Abstract
Product analysis from the iron Fenton oxidation of 2′-deoxyguanosine found reactions in bicarbonate buffer yield 8-oxo-2′-deoxyguanosine and spiroiminodihyantoin consistent with CO3•−. Reactions in phosphate buffer furnished high yields of sugar oxidation products consistent with HO•. These observations change the view of DNA oxidation products from the iron-Fenton reaction.
The Fenton reaction between ferrous ion and H2O2 in the context of biological conditions is claimed to generate HO• based on many decades of model studies.1 This thinking changed in 2019 when the Meyerstein laboratory determined the Fenton reaction catalyzed by Fe(II) in physiologically relevant concentrations of bicarbonate buffer does not yield HO• but instead generates CO3•−.2 They extended this observation to include the Fe(II)-citrate complex catalysing the Fenton reaction in bicarbonate buffer to also yield CO3•− instead of HO•.3 Their observations have immediate implications in biology because bicarbonate is part of the intracellular buffer system, and it exists at >10 mM concentration.4 Additionally, the oxidation products from the highly reactive and non-selective HO• (Ered = 2.3 V) are different than those from the more selective CO3•− (Ered = 1.6 V).5
Oxidative modification of DNA by reactive oxygen species (ROS) has been studied to elucidate reaction pathways and products formed,5,6 address the biology of oxidative stress and inflammation,7 and identify molecular details of disease, aging, and the cellular response to stress.7,8 In vitro experiments under model conditions are an approach to begin to understand these phenomena. When DNA is exposed to ROS, 2′-deoxyguanosine (dG) is the major site of oxidative modification as a result of its low redox potential (Ered = 1.3 V).5,6 Reactions on nucleoside models have identified a wide array of oxidation products leading to proposed mechanisms for their formation that have been supported with computational modeling.5,6,9 When conducting in vitro reactions to model biochemical ones, the physical parameters of the reaction can influence the answers identified. The community has learned dG oxidation products are influenced by pH, concentration of O2, and reaction context (i.e., nucleoside vs. single-stranded DNA vs. duplex DNA vs. G-quadruplex DNA),5,6 thus, illustrating the dependency of the reaction conditions on the outcome that may introduce biases to the conclusions drawn.
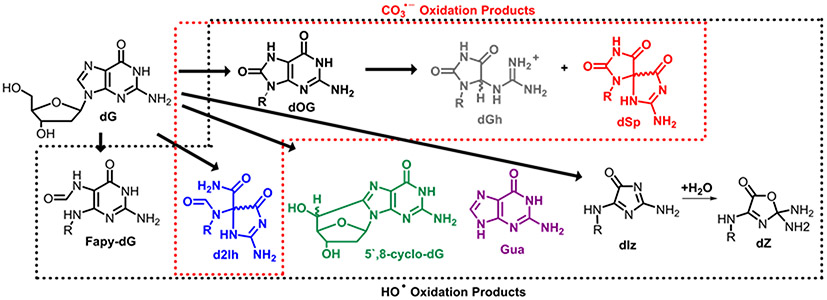
More importantly, dG oxidation products and their yields are highly dependent on the nature of the oxidant. Intracellular ROS generation occurs by both the incomplete reduction of O2 to H2O during metabolism to yield O2•− and the inflammatory response yielding O2•−.5 Superoxide is a poor oxidant and further reacts by either enzymatic dismutation to yield H2O2 plus O2, or it combines with NO to yield ONOO−.5,6 Previous thinking suggested H2O2 generates a strong oxidant when allowed to react with Fe(II) in the Fenton reaction to yield HO•, an indiscriminate and powerful one-electron oxidant.5,6 Reaction of dG by HO• can occur on the sugar predominantly yielding the diastereomers of 5′,8-cyclo-dG (from H5’ abstraction) or release of the free base guanine (Gua) after H1’ abstraction,10,11 while oxidation of the heterocyclic base yields 8-oxo-7,8-dihydro-2′-deoxyguanosine (dOG; Ered = 0.7 V), and its further oxidation products spiroiminodihydantoin (dSp) and 5-guanidinohydantoin (dGh), along with 2-iminohydantoin (d2Ih), 2,6-diamino-4-hydroxy-5-formamidopyrimidine (Fapy-dG), and imidazolone/oxazolone (dIz/dZ; Scheme 1) all as 2′-deoxyribose nucleosides.5,6 The products resulting from ONOO− oxidation of dG significantly differ because this nucleophile reacts with dissolved CO2 to yield an unstable intermediate that degrades to furnish the more selective one-electron oxidant CO3•−.7 Oxidation of dG by CO3•− yields dOG, which can be further oxidized to yield dSp or dGh, or at a higher flux of CO3•−, can also yield d2Ih besides dOG and the hydantoins (Scheme 1).12,13 Thus, the identity of the oxidant dictates the dG oxidation products formed. The product in turn determines the principal DNA repair pathway activated. Base excision repair predominantly repairs dOG and the hydantoins, while the sugar oxidation products are substrates for nucleotide excision repair or single-strand break repair.14-16
Scheme 1.
