Abstract
Although influenza is primarily considered a respiratory infection and causes significant respiratory mortality, evidence suggests that influenza has an additional burden due to broader consequences of the illness. Some of these broader consequences include cardiovascular events, exacerbations of chronic underlying conditions, increased susceptibility to secondary bacterial infections, functional decline, and poor pregnancy outcomes, all of which may lead to an increased risk for hospitalization and death. Although it is methodologically difficult to measure these impacts, epidemiological and interventional study designs have evolved over recent decades to better take them into account. Recognizing these broader consequences of influenza virus infection is essential to determine the full burden of influenza among different subpopulations and the value of preventive approaches. In this review, we outline the main influenza complications and societal impacts beyond the classical respiratory symptoms of the disease.
Keywords: Burden of disease, Comorbidity, Vaccination, Influenza virus, Complications, Cardiovascular events
1. Influenza: A significant respiratory pathogen
Influenza is an acute infectious viral respiratory disease that causes annual epidemics and occasionally, pandemics [1]. Influenza viruses spread easily, through aerosolized droplets produced by coughing and sneezing, or by hands and fomites contaminated with influenza viruses. Transmission occurs predominantly during the winter seasons in temperate regions and year-round in tropical regions, especially in crowded areas such as schools, nursing homes, or on public transport [2]. Illness is normally characterized by fever, cough, headache, muscle and joint pain, malaise, sore throat, and a runny nose – symptoms that have an abrupt onset, and can last for more than 2 weeks [2], [3]. Although most people recover within a week without requiring medical attention, influenza can lead to severe illness, hospitalization, and death, especially in older adults, infants, pregnant women, overweight individuals, and individuals with chronic medical conditions [1], [2], [4].
Influenza viruses continually evolve, allowing them to evade immune memory responses and infect individuals previously exposed to similar virus strains [1]. Occasionally, an influenza virus from a non-human reservoir infects humans and acquires the potential to spread from person to person, as illustrated by the 1918–1919 pandemic that resulted in an estimated 500 million infections and 50 million deaths worldwide [5]. This level of devastation from influenza has thankfully not been seen since, possibly due to better public health measures and surveillance systems, the advent of vaccines, and the development of antibiotics and antiviral treatments. Even so, influenza causes epidemics affecting millions of people worldwide every year and, especially in temperate countries, the surges in medical visits and increase in healthcare utilization during the influenza months have been well described [4]. Nonetheless, vaccination rates in most countries remain well below the 75% recommended by the World Health Organization (WHO2 ) [1], [6]. In addition to encouraging vaccination of high-risk populations, the WHO encourages countries to maintain up-to-date pandemic preparedness and response plans to prevent emerging novel strains from overwhelming healthcare systems [7], as illustrated by the current worldwide COVID-19 pandemic [8].
Reliable global disease burden data have historically been absent, but an important estimate was provided by the Global Burden of Disease Study [4]. It estimated that 54.5 million lower respiratory tract infections (LRTIs) attributable to influenza occurred in 2017, of which 8.2 million were severe and resulted in 145,000 (95% uncertainty interval: 99,000–200,000) deaths [4]. In contrast, the global annual number of deaths was estimated to be much higher in a study by Iuliano et al. Using a less conservative method based on respiratory death codes, they calculated a global annual burden of ~ 290,000–650,000 influenza-associated respiratory deaths (4.0–8.8 per 100,000 individuals) occurring during the influenza seasons between 1999 and 2015 [9].
Specific attempts to monitor influenza contribution to the winter respiratory disease burden were only initiated since the 1970s when specific laboratory tests became available to confirm infection. In the United States (US), initial estimates of influenza disease burden suggested an annual influenza attack rate (i.e., incidence) of 9–20% of the general population, with rates of more than 30% in children [10], [11], confirming the virus as a significant contributor to morbidity. More recent estimates are slightly lower: it was estimated that between 2010 and 2016, the incidence of symptomatic influenza varied from 3% to 11% among seasons [12].
Determining accurate laboratory-confirmed influenza attack rates is often difficult because the cohort studies required to do this are costly and complex. The placebo arm of a clinical trial can mimic a cohort study because unvaccinated individuals are closely followed up for disease, and trials are typically accompanied by high-quality diagnostics. A systematic review of 32 controlled influenza vaccine trials conducted between 1974 and 2011 estimated that approximately 1 in 5 unvaccinated children and 1 in 10 unvaccinated adults were infected with seasonal influenza annually [13]. These summary rates obscure significant variability between studies and seasons. Despite close monitoring and active surveillance, symptomatic attack rates may be higher than that measured in those trials where only patients with respiratory illness are tested for influenza, and atypical presentations that do not conform to strict case definitions (e.g., exacerbations of underlying conditions) are likely missed.
