA after functionalizing it with APTES, N-ethyl-N′-dimethyl aminopropyl carbodiimide (EDC), and N-hydroxysuccinimide (NHS) [70].
3.4. Alzheimer's disease (AD)
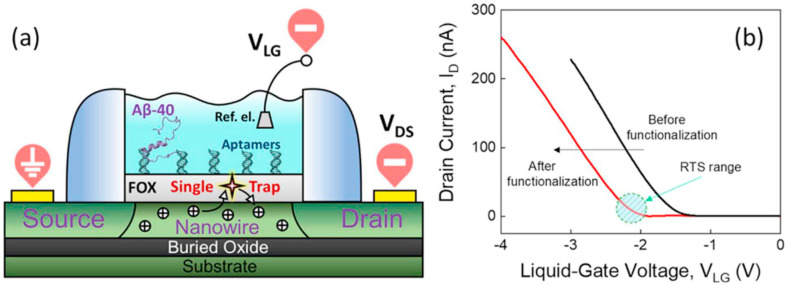
AD is the most prevalent type of dementia, representing over 60% of cases of dementia in people over 65 [71]. This neurodegenerative disease causes advanced damage to cognitive functions such as language, attention, visuospatial orientation, memory, comprehension, judgment, and reasoning [72]. Irreparable and gradual molecular processes that occur in the brain of AD patients are resulting in fatal outcomes generally [73]. Therefore, it is a very crucial health disorder especially for aged people [74]. Much research has been conducted to determine risk factors along with finding the most important processes that give rise to the commencement and progression of AD [75]. Studies supported by clinical trials related to early detection and treatment of AD include targeting aggregations of amyloid-beta (Aβ) plaques [76]. It is approved that the existence of these biomarkers is interconnected with the pathological changes taking place in the brain throughout AD progress [77]. So, it is essential to measure the concentration of Aβ-42 to recognize AD in the early stages [78]. Its level in the serum of healthy people is in the range of 20 pg/ml, whereas, this amount in AD or pre-AD patients is over 40 pg/ml [78]. For this reason, designing novel biosensors with lower limits of detection for swift and reliable detection of Aβ is of high importance [79]. Up to now, several techniques have been presented for the measurement of Aβ-42. As it is depicted in Fig. 5 , Kutovyi and coworkers developed an innovative strategy for detecting Aβ peptides by immobilizing aptamers onto the SiO2 surface of the manufactured sensors via GPTS (3- glycidyloxypropyltrimethoxysilane). To target the Aβ-40 (amyloid beta-40) sequence, ssDNA aptamer was designed, synthesized, and immobilized on silicon (Si) nanowire (NW) FETs altered with a thin layer of SiO2. The binding of Aβ peptides of different concentrations in the buffer solution to the sensors' surface was in the range from 0.1 pg/ml to 10 μg/ml [80].
Fig. 5.
(a) Illustration of a SiNW-FET biosensor. (b) Transfer curves of SiNW-FET before and after modification with Aβ-40-specific aptamers. The dashed circle demonstrates the range of voltages at which noticeable RTS noise was detected [80].
3.5. Cancer
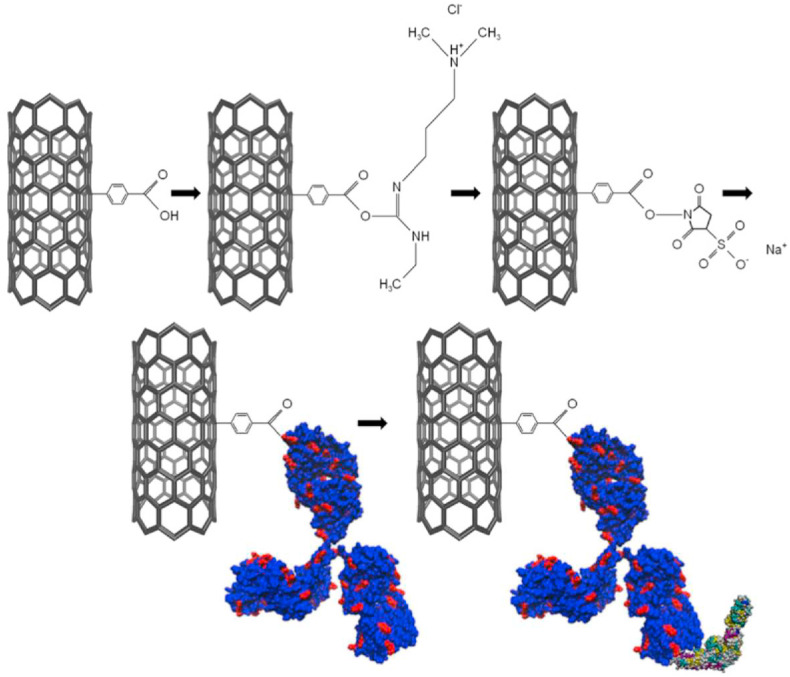
Cancer is the second dominant fatal disease globally with above 200 known categories and more than 1600 deaths taking place each day [81]. Despite the ever-growing technology, the number of survived cancer patients is still low as a consequence of detection at advanced levels and the unsatisfactory prognosis of cancer [82]. The common diagnostic approaches, including ultrasound, CT scan, PET, magnetic resonance imaging, and biopsy are incompetent for early cancer detection [83]. The outset and development of this multilevel disease, are connected with an intricate series of genetic or epigenetic conversions which cause cellular signaling disruption and tumorigenic alteration and malignancy [84]. Biomarkers are the molecules which experience conspicuous modifications during cancer. These molecules which may be nucleic acids, proteins, metabolites, isoenzymes, or hormones have high clinical importance [85]. The presence, absence, or variation in the concentration of a particular biomarker in a cell usually demonstrates cancer progression [86]. Recognition and measurement of these oncobiomarkers could help in early detection and monitoring disease development [87]. Recently, there is increasing attention to FET-based cancer biosensors as they show superb analytical efficiency and real-time measurement. A wide range of cancer biomarkers has been monitored by these kinds of biosensors, including, CEACAM, PSA, cDNA, SSAT, CEA, DTC, miRNA, IL-8, TNF-α, AFP, VEGF, CRP, CYFRA21-1, IP-10, APOA2, and ALCAM. Table 2 shows a list of recently developed biosensors for early cancer detection. Between diverse approaches of assembling biosensing systems, geno and aptasensors display advanced preciseness as compared to immunosensing assays. The stated LODs vary between 12.5 aM–0.2 μM. It is demonstrated in Fig. 6 that, Lu et al. proposed a SiNW-FET biosensor for detecting miR-21 and miR-205, which offers high sensitivity along with lower manufacturing cost. The response time of this nanosensor was less than 1 min and its LOD was 1 zmol [88]. In another research work done by Bao et al. a pioneering method was used to detect a specific cancer biomarker (CEA). The top-down construction technique of this FET-based biosensor made the researcher have control over its electrical characteristics. Furthermore, this approach is CMOS-compatible which enables mass production of the proposed biosensor for high throughput, real-time, cheap, and precise assays. The surface of the device was modified with APTES, GA, and anti-CEA, and a microfluidic channel was added on top of it. This approach was successful enough to detect down to 10 pg/mL of the analyte [89]. Or Gao and colleagues designed a microfluidic sensing platform based on CMOS-compatible SiNW-FET for the identification of lung cancer biomarkers ((miRNA)−126 and CEA). Utilizing nanowires enabled single-molecule level detection with high sensitivity and specificity in biofluids [90]. The other CMOS-compatible silicon nanowire tunneling field-effect transistor (SiNW-TFET) biosensor was reported by Gao et al. for pH and cytokeratin 19 fragment (CYFRA21-1) detection. It was capable of recognizing down to 12.5 aM of the target protein in the serum sample. These successful studies demonstrate the power of FET-based platforms for a wide range of POC applications [91].
Table 2.
FET-base biosensors for early cancer detection.