Products of dG oxidation by HO• (black box) or CO3•− (red box).
In the present report, we determined the products from dG oxidation by the Fe(II)-Fenton reaction in bicarbonate buffer and compared the results to oxidations conducted in the classically studied phosphate buffer (ESI†). Initially, dG was oxidized by the Fenton reaction catalyzed by either the hexaaqua Fe(II) ion used by Meyerstein and coworkers2 or the Fe(II)-EDTA complex typical of DNA oxidation studies.17 The reactions were set up with dG (1 mM) being allowed to react with H2O2 (1-3 mM) and the Fe(II) catalyst (50 μM) in the presence of ascorbate (3 mM) under aerobic reaction conditions at 22 °C for 30 min. The buffer was either bicarbonate or phosphate at 25 mM that is in the range of the physiological concentration of bicarbonate in cells.4 The reaction products were quantified by HPLC, using an internal standard added post-reaction, to resolve the products and measure their absorbance for quantification via a method previously established in our laboratory (ESI† Fig. S1).18
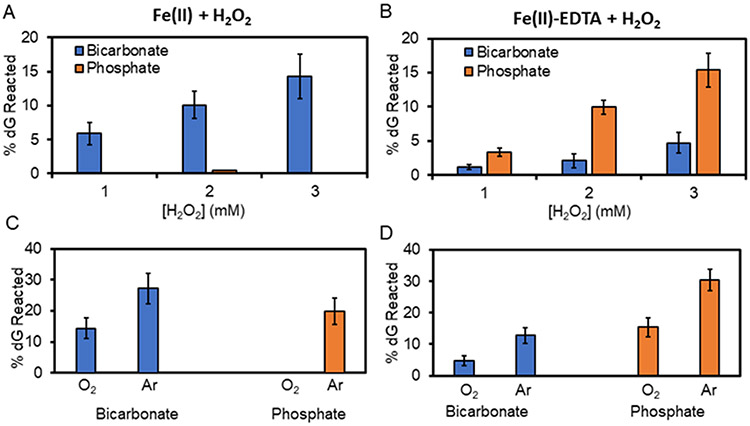
The Fenton reaction catalysed by Fe(II) in bicarbonate buffer furnished low reaction of dG with dependency on the [H2O2]; in contrast, the reaction gave undetectable oxidation of dG in phosphate buffer (Fig. 1A). When Fe(II) was stabilized by coordination with EDTA before initiation of the reaction (30 min at pH 7 in an Ar atmosphere), the Fenton-mediated oxidation in both buffers led to dG oxidation (Fig. 1B). The Fe(II)-EDTA catalyzed dG oxidation that was quantified increased with the [H2O2], and reaction conversions in phosphate buffer were consistently ~3-fold greater than those in bicarbonate buffer (Fig. 1B). Finally, the reactions were studied under low O2 concentrations by bubbling Ar gas through them for 10 min before the addition of H2O2. In all cases, the amount of dG reacted under decreased O2 conditions increased, and the percent conversion was greatest for the Fe(II)-catalysed reaction in phosphate buffer (Figs. 1C and 1D).