Irrespective of the methodological approach used to evaluate the burden of influenza, cases are disproportionately common among the youngest and oldest individuals [1], [4], [14], [15]. Iuliano et al. estimated a mean annual influenza-associated excess respiratory mortality rate per 100,000 individuals of 2.9–44.0 in people aged 65–74 years and 17.9–223.5 in people aged ≥ 75 years, compared to 0.1–6.4 in people aged < 65 years, and to an overall rate of influenza-associated respiratory deaths of 2.1–23.8 per 100,000 in children aged < 5 years [9].
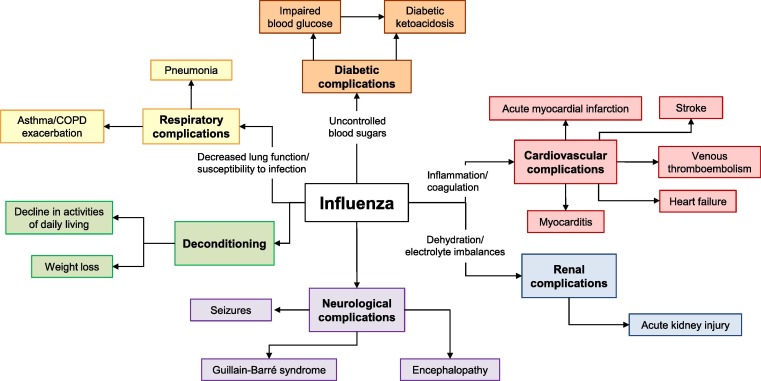
Individuals with chronic medical conditions are also affected disproportionately by influenza. They account for as many as 80% of all hospital admissions with laboratory-confirmed influenza in adults, and approximately 50% of those in children [16], [17]. Severe influenza and influenza complications are much more likely to develop in these at-risk individuals [1], often because they are less able to immunologically control infections and because the infection itself can exacerbate the underlying condition leading to an increased risk for hospitalization and death (Fig. 1 ). Specific populations, including those with chronic heart or respiratory conditions, are at highest risk of both severe influenza illness and exacerbated comorbidities, and this risk increases with age.
Fig. 1.
Domino effect of influenza. The influenza virus infection triggers various effects that can exacerbate underlying chronic medical conditions, leading to an increased risk for hospitalization and death [15], [34], [36], [57], [65], [74], [77], [91], [92], [109], [110], [111], [112], [113].
Pregnant women also represent a group at greatest risk of severe illness, complications, and death from influenza; and in addition to maternal illness, influenza can lead to complications such as stillbirth, pre-term delivery, and decreased birth weight [18], [19]. Importantly, in four large, controlled, randomized trials conducted in developing countries, influenza vaccination during pregnancy reduced laboratory-confirmed influenza by 50–58% in mothers and 27–63% in children; and acute respiratory disease by 19–36% in mothers and 29% in children [20], [21], [22], [23]. For these reasons, the WHO recommends that pregnant women should be the highest priority for countries planning to initiate or extend their seasonal influenza immunization programs [18].
2. Influenza complications beyond classic respiratory illness
Most estimates of the global influenza disease burden focus on the burden resulting from respiratory symptoms of the disease. However, clinical evidence and epidemiological studies have shown an additional hidden burden of influenza disease from broader consequences of the illness. Such consequences include exacerbation of chronic underlying conditions, increased susceptibility to secondary microbial infections, cardiovascular events, and functional decline (Fig. 1).
3. Cardiovascular disease
An estimated 422 million people had cardiovascular disease worldwide in 2015 [24]. Associations between seasonal influenza and cardiovascular events have been observed using time-series analyses, which quantify increases in cardiovascular events occurring concurrently with, or immediately following, an influenza epidemic [25], [26], [27]. Increased winter mortality from cardiovascular events coincides with peaks in influenza circulation rather than with weather patterns [27], and the associations seem especially strong in older adults [26].
These associations may be caused by several mechanistic pathways whereby an influenza virus infection alters the cardiovascular system to raise the risk of events, even in otherwise healthy individuals. Experimental and observational reports found that influenza virus infections can cause direct cardiac changes, ranging in severity from asymptomatic electrocardiogram abnormalities, myopericarditis, to acute myocardial infarction (AMI) [28], [29]. Influenza may also elicit systemic effects via inflammatory cytokines and prothrombotic changes, and these effects are associated with population-level increases in cardiovascular hospitalizations and deaths from AMI, heart failure, and cerebrovascular events [28], [30].
