Highlights
-
•
NLRP3 inflammasome inhibition reduces TREM-1 expression in the lungs of mice with ALI.
-
•
Activation of NLRP3 inflammasome up-regulates TREM-1 expression in murine macrophages via HMGB1 and IL-18.
-
•
NLRP3 inflammasome activation induces TREM-1 expression, contributing to the inflammatory network in the lungs of ALI mice.
Keywords: TREM-1, NLRP3 inflammasome, Acute lung injury, HMGB1, IL-18
Abbreviations: NLRP3, NOD-, LRR- and pyrin domain-containing 3; TREM-1, triggering receptor expressed on myeloid cells-1; ALI, acute lung injury; ARDS, acute respiratory distress syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; LPS, lipopolysaccharide; HMGB1, high-mobility group protein B1; IL-18, interleukin-18; IL-1β, interleukin-1 beta; ASC, apoptosis-associated speck-like protein; CM, conditioned medium; NF-κB, nuclear factor kappa-B; p-IκBα, inhibitor κB kinase protein phosphorylation; IκB, inhibitor κB kinase; ROS, reactive oxygen species; ATP, adenosine triphosphate; DAMPs, danger-associated molecular patterns
Abstract
NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome and triggering receptor expressed on myeloid cells-1 (TREM-1) are considered critical orchestrators of the inflammatory response in acute lung injury (ALI). However, few assumptions are based on the relationship between them. Here, we investigated the effect of NLRP3 inflammasome activation on the TREM-1 expression in lipopolysaccharide (LPS)-induced ALI and macrophages. We found that inhibition of the NLRP3 inflammasome reduced the TREM-1 expression and pathological lung injury in mice with ALI. Then, primary murine macrophages were used to dissect the underlying mechanistic events of the activation NLRP3 inflammasome involved in the TREM-1 expression. Our results demonstrated that the conditioned medium (CM) from NLRP3 inflammasome-activated-macrophages up-regulated the TREM-1 expression in macrophages, while this effect was reversed by an NLRP3 inflammasome inhibitor MCC950. Furthermore, neutralizing antibodies anti-IL-18 and anti-HMGB1 reduced the TREM-1 expression induced by NLRP3 inflammasome activation. Mechanistically, we found that CM from NLRP3 inflammasome-activated-macrophages increased the level of inhibitor κB kinase protein phosphorylation (p-IκBα) and reactive oxygen species (ROS) content, and promoted IκBα protein degradation in macrophages. While the inhibition of nuclear factor kappa-B (NF-κB) and scavenging ROS eliminated the up-regulation of TREM-1 induced by the NLRP3 inflammasome activation in macrophages. In summary, our study confers NLRP3 inflammasome as a new trigger of TREM-1 signing, which allows additional insight into the pathological of the inflammatory response in ALI.
1. Introduction
Acute respiratory distress syndrome (ARDS) is a progressive life-threatening form of respiratory failure. It is characterized by an uncontrolled inflammatory response, alveolar-capillary barrier damage, and non-cardiogenic pulmonary edema [1]. Recently, a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected over 200 countries [2]. ARDS is the leading cause of mortality of severe patients with SARS-CoV-2 infection [3]. Although the term acute lung injury (ALI) is no longer used clinically, it is widely used in experimental settings [4]. One hallmark of ARDS is the accumulation of immune cells in the lungs, resulting in tremendous inflammatory cytokine release [5]. Other groups and we have identified that NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome and triggering receptor expressed on myeloid cells-1 (TREM-1) as crucial effector molecules in ARDS [6], [7], [8], [9].
The NLRP3 inflammasome is a crucial intracellular multiprotein complex, consisting of NLRP3, an adaptor apoptosis-associated speck-like protein (ASC), and an effector (caspase-1) [10]. The NLRP3 inflammasome mediates the secretion of potent inflammatory mediators [11], and its activation requires two steps. The first step is a priming event mediating the transcription of pro-caspase-1, pro-interleukin (IL)‐1β, and pro-IL-18 mainly via nuclear factor kappa-B (NF-κB). The second step is the activation, which is recognized with danger-associated molecular patterns (DAMPs), such as nigericin and extracellular adenosine triphosphate (ATP) [12]. The NLRP3 inflammasome activation facilitates pro-caspase-1 self-cleavage into active caspase-1 and mediates the release of IL-1β, IL-18, and high-mobility group protein B1 (HMGB1) [13]. These cytokines are crucial in patients with ALI/ARDS.
TREM-1 is known as an activating receptor primarily expressed on macrophages and neutrophils [14]. The activation of TREM-1 initiates inflammatory responses independently, as well as by cross-talking with Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) [15]. The agonistic antibody for TREM-1 results in the production of various pro-inflammatory cytokines (such as TNF-α and IL-1β) and chemokines (such as IL-8). TREM-1 also regulates neutrophil and monocyte migration to inflammatory sites [16]. Although the natural ligands for TREM-1 remain elusive, its crucial role in acute inflammation has been demonstrated. We have reported that antagonistic peptide of TRME-1 (LR12) inhibits the NLRP3 inflammasome activation in a murine model of ALI induced by lipopolysaccharide (LPS) [7], indicating that activation of TREM-1 could activate the NLRP3 inflammasome. However, the effect of NLRP3 inflammasome activation on TREM-1 expression remains elusive. Here, we investigated whether activation of NLRP3 inflammasome up-regulated TREM-1 expression in the lungs of ALI mice.
2. Materials and methods
2.1. Mice
Male C57BL/6 mice (18 ± 2 g) were purchased from the Hunan SJA Laboratory Animal Co., Ltd (Hunan, China). The mice were kept in a specific pathogen-free environment with 50%-60% humidity, 24–26 °C, and 12-h light/dark cycle. All animal procedures in the present study were approved by the Ethics Committee Institute of Clinical Pharmacology at Central South University (Changsha, China).