Fig 1.
Percent dG oxidized by the iron-Fenton reaction in bicarbonate (blue) or phosphate (orange) buffer. The top panels plot [H2O2] dependency for the (A) Fe(II) or (B) Fe(II)-EDTA initiated reactions and the bottom panels illustrates the O2 dependency for the (C) Fe(II) or (D) Fe(II)-EDTA initiated reactions.
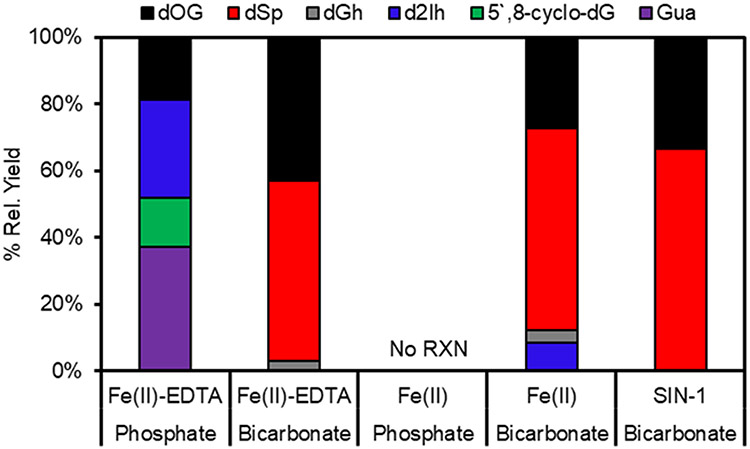
The reactions driven by 3 mM H2O2 were then inspected more closely to determine the product distribution using a dual HPLC method previously developed in our laboratory that readily resolves dOG, Gua, Fapy-dG, and dIz/dZ, as well as the diastereomers of 5′,8-cyclo-dG, dSp, dGh, and d2Ih.18 Products were first determined for the Fe(II)-EDTA reaction in phosphate buffer because prior work has found most of the dG oxidation products are observed from this reaction.17 Consistent with the previous work at low conversion to product,17 the modifications dOG, d2Ih, Gua, and 5′,8-cyclo-dG dominated the product distribution (Fig. 2). The yields of the two products resulting from sugar oxidation were ~50%; this product distribution displays the signature of HO• as the active oxidant in the reaction. In contrast, reactions in bicarbonate buffer furnished predominantly dOG and dSp (Fig. 2). For verification that this product distribution was characteristic of CO3•−, the dG oxidation products were determined via a reaction previously shown to generate CO3•− in high yield with minimal side reactions. Thermal decomposition of SIN-1 in bicarbonate buffer yields ONOO− that reacts with dissolved CO2 to yield peroxynitrosocarbonate that degrades to CO3•−.19 SIN-1-mediated oxidation of dG in bicarbonate buffer produced dOG and dSp as the only detectable products, consistent with prior work.20 More importantly, the SIN-1 oxidation and Fe(II)-EDTA Fenton reaction in bicarbonate buffer both produced similar product distributions. This observation is consistent with Meyerstein and coworkers' observation2 that the Fe(II)-Fenton reaction in bicarbonate buffer does not yield HO• and instead forms CO3•−.
Fig. 2.
Product yields of dG oxidation from the Fe(II) or Fe(II)-EDTA catalysed Fenton reaction in 25 mM bicarbonate or phosphate buffer.