Time-series studies provide strong evidence for an association. At the population level, it has been reported that influenza has a small but significant impact on cardiovascular events; for instance in England, 4% of AMI hospital admissions in adults aged ≥ 75 years were attributable to influenza during the peak of the transmission season [26]. In Singapore, influenza was significantly associated with excess hospitalizations in adults aged ≥ 80 years, with an excess hospitalization rate of 497.2 per 100,000 person-years from overall cardiovascular disease [31] and; in the US, annual influenza-associated mortality rates per 100,000 individuals were estimated to be 3.82 (95% CI: 3.21, 4.4) for heart disease and 4.6 (3.79, 5.39) for all circulatory causes [32].
More recent studies have used self-controlled case-series methods, which measure the association between influenza (as a transient exposure) and a separate outcome event and, therefore, offer some advantages in reducing study bias because individuals act as their own control. One early case-series study from the United Kingdom found the risk of AMI and stroke to be substantially higher after a diagnosis of systemic respiratory tract infection [33]. This risk was highest during the first 3 days after infection diagnosis before decreasing gradually over the following weeks. This association was later confirmed in other studies with the more specific diagnosis of laboratory-confirmed influenza, which found a 6–10-fold elevated risk of AMI within 1 week of an influenza virus infection [34], [35] and a 3–8-fold elevated risk of stroke for several weeks after an influenza virus infection [35], [36]. In a study of 1.9 million hospital admissions for AMI, patients with AMI and concomitant influenza (1% of patients) had worse outcomes than patients with AMI alone in terms of in-hospital death and development of shock, acute respiratory failure, and acute kidney injury [37].
Influenza vaccines represent another tool to probe the impact of influenza on cardiovascular complications. Assessing reductions in rates of serious outcomes in vaccine recipients can provide insights into the broader impact of influenza – because the vaccine can only reasonably be assumed to prevent infection with influenza – regardless of whether the illness was directly or indirectly attributable to influenza. For example, in a Danish retrospective cohort study, influenza vaccination was found to reduce the risk of all-cause and cardiovascular death in patients with newly diagnosed heart failure, especially if the vaccine was received annually and early before the influenza season [38]. Similarly, in a self-controlled case series study in England, influenza vaccination of heart failure patients was associated with a lower risk of hospitalization due to cardiovascular disease (incidence rate ratio, 0.73 [95% CI: 0.71, 0.76]), and modestly reduced risk of hospitalizations from respiratory infections (0.83 [95% CI: 0.77, 0.90]) [39]. MacIntyre et al. indicated that influenza vaccine efficacy/effectiveness in preventing AMI was roughly equivalent to the one seen with statins or antihypertensive treatments, or smoking cessation, suggesting that influenza vaccination should be considered as an integral part of cardiovascular disease management and prevention [40].
Randomized clinical vaccine trials provide an alternative method for assessing how influenza may exacerbate chronic health conditions [41]. Because randomization assures that treatment groups are otherwise similar, differences between vaccinated and unvaccinated populations can be attributed to the vaccine-prevented pathogen (“vaccine probe” approach). A meta-analysis of five randomized trials indicated a reduced frequency of a composite indicator of cardiovascular events in vaccinated subjects vs. control subjects (risk ratio, 0.64 [95% CI: 0.48, 0.86]) [42]. A randomized phase III trial of a high-dose influenza vaccine in approximately 32,000 subjects ≥ 65 years of age showed that it is more immunogenic and efficacious than a standard-dose vaccine in this population [43], [44]. In addition, it further reduced serious cardio-respiratory events by 17.7% (95% CI: 6.6, 27.4%; from 35.5 to 26.8 events per 1000 participant-seasons), pneumonia events by 39.8% (95% CI: 19.3, 55.1%; from 7.4 to 4.4 events per 1000 participant-seasons), and congestive heart failure by 24.0% (95% CI: −7.2, 46.1%; from 4.7 to 3.6 events per 1000 participant-seasons) [45]. These findings have been broadly matched by observational studies [46]. Dedicated larger randomized studies are ongoing to confirm these associations in patients with high-risk cardiovascular disease and evaluate the impact of influenza vaccines on cardiovascular outcomes (e.g., clinicaltrials.gov, NCT04137887) [47], [48].