| Application | Target | RE | Linker | Surface | Sensor | Readout | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Disease antigen detection | CEACAM5 CEACAM1 |
Antibody | GTA | SiO2/SiNW | Potentiometric | Off-chip | 18 ng/mL and 21.6 ng/mL | Buffer solution | [92] |
| Prostate cancer diagnosis | PSA | PSA antibodies | APTES GA AuNPs |
SiO2/SiNW | Potentiometric | Off-chip | 90 pg/mL | Serum | [93] |
| Oncological diseases diagnosis | oDNA | Complementary oDNA probe molecule | APTES DTSSP cross-linker |
a stack of h-k PEALD dielectrics, HfO2 and Al2O3 | Amperometric | Off-chip | 10−16 M | Buffer solution (potassium phosphate buffer) | [94] |
| CEA detection | CEA | Antibody | APTES GA |
SiNx/SiNRs | CMOS-compatible FET | Off-chip | 10 pg/mL | Buffer solution (PBS) | [95] |
| Lung cancer diagnosis | miRNA−126 and CEA | probe DNA and anti-CEA antibody | APTES GA |
SiNW | CMOS-compatible FET | Off-chip | 0.1 fM and 1 fg/ml | Buffer solution (PBS) | [96] |
| PSA detection | PSA | DNA aptamers | PYCOOH EDC/NHS PEG ETA |
SiO2/graphene FET channel/Si3N4 | FET | Off-chip | 1 nM | Buffer solution (PBS) | [97] |
| Lung cancer diagnosis | CYFRA21-1 | Antibody | ETA | SiNW | CMOS-compatible SiNW-TFET | Off-chip | 12.5 aM | Serum sample | [98] |
| Colorectal Cancer Diagnosis |
Cytokeratin-19 as a marker of DTC | Anti-KRT antibodies | APTES GA |
SiO2/Si3N4/p-type SiNWs | CMOS-compatible FET | On-chip | 83 fM (~1.3 cancer cells/μL) | 10% serum | [99] |
| Bladder cancer diagnosis | APOA2 | APOA2 antibody | Biotin APTES GA EDC/NHS |
SiNx/MGLA | poly-SiNW-FET | Off-chip | 6.7 pgmL−1 | Urine sample | [100] |
| Prostate cancer diagnosis | PSA | Antibody | APTES GA silane-PEG |
P-type SiNW | SiNW-FET | Off-chip | 10 nM | Buffer solution (PBS) | [101] |
| Cancer diagnosis | CYFRA21-1 and PSA | Antibody | APTES GA |
SiNW | CMOS-compatible FET | Off-chip | 1 fg/mL-10 fg/mL | Buffer solution (PBS) –human serums | [102] |
| Cancer diagnosis | ALCAM | Antibody | APTES GA |
SiO2/Si3N4 | CMOS-compatible FET | Off-chip | 15.5 pg/ml | Serum | [103] |
| OSCC diagnosis | IL-8 and TNF-α | Antibody | APTES GA |
SiO2/Si3N4/SiNW | CMOS-compatible FET | Off-chip | 10 fg/mL-100 fg/mL | Buffer solution (PBS) –artificial saliva | [104] |
| Cancer diagnosis | AFP | Antibody | APTES GA |
SiO2/SiN x/SiNW | SiNW-FET | Off-chip | 0.1 ng/mL | Buffer solution (PBS) | [105] |
| Cervical carcinoma diagnosis | VEGF | Avastin | APTES GA EDC/NHS SPAnH- Fe3O4 |
n-type polycrystalline silicon nanowire | SiNW-FET EIS |
Off-chip | 1.25 pg/mL | Serum samples | [106] |
| Early cytokine detection | IL-10 | Anti- IL-10 | Hydroxyl group OTS APTES EDC/NHS |
Si/Al2O3/multi-walled carbon nanotubes–COOH | FET | Off-chip | 0.5 pg/mL | standard samples | [107] |
| Cancer diagnosis | miR-21 and miR-205 | Probe DNA (Prb21) | APTES EDC/NHS |
P-type SiNW | CMOS-compatible SiNWFET | Off-chip | 1 zmol | Human serum samples | [88] |
| Prostatic hyperplasia and prostate cancer diagnosis | MMP-2 | Synthetic peptide | O2 Plasma TESBA SH-PEG |
P-type SiNW | FET | Off-chip | 1 pM | Standard sample | [108] |
| Prostatic hyperplasia and prostate cancer diagnosis | MMP-2 | Synthetic peptide | O2 Plasma TESBA SH-PEG Streptavidin |
P–SiNW/AuNP | FET | Off-chip | 100 fM | Standard sample | [109] |
| CRP detection | CRP | Anti-CRP | SBP protein A 6-His |
SiO2 | nanogap-embedded FET CMOS |
On-chip | 0.1 ng/ml | Human serum | [110] |
| Multiplexed detection of protein markers | CRP and PSA | Anti-CRP and anti-PSA | APTES silicate monomer, buffer solution, and SolB reagent mixture to form sol-gel | SiNW | SiNW FET | Off-chip | 0.12 ng/ml and 0.18 ng/ml | Serum | [111] |
CEACAM: carcinoembryonic antigen-related cell adhesion molecule, GTA: Glutaraldehyde, oDNA: DNA oligonucleotide, PYCOOH: pyrene butyric acid, DTC: disseminated tumor cell, APOA2: apolipoprotein A-II, MGLA: magnetic graphene with long-chain acid groups, ALCAM: Activated leukocyte cell adhesion molecule, IL-8: interleukin-8, TNF-α: tumor necrosis factor α, OSCC: oral squamous cell carcinoma, AFP: Alpha-fetoprotein, VEGF: vascular endothelial growth factor, SPAnH- Fe3O4: Magnetic nanoparticles with poly(aniline-co-N-(1-one-butyric acid) aniline)- Fe3O4, EIS: electrochemical impedance spectroscopy, IL-10: interleukin-10, MMP-2: Matrix metalloproteinases, SH-PEG: polyethylene glycol functionalized thiol group, TESBA: 3-(triethoxysilyl)butyl aldehyde, 6-His: six-histidine, OTS: Octadecyltrichlorosilane.
Fig. 6.
Illustration of detecting miRNA by SiNW which is functionalized by APTES, EDC, and NHS [88].
3.6. Acute myocardial infarction (AMI)
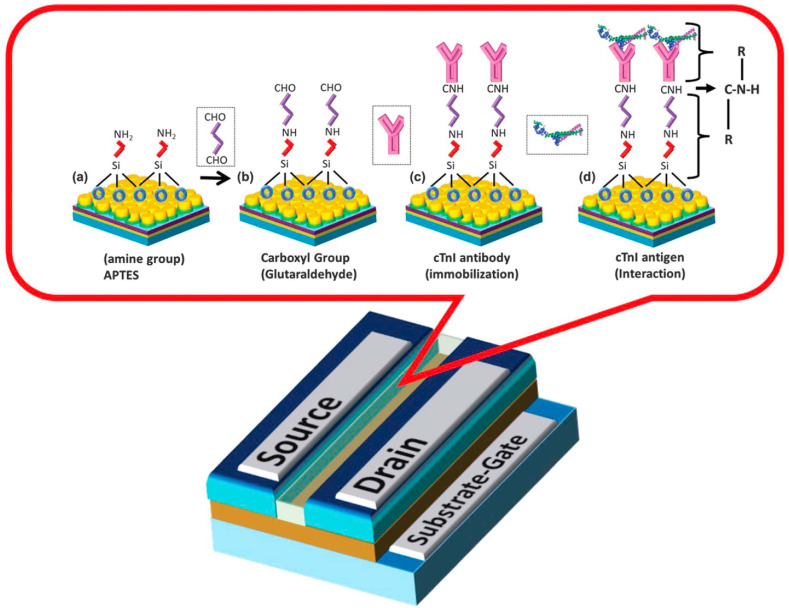
AMI is the primary reason for demise worldwide among heart-related disorders [112]. It is usually initiated by a decrease or blockage of blood flow to a section of the heart, resulting in necrosis of heart muscle [113]. This is generally the result of a blood clot or fat that obstructs cardiac muscle, which contains a coronary artery that carries oxygenated blood [114]. Accordingly, due to the insufficient circulation of the oxygenated blood within the organ itself, the cardiac muscle gradually damages and it secretes a protein-based molecule called cardiac troponin I (cTnI) [115]. Therefore, cTnI is an excellent biomarker for AMI detection and its concentration level can be associated with the stage of AMI [116]. Its durability and response capability after interaction with its complementary antibody turn it to be the reference point for early diagnosis of AMI [117]. To treat AMI early and prevent permanent damage to the heart, early detection is necessary [118]. Conventional detection methods like electrocardiogram (ECG) are not efficient enough because of their low sensitivity. Also, they require capturing the unusual signal and therefore, cannot be considered as early detection [119]. But detecting cTnI as a biomarker of AMI offers higher sensitivity, and selectivity [120]. Commonly used assays to detect this biomarker, including ELISA, fluorescence, electrochemiluminescence immunoassay, and surface plasmon resonance (SPR) are not cost-effective, and swift since they necessitate using highly sophisticated instruments and skilled laboratory personal [121]. So, they lack the capacity of being used as point-of-care (POC) testing [122]. Hitherto, several different strategies based on FET have been developed for detecting AMI, which is listed in Table 3 . Amongst different approaches, the most proficient method is established by Arshad and coworkers who developed an alternative technique for signal amplification. As it is illustrated in Fig. 7 , they designed a FET biosensor for achieving a boosted sensitivity and lower limit of detection. In this regard, a silicon-on-insulator wafer with top silicon and buried oxide (BOX) layers, and titanium dioxide (TiO2) nanomaterial were used to facilitate an efficient immobilization of specific antibody to selectively bind to cardiac troponin I (cTnI). The fabricated device presented a high capability in detecting lower levels (LOD = 1 fg/ml) of cTnI [123]. However, Kong et al. assembled a biosensing technique for identifying cTnI, using SiNW-based FETs. This way, monoclonal antibodies against CTnI were successfully immobilized on the surfaces of nanowires. The designed arrangement displayed a fast and sensitive response to target analytes which was recorded by a homemade biosensor measurement system. Additionally, its construction process enables its mass-production which paves the way for practical applications [124].
Table 3.
FET-base biosensors for early detection of AMI.
| Application | Target | RE | Linker | Surface | Sensor | Readout | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Acute myocardial infarction disease diagnosis | cTn1 | Anti-cardiac troponin I antibody | APTES GA |
TiO2 | FET | Off-chip | 1 fg/ml | Blood | [123] |
| CTnI detection | cTnI | Anti-cTnI | APTES GA |
Poly-SiNW | CMOS-compatible SiNWFET | On-chip | 7.6 pg/ml | Serum samples | [125] |
| AMI diagnosis | cTnT | Anti-cTnT | APTES GA |
Si/SiO2/SiNW | P-type SiNW-FET |
Off-chip | 27pM | serum | [126] |
| AMI diagnosis | cTnI | Anti- cTnI | APTES GA |
SiNW/SiO2/SiNx | CMOS-compatible SiNW FET | Off-chip | 0.092 ng/mL | Buffer solution (PBS) | [124] |
cTnT: cardiac Troponin T.
Fig. 7.