2.2. ALI mouse model
Mice were randomly divided into the control, ALI, and ALI + MCC950 groups (8 mice per group). ALI was induced by tracheal injection of 5 mg/kg LPS (from E. coli O111:B4, Sigma-Aldrich, USA) in 50 μL of sterile saline as our previous report [17]. Mice in the control group received 50 μL saline intratracheally. Mice in the ALI + MCC950 and control groups were intraperitoneally injected (i.p.) with MCC950 (20 mg/kg) or vehicle (PBS) 2 h before the LPS injection. Mice were euthanized 6 h after LPS injection. All surgeries were performed under anesthesia with sodium pentobarbital (80 mg/kg, i.p.).
2.3. Histological analysis
Lung tissue from LPS-treated mice with or without MCC950 administration was collected. The lungs were fixed in 10% formalin and embedded in paraffin. Multiple sections (5-µm) were stained with Hematoxylin-Eosin (H&E).
2.4. Peritoneal macrophages isolation and culture
The isolation of peritoneal macrophages was described previously [6]. C57BL/6 mice received an intraperitoneal injection of 3% sterile thioglycollate (3 mL/mouse, Sigma-Aldrich, USA). Three days later, cells were isolated and cultured in RPMI-1640 (Gibco, Life Technologies, Carlsbad, CA), contenting 10% fetal bovine serum at 37 °C. Two hours later, non-adherent cells were washed.
2.5. Conditioned media preparation and treatment of macrophages
Primary murine peritoneal macrophages were seeded at 1 × 106 cells/mL in culture plate and cultured overnight. All cells were primed with LPS (100 ng/mL, Sigma-Aldrich, USA) for 135 min. The medium was then removed and replaced with fresh medium containing DMSO (1:1000) or a selective NLRP3 inflammasome inhibitor MCC950 (5 μM, Sigma-Aldrich, USA) for 30 min. Cells were then stimulated with NLRP3 inflammasome activator ATP (5 mM, Solarbio, China) for 45 min. The supernatant as a conditioned medium (CM-CON, CM-LA, and CM-LA + MCC950) was collected by centrifugation at 12000g for 10 min, and then diluted at 1:20, 1:10, and 1:5 with RPMI-1640 (containing serum).
Macrophages were treated with CM for 6 h or 24 h for total RNA extraction or Western-blotting. To estimate the mechanism by which NLRP3 inflammasome activation promoted TREM-1 expression, we treated macrophages with an NF-κB selective inhibitor (BAY 11-7082, 5 μM, Sigma-Aldrich) or ROS scavenger NAC (100 μM, Sigma-Aldrich, USA) for 30 min then exposed to 20% CM-LA for 6 h or 24 h. Peritoneal macrophages also were treated with different doses of IL-1β (Sigma-Aldrich, USA), HMGB1 (Biolegend, USA), or IL-18 (Biolegend, USA). Furthermore, to identify whether the effect of NLRP3 inflammasome on TREM-1 expression is via HMGB1 and IL-18, neutralizing antibodies of anti-IL-18 (20 μg/mL, R&D Systems, USA) and anti-HMGB1 (20 μg/mL, NOVUS, USA) were applied in CM-LA-stimulated macrophages.
2.6. Western blotting analysis
Cell lysates and lung tissue homogenates were prepared in RIPA buffer (Beyotime, Jiangsu, China) supplemented with protease inhibitors (Roche, Mannheim, Germany). Equal amounts of protein were subjected to SDS-PAGE gels and transferred to polyvinylidene difluoride membranes (Millipore, USA). After blocked in 5% fat-free milk or bovine serum albumin at room temperature for 1.5 h, the membranes were probed with primary antibody against TREM-1 (1:1000; Proteintech, China), β-actin (1:7500; SAB, USA), α-tubulin (1:5000; Servicebio, China), inhibitory κB kinase (IκB) (1:2000; Beyotime Biotechnology, China) and p-IκB (1:1000; immunoway, China). Horseradish peroxidase-conjugated secondary antibodies (1:1000; Cell Signaling Technology, USA) and enhanced chemiluminescence (Millipore, USA) were applied to detect protein content. Images were collected using ChemiDoc XRS (Bio-Rad, USA). Bands were quantified using the Image Lab Analyzer software (Bio-Rad). β-actin and α-tubulin were used as internal control.
2.7. Real-time PCR
Total RNA from macrophage or lung tissue was extracted with RNAiso (TaKaRa, Japan). Approximately 1 μg of RNA was synthesized into cDNA via the PrimeScript RT reagent Kit (TaKaRa). Gene expression was measured by qPCR using SYBR Green Ultra Mixture (TaKaRa) on a Bio-Rad real-time PCR system (CFX96 Touch™, Bio-Rad, USA). The cycling program was initiated with 30 s at 95 °C, 15 s at 95 °C for 40 cycles, and 30 s at 60 °C. Gene expression profiles were normalized to β-actin. Relative expression of genes was calculated by the 2−ΔΔCt method according to our previous study [18]. Primer sequences are in Table1 .
Table 1.
Sequences of the primers used in this study.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| Trem-1 | CTGTGCGTGTTCTTTGTC | CTTCCCGTCTGGTAGTCT |
| Nf-κb | GGAGGCATGTTCGGTAGTGG | CCCTGCGTTGGATTTCGTG |
| β-actin | TTCCAGCCTTCCTTCTTG | GGAGCCAGAGCAGTAATC |
2.8. Mitochondrial ROS measurement
Mitochondrial ROS was measured by staining with MitoSOX™ Red mitochondrial superoxide indicator (Invitrogen Life Technologies). Macrophages were seeded in 96-well plates and stimulated with CM-CON, CM-LA, or CM-LA + MCC950 for 3 h. Then cells were incubated with 5 µM MitoSOX reagent for 10 min at 37 °C in the dark. The cells were then gently washed twice with Hank’s. The fluorescence intensity was detected by Varioskan Flash (Thermo Fisher Scientific, USA) to estimate mitochondrial ROS levels.
2.9. Statistical analysis
All values were obtained from three independent experiments and expressed as the mean ± standard deviation. Statistical analyses were carried out using GraphPad Prism 7.0 (San Diego, CA, USA). Differences between the two groups were made with unpaired t-test. For comparisons among multiple groups, a one-way analysis of variance (ANOVA) was used. P-value < 0.05 was regarded statistically significant.