4. Chronic respiratory conditions
Because of the high burden of influenza in individuals with chronic respiratory conditions [15], many national immunization technical advisory groups recommend those living with pulmonary disorders and chronic lung disease as a high priority group for annual immunization [15], [49].
Influenza virus infection of the lungs can trigger asthma attacks and a worsening of asthma symptoms, even in individuals with mild asthma or asthma that is otherwise well controlled by medication [50]. Asthma is one of the most common underlying diseases in patients admitted to hospital with influenza, both in adults (7.6–46% of admissions) and children (8.3–42%) [51]. Although spatiotemporal association has been found in adults between influenza and increased asthma hospitalizations [52], several clinical reports, particularly during the 2009 influenza A/H1N1 pandemic, showed that hospitalized asthma patients may have less severe influenza morbidity compared to non-asthmatic patients, with decreased risk of intensive care unit (ICU) admission, mechanical ventilation, and death [53], [54], [55], [56]. Different hypotheses have been proposed to try to explain this outcome, including corticosteroid use [55], pulmonary immune responses, and lung changes in asthma patients that may limit the severity of infection [56]. Another hypothesis may be that the threshold for hospital admission is lower for these at-risk patients, with earlier presentation and lower degree of respiratory dysfunctions at admission. Nevertheless, because influenza virus infection can dangerously exacerbate asthma symptoms, individuals with asthma are strongly recommended to receive their annual influenza vaccination [18], [51].
Influenza and other respiratory viruses are important triggers of exacerbations in chronic obstructive pulmonary disease (COPD) [57], [58]. In a case-control study, influenza A virus was found more often in respiratory specimens from patients hospitalized for COPD exacerbations than in specimens from patients with stable COPD [59]. Influenza is also associated with significant excess hospital admissions among older adults [60], [61], and a systematic review of randomized vaccine controlled trials found patients to have significantly reduced exacerbations of COPD if they had been vaccinated against influenza compared to patients who had received placebo [62].
5. Diabetes
Between 2017 and 2045, the global prevalence of diabetes is expected to increase by 48%, from 425 million people (8.8% of adults 20–79 years of age) to 629 million [63]. Most of this increase will be due to type II diabetes resulting from rising obesity, unhealthy diets, and physical inactivity.
Individuals living with type I or type II diabetes are more likely to suffer from severe and fatal influenza complications [64]. The association between influenza and diabetes was initially suggested in the 1930s and confirmed several decades later by several studies showing that patients with diabetes were more likely to be hospitalized, admitted to ICU, and die from pneumonia and influenza compared to people without diabetes [65]. However, the most extensive evidence emerged following the 2009 influenza A/H1N1 pandemic, for which numerous studies worldwide confirmed that patients with diabetes were a susceptible group for severe infections [66].
A systematic review and meta-analysis of populations at risk of developing severe or complicated influenza found that diabetes was associated with higher risks of hospital admission from seasonal influenza A/H3N2 infection and death from pandemic influenza A/H1N1pdm09 virus infection [67]. In one study of 162 hospitalized patients with influenza, the odds of being admitted to an ICU were four times higher for patients with diabetes compared to those without (adjusted odds ratio, 4.29 [95% CI: 1.29, 14.3]), making this a greater risk than that from cardiac disease (1.77 [95% CI: 0.61, 5.16]) [68]. Several case reports and case series have also identified that influenza virus infection can aggravate diabetic ketoacidosis [69], [70]. Influenza not only increases abnormal glucose levels by 75% but also impacts daily life of diabetic patients, with reduced sleep and physical activity [71]. Based on the collective data, the WHO considers individuals with diabetes a high-risk group with a greater susceptibility for developing more severe and complicated influenza virus infections [18] and, accordingly, many national immunization technical advisory groups recommend that those living with diabetes and other metabolic disorders represent high priority groups for immunization [15], [49].
Influenza complications are thought to be caused by chronic hyperglycemia in individuals with diabetes. Hyperglycemia can reduce immune cell recruitment, neutrophil degranulation, complement activation, and immune cell phagocytosis, which together can inhibit the immune response against influenza virus infection [66], [72]. Elevated glucose levels can also increase influenza virus replication in pulmonary epithelial cells, cause structural changes to the lungs that reduce pulmonary function, and triggers micro- and macrovascular complications that influenza can further impact [66], [73].