Surface functionalization process involving binding of linkers ((a)APTES and (B)GA) and (c) immobilizing antibody (d) Ab-Ag interaction [123].
3.7. Malaria
Malaria is among the serious infectious maladies in over 100 countries in the tropical and subtropical regions [127]. Each year, about 500 million people especially children under 5 years become infected and 1 to 3 million people lose their lives [128]. The pathogenesis of human malaria clinical illness is complicated since it has several settings, and possible outcomes [129]. Generally, the main cause of Malaria is the Plasmodium parasite known to pass on a disease to the human population [130]. Plasmodium falciparum is the most predominant of them all [131]. Plasmodium falciparum malaria parasites' biology, is definite, foreseeable, and simply distressed during their life cycle [132]. Furthermore, by the appearance of some strains that are not sensitive to drugs, the efficacy of existing malaria medications is decreasing [133]. Accordingly, the need to precisely spot this disorder for appropriate medicament and monitoring cannot be denied [134]. To control transmission dynamics, it is necessary to detect this disease early even in patients without any symptoms [135]. Up to the present time, a large number of researchers have tried to overcome the difficulties in malaria diagnostics with technologies that consider point-of-care demands and early-stage detection [136]. But amongst these novel techniques, biosensors seem to be the most sensitive and rapid ones. Specifically, Lab-on-Chip (LOC) platforms based on CMOS are precise and efficient for targeting this goal. Fig. 8 depicts a particular, and quantifiable detection method for this parasite, using a LOC system proposed by Malpartida-Cardenas and coworkers. They established reliable detection of a single-nucleotide related to drug-resistant strain of malaria. This DNA sensing was enabled by utilizing ISFETs, which were constructed through standard CMOS technology. This research study is capable of being developed to a handy and assessable POC assay of malaria [37].
Fig. 8.
Transverse profile of an ISFET manufactured in standard CMOS technology and the analogous circuit macro model [37].
3.8. Bacteria-related disease
Rapid and reliable detecting bacterial pathogens are imperative in different fields such as public health, medicine, food poisoning, bioterrorism, and security [137]. Bacterial infections are causing millions of deaths and hospitalizations yearly all around the world [138]. These types of contagious or transmittable illnesses are most challenging in poor nations, such as African countries, where therapeutic amenities and diagnostic methods are not at an advanced level [139]. However, in wealthy and developed countries such as western nations, food-borne pathogens are considered a major risk as well [140]. So it is vital to detect these bacteria early to prevent disease development and subsequent treatment. A time-consuming process, lack of sensitivity and selectivity, the need for specialized laboratory equipment, expert personnel, and high cost of conventional methods of bacterial detection, made scientists look for a more efficient alternative [141]. A rapid investigation that could sense and identify the cause of bacterial infection within minutes is needed immediately. Recently developed FET-based biosensors are very appropriate for handy POC and LOC devices [10]. Table 4 summarizes the recently fabricated biosensors for bacterial infections. For instance, Nikkhoo and coworkers, an innovative all-electronic biosensor that can detect bacteria in below ten minutes was introduced. They selected bacteriocins as the potential BREs were which were incorporated with potassium-selective sensors in CMOS technology to offer a cost-effective biosensing tool [142]. As can be seen in Fig. 9 , Lerner and colleagues evaluated the capability of a FET device based on single-walled carbon nanotubes for speedy and precise detection of specific antigen of Lyme disease. They were successful to effectively detect this antigen with a LOD of 1 ng/ml [143].
Table 4.
FET-base biosensors for early detection of bacteria-related diseases.
| Application | Target | RE | Linker | Surface | Sensor | Readout | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Grampositive and Gram-Negative bacteria detection | E. coli, S. aureus or P. aeruginosa | Bacteriocins potassium ions | Valinomycin- polyvinylchloride (PVC) | SiO2/Al/methylene blue/potassium-sensitive electrode | CMOS integrated ISFETs | On-chip | 107 bacteria/mL | Buffer solution | [142] |
| Lyme disease diagnosis | B. burgdorferi flagellar antigen | Borrelia burgdorferi (Lyme) flagellar antibody | Diazonium salts Histidine EDC/NHS |
Si/SiO2/single-wall nanotube (SWNT) | SWNT FET | Off-chip | 1 ng/ml | Buffer solution | [143] |
Fig. 9.
Modification chemistry for Lyme antibody and its interaction with a flagellar antigen [143].
3.9. Other potential diseases
3.9.1. Nucleic acid detection
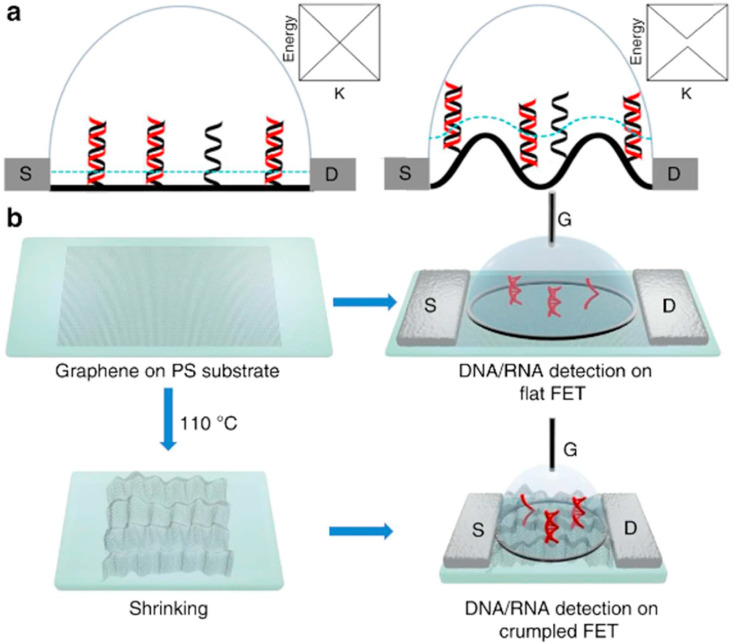
Nucleic acid diagnostics are of significant importance in numerous areas, such as early detection of genetic diseases, identifying pathogens, and drug development studies [144]. DNA hybridization is the key strategy of most DNA detection methods, in which a particular single-stranded DNA (ssDNA) molecules identify the reciprocal strand mediated by typically Watson and Crick base pairing procedures [145]. By immobilizing probe DNAs onto the sensor's surface, this hybridization is generally sensed [146]. DNA's phosphate backbone is the reason for the shift in-charge near the sensor's surface yielding a response after attachment [147]. One of the most essential features to take into consideration is the DNA probe density on the sensing area that should be high enough to produce measurable hybridized charge and boosted sensor signal. But to prevent electrostatic repulsions and the resulting reduction in hybridization efficacy, it should not be too high [148]. In recent years, FET devices have attracted attention amid the abundance of electrical DNA detection platforms, owing to their several benefits, including reduced size, rapid response, incorporation into arrays, and the probability of affordable large-scale manufacture [149]. The capability of concurrent analysis of several DNA/RNA targets in down-sized analytical structures has paved the way for the improvement of all-inclusive LOC platforms [150]. Between the currently developed FET-based genosensors that are presented in Table 5 , the device fabricated by Hu and coworkers showed an extraordinary performance. They functionalized the surface of polycrystalline silicon nanowire FET devices with DNA probes and attained a LOD of 0.1 fM [151]. In another successful work conducted by Hwang et al. FETs with a monolayer of graphene channel were utilized for the detection of DNA. Fig. 10 demonstrates that these miniaturized devices showed exceptional sensitivity in both buffer and human serum samples. The reported LODs were 600 zM and 20 aM, respectively [152]. These results depict that FET biosensors are offering a favorable methodology sensitive and specific for nucleic acid detection.
Table 5.
FET-base biosensors for early detection of nucleic acids.
| Application | Target | RE | Linker | Surface | Sensor | Readout | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Optimize the ionic strength and the debye length of the detection | Target oligomers | DNA oligomers | APTES GA |
Poly-Si NW | FET | Off-chip | 0.1 fM | Buffer solution (BTP) | [151] |
| DNA detection | DNA | PNA | APTES GA PASE ETA |
SiO2/Ti/Au/PMMA/SLG/copper/CVD-grown SLG | G-FET | Off-chip | 10 fM | Buffer solution (PBS) | [153] |
| Nucleic acid analysis | Target DNA | Probe DNA | APTES EDC/NHS |
SiO2/Si3N4/SWNT | CMOS-compatible SiNW-FET | Off-chip | 1 fM | Buffer solution (PBS) | [154] |
| DNA hybridization sensing | Target DNA | Probe DNA | APTES GA |
SiO2/Poly SiNW | CMOS-compatible poly-Si NW FET | Off-chip | 1 fM | Buffer solution (PBS) | [155] |
| DNA detection | Target DNA | Probe DNA | PASE ETA |
PS/Graphene/PMMA/Silicone rubber | FET | Off-chip | 20 aM | Serum | [152] |
ETA: ethanolamine, SLG: single-layer graphene, PNA: peptide nucleic acid, PASE: 1-Pyrenebutanoic acid succinimidyl ester, PS: polystyrene.
Fig. 10.
Illustration of the flat and crumpled graphene FET genosensor. b fabrication of FETs and investigational flow of the procedure [152].