3. Results
3.1. NLRP3 inflammasome inhibition reduces the lung injury and TREM-1 expression in the lungs
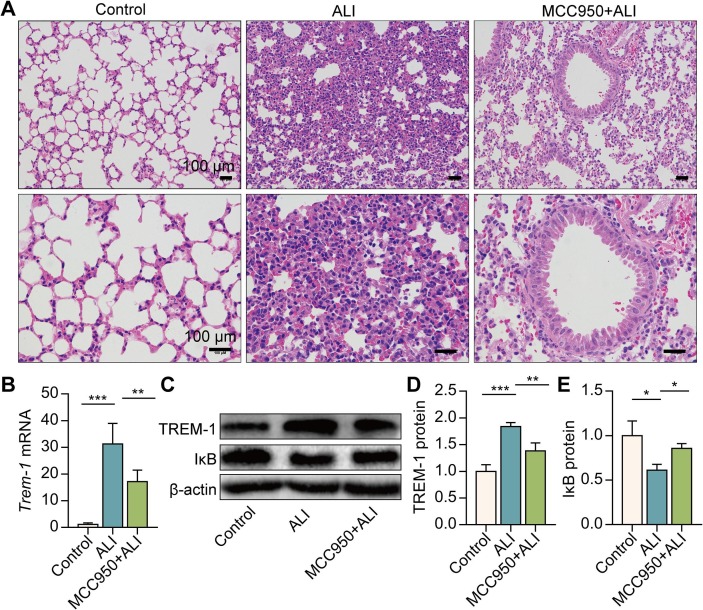
We first investigated whether NLRP3 inflammasome activation contributed to the progression of LPS-induced acute lung injury. NLRP3 inflammasome inhibitor MCC950 (20 mg/kg, i.p) was employed at 2 h before LPS administration. In association with the collapse of the alveoli, inflammatory cell influx and a thickened septum were observed in ALI mice’s lungs. These characteristic features of ALI were remarkably alleviated by MCC950 pre-treatment (Fig. 1 A). Our previous study indicated that TREM-1 acts as a crucial amplifier of inflammation in ALI [7]. We then evaluated the effect of NLRP3 inflammasome activation on TREM-1 expression in vivo. The results showed that MCC950 pre-treatment remarkably reduced TREM-1 mRNA and protein expression in the lungs of ALI mice (Fig. 1B–D). Additionally, MCC950 pre-treatment significantly inhibited IκB protein degradation and decreased NF-κB activation (Fig. 1C, E). These results demonstrate that inhibition of NLRP3 inflammasome activation reduces the lung injury and TREM-1 expression in vivo.
Fig. 1.
NLRP3 inflammasome inhibition reduces pathological lung injury and TREM-1 expression in the lungs of ALI mice. C57BL/6 mice were intraperitoneally injected with MCC950 2 h before the LPS administration (5 mg/kg, i.t.). Six hours after the LPS exposure, H&E staining of lung histopathology was performed (A). Trem-1 mRNA in the lungs was determined by real-time PCR (B, n = 8). TREM-1 and IκB protein in the lungs were detected by Western blotting (C-E, n = 8). The data are expressed as the mean ± SD. * P < 0.05, ** P < 0.01, and *** P < 0.001.
3.2. CM from NLRP3 inflammasome-activated macrophages induces TREM-1 expression
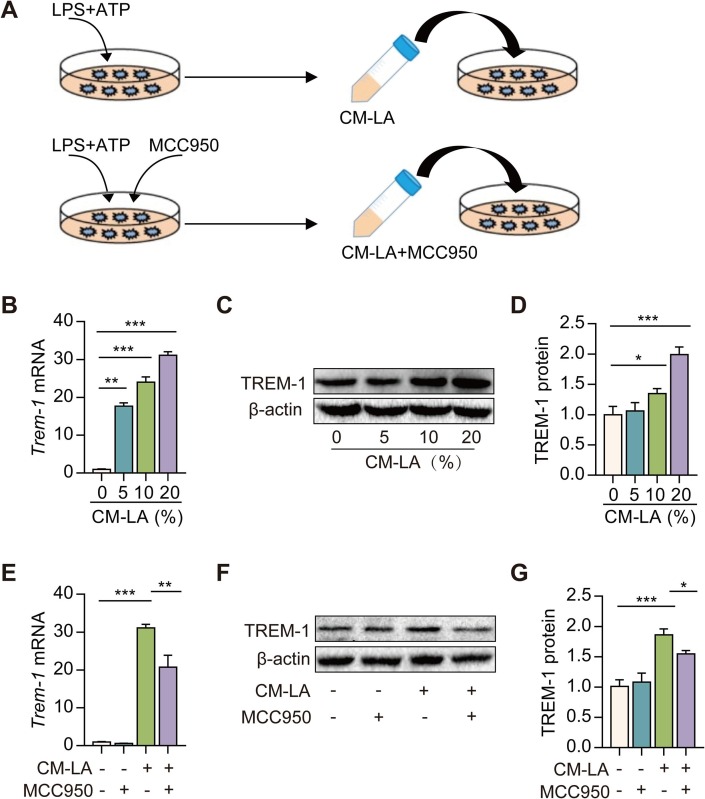
Macrophages are considered as crucial orchestrators of the inflammatory response in ALI [19]. We used peritoneal macrophages to evaluate the effect of CM derived from NLRP3 inflammasome-activated macrophages on TREM-1 expression in vitro. Macrophages were stimulated with or without LPS + ATP to collect the cell culture supernatants as CM (CM-CON and CM-LA). Then, the CM was used to incubate macrophages. An increase in TREM-1 mRNA and protein expression was observed after exposure to CM-LA in macrophages (Fig. 2 A-C). Besides, NLRP3 inflammasome inhibitor (MCC950, 5 μM) was applied 5 min before ATP treatment, and the conditioned medium (CM-LA + MCC950) was collected. A decrease in TREM-1 mRNA and protein expression was observed after exposure to CM-LA + MCC950 in macrophages (Fig. 2D–E). These data indicate that NLRP3 inflammasome activation induces TREM-1 expression via CM-LA stimulation in macrophages.
Fig. 2.