Completion of the product analysis found the Fe(II)-catalyzed Fenton reaction in bicarbonate buffer predominantly produced dOG and dSp, again supporting CO3•− as the active oxidant. In the reactions in which O2 was minimized with Ar gas, the overall picture of products in bicarbonate buffer did not change; on the other hand, in phosphate buffer, products were detected with the Fe(II)-catalyzed Fenton reaction to identify a product profile consistent with HO• as the active oxidant (ESI† Fig. S2). Prior work has detected Fapy-dG as a major product arising from HO• addition to C8 of dG under hypoxic conditions.21,22 Our HPLC method can resolve Fapy-dG from a reaction mixture17 but in the present studies, no Fapy-dG was detected. The reason Fapy-dG was not observed might be the presence of low levels of O2 during the reaction; alternatively, this product is perhaps not formed when physiologically relevant concentrations of bicarbonate buffer are present in the reaction mixture.
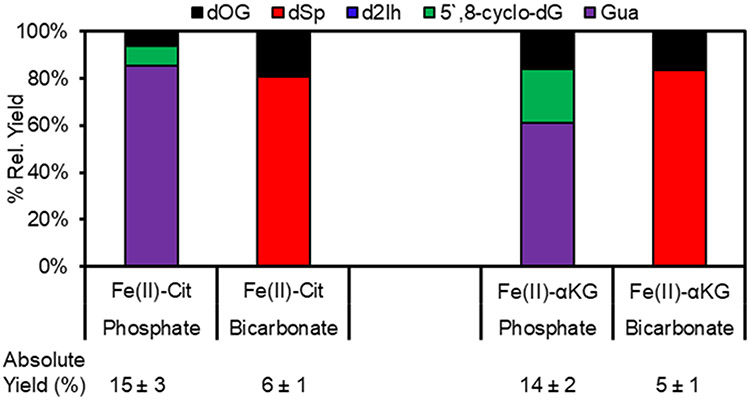
In cellular contexts, Fe(II) would be coordinated to a protein or metabolite and not exist in the hexa-aqua form or coordinated to EDTA; therefore, the metabolites citrate (Cit) or alpha-ketoglutarate (αKG) were studied next as Fe(II) ligands to drive the Fenton reaction in either bicarbonate or phosphate buffers. The reaction yields with Cit or αKG as Fe(II) ligands were similar to those found for the Fe(II)-EDTA Fenton reaction in phosphate and bicarbonate buffers. In the phosphate buffer reactions, the major dG oxidation product was release of the free base Gua that is >2-fold more than observed when EDTA was the Fe(II) ligand (Fig. 3). The reason for this difference is not immediately known; nonetheless, these product yields further support HO• as the active oxidant in phosphate buffer because Gua release is common from the 1’-hydroxy or 1’-hydroperoxy intermediate formed when the ribose H1’ is abstracted.11 When bicarbonate was the buffer for the same Fenton reactions, the products observed were dOG and dSp, and the yields were similar to those found from SIN-1 as the common approach to generate CO3•− in high yields (Fig. 3). To reiterate, the Fenton reaction catalysed by Fe(II) coordinated to either metabolite Cit or αKG in bicarbonate buffer yields CO3•− instead of HO• based on the products of dG oxidation.
Fig 3.
Product yields of dG oxidation from the Fe(II)-Fenton reaction when the metal ion was coordinated to the metabolites Cit or αKG in bicarbonate or phosphate buffer.
A final reaction was conducted to reinforce that it is the presence of bicarbonate and not the absence of phosphate that results in CO3•− formation. The Fe(II)-EDTA or Fe(II)-citrate Fenton reactions were performed in a mixed buffer system of 12.5 mM each of phosphate and bicarbonate. The product analysis of this mixed buffer system found dOG and dSp as the only detectable products (ESI† Fig. S3); thus, the presence of bicarbonate during the Fenton reaction results in CO3•− at the expense of HO• formation.
A final point regarding these data pertains to the yields of dOG and dSp. The product distributions were evaluated in reactions that converted 10-15% of the dG to product. As a result of the 0.6 V lower Ered of dOG compared to dG,5 once dOG is formed in the reaction vessel, any additional oxidation will favour dOG oxidation over dG by a factor of nearly 106.5 Therefore, during cellular reactions where oxidations are rare events, the dG oxidation product of the Fe(II)-Fenton reaction in bicarbonate buffer will be dOG with near exclusivity.