3.9.2. Other biomarkers
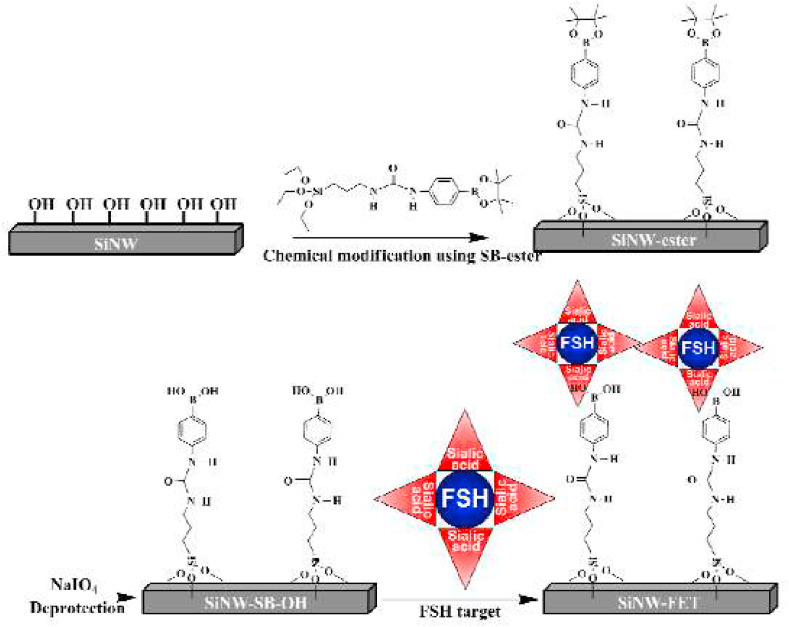
FET biosensors are used for some other applications as well. For instance, measuring the amount of l-carnitine [156]. Its deficiency that is caused by chronic disorders is generally associated with genetic diseases, cardiomyopathy, and hypoglycemia in newborns, isovaleric acidaemia, chronic fatigue syndrome, methylmalonic, and hypoketotic hypoglycaemic encephalopathy [157]. So, measuring the serum concentration of l-carnitine for monitoring anomalies in the oxidation of fatty acid is significantly imperative in therapeutic applications. Or follicle-stimulating hormone (FSH) which is secreted by the pituitary gland and has a part in body growth [158]. By planning a sensor sensitive to sialic acids, one can directly measure the concentration of FSH. Another target analyte is phosphate -one of the most fundamental nourishing materials. Its normal physiological range is between 0.80 and 1.45 mM. To maintain a healthy body, an average human must consume 700–1250 mg phosphorus every day. It is noteworthy that, excessive consumption accelerates the aging processes [159]. Hence, checking phosphate levels in nutrients is necessary to avoid unfavorable effects. Real-time inspection of glycemic levels can affect diabetic outcomes, such as amended hemoglobin A1C levels and lower long term complications [160]. Therefore, glucose-sensitive biosensors are amongst the most popular and well-established FET devices. In brain tissue, the neurotransmitter acetylcholine which is one of the neurochemically active molecules is engaged in a variety of behavioral aspects such as, reasoning, attention, motivation, and reward in the central nervous system. As a result, its monitoring is vital for revealing brain disorders [161]. Transplanting isolated islets from a donor pancreas into another person is a form of treatment for type 1 diabetes. After transplantation, the islets start producing insulin and regulating the level of glucose in the blood. The average concentration of pancreatic insulin is ~10 μM. This value is contingent upon several parameters such as the number and size of islets, extent, and length of glucose stimulation, etc. Thus, it is important to monitor the level of insulin secreted from islets [162]. Thyroid-stimulating hormone (TSH), is a glycoprotein secreted from the pituitary gland, which controls the endocrine role of the thyroid gland. Its level is an indicator of the metabolic actions in the human body [163]. That is why TSH biosensors are being developed in recent years. To have a successful treatment without side effects, it is imperative to maintain the drug's proper concentration in the blood. For this reason, tracking the drug level and regulating its effective dosage is of considerable importance [164]. A list of these newly developed FET biosensors is summarized in Table 6 . For example, Lee et al. came up with a pioneering recognizing method for FSH using SiNW-FET devices modified with boronic acid. The detection process was effectively done in both buffer solution and 20% serum condition with a LOD of 0.72 fM and 1.1 fM, respectively [165]. Fig. 11 clarifies the functionalization process of the SiNW-FET device.
Table 6.
FET-base biosensors for early detection of other biomarkers.
| Application | Target | RE | Linker | Surface | Sensor | Readout | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Direct protein sensing beyond the debye length limit | Streptavidin | Biotin | Magnetic beads | Ta2O5/SiO2 | ISFET | Off-chip | 2.3 μM | Buffer solution | [166] |
| Screening disorders in fatty acid oxidation | l-carnitine | Carnitine acetyltransferase | APTES GA |
Ta2O5 | ISFET | Off-chip | 0.2 μM | Artificial urine | [167] |
| FSH detection | Sialic acids | Boronic acid | SB-ester NaIO4 |
SiNW | FET | Off-chip | 0.72 fM 1.1 fM |
Buffer solution (PBS) and 20% serum solution | [165] |
| Phosphate detection | Phosphate | Pyruvate oxidase | ZnO NRs | Si/SiO2 | FET | Off-chip | 0.5 μM | Buffer solution (HEPES) | [168] |
| Thyroid diagnosis | hTSH | Anti-hTSH | APTES GA |
SiNW/SiO2 | CMOS-compatible SiNWFET | Off-chip | 0.11 pM | Buffer solution (PBS) | [169] |
| Lead and potassium ions detection | K+ and Pb2+ | TBA | MB | Si/SiO2/graphene | FET-like | Off-chip | 100 μM–10 μM | Standard sample | [170] |
| Biomolecular recognition | Avidin | Biotin | APTMS | SiNW | SiNW-FET | Off-chip | 15 ± 1 fM | Buffer solution (PBS) | [171] |
ZnO NRs: ZnO nanorods, hTSH: human thyroid-stimulating hormone, TBA: thrombin binding aptamer, APTMS: 3-Aminopropyl)trimethoxysilane.
Fig. 11.
Illustration of the functionalization process of the SiNW-FET sensor using SB-ester [165].
4. Non- selective adhesion for cell-based screening applications
It can be inferred from Table 7 that a wide variety of cell-based biosensing platforms are designed and used for different applications in recent years. For example, Anand et al. established a FET device based on SiNW for the instantaneous sensing of the cultured cortical neurons' K+ efflux. They functionalized the surface of the system by K+-specific DNA-aptamers. After introducing the cells over the FET in a buffer free of Na+/K+ ions, the extracellular level of K+ varied according to the environmental status. The results validated the sensitivity of the arrangement for detecting cations and measuring the intra- and extracellular concentrations of the K+ [172]. Or in another work done by Li and colleagues, a bottom-up technique was used to detect the secreted dopamine from PC12 cells. They modified the surface of Si NWs with APTMS. The selective surface-modified SiNW-FETs presented favorable electrical properties such as high trans-conductance. It was confirmed that employing the interaction between biotin and avidin in the surface amendment of the SiNW-FETs significantly boosted the detection sensitivity [171]. The same research group designed another multiple-parallel-connected SiNW-FET device for identifying dopamine by utilizing DNA-aptamers as BREs. It was demonstrated that the LOD improved to below 10−11 M in comparison with conventional electrochemical approaches. Also, the high selectivity of the device was such that it could differentiate dopamine from its other chemical analogs. This FET aptasensor was also used to screen the release of dopamine from living cells in a milieu which had poor oxygen concentration [173]. In a different research work conducted by Susloparova et al., open-gate FET devices were employed to investigate the cancerous cells' adhesion status in the presence of an anti-cancer drug. The proposed approach has the potential to be used for the examination of the effectiveness of new medications in concurrent evaluations [174]. Another application can be seen in Lim and coworkers' research, which used SWCNT-FET systems coupled with nanovesicles for designing an olfactory receptor (OR) genes screening test. The ORs on the surface membrane of nanovesicles induced the increase of calcium ions. Consequently, a variation in the current occurred alongside the carbon nanotube which was monitored by the fabricated biosensor [175]. These successful research works confirm the significant potential of FET-base devices for cell-based screening applications. Despite the improvements in this field, researchers face many challenges that should address in future studies. For example, to solve packaging issues, upcoming designs can have a more compact structure. Besides, a shared readout system can be huge save in power consumption and area. It is imperative to keep the power factor as low as possible to prevent temperature increase and accordingly cell death. Or to construct a wide-ranging biological micro-device that can identify cellular reactions and process the gathered data, digitization of blocks in conjunction with multi-array electrode systems might be needed [176,177]. In addition, implantable systems that are capable of real-time reporting of cellular activities set the stage for the emergence of a new category of sensing platforms. Compactness and flexibility are the two important features of theses novel sensors. Tackling these problems requires the collaboration of researchers from different backgrounds. If the abovementioned confrontations are resolved, FET-based devices may be expected to function as an efficient platform for biological and biomedical applications [178,179].
Table 7.