Conditioned medium from NLRP3 inflammasome-activated macrophages induces TREM-1 expression. A: The protocol for conditioned medium experimentation. LPS-primed peritoneal macrophages were subsequently stimulated for 30 min with 5 mM of ATP. NLRP3 inflammasome inhibitor (MCC950, 5 μM) was applied 5 min before ATP treatment. Cell culture supernatants (CM-CON, CM-LA, and CM-LA + MCC950) were collected and diluted at 1:20, 1:10, and 1:5 with RPMI-1640. Then, the CM was used to incubate macrophages. Real-time PCR and western blotting were used to detect the TREM-1 expression in macrophage treated with CM-CON or CM-LA (B-D, n = 3). Real-time PCR and western blotting were used to detect the TREM-1 expression in macrophage treated with CM-CON, CM-LA, or CM-LA + MCC950 (20%, v/v) (E-G, n = 3). The data are expressed as the mean ± SD. * P < 0.05, ** P < 0.01, and *** P < 0.001.
3.3. TREM-1 expression is up-regulated by CM from NLRP3 inflammasome-activated macrophages via the NF-κB pathway
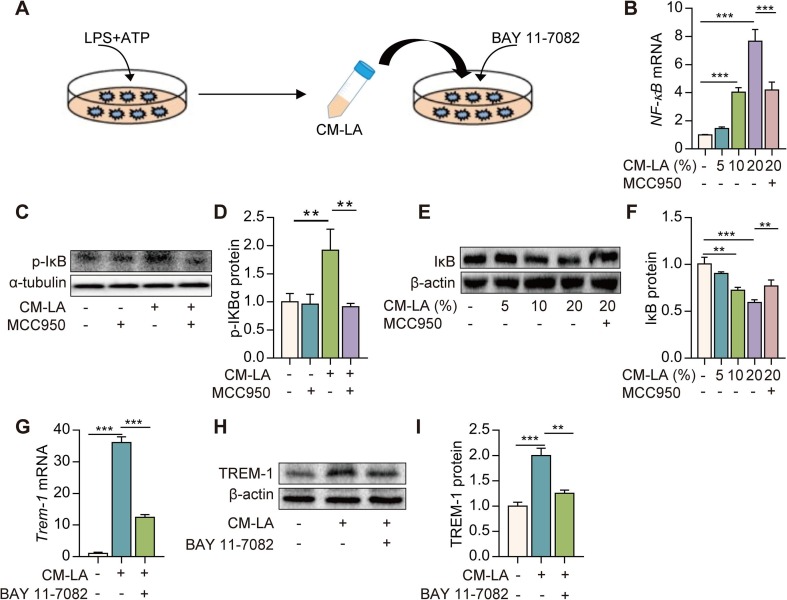
NF-κB is the most critical transcription factor mediating the expression of TREM-1. We found that NF-κB mRNA expression, p-IκBα lever, and IκB protein degradation were significantly increased in macrophages exposed to CM-LA in a dose-dependent manner (Fig. 3 B–F). While CM-LA + MCC950 treatment reversed these alterations and inhibited the NF-κB activation (Fig. 3B–F). Furthermore, Macrophages were treated with NF-κB selective inhibitor (BAY 11–7082, 5 μM) for 30 min then exposed to CM-LA (20%, v/v) for 6 h (Fig. 3A). The up-regulation of TREM-1 expression induced by CM-LA is remarkably suppressed by BAY 11-7082 (Fig. 3G–I). Altogether, these data indicate that CM from NLRP3 inflammasome-activated macrophages up-regulates TREM-1 expression via the NF-κB pathway.
Fig. 3.
TREM-1 expression is up-regulated by conditioned medium from NLRP3 inflammasome-activated macrophages via the NF-κB pathway. A: The protocol for conditioned medium experimentation. Macrophages were incubated with CM-CON, CM-LA, or CM-LA + MCC950. NF-κB mRNA expression was determined by real-time PCR 6 h later (B, n = 3), and p-IκBα, IκB protein was detected by western blotting 24 h later (C-F, n = 3). Macrophages were treated with NF-κB selective inhibitor (BAY 11–7082, 5 μM) for 30 min, then exposed to 20% CM-LA. Trem-1 mRNA expression was detected by real-time PCR 6 h later (G, n = 3), and TREM-1 protein expression was determined by western blotting 24 h later (H-I, n = 3). The data are expressed as the mean ± SD. ** P < 0.01 and *** P < 0.001.
3.4. TREM-1 expression is up-regulated by CM from NLRP3 inflammasome-activated macrophages through ROS/NF-κB signaling
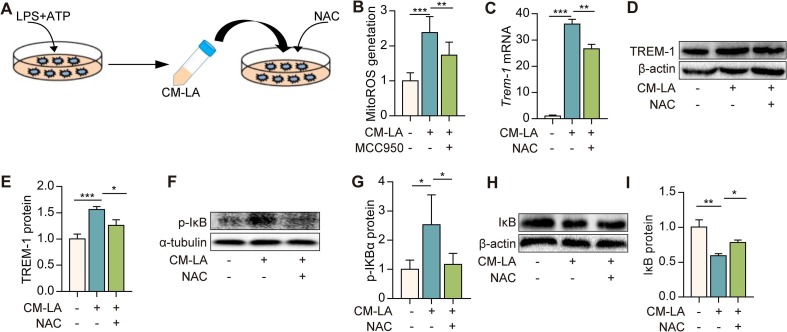
Next, we investigated the mechanism underlying the activation of NF-κB induced by CM-LA. We found that mitochondria ROS (mitoROS) production was significantly increased in macrophages treated to 20% CM-LA, while CM-LA + MCC950 treatment reduced the mitoROS production (Fig. 4 B). Furthermore, ROS scavenger NAC (100 μM) pre-treatment significantly reversed the expression of TREM-1 induced by CM-LA (Fig. 4C–E). Since ROS is an upstream signal for NF-κB, we found that NAC pre-treatment significantly diminished p-IκBα lever and IκB protein degradation induced by CM-LA (Fig. 4F–I). These results imply that NLRP3 inflammasome activation induces TREM-1 expression via ROS/NF-κB signaling.
Fig. 4.