Significant points are derived from the observation that CO3•− is the likely active oxidant generated by the Fe(II)-Fenton reaction in biology. (1) When oxidation of duplex DNA occurs by CO3•− an electron hole is generated that migrates via the π-stacked bases to low energy sites to complete the oxidation that we and others have studied.20,23 These low energy sites are runs of dG in which the 5′ most dG is the lowest in energy and is the major site at which dG•+ leads to product formation.24,25 This allows oxidations to occur remotely and be funnelled to dG-rich sites, which in the mammalian genome are located in regulatory gene promoters, introns, and telomeres, often corresponding to G-quadruplex-forming sequences.8,26 The product formed from reaction of the dG•+ with H2O in duplex DNA is dOG.20,27 The chemistry of funnelling electron holes to promoters is not possible with HO• because it is too reactive and favours local oxidation to yield a broad spectrum of both ribose and base-derived products.17,20,23 (2) The main dG oxidation product being dOG from the Fe(II)-Fenton reaction in physiological bicarbonate buffer reinforces the significance of this heterocycle in biology and points to why dOG-specific glycosylases exist in the base excision repair system.28
Oxidation of DNA by CO3•− provides a viable means by which dG in regulatory regions of DNA, such as gene promoters, can be selectively oxidized to yield dOG specifically to regulate transcription.8,29 Currently, the only viable mechanism to site-selectively form dOG in the genome occurs during chromatin remodelling via LSD1 that is a flavin-dependent demethylase yielding H2O2 in close proximity to a target DNA sequence.30 The H2O2 formed could react via the Fe(II)-Fenton reaction in bicarbonate buffer to yield CO3•− that would effect DNA oxidation to yield exclusively dOG at dG-runs in dG-rich gene promoters.8,29,30 The present evidence builds a stronger case for dOG as an epigenetic DNA modification,8,31,32 in which the reader and eraser protein is OGG1 and the writer could be LSD1 via H2O2 release and the Fe(II)-Fenton reaction in bicarbonate buffer.8,30-32
In conclusion, we have applied the observations of Meyerstein and co-workers that the Fe(II)-Fenton reaction in physiological concentrations of bicarbonate buffer generates CO3•− and not HO•.2 By following the products of dG oxidation, the nature of the active oxidant could be verified. Oxidation of dG by the Fe(II)-Fenton reaction in bicarbonate buffer found dOG and dSp as products, consistent with CO3•− as the active oxidant; this observation contrasts with oxidations in phosphate buffer that generated 5′,8-cyclo-dG and Gua that are products arising from HO• as the active oxidant (Fig. 2). These data provide the DNA oxidation community a new way to think about the role of the buffer in oxidation reactions, demonstrating that the buffer can impact the reaction products and their yields (Figs. 1-3). How the presence of bicarbonate buffer influences oxidation of DNA by ionizing radiation, non-Fe(II)-mediated Fenton reactions, or the anticancer drug Fe(II)-bleomycin, for example, is of considerable future interest.
Supplementary Material
Acknowledgments
We acknowledge financial support from the National Cancer Institute (R01 CA090689) and the National Science Foundation (CHE1808475).