FET-base biosensors for cell-based screening applications.
| Application | Cell | AL | Surface | Sensor | Readout | Specification | Culture? | Sample | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Detection of dopamine released from PC12 cells | Living PC12 cells | Boronic acid/dopamine APTMS EDC/NHS |
SiNW | SiNW-FET | Off-chip | 33 ± 8 fM | Yes | Buffer solution (PBS) | [171] |
| Detection of the K+ efflux | Cortical neurons | Aptamers | SiNW/APTM/DSC | FET | Off-chip | AMPA stimulation boosted the K+ level to ~800 nM (EC50 = 10.3 μM) | Yes | Tris buffer solution | [172] |
| Detection of dopamine released from PC12 cells | Living PC12 cells | APTMS PTMS BMS DNA-aptamers |
SiNW | SiNW-FET | Off-chip | <10–11 M | Yes | Buffer solution (PBS) | [173] |
| OR screening assay | HEK-293 | PDL | SWNT | FET | Off-chip | Calcium influx (1 mM Ca2+ ions) | Yes | Buffer solution (PBS) | [175] |
| Anti-cancer drug (topotecan hydrochloride) action analysis | HEK293 H441 |
Fibronectin | SiO2 | Impedance spectroscopy (open gate FET) | Off-chip | 20% change in the amplitude of the impedance spectra in the presence of the drug | Yes | Buffer solution (PBS) | [174] |
| Detection of the K+-efflux | Chromaffin cells | VAL-PVC | SiO2 | SiNW-FET | Off-chip | Increase of the K+ level under the stimulation of nicotine | Yes | Buffer solution (Tris buffer) | [180] |
HEK-293: human embryonic kidney cells, AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid, DSC: N, N′-disuccinimidyl carbonate, OR: Olfactory receptor genes, PDL: Poly- d –lysine, PTMS: propyltrimethoxysilane, BMS: 3-maleimidobenzoic acid, H441: human lung adenocarcinoma epithelial cells.
5. Conclusion
The most recent and innovative approaches in the field of biologically sensitive field-effect transistors for early diagnosis of diseases and cell-based assays for drug screening studies were highlighted in this article. Since the past few decades, several research works have focused on FET-based biosensors owing to their favorable potentials including, sensitivity, specificity, compactness, portability, speed, cost-effectiveness, ease of use, and the possibility of high-throughput analysis. The fact that most of them are constructed using standard, commercialized, and mass-produced devices, turn them into one of the most preferred and successful approaches. Furthermore, the feasibility to study low volumes of samples makes them exceptionally efficient. It cannot be denied that the constant enhancement of nanotechnology and amendment of readout systems further improve the biosensing platforms that facilitate high throughput screening for biomolecular assays and address the need for rapid, robust, and economical POC detection. However, there are still numerous important challenges that need to be addressed before they become validated as POC assessments. For instance, BREs should be immobilized in a firm and reproducible manner onto the functionalized FET channel. Also, the ability to be massively produced with high accuracy of fundamental representative properties, including rate, reliability, and shelf-life is of high significance. In addition, a FET type biosensor should be highly selective to have a minimum amount of cross-reactions, in order to be functional as a POC tool. Though, recently done research works such as the ones studied here acknowledge that FET-based biosensors will soon find their way from the laboratory to the market specifically for applications in the early detection of disease biomarkers. Integrating nanomaterials and nanostructures such as SiNWs, CNTs, graphene, and metal nanoparticles as sensing channels improve the performance of FET type biosensing devices considerably owing to high surface to volume ratio, chemical firmness, and biocompatibility. Standard biosensing tools like SiNWFETs are predicted to contribute significantly to the improvement of biomedical applications and provide extraordinary sensitivity and specificity for identifying biomolecules binding acts or metabolic reactions at the touchpoint of living organisms and electronics. But, it is not easy to judge which one is the most appropriate choice for further progression due to the discrepancy in results from one lab to another. In addition, it is noteworthy that, incorporation of microfluidics with biosensing devices will be vital for constructing channelized integrated analytical arrangements. Consequently, there is a laborious way in front of researchers to come up with advanced policies for improving the efficacy of FET devices.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Bhalla N., Jolly P., Formisano N., Estrela P. Essays Biochem. 2016;60:1. doi: 10.1042/EBC20150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carpenter A.C., Paulsen I.T., Williams T.C. Genes (Basel) 2018;9 doi: 10.3390/genes9080375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel S., Nanda R., Sahoo S., Mohapatra E. Biochem. Res. Int. 2016;2016 doi: 10.1155/2016/3130469. 3130469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehrotra P. J. Oral Biol. Craniofac. Res. 2016;6:153. doi: 10.1016/j.jobcr.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syedmoradi L., Ahmadi A., Norton M.L., Omidfar K. Mikrochim. Acta. 2019;186:739. doi: 10.1007/s00604-019-3850-6. [DOI] [PubMed] [Google Scholar]
- 6.Bergveld P. IEEE Tran. Biomed. Eng. BME-17. 1970:70. doi: 10.1109/tbme.1970.4502688. [DOI] [PubMed] [Google Scholar]
- 7.Duroux P., Emde C., Bauerfeind P., Francis C., Grisel A., Thybaud L., Arstrong D., Depeursinge C., Blum A.L. Gut. 1991;32:240. doi: 10.1136/gut.32.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X., Jiang H. Sensors (Basel) 2017;17 doi: 10.3390/s17122805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai X., Vo R., Hsu H.H., Deng P., Zhang Y., Jiang X. Nano Lett. 2019;19:6658. doi: 10.1021/acs.nanolett.9b02939. [DOI] [PubMed] [Google Scholar]
- 10.Vu C.A., Chen W.Y. Sensors (Basel) 2019;19 doi: 10.3390/s19194214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pachauri V., Ingebrandt S. Essays Biochem. 2016;60:81. doi: 10.1042/EBC20150009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wadhera T., Kakkar D., Wadhwa G., Raj B. J. Electron. Mater. 2019;48:7635. [Google Scholar]
- 13.Huang W., Diallo A.K., Dailey J.L., Besar K., Katz H.E. J. Mater. Chem. C Mater. 2015;3:6445. doi: 10.1039/C5TC00755K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee C.S., Kim S.K., Kim M. Sensors (Basel) 2009;9:7111. doi: 10.3390/s90907111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mu L., Chang Y., Sawtelle S.D., Wipf M., Duan X., Reed M.A. IEEE Access. 2015;3:287. [Google Scholar]
- 16.Lowe B.M., Sun K., Zeimpekis I., Skylaris C.K., Green N.G. Analyst. 2017;142:4173. doi: 10.1039/c7an00455a. [DOI] [PubMed] [Google Scholar]
- 17.Rajan N.K., Duan X., Reed M.A. Wiley Interdiscipl. Rev. Nanomed. Nanobiotechnol. 2013;5:629. doi: 10.1002/wnan.1235. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad R., Mahmoudi T., Ahn M.S., Hahn Y.B. Biosens. Bioelectron. 2018;100:312. doi: 10.1016/j.bios.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lage O.M., Ramos M.C., Calisto R., Almeida E., Vasconcelos V., Vicente F. Mar. Drugs. 2018;16 doi: 10.3390/md16080279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carnero A. Clin. Transl. Oncol. 2006;8:482. doi: 10.1007/s12094-006-0048-2. [DOI] [PubMed] [Google Scholar]
- 21.Hierlemann A., Baltes H. Analyst. 2003;128:15. doi: 10.1039/b208563c. [DOI] [PubMed] [Google Scholar]
- 22.Nakazato K. Sensors (Basel) 2009;9:8831. doi: 10.3390/s91108831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Risveden K., Ponten J.F., Calander N., Willander M., Danielsson B. Biosens. Bioelectron. 2007;22:3105. doi: 10.1016/j.bios.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Dinar A.M., Zain A.S.M., Salehuddin F. Int. J. Electr. Comput. Eng. 2019;9 [Google Scholar]
- 25.Jimenez-Jorquera C., Orozco J., Baldi A. Sensors (Basel) 2010;10:61. doi: 10.3390/s100100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parizi K.B., Xu X., Pal A., Hu X., Wong H.S. Sci. Rep. 2017;7:41305. doi: 10.1038/srep41305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.M. Meyyappan, J.-S. Lee, (2014) 225.
- 28.Morales M.A., Halpern J.M. Bioconjugate Chem. 2018;29:3231. doi: 10.1021/acs.bioconjchem.8b00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J.J., Luo X.L., Chen H.Y. Front. Biosci. 2005;10:420. doi: 10.2741/1538. [DOI] [PubMed] [Google Scholar]
- 30.Synhaivska O., Mermoud Y., Baghernejad M., Alshanski I., Hurevich M., Yitzchaik S., Wipf M., Calame M. Sensors. 2019;19:4022. doi: 10.3390/s19184022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H., Liu X., Li L., Mu X., Genov R., Mason A.J. Sensors (Basel) 2016;17 doi: 10.3390/s17010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang A.Y., Lu M.S. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2013. 2013:4102. doi: 10.1109/EMBC.2013.6610447. [DOI] [PubMed] [Google Scholar]
- 33.Hussain A.M., Hussain M.M. Adv. Mater. 2016;28:4219. doi: 10.1002/adma.201504236. [DOI] [PubMed] [Google Scholar]
- 34.Adiguzel Y., Kulah H. Sensors (Basel) 2012;12:10042. doi: 10.3390/s120810042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radamson H.H., He X., Zhang Q., Liu J., Cui H., Xiang J., Kong Z., Xiong W., Li J., Gao J., Yang H., Gu S., Zhao X., Du Y., Yu J., Wang G. Micromachines (Basel) 2019;10 doi: 10.3390/mi10050293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lei K.M., Mak P.I., Law M.K., Martins R.P. Lab Chip. 2016;16:3664. doi: 10.1039/c6lc01002d. [DOI] [PubMed] [Google Scholar]
- 37.Malpartida-Cardenas K., Miscourides N., Rodriguez-Manzano J., Yu L.S., Moser N., Baum J., Georgiou P. Biosens. Bioelectron. 2019;145:111678. doi: 10.1016/j.bios.2019.111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferain I., Colinge C.A., Colinge J.P. Nature. 2011;479:310. doi: 10.1038/nature10676. [DOI] [PubMed] [Google Scholar]
- 39.Sridhar M., Xu D., Kang Y., Hmelo A.B., Feldman L.C., Li D., Li D. J. Appl. Phys. 2008;103:104701. doi: 10.1063/1.2931026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kudo T., Ito T., Nakajima A. J. Vac. Sci. Technol. B. 2013;31 [Google Scholar]
- 41.B.M. Cullum, H.-K. Lyu, D.M. Porterfield, Y.-S. Choi, J.-K. Shin, J.-H. Kim, 7313 (2009) 73130S.