TREM-1 expression is up-regulated by CM from NLRP3 inflammasome-activated macrophages through ROS-NF-κB signaling. A: The protocol for conditioned medium experimentation. Macrophages were treated with ROS scavenger NAC (100 μM) for 30 min, then exposed to 20% CM-LA. Fluorescence density of mitoROS was measured in macrophages treated with CM-CON, CM-LA, or CM-LA + MCC950 for 3 h (B, n = 3). Trem-1 mRNA expression was determined by real-time PCR (C, n = 3). TREM-1 (D-E, n = 3), p-IκB, and IκB (F-I, n = 3) protein expressions were detected by western blotting 24 h later. The data are expressed as the mean ± SD. * P < 0.05, ** P < 0.01, and *** P < 0.001.
3.5. HMGB1 and IL-18 mediate the up-regulation of TREM-1 expression induced by CM-LA in macrophages
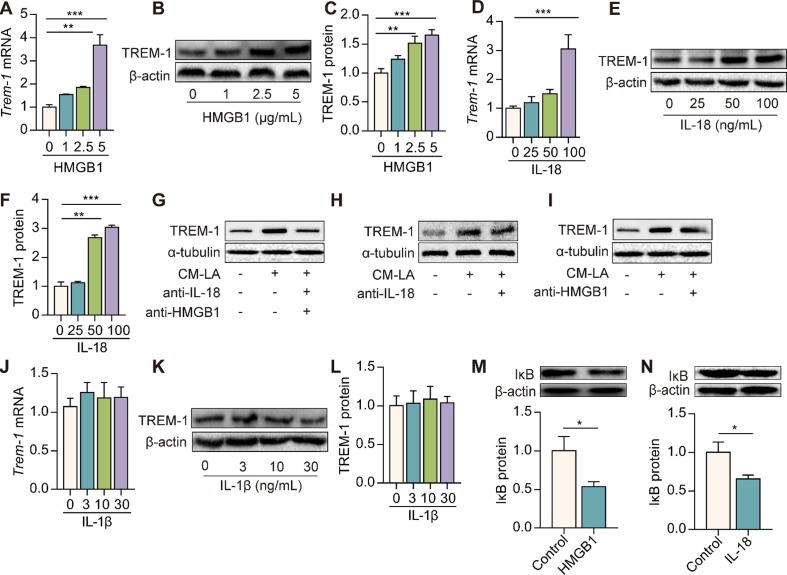
Lastly, we investigated the molecules in CM-LA mediating TREM-1 expression. We found that IL-β, IL-18, and HMGB1 were significantly increased in the CM of macrophages stimulated by LPS + ATP (Figure S1 and Figure S2). HMGB1 and IL-18 remarkably up-regulated TREM-1 mRNA and protein expressions in macrophages in a dose-dependent manner (Fig. 5 A–F). However, IL-1β did not affect TREM-1 expression (Fig. 5J–L). To identify whether HMGB1 and IL-18 mediate the effect of NLRP3 inflammasome on TREM-1 expression, we used neutralization antibodies of anti-IL-18 and anti-HMGB1. We found that CM-LA-induced TREM-1 expression was significantly reduced in the presence of neutralization antibodies of anti-IL-18 or/and anti-HMGB1 (Fig. 5G–I). Furthermore, HMGB1 and IL-18 treatment also promoted IκB protein degradation in macrophages (Fig. 5M, N). These data indicate that HMGB1 and IL-18 but not IL-1β in CM-LA mediate the up-regulation of TREM-1 expression in macrophages.
Fig. 5.
HMGB1 and IL-18 but not IL-1β mediate the up-regulation of TREM-1 in macrophages. Peritoneal macrophages were treated with different doses of HMGB1, IL-18, or IL-1β. Trem-1 mRNA expression was detected by Real-time PCR 6 h later (A, D, J, n = 3). TREM-1 protein expression was detected by western blotting 24 h later (B, C, E, F, K, L, n = 3). Macrophages were treated with neutralizing antibodies of anti-IL-18 (20 μg/mL) and anti-HMGB1 (20 μg/mL) for 30 min, then exposed to 20% CM-LA. TREM-1 protein expression was detected by western blotting 24 h later (G-I, n = 3). Macrophages were stimulated with HMGB1 (2.5 μg/mL) or IL-18 (50 ng/mL) for 24 h. IκB protein expression was detected by western blotting (M−N, n = 3). The data are expressed as the mean ± SD. * P < 0.05, ** P < 0.01, and *** P < 0.001.
4. Discussion
Although NLRP3 inflammasome and TREM-1 are assumed to play causative roles in ALI inflammatory progression, their relationship remains elusive. In this work, we investigated the effects of NLRP3 inflammasome activation on the expression of TREM-1 in primary macrophages and dissected the underlying mechanisms. Our results demonstrated that conditioned media from NLRP3 inflammasome-activated macrophages up-regulated TREM-1 expression by secreting soluble molecules HMGB1 and IL-18, which activated a ROS-NF-κB signaling. We further extended these findings in showing NLRP3 inflammasome inhibition reduced the pathological lung injury and TREM-1 expression in mice. Our study provides a novel mechanism of NLRP3 inflammasome on the inflammatory cascade.
NLRP3 inflammasome is a cytoplasmic receptor and mediates the release of pro-inflammatory cytokines, such as IL-1β, IL-18, and HMGB1 [20], [21]. Elevated IL-18 and HMGB1 occur in ALI patients and have been associated with a poor long-term prognosis in ALI [22], [23]. NLRP3 inflammasome has emerged as a novel strategy for the prevention and treatment of ALI [24]. NLRP3 inflammasome-amplified production of pro-inflammatory cytokines represents a key pathogenic event. However, the mechanical events of NLRP3 inflammasome promoting inflammatory cascade have not been understood completely. Our previous study found that blocking TREM-1 inhibits NLRP3 inflammasome activation in LPS-induced ALI [7]. Although the specific ligands for TREM-1 are not well elucidated, HMGB1 released by NLRP3 inflammasome activation has been identified as a TREM-1 binding protein [25]. Therefore, we hypothesized that NLRP3 inflammasome activation could induce TREM-1-amplified responses. Here, our results showed that inhibition of NLRP3 inflammasome reduced TREM-1 expression in ALI. Additionally, conditioned media from NLRP3 inflammasome-activated macrophages induced TREM-1 expression, which was reversed by an NLRP3 inflammasome inhibitor MCC950. We also found that HMGB1 and IL-18 induced TREM-1 expression in macrophages. Pre-treatment with neutralization antibodies of anti-IL-18 and anti-HMGB1 reduced TREM-1 expression induced by NLRP3 inflammasome activation. Our results indicate that NLRP3 inflammasome activation induces TREM-1 expression by secreting HMGB1 and IL-18.