6. Neurologic complications
In rare cases, influenza virus infection may result in neurologic complications including febrile seizures, influenza-associated encephalitis or encephalopathy, Guillain-Barré syndrome, and exacerbations in patients with epilepsy [29], [74]. Although the causes of these complications have not been clearly elucidated, molecular mimicry between viral antigens and self-antigens has been suggested as a potential mechanism [29]. The severity of these rare events appears variable, and many diagnostic tests such as electroencephalogram and cerebrospinal fluid studies often fail to reliably associate severity with long-term outcomes [74]. Neurologic complications of influenza appear to be more frequently reported in children, and some (e.g., encephalitis) may have a genetic predisposition, given that more cases are reported in some Asian populations [29]. An association has also been suggested between influenza vaccines and Guillain-Barré syndrome (annual incidence of 0.4–4.0 cases per 100,000 population), although the magnitude of risk is several times lower than after influenza virus infection [75], [76]. Therefore, the benefits of influenza vaccination are significantly greater than any potential associated risks.
7. Co-infections and secondary infections
Bacterial co-infection or secondary infections greatly increase morbidity and mortality among patients with influenza, both with seasonal and pandemic viruses. A systematic review found that, in most studies published since 1982, bacterial co-infections occurred in 11–35% of laboratory-confirmed influenza patients of all ages [77]. The greatest mortality risk is typically seen in persons with underlying chronic conditions, especially immunocompromised populations [78].
Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus are the most commonly reported causes of co-infection or secondary infection associated with influenza, and usually result in bacterial pneumonia [78]. Indeed, it is commonly accepted that most of the deaths attributed to the 1918–1919 influenza pandemic were due to secondary bacterial pneumonia, especially caused by S. pneumoniae [78]. Other associations have also been shown with invasive meningococcal disease in temperate countries and with Clostridium difficile infections [79], [80]. Some studies have shown these associations decrease after controlling for seasonal patterns and other temporal effects [81], [82], and additional well-controlled studies across temperate and tropical climates will be needed to accurately quantify these relationships.
Influenza virus infection is believed to facilitate bacterial infection by these and other commensals of the respiratory tract by revealing and providing more sites for bacterial adhesion, impairing host immune responses (notably innate responses), and destroying tissues to allow bacterial colonization and spread [83], [84]. For example, influenza promotes S. pneumoniae growth by increasing host-derived sialic acid availability as a nutrient source [85]. Also, animal models of influenza and H. influenzae co-infection show bronchial necrosis, bronchial inflammation, and bronchitis that are greater than those caused by either infection alone; the heightened pathology is characterized by extensive epithelial cell destruction caused by a synergistic effect from the two pathogens [86]. Finally, although the association between influenza and C. difficile infections is likely due to frequent antibiotic use during influenza seasons [80], it highlights the importance of influenza virus infection in different aspects of health.
By limiting primary viral infection, influenza vaccination has the ability to decrease secondary bacterial infections [83]. Similarly, prevention and treatment of secondary bacterial infections may help decrease the burden of influenza. Notably, a prospective study conducted in Sweden showed that influenza and pneumococcal vaccinations in individuals aged ≥ 65 years substantially reduced influenza- and pneumonia-related hospitalizations, and reduced mortality from all causes by 57% in this age group [87].
8. Functional decline in activities and daily living
Aging is often associated with functional changes, such as a decline in muscle strength, aerobic capacity, bone density, and pulmonary ventilation, all of which may impact daily activities [88]. Because the physical and physiological impact of hospitalization and bed rest includes reduction of plasma volume, accelerated bone loss, increased lung closing volume, and sensory deprivation, vulnerable older adults who are hospitalized or have prolonged bed rest are generally at higher risk of irreversible functional decline [89]. Even mild illnesses may be sufficient to lead independent older adults into a cascade of functional decline and dependence.
Given that influenza illness may lead to prolonged bed rest and, for many older adults, hospitalization, it is not surprising that functional decline among older adults may be a critical, but highly underappreciated, consequence of influenza. In a Canadian survey of 5014 adults aged ≥ 65 years, 21.5% reported having experienced influenza or influenza-like illness (ILI) in the previous season [90]. Of these, 39.3% indicated that they took longer than 2 weeks to recover and 3.1% indicated they never fully recovered. Additionally, ~20% had health and functional decline during acute illness.