- 42.Mostafa S., Lee I., Islam S.K., Eliza S.A., Shekhawat G., Dravid V.P., Tulip F.S. IEEE Electron. Device Lett. 2011;32:408. [Google Scholar]
- 43.Li H., Liu S.M., Yu X.H., Tang S.L., Tang C.K. Int. J. Antimicrob. Agents. 2020:105951. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He F., Deng Y., Li W. J. Med. Virol. 2020;92:719–725. doi: 10.1002/jmv.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ge H., Wang X., Yuan X., Xiao G., Wang C., Deng T., Yuan Q., Xiao X. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39(6):1011–1019. doi: 10.1007/s10096-020-03874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. Int. J. Antimicrob. Agents. 2020:105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seo G., Lee G., Kim M.J., Baek S.H., Choi M., Ku K.B., Lee C.S., Jun S., Park D., Kim H.G., Kim S.J., Lee J.O., Kim B.T., Park E.C., Kim S.I. ACS Nano. 2020;14:5135. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- 48.Paget J., Spreeuwenberg P., Charu V., Taylor R.J., Iuliano A.D., Bresee J., Simonsen L., Viboud C., Global N. Seasonal influenza-associated mortality collaborator, G.L.C. Teams∗. J Glob Health. 2019;9 doi: 10.7189/jogh.09.020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghebrehewet S., MacPherson P., Ho A. BMJ. 2016;355:i6258. doi: 10.1136/bmj.i6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beigel J.H. Crit. Care Med. 2008;36:2660. doi: 10.1097/CCM.0b013e318180b039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pleschka S. Curr. Top. Microbiol. Immunol. 2013;370:1. doi: 10.1007/82_2012_272. [DOI] [PubMed] [Google Scholar]
- 52.Fukuyama S., Kawaoka Y. Curr. Opin. Immunol. 2011;23:481. doi: 10.1016/j.coi.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Otto C.C., Kaplan S.E., Stiles J., Mikhlina A., Lee C., Babady N.E., Tang Y.W. J. Vis. Exp. 2017;119:e54312. doi: 10.3791/54312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kontou P.I., Braliou G.G., Dimou N.L., Nikolopoulos G., Bagos P.G. Diagnostics (Basel) 2020;10 doi: 10.3390/diagnostics10050319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee T., Ahn J.H., Park S.Y., Kim G.H., Kim J., Kim T.H., Nam I., Park C., Lee M.H. Micromachines (Basel) 2018;9 doi: 10.3390/mi9120651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim D.K., Poudel B. Yonsei Med. J. 2013;54:560. doi: 10.3349/ymj.2013.54.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.George K.S. Methods Mol. Biol. 2012;865:53. doi: 10.1007/978-1-61779-621-0_4. [DOI] [PubMed] [Google Scholar]
- 58.Chiang P.L., Chou T.C., Wu T.H., Li C.C., Liao C.D., Lin J.Y., Tsai M.H., Tsai C.C., Sun C.J., Wang C.H., Fang J.M., Chen Y.T. Chem. Asian J. 2012;7:2073. doi: 10.1002/asia.201200222. [DOI] [PubMed] [Google Scholar]
- 59.Lee K.H., Lee J.O., Choi S., Yoon J.B., Cho G.H. Biosens. Bioelectron. 2012;31:343. doi: 10.1016/j.bios.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 60.Uhm M., Lee J.M., Lee J., Lee J.H., Choi S., Park B.G., Kim D.M., Choi S.J., Mo H.S., Jeong Y.J., Kim D.H. Sensors (Basel) 2019;19 doi: 10.3390/s19204502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J.Y., Choi K., Moon D.I., Ahn J.H., Park T.J., Lee S.Y., Choi Y.K. Biosens. Bioelectron. 2013;41:867. doi: 10.1016/j.bios.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 62.Si J., Yu C., Guo Y., Bian Z., Meng R., Yang L., Chen Y., Jin J., Liu J., Guo Z., Chen J., Chen Z., Lv J., Li L., China Kadoorie Biobank Collaborative G. BMJ Open. 2019;9 [Google Scholar]
- 63.Stasi C., Silvestri C., Voller F. J Clin Transl Hepatol. 2017;5:272. doi: 10.14218/JCTH.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kushner T., Sarkar M. Clin. Liver Dis. (Hoboken) 2018;12:24. doi: 10.1002/cld.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang M.H., Chen D.S. Cold Spring Harb. Perspect. Med. 2015;5:a021493. doi: 10.1101/cshperspect.a021493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aspinall E.J., Hawkins G., Fraser A., Hutchinson S.J., Goldberg D. Occup. Med. (Lond.) 2011;61:531. doi: 10.1093/occmed/kqr136. [DOI] [PubMed] [Google Scholar]
- 67.Wilkins T., Sams R., Carpenter M. Am. Fam. Physician. 2019;99:314. [PubMed] [Google Scholar]
- 68.Yao C.Y., Fu W.L. World J. Gastroenterol. 2014;20:12485. doi: 10.3748/wjg.v20.i35.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shariati M. Biosens. Bioelectron. 2018;105:58. doi: 10.1016/j.bios.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 70.Gao A., Zou N., Dai P., Lu N., Li T., Wang Y., Zhao J., Mao H. Nano Lett. 2013;13:4123. doi: 10.1021/nl401628y. [DOI] [PubMed] [Google Scholar]
- 71.Kumar A., Sidhu J., Goyal A., Tsao J.W. StatPearls Publishing StatPearls Publishing LLC. Treasure Island (FL); 2020. Alzheimer Disease. [Google Scholar]
- 72.Schachter A.S., Davis K.L. Dialogues Clin. Neurosci. 2000;2:91. doi: 10.31887/DCNS.2000.2.2/asschachter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bondi M.W., Edmonds E.C., Salmon D.P. J. Int. Neuropsychol. Soc. 2017;23:818. doi: 10.1017/S135561771700100X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Apostolova L.G. Continuum (Minneap Minn) 2016;22:419. doi: 10.1212/CON.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neugroschl J., Wang S. Mt. Sinai J. Med. 2011;78:596. doi: 10.1002/msj.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weller J., Budson A. F1000Res. 2018;7 doi: 10.12688/f1000research.14506.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rasmussen J., Langerman H. Degener. Neurol. Neuromuscul. Dis. 2019;9:123. doi: 10.2147/DNND.S228939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee M., Guo J.P., Kennedy K., McGeer E.G., McGeer P.L. J Alzheimers Dis. 2017;55:1175. doi: 10.3233/JAD-160748. [DOI] [PubMed] [Google Scholar]
- 79.Lee J.C., Kim S.J., Hong S., Kim Y. Exp. Mol. Med. 2019;51:1. doi: 10.1038/s12276-019-0250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kutovyi Y., Hlukhova H., Boichuk N., Menger M., Offenhausser A., Vitusevich S. Biosens. Bioelectron. 2020;154:112053. doi: 10.1016/j.bios.2020.112053. [DOI] [PubMed] [Google Scholar]
- 81.Siegel R.L., Miller K.D., Jemal A. CA cancer. J Clin. 2020;70:7. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 82.Loud J.T., Murphy J. Semin. Oncol. Nurs. 2017;33:121. doi: 10.1016/j.soncn.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schiffman J.D., Fisher P.G., Gibbs P. Am Soc Clin Oncol Educ Book. 2015:57. doi: 10.14694/EdBook_AM.2015.35.57. [DOI] [PubMed] [Google Scholar]
- 84.Sarkar S., Horn G., Moulton K., Oza A., Byler S., Kokolus S., Longacre M. Int. J. Mol. Sci. 2013;14:21087. doi: 10.3390/ijms141021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sauter E.R. Eur. J. Breast Health. 2017;13:162. doi: 10.5152/ejbh.2017.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hazelton W.D., Luebeck E.G. Sci. Transl. Med. 2011;3:109fs9. doi: 10.1126/scitranslmed.3003272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tobore T.O. Future Sci. OA. 2019;6:FSO439. doi: 10.2144/fsoa-2019-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu N., Gao A., Dai P., Song S., Fan C., Wang Y., Li T. Small. 2014;10:2022. doi: 10.1002/smll.201302990. [DOI] [PubMed] [Google Scholar]
- 89.Bao Z., Sun J., Zhao X., Li Z., Cui S., Meng Q., Zhang Y., Wang T., Jiang Y. Int. J. Nanomed. 2017;12:4623. doi: 10.2147/IJN.S135985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao A., Yang X., Tong J., Zhou L., Wang Y., Zhao J., Mao H., Li T. Biosens. Bioelectron. 2017;91:482. doi: 10.1016/j.bios.2016.12.072. [DOI] [PubMed] [Google Scholar]
- 91.Gao A., Lu N., Wang Y., Li T. Sci. Rep. 2016;6 doi: 10.1038/srep22554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su D.S., Chen P.Y., Chiu H.C., Han C.C., Yen T.J., Chen H.M. Biosens. Bioelectron. 2019;141:111209. doi: 10.1016/j.bios.2019.03.042. [DOI] [PubMed] [Google Scholar]