Regulating TREM-1 expression has emerged as a novel therapeutic target for ALI [9]. As an inflammatory amplifier, TREM-1 plays a crucial role in the pathophysiology of inflammatory diseases, such as sepsis and neuroinflammatory injury [26], [27]. TREM-1 blockade attenuates inflammatory disease’s clinical manifestation and progression [28], [29]. TREM-1 signaling also is the central component of the pulmonary inflammatory response during ALI. Therefore, the mechanism to trigger TREM-1 signaling has attracted our attention. For example, TREM-1 expression in monocytes is dependent on the cyclooxygenase (COX)-2 pathway and is mediated by prostaglandin E2 (PGE2) [30]. Our previous studies found that transforming growth factor-β1 (TGF-β1) up-regulates TREM-1 expression [31], while vasoactive intestinal peptide (VIP) and epoxyeicosatrienoic acids (EETs) down-regulate TREM-1 expression induced by LPS in macrophages [32], [33]. In this study, we established a novel link between NLRP3 inflammasome and TREM-1 for the first time. Our previous study indicated that TREM-1-amplified signals facilitate NLRP3 inflammasome priming and activation in LPS-induced ALI [7]. And another study proposed that TREM-1 could activate NLRP3 inflammasome through interacting with spleen tyrosine kinase (Syk) in experimental ischemic stroke [26]. Our study demonstrated that the NLRP3 inflammasome activation induced TREM-1-amplified response, forming a positive feedback loop and enhancing the inflammatory response.
We also provide a mechanism by which NLRP3 inflammasome activation promotes the expression of TREM-1 in macrophages. Previous studies indicate that NF-κB modulates the TREM-1 expression. Viral proteins from human immunodeficiency virus up-regulate TREM-1 expression though NF-κB signaling [34]. When NF-κB and IKBα are depolymerized, IKBα is phosphorylated and subsequently degraded by the ubiquitin–proteasome pathway. Then, NF-κB is transported into the nucleus to facilitate NF-κB dependent gene transcription [35]. Here, we showed that inhibition of NLRP3 inflammasome activation reduced IκB degradation in ALI mice’s lungs. Inhibition of NF-κB attenuated TREM-1 expression induced by CM from NLRP3 inflammasome-activated macrophages. Furthermore, we found that HMGB1 and IL-18 alone induced IκB degradation and activated NF-κB. Taken together, these findings suggest that TREM-1 expression is up-regulated by NLRP3 inflammasome activation via the NF-κB pathway. It is known that ROS triggers NF-κB activation in several ways [36]. ROS influences the degradation of IκB, promoting NF-κB nuclear translocation [37]. Our findings indicate that TREM-1 expression is up-regulated by NLRP3 inflammasome through ROS-NF-κB signaling in macrophages.
Our study has several limitations. First, the mechanistic link between NLRP3 inflammasome and TREM-1 signing in macrophages requires further investigation. Our data suggest that NLRP3 inflammasome activation triggers TREM-1 expression via ROS-NF-κB signaling. Other molecular events and regulators require further exploration. Secondly, TREM-1 is known expressed primarily on macrophages and neutrophils. Several studies prove its presence in endothelial cells [28]. Moreover, endothelial dysfunction and neutrophils migration are also associated with ALI’s pathological process [38], [39]. We just explored the NLRP3-TREM-1-amplified inflammation response in macrophages. Further studies on the mechanism of NLRP3 inflammasome activation as a trigger of TREM-1 response in endothelial dysfunction or neutrophils infiltration are required.
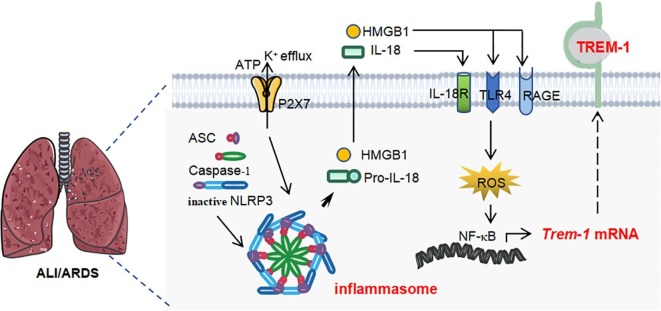
In summary, our study provides a novel link between NLRP3 inflammasome activation and TREM-1. We have found that NLRP3 inflammasome activation induces TREM-1-amplified response, forming a positive feedback loop and promoting the inflammatory cascade. HMGB1 and IL-18 released by NLRP3 inflammasome activation up-regulate TREM-1 expression in macrophages (see Fig. 6 ).
Fig. 6.
Schematic illustration. Activation of NLRP3 inflammasome induces TREM-1 expression in macrophages by secreting soluble molecules HMGB1 and IL-18.
Acknowledgments
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81670014, 91949110), the Hunan Provincial Natural Science Foundation of China (2019JJ50975), the Research Foundation of Education Bureau of Hunan Province, China (18A491), and Fundamental Research Funds for the Central Universities of Central South University (2020zzts219).
Author contributions
W.J.Z., J.X.D., T.L., H.H.Y., X.X.G., C.Y.Z., and J.T.Y. performed the experiments; J.X.D., Q.L., and J.B.X. analyzed the data. Y.Z. and C.X.G. contributed reagents/materials/analysis tools. W.J.Z. and J.X.D. wrote the paper. Qing Li conceived, designed the experiments, and critically reviewed the manuscript. All authors had final approval of the submitted versions.