Footnotes
Conflicts of interest
There are no conflicts to declare
Notes and references
- 1.Poprac P, Jomova K, Simunkova M, Kollar V, et al. , Trends Pharmacol. Sci, 2017, 38, 592. [DOI] [PubMed] [Google Scholar]
- 2.Illes E, Mizrahi A, Marks V and Meyerstein D, Free Radic. Biol. Med, 2019, 131, 1. [DOI] [PubMed] [Google Scholar]
- 3.Illés E, Patra SG, Marks V, Mizrahi A, et al. , J. Inorg. Biochem, 2020, 206, 111018. [DOI] [PubMed] [Google Scholar]
- 4.Medinas DB, Cerchiaro G, Trindade DF and Augusto O, IUBMB Life, 2007, 59, 255. [DOI] [PubMed] [Google Scholar]
- 5.Fleming AM and Burrows CJ, Free Radic. Biol. Med, 2017, 107, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cadet J, Wagner JR, Shafirovich V and Geacintov NE, Int. J. Radiat. Biol, 2014, 90, 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lonkar P and Dedon PC, Int. J. Cancer, 2011, 128, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming AM and Burrows CJ, J. Am. Chem. Soc, 2020, 142, 1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hebert SP and Schlegel HB, Chem. Res. Toxicol, 2019, 32, 195. [DOI] [PubMed] [Google Scholar]
- 10.Chatgilialoglu C, Eriksson LA, Krokidis MG, Masi A, et al. , J. Am. Chem. Soc, 2020, 142, 5825. [DOI] [PubMed] [Google Scholar]
- 11.Pogozelski WK and Tullius TD, Chem. Rev, 1998, 98, 1089. [DOI] [PubMed] [Google Scholar]
- 12.Crean C, Geacintov NE and Shafirovich V, Angew. Chem., Int. Ed, 2005, 44, 5057. [DOI] [PubMed] [Google Scholar]
- 13.Rokhlenko Y, Geacintov NE and Shafirovich V, J. Am. Chem. Soc, 2012, 134, 4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shafirovich V, Kropachev K, Kolbanovskiy M and Geacintov NE, Chem. Res. Toxicol, 2019, 32, 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKibbin PL, Fleming AM, Towheed MA, Van Houten B, et al. , J. Am. Chem. Soc, 2013, 135, 13851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shafirovich V, Kolbanovskiy M, Kropachev K, Liu Z, et al. , Biochemistry, 2019, 58, 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alshykhly OR, Fleming AM and Burrows CJ, J. Org. Chem, 2015, 80, 6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming AM, Muller JG, Ji I and Burrows CJ, Org. Biomol. Chem, 2011, 9, 3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim MY, Dong M, Dedon PC and Wogan GN, Chem. Res. Toxicol, 2005, 18, 76. [DOI] [PubMed] [Google Scholar]
- 20.Fleming AM and Burrows CJ, Chem. Res. Toxicol, 2013, 26, 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douki T, Martini R, Ravanat JL, Turesky RJ, et al. , Carcinogenesis, 1997, 18, 2385. [DOI] [PubMed] [Google Scholar]
- 22.Dizdaroglu M, Mutat. Res. Rev. Mutat. Res, 2015, 763, 212. [DOI] [PubMed] [Google Scholar]
- 23.Margolin Y, Shafirovich V, Geacintov NE, DeMott MS, et al. , J. Biol. Chem, 2008, 283, 35569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito I, Takayama M, Sugiyama H, Nakatani K, et al. , J. Am. Chem. Soc, 1995, 117, 6406. [Google Scholar]
- 25.Delaney S and Barton JK, J. Org. Chem, 2003, 68, 6475. [DOI] [PubMed] [Google Scholar]
- 26.Heller A, Faraday Discuss., 2000, 116, 1. [DOI] [PubMed] [Google Scholar]
- 27.Rokhlenko Y, Cadet J, Geacintov NE and Shafirovich V, J. Am. Chem. Soc, 2014, 136, 5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.David SS, O'Shea VL and Kundu S, Nature, 2007, 447, 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang R, Hao W, Pan L, Boldogh I, et al. , Cell. Mol. Life Sci, 2018, 75, 3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perillo B, Ombra MN, Bertoni A, Cuozzo C, et al. , Science, 2008, 319, 202. [DOI] [PubMed] [Google Scholar]
- 31.Pan L, Zhu B, Hao W, Zeng X, et al. , J. Biol. Chem, 2016, 291, 25553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleming AM, Ding Y and Burrows CJ, Proc. Natl. Acad. Sci. U.S.A, 2017, 114, 2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.