- 93.Mirsian S., Khodadadian A., Hedayati M., Manzour-Ol-Ajdad A., Kalantarinejad R., Heitzinger C. Biosens. Bioelectron. 2019;142:111527. doi: 10.1016/j.bios.2019.111527. [DOI] [PubMed] [Google Scholar]
- 94.Malsagova K.A., Pleshakova T.O., Kozlov A.F., Shumov I.D., Ilnitskii M.A., Miakonkikh A.V., Popov V.P., Rudenko K.V., Glukhov A.V., Kupriyanov I.N., Ivanova N.D., Rogozhin A.E., Archakov A.I., Ivanov Y.D. Biosensors (Basel) 2018;8 doi: 10.3390/bios8030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bao Z., Sun J., Zhao X., Li Z., Cui S., Meng Q., Zhang Y., Wang T., Jiang Y. Int. J. Nanomed. 2017;12:4623. doi: 10.2147/IJN.S135985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao A., Yang X., Tong J., Zhou L., Wang Y., Zhao J., Mao H., Li T. Biosens. Bioelectron. 2017;91:482. doi: 10.1016/j.bios.2016.12.072. [DOI] [PubMed] [Google Scholar]
- 97.Gao N., Gao T., Yang X., Dai X., Zhou W., Zhang A., Lieber C.M. Proc. Natl. Acad. Sci. U. S. A. 2016;113:14633. doi: 10.1073/pnas.1625010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao A., Lu N., Wang Y., Li T. Sci. Rep. 2016;6:22554. doi: 10.1038/srep22554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tran D.P., Winter M.A., Wolfrum B., Stockmann R., Yang C.T., Pourhassan-Moghaddam M., Offenhausser A., Thierry B. ACS Nano. 2016;10:2357. doi: 10.1021/acsnano.5b07136. [DOI] [PubMed] [Google Scholar]
- 100.Chen H.C., Chen Y.T., Tsai R.Y., Chen M.C., Chen S.L., Xiao M.C., Chen C.L., Hua M.Y. Biosens. Bioelectron. 2015;66:198. doi: 10.1016/j.bios.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 101.Gao N., Zhou W., Jiang X., Hong G., Fu T.M., Lieber C.M. Nano Lett. 2015;15:2143. doi: 10.1021/acs.nanolett.5b00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu N., Gao A., Dai P., Mao H., Zuo X., Fan C., Wang Y., Li T. Anal. Chem. 2015;87:11203. doi: 10.1021/acs.analchem.5b01729. [DOI] [PubMed] [Google Scholar]
- 103.Tran D.P., Wolfrum B., Stockmann R., Pai J.H., Pourhassan-Moghaddam M., Offenhausser A., Thierry B. Anal. Chem. 2015;87:1662. doi: 10.1021/ac503374j. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Y., Chen R., Xu L., Ning Y., Xie S., Zhang G.J. Anal. Sci. 2015;31:73. doi: 10.2116/analsci.31.73. [DOI] [PubMed] [Google Scholar]
- 105.Zhou F., Li Z., Bao Z., Feng K., Zhang Y., Wang T. Scand. J. Clin. Lab. Invest. 2015;75:578. doi: 10.3109/00365513.2015.1060519. [DOI] [PubMed] [Google Scholar]
- 106.Chen H.C., Qiu J.T., Yang F.L., Liu Y.C., Chen M.C., Tsai R.Y., Yang H.W., Lin C.Y., Lin C.C., Wu T.S., Tu Y.M., Xiao M.C., Ho C.H., Huang C.C., Lai C.S., Hua M.Y. Anal. Chem. 2014;86:9443. doi: 10.1021/ac5001898. [DOI] [PubMed] [Google Scholar]
- 107.Lee M., Baraket A., Zine N., Zabala M., Campabadal F., Caruso R., Trivella M.G., Jaffrezic-Renault N., Errachid A. Methods Mol. Biol. 2014;1172:49. doi: 10.1007/978-1-4939-0928-5_5. [DOI] [PubMed] [Google Scholar]
- 108.Choi J.H., Kim H., Kim H.S., Um S.H., Choi J.W., Oh B.K. J. Biomed. Nanotechnol. 2013;9:732. doi: 10.1166/jbn.2013.1541. [DOI] [PubMed] [Google Scholar]
- 109.Choi J.H., Kim H., Choi J.H., Choi J.W., Oh B.K. ACS Appl. Mater. Interfaces. 2013;5:12023. doi: 10.1021/am403816x. [DOI] [PubMed] [Google Scholar]
- 110.Kim C.H., Ahn J.H., Kim J.Y., Choi J.M., Lim K.C., Park T.J., Heo N.S., Lee H.G., Kim J.W., Choi Y.K. Biosens. Bioelectron. 2013;41:322. doi: 10.1016/j.bios.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 111.Lee M.H., Lee K., Jung S.W. Conf Proc IEEE Eng Med Biol Soc 2012. 2012:570. doi: 10.1109/EMBC.2012.6345995. [DOI] [PubMed] [Google Scholar]
- 112.Lee T., Ahn J.H., Choi J., Lee Y., Kim J.M., Park C., Jang H., Kim T.H., Lee M.H. Micromachines (Basel) 2019;10 doi: 10.3390/mi10030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu L., Liu M., Sun R., Zheng Y., Zhang P. Cell Biochem. Biophys. 2015;72:865. doi: 10.1007/s12013-015-0553-4. [DOI] [PubMed] [Google Scholar]
- 114.Bruyninckx R., Aertgeerts B., Bruyninckx P., Buntinx F. Br. J. Gen. Pract. 2008;58:105. doi: 10.3399/bjgp08X277014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Braunwald E. Eur Heart J Acute Cardiovasc Care. 2012;1:9. doi: 10.1177/2048872612438026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aydin S., Ugur K., Aydin S., Sahin I., Yardim M. Vasc. Health Risk Manag. 2019;15:1. doi: 10.2147/VHRM.S166157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park K.C., Gaze D.C., Collinson P.O., Marber M.S. Cardiovasc. Res. 2017;113:1708. doi: 10.1093/cvr/cvx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tiwari R.P., Jain A., Khan Z., Kohli V., Bharmal R.N., Kartikeyan S., Bisen P.S. Mol. Diagn. Ther. 2012;16:371. doi: 10.1007/s40291-012-0011-6. [DOI] [PubMed] [Google Scholar]
- 119.Boeddinghaus J., Reichlin T., Nestelberger T., Twerenbold R., Meili Y., Wildi K., Hillinger P., Gimenez M.R., Cupa J., Schumacher L., Schubera M., Badertscher P., Corbiere S., Grimm K., Puelacher C., Sabti Z., Widmer D.F., Schaerli N., Kozhuharov N., Shrestha S., Burge T., Machler P., Buchi M., Rentsch K., Miro O., Lopez B., Martin-Sanchez F.J., Rodriguez-Adrada E., Morawiec B., Kawecki D., Ganovska E., Parenica J., Lohrmann J., Buser A., Keller D.I., Osswald S., Mueller C. Clin. Res. Cardiol. 2017;106:457. doi: 10.1007/s00392-016-1075-9. [DOI] [PubMed] [Google Scholar]
- 120.Hasic S., Kiseljakovic E., Jadric R., Radovanovic J., Winterhalter-Jadric M. Bosn. J. Basic Med. Sci. 2003;3:41. doi: 10.17305/bjbms.2003.3527. [DOI] [PubMed] [Google Scholar]
- 121.Freund Y., Chenevier-Gobeaux C., Bonnet P., Claessens Y.E., Allo J.C., Doumenc B., Leumani F., Cosson C., Riou B., Ray P. Crit. Care. 2011;15:R147. doi: 10.1186/cc10270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Garg P., Morris P., Fazlanie A.L., Vijayan S., Dancso B., Dastidar A.G., Plein S., Mueller C., Haaf P. Intern Emerg Med. 2017;12:147. doi: 10.1007/s11739-017-1612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arshad M.K.M., Adzhri R., Fathil M.F.M., Gopinath S.C.B., N M.N. J. Nanosci. Nanotechnol. 2018;18:5283. doi: 10.1166/jnn.2018.15419. [DOI] [PubMed] [Google Scholar]
- 124.Kong T., Su R., Zhang B., Zhang Q., Cheng G. Biosens. Bioelectron. 2012;34:267. doi: 10.1016/j.bios.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 125.Pei-Wen Y., Che-Wei H., Yu-Jie H., Min-Cheng C., Hsin-Hao L., Shey-Shi L., Chih-Ting L. Biosens. Bioelectron. 2014;61:112. doi: 10.1016/j.bios.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 126.Elnathan R., Kwiat M., Pevzner A., Engel Y., Burstein L., Khatchtourints A., Lichtenstein A., Kantaev R., Patolsky F. Nano Lett. 2012;12:5245. doi: 10.1021/nl302434w. [DOI] [PubMed] [Google Scholar]
- 127.Hay S.I., Guerra C.A., Tatem A.J., Noor A.M., Snow R.W. Lancet Infect. Dis. 2004;4:327. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Autino B., Noris A., Russo R., Castelli F. Mediterr J Hematol Infect Dis. 2012;4 doi: 10.4084/MJHID.2012.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mawson A.R. Pathog. Glob. Health. 2013;107:122. doi: 10.1179/2047773213Y.0000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Laishram D.D., Sutton P.L., Nanda N., Sharma V.L., Sobti R.C., Carlton J.M., Joshi H. Malar. J. 2012;11:29. doi: 10.1186/1475-2875-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Autino B., Corbett Y., Castelli F., Taramelli D. Mediterr J Hematol Infect Dis. 2012;4 doi: 10.4084/MJHID.2012.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Day K.P., Hayward R.E., Dyer M. Parasitology. 1998;116(Suppl):S95. doi: 10.1017/s0031182000084985. [DOI] [PubMed] [Google Scholar]