Declaration of Competing Interest
The authors have declared that no competing interest exists.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.107045.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Fan E., Brodie D., Slutsky A.S. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 2.Wu Y., Ho W., Huang Y., Jin D.Y., Li S., Liu S.L., Liu X., Qiu J., Sang Y., Wang Q., Yuen K.Y., Zheng Z.M. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet. 2020;395:949–950. doi: 10.1016/S0140-6736(20)30557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet. Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Force A.D.T., Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307 doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 5.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., Herridge M., Randolph A.G., Calfee C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang H.H., Duan J.X., Liu S.K., Xiong J.B., Guan X.X., Zhong W.J., Sun C.C., Zhang C.Y., Luo X.Q., Zhang Y.F., Chen P., Hammock B.D., Hwang S.H., Jiang J.X., Zhou Y., Guan C.X. A COX-2/sEH dual inhibitor PTUPB alleviates lipopolysaccharide-induced acute lung injury in mice by inhibiting NLRP3 inflammasome activation. Theranostics. 2020;10:4749–4761. doi: 10.7150/thno.43108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu T., Zhou Y., Li P., Duan J.X., Liu Y.P., Sun G.Y., Wan L., Dong L., Fang X., Jiang J.X., Guan C.X. Blocking triggering receptor expressed on myeloid cells-1 attenuates lipopolysaccharide-induced acute lung injury via inhibiting NLRP3 inflammasome activation. Sci. Rep. 2016;6 doi: 10.1038/srep39473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M., Hua Q., Shao Y., Zeng H., Liu Y., Diao Q., Zhang H., Qiu M., Zhu J., Li X., Ling Y., Zhang R., Jiang Y. Circular RNA circBbs9 promotes PM2.5-induced lung inflammation in mice via NLRP3 inflammasome activation. Environ. Int. 2020;143 doi: 10.1016/j.envint.2020.105976. [DOI] [PubMed] [Google Scholar]
- 9.Su, V. Y., Yang, K. Y., Chiou, S. H., Chen, N. J., Mo, M. H., Lin, C. S. & Wang, C. T. (2019) Induced Pluripotent Stem Cells Regulate Triggering Receptor Expressed on Myeloid Cell-1 Expression and the p38 Mitogen-Activated Protein Kinase Pathway in Endotoxin-Induced Acute Lung Injury, Stem Cells. 37, 631–639. [DOI] [PubMed]
- 10.Moossavi M., Parsamanesh N., Bahrami A., Atkin S.L., Sahebkar A. Role of the NLRP3 inflammasome in cancer. Mol. Can. 2018;17:158. doi: 10.1186/s12943-018-0900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinar A.A., Scott T.E., Huuskes B.M., Tapia Caceres F.E., Kemp-Harper B.K., Samuel C.S. Targeting the NLRP3 inflammasome to treat cardiovascular fibrosis. Pharmacol. Ther. 2020;209 doi: 10.1016/j.pharmthera.2020.107511. [DOI] [PubMed] [Google Scholar]
- 13.Mangan M.S.J., Olhava E.J., Roush W.R., Seidel H.M., Glick G.D., Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug. Discov. 2018;17:588–606. doi: 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- 14.Carrasco K., Boufenzer A., Jolly L., Le Cordier H., Wang G., Heck A.J., Cerwenka A., Vinolo E., Nazabal A., Kriznik A., Launay P., Gibot S., Derive M. TREM-1 multimerization is essential for its activation on monocytes and neutrophils. Cell Mol. Immunol. 2019;16:460–472. doi: 10.1038/s41423-018-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao S., Yi Y., Xia G., Yu C., Ye C., Tu F., Shen L., Wang W., Hua C. The characteristics and pivotal roles of triggering receptor expressed on myeloid cells-1 in autoimmune diseases. Autoimmun. Rev. 2019;18:25–35. doi: 10.1016/j.autrev.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Baruah S., Murthy S., Keck K., Galvan I., Prichard A., Allen L.H., Farrelly M., Klesney-Tait J. TREM-1 regulates neutrophil chemotaxis by promoting NOX-dependent superoxide production. J. Leukoc. Biol. 2019;105:1195–1207. doi: 10.1002/JLB.3VMA0918-375R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong W.J., Yang H.H., Guan X.X., Xiong J.B., Sun C.C., Zhang C.Y., Luo X.Q., Zhang Y.F., Zhang J., Duan J.X., Zhou Y., Guan C.X. Inhibition of glycolysis alleviates lipopolysaccharide-induced acute lung injury in a mouse model. J. Cell Physiol. 2019;234:4641–4654. doi: 10.1002/jcp.27261. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C.Y., Duan J.X., Yang H.H., Sun C.C., Zhong W.J., Tao J.H., Guan X.X., Jiang H.L., Hammock B.D., Hwang S.H., Zhou Y., Guan C.X. COX-2/sEH dual inhibitor PTUPB alleviates bleomycin-induced pulmonary fibrosis in mice via inhibiting senescence. FEBS J. 2020;287:1666–1680. doi: 10.1111/febs.15105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrell E.D., Bhatraju P.K., Mikacenic C.R., Radella F., 2nd, Manicone A.M., Stapleton R.D., Wurfel M.M., Gharib S.A. Alveolar macrophage transcriptional programs are associated with outcomes in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2019;200:732–741. doi: 10.1164/rccm.201807-1381OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding Z., Wang X., Liu S., Zhou S., Kore R.A., Mu S., Deng X., Fan Y., Mehta J.L. NLRP3 inflammasome via IL-1beta regulates PCSK9 secretion. Theranostics. 2020;10:7100–7110. doi: 10.7150/thno.45939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang K., Fan C., Cai D., Zhang Y., Zuo R., Zhu L., Cao Y., Zhang J., Liu C., Chen Y., Liang H. Contribution of TGF-beta-mediated NLRP3-HMGB1 activation to tubulointerstitial fibrosis in rat with angiotensin ii-induced chronic kidney disease. Front. Cell Dev. Biol. 2020;8:1. doi: 10.3389/fcell.