Deterioration in the ability to perform basic activities of daily living (e.g., dressing, eating, toileting, personal hygiene, and ambulation) is usually more severe in older adults with influenza, especially in frail older people [91]. A study of nursing home residents in the US from 1999 to 2005 found that daily activity decline, weight loss, and new or worsening pressure ulcers were impacted by influenza [92]. In a study in Canada, 15% of adults ≥ 65 years of age experienced catastrophic disability (loss of independence in at least two self-care activities) following hospitalization for influenza [93]. Influenza illness may also increase the risk of falls and fractures due to unsteady gait or dizziness. ILI hospitalizations are associated with a 13% average increase in the risk of hip fracture hospitalization among long-stay and nursing home residents [94]. Influenza has also been temporally linked to increased hip fracture incidence during winter, an effect that was reversed by influenza vaccination [95]. Some of the mobility impairments in older individuals with influenza have been linked to upregulated inflammatory and muscle degradation genes, and downregulated positive regulators of muscle function [96].
Many estimates of the impact of influenza impact on health-related quality of life are biased towards more severe illness because they are usually based on medically attended cases or those meeting ILI case definitions. By contrast, the Flu Watch study investigated the burden of milder, community influenza cases among a cohort of 5484 participants of all ages in England from 2006 to 2011 [97]; these cases did not have to meet ILI definitions or result in healthcare consultations. Community influenza cases lost 24,300 quality-adjusted life years (95% CI: 16,600, 34,700) and caused 2.9 million absences per season (based on data from 2006−07 to 2009–10). Around 40% of influenza A cases and 24% of influenza B cases resulted in absenteeism from work (average duration of 3.6 days) or school (2.4 days). Milder influenza illness therefore also contributes to lost health-related quality of life on a population level. Overall, the impact of influenza on functional activity, frailty, dependence, and quality of life are critical areas of research needs because preservation of health, active living, and independence represent important objectives for older adults.
9. Societal impacts
Compared to other major vaccine-preventable diseases in adults (pertussis, pneumococcal disease, and herpes zoster), influenza represents the most significant contributor in terms of incidence of disease and economic costs [98]. In the US, the total annual economic burden of seasonal influenza has been estimated at $11.2 billion ($6.3–$25.3 billion), of which $8.0 billion ($4.8–$13.6 billion) were indirect costs, such as absenteeism from work [99]. Similarly, in Europe, the total costs of influenza may range from €6 to €14 billion per year [100]. Yet the total economic cost of influenza is likely underestimated because other factors, for example poor performance at work due to influenza illness, are not easy to measure.
Evaluations of the economic value of vaccines may provide a good insight into societal impacts. Such evaluations are informed by multiple, overlapping data sources that include data from clinical trials, published literature, mathematical models, information regarding the costs of disease episodes, and specifically designed epidemiological studies. However, these assessments are often narrow, excluding many aspects of social value and externalities, such as hospital congestion, follow-on effects, aborted operations, and emergency room visits. Based on a retrospective database analysis in Canada, influenza seems to have a much larger effect on emergency room visits than captured by influenza or ILI clinical diagnoses alone, as evidenced by increased proportion of persons who registered for non-respiratory complaints during periods of elevated ILI activity [101]. Barnighausen et al. suggested that the value of vaccination may be divided into ‘narrow’ and ‘broad’ benefits [102]. Narrow benefits include direct gains in health, healthcare cost savings, and protection against productivity losses directly in vaccine recipients and indirectly at the community level via herd protection. Broad benefits include longer-term improvements in child health, educational outcomes, and macroeconomic stability. Because these broader benefits are not normally measured in microeconomic evaluations, there are gaps in the evidence base behind broader economic and societal impacts of vaccination. Recommendations have been made to collect more of this type of data – for example, data on influenza-related household choices around consumption and savings could be collected in vaccine effectiveness trials or in standard demographic and health surveys [103].
One recent Canadian study revealed that the mean costs associated with influenza-related hospitalizations ($14,612 CAD) and ICU admissions ($39,477 CAD) are much higher than previously estimated [104]. Beyond reducing outcomes and their associated costs from third-party payer and societal perspectives, significant decreases in healthcare utilization are possible through vaccination [105]. For example, individuals with influenza are frequently prescribed antibiotics, either for bacterial co-infections or sometimes inappropriately, which contribute to antibiotic resistance [106]. Hence, by preventing illness, influenza vaccination likely reduces antibiotic use and the threat of new resistant microbes [107].
10. Discussion and conclusions
Influenza has for several decades been considered a common winter respiratory disease that can progress to more severe secondary pneumonia in a subset of high-risk individuals. Historical and more recent data have, however, underlined the impact of influenza as a trigger for severe conditions and a contributor to broader morbidity and mortality in general