- 133.Castelli F., Tomasoni L.R., Matteelli A. Mediterr J Hematol Infect Dis. 2012;4 doi: 10.4084/MJHID.2012.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ragavan K.V., Kumar S., Swaraj S., Neethirajan S. Biosens. Bioelectron. 2018;105:188. doi: 10.1016/j.bios.2018.01.037. [DOI] [PubMed] [Google Scholar]
- 135.Ittarat W., Chomean S., Sanchomphu C., Wangmaung N., Promptmas C., Ngrenngarmlert W. Clin. Chim. Acta. 2013;419:47. doi: 10.1016/j.cca.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 136.Krampa F.D., Aniweh Y., Kanyong P., Awandare G.A. Sensors (Basel) 2020;20 doi: 10.3390/s20030799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Varadi L., Luo J.L., Hibbs D.E., Perry J.D., Anderson R.J., Orenga S., Groundwater P.W. Chem. Soc. Rev. 2017;46:4818. doi: 10.1039/c6cs00693k. [DOI] [PubMed] [Google Scholar]
- 138.Murray T.S., Groth M.E., Weitzman C., Cappello M. Clin. Microbiol. Rev. 2005;18:510. doi: 10.1128/CMR.18.3.510-520.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fenollar F., Mediannikov O. New Microbes New Infect. 2018;26:S10. doi: 10.1016/j.nmni.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Toone E.J. Adv. Enzymol. Relat. Area Mol. Biol. 2011;77:xi. [PubMed] [Google Scholar]
- 141.Tsalik E.L., Bonomo R.A., Fowler V.G., Jr. Annu. Rev. Med. 2018;69:379. doi: 10.1146/annurev-med-052716-030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nikkhoo N., Cumby N., Gulak P.G., Maxwell K.L. PloS One. 2016;11 doi: 10.1371/journal.pone.0162438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lerner M.B., Dailey J., Goldsmith B.R., Brisson D., Johnson A.T. Biosens. Bioelectron. 2013;45:163. doi: 10.1016/j.bios.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang J. Nucleic Acids Res. 2000;28:3011. doi: 10.1093/nar/28.16.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Teles F., Fonseca L. Talanta. 2008;77:606. [Google Scholar]
- 146.Ping J., Vishnubhotla R., Vrudhula A., Johnson A.T. ACS Nano. 2016;10:8700. doi: 10.1021/acsnano.6b04110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kaisti M., Kerko A., Aarikka E., Saviranta P., Boeva Z., Soukka T., Lehmusvuori A. Sci. Rep. 2017;7:15734. doi: 10.1038/s41598-017-16028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gao Z., Xia H., Zauberman J., Tomaiuolo M., Ping J., Zhang Q., Ducos P., Ye H., Wang S., Yang X., Lubna F., Luo Z., Ren L., Johnson A.T.C. Nano Lett. 2018;18:3509. doi: 10.1021/acs.nanolett.8b00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Veigas B., Fortunato E., Baptista P.V. Sensors (Basel) 2015;15:10380. doi: 10.3390/s150510380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li B.R., Chen C.C., Kumar U.R., Chen Y.T. Analyst. 2014;139:1589. doi: 10.1039/c3an01861j. [DOI] [PubMed] [Google Scholar]
- 151.Hu W.P., Tsai C.C., Yang Y.S., Chan H.W., Chen W.Y. Sci. Rep. 2018;8:12598. doi: 10.1038/s41598-018-30996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hwang M.T., Heiranian M., Kim Y., You S., Leem J., Taqieddin A., Faramarzi V., Jing Y., Park I., van der Zande A.M., Nam S., Aluru N.R., Bashir R. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-15330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zheng C., Huang L., Zhang H., Sun Z., Zhang Z., Zhang G.J. ACS Appl. Mater. Interfaces. 2015;7:16953. doi: 10.1021/acsami.5b03941. [DOI] [PubMed] [Google Scholar]
- 154.Lu N., Gao A., Dai P., Li T., Wang Y., Gao X., Song S., Fan C., Wang Y. Methods. 2013;63:212. doi: 10.1016/j.ymeth.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 155.Wenga G., Jacques E., Salaun A.C., Rogel R., Pichon L., Geneste F. Biosens. Bioelectron. 2013;40:141. doi: 10.1016/j.bios.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 156.Ferreira G.C., McKenna M.C. Neurochem. Res. 2017;42:1661. doi: 10.1007/s11064-017-2288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pekala J., Patkowska-Sokola B., Bodkowski R., Jamroz D., Nowakowski P., Lochynski S., Librowski T. Curr. Drug Metabol. 2011;12:667. doi: 10.2174/138920011796504536. [DOI] [PubMed] [Google Scholar]
- 158.Zaidi M., Lizneva D., Kim S.M., Sun L., Iqbal J., New M.I., Rosen C.J., Yuen T. Endocrinology. 2018;159:3503. doi: 10.1210/en.2018-00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Takeda E., Taketani Y., Sawada N., Sato T., Yamamoto H. Biofactors. 2004;21:345. doi: 10.1002/biof.552210167. [DOI] [PubMed] [Google Scholar]
- 160.Mergenthaler P., Lindauer U., Dienel G.A., Meisel A. Trends Neurosci. 2013;36:587. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Picciotto M.R., Higley M.J., Mineur Y.S. Neuron. 2012;76:116. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ryan E.A., Lakey J.R., Rajotte R.V., Korbutt G.S., Kin T., Imes S., Rabinovitch A., Elliott J.F., Bigam D., Kneteman N.M., Warnock G.L., Larsen I., Shapiro A.M. Diabetes. 2001;50:710. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 163.Mullur R., Liu Y.Y., Brent G.A. Physiol. Rev. 2014;94:355. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McKeating K.S., Aube A., Masson J.F. Analyst. 2016;141:429. doi: 10.1039/c5an01861g. [DOI] [PubMed] [Google Scholar]
- 165.Lee M., Palanisamy S., Zhou B.H., Wang L.Y., Chen C.Y., Lee C.Y., Yuan S.F., Wang Y.M. ACS Appl. Mater. Interfaces. 2018;10:36120. doi: 10.1021/acsami.8b11882. [DOI] [PubMed] [Google Scholar]
- 166.Yang H., Sakata T. Sensors (Basel) 2019;19 doi: 10.3390/s19153393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Andrianova M.S., Kuznetsov E.V., Grudtsov V.P., Kuznetsov A.E. Biosens. Bioelectron. 2018;119:48. doi: 10.1016/j.bios.2018.07.044. [DOI] [PubMed] [Google Scholar]
- 168.Ahmad R., Ahn M.S., Hahn Y.B. J. Colloid Interface Sci. 2017;498:292. doi: 10.1016/j.jcis.2017.03.069. [DOI] [PubMed] [Google Scholar]
- 169.Lu N., Dai P., Gao A., Valiaho J., Kallio P., Wang Y., Li T. ACS Appl. Mater. Interfaces. 2014;6:20378. doi: 10.1021/am505915y. [DOI] [PubMed] [Google Scholar]
- 170.Xu K., Meshik X., Nichols B.M., Zakar E., Dutta M., Stroscio M.A. Nanotechnology. 2014;25:205501. doi: 10.1088/0957-4484/25/20/205501. [DOI] [PubMed] [Google Scholar]
- 171.Li B.R., Chen C.W., Yang W.L., Lin T.Y., Pan C.Y., Chen Y.T. Biosens. Bioelectron. 2013;45:252. doi: 10.1016/j.bios.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 172.Anand A., Liu C.R., Chou A.C., Hsu W.H., Ulaganathan R.K., Lin Y.C., Dai C.A., Tseng F.G., Pan C.Y., Chen Y.T. ACS Sens. 2017;2:69. doi: 10.1021/acssensors.6b00505. [DOI] [PubMed] [Google Scholar]
- 173.Li B.R., Hsieh Y.J., Chen Y.X., Chung Y.T., Pan C.Y., Chen Y.T. J. Am. Chem. Soc. 2013;135:16034. doi: 10.1021/ja408485m. [DOI] [PubMed] [Google Scholar]
- 174.Susloparova A., Koppenhofer D., Vu X.T., Weil M., Ingebrandt S. Biosens. Bioelectron. 2013;40:50. doi: 10.1016/j.bios.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 175.Lim J.H., Park J., Hong S., Park T.H. Methods Mol. Biol. 2015;1272:189. doi: 10.1007/978-1-4939-2336-6_13. [DOI] [PubMed] [Google Scholar]
- 176.Matsumoto A., Miyahara Y. Nanoscale. 2013;5:10702. doi: 10.1039/c3nr02703a. [DOI] [PubMed] [Google Scholar]
- 177.Rollo S., Rani D., Olthuis W., Pascual Garcia C. Biophys Rev. 2019;11:757. doi: 10.1007/s12551-019-00592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pullano S.A., Critello C.D., Mahbub I., Tasneem N.T., Shamsir S., Islam S.K., Greco M., Fiorillo A.S. Sensors (Basel) 2018;18 doi: 10.3390/s18114042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.A. Zhang, G. Zheng, C.M. Lieber, (2016) 255.
- 180.Chang K.S., Sun C.J., Chiang P.L., Chou A.C., Lin M.C., Liang C., Hung H.H., Yeh Y.H., Chen C.D., Pan C.Y., Chen Y.T. Biosens. Bioelectron. 2012;31:137. doi: 10.1016/j.bios.2011.10.005. [DOI] [PubMed] [Google Scholar]