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers A.J., Guan J., Trtchounian A., Hunninghake G.M., Kaimal R., Desai M., Kozikowski L.-A., DeSouza L., Mogan S., Liu K.D., Matthay M.A., Steingrub J., Wheeler A., Yoon J.H., Nakahira K., Choi A.M., Baron R.M. Association of elevated plasma interleukin-18 level with increased mortality in a clinical trial of statin treatment for acute respiratory distress syndrome. Crit. Care Med. 2019;47(8):1089–1096. doi: 10.1097/CCM.0000000000003816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang K., Jin Y., Lai D., Wang J., Wang Y., Wu X., Scott M., Li Y., Hou J., Billiar T., Wilson M., Shu Q., Fang X., Fan J. RAGE-induced ILC2 expansion in acute lung injury due to haemorrhagic shock. Thorax. 2020;75:209–219. doi: 10.1136/thoraxjnl-2019-213613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ying Y., Mao Y., Yao M. NLRP3 Inflammasome activation by Microrna-495 promoter methylation may contribute to the progression of acute lung injury. Mol. Ther. Nucleic Acids. 2019;18:801–814. doi: 10.1016/j.omtn.2019.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tammaro A., Derive M., Gibot S., Leemans J.C., Florquin S., Dessing M.C. TREM-1 and its potential ligands in non-infectious diseases: from biology to clinical perspectives. Pharmacol. Ther. 2017;177:81–95. doi: 10.1016/j.pharmthera.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 26.Xu P., Zhang X., Liu Q., Xie Y., Shi X., Chen J., Li Y., Guo H., Sun R., Hong Y., Liu X., Xu G. Microglial TREM-1 receptor mediates neuroinflammatory injury via interaction with SYK in experimental ischemic stroke. Cell Death Dis. 2019;10:555. doi: 10.1038/s41419-019-1777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sigalov A.B. Commentary: triggering receptor expressed on myeloid cells-1 inhibitor targeted to endothelium decreases cell activation. Front. Immunol. 2020;11:173. doi: 10.3389/fimmu.2020.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jolly L., Carrasco K., Derive M., Lemarie J., Boufenzer A., Gibot S. Targeted endothelial gene deletion of triggering receptor expressed on myeloid cells-1 protects mice during septic shock. Cardiovasc Res. 2018;114:907–918. doi: 10.1093/cvr/cvy018. [DOI] [PubMed] [Google Scholar]
- 29.Kokten T., Gibot S., Lepage P., D'Alessio S., Hablot J., Ndiaye N.C., Busby-Venner H., Monot C., Garnier B., Moulin D., Jouzeau J.Y., Hansmannel F., Danese S., Gueant J.L., Muller S., Peyrin-Biroulet L. TREM-1 inhibition restores impaired autophagy activity and reduces colitis in mice. J. Crohns Colitis. 2018;12:230–244. doi: 10.1093/ecco-jcc/jjx129. [DOI] [PubMed] [Google Scholar]
- 30.Peng A., Lu X., Huang J., He M., Xu J., Huang H., Chen Q. Rheumatoid arthritis synovial fibroblasts promote TREM-1 expression in monocytes via COX-2/PGE2 pathway. Arthritis. Res. Ther. 2019;21:169. doi: 10.1186/s13075-019-1954-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng L., Zhou Y., Dong L., Chen R.Q., Sun G.Y., Liu T., Ran W.Z., Fang X., Jiang J.X., Guan C.X. TGF-beta1 upregulates the expression of triggering receptor expressed on myeloid cells 1 in murine lungs. Sci. Rep. 2016;6 doi: 10.1038/srep18946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y., Yang J., Sun G.Y., Liu T., Duan J.X., Zhou H.F., Lee K.S., Hammock B.D., Fang X., Jiang J.X., Guan C.X. Soluble epoxide hydrolase inhibitor 1-trifluoromethoxyphenyl-3- (1-propionylpiperidin-4-yl) urea attenuates bleomycin-induced pulmonary fibrosis in mice. Cell Tissue Res. 2016;363:399–409. doi: 10.1007/s00441-015-2262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong L., Zhou Y., Zhu Z.-Q., Liu T., Duan J.-X., Zhang J., Li P., Hammcok B.D., Guan C.-X. Soluble epoxide hydrolase inhibitor suppresses the expression of triggering receptor expressed on myeloid cells-1 by inhibiting NF-kB activation in murine macrophage. Inflammation. 2017;40:13–20. doi: 10.1007/s10753-016-0448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyun J., McMahon R.S., Lang A.L., Edwards J.S., Badilla A.D., Greene M.E., Stone G.W., Pallikkuth S., Stevenson M., Dykxhoorn D.M., Kottilil S., Pahwa S., Thomas E. HIV and HCV augments inflammatory responses through increased TREM-1 expression and signaling in Kupffer and Myeloid cells. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.S. Singh, T.G. Singh, Role of Nuclear factor kappa B (NF-kappaB) Signalling in Neurodegenerative Diseases: An Mechanistic Approach, Curr Neuropharmacol. (2020). [DOI] [PMC free article] [PubMed]
- 36.Chen Y., Zhou Z., Min W. Mitochondria, Oxidative Stress and Innate Immunity. Front Physiol. 2018;9:1487. doi: 10.3389/fphys.2018.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.N. Kumar Rajendran, B.P. George, R. Chandran, I.M. Tynga, N. Houreld, H. Abrahamse, The influence of light on reactive oxygen species and NF-small ka, CyrillicB in Disease Progression, Antioxidants (Basel). 8 (2019). [DOI] [PMC free article] [PubMed]
- 38.A. Huertas, C. Guignabert, J. A. Barbera, P. Bartsch, J. Bhattacharya, S. Bhattacharya, M. R. Bonsignore, L. Dewachter, A. T. Dinh-Xuan, P. Dorfmuller, M. T. Gladwin, M. Humbert, T. Kotsimbos, T. Vassilakopoulos, O. Sanchez, L. Savale, U. Testa, M.R. Wilkins Pulmonary vascular endothelium: the orchestra conductor in respiratory diseases: Highlights from basic research to therapy, Eur. Respir. J. 51 (2018). [DOI] [PubMed]
- 39.Kurdowska A.K., Florence J.M. Promoting neutrophil apoptosis to treat acute lung injury. Am. J. Respir. Crit. Care Med. 2019;200:399–400. doi: 10.1164/rccm.201903-0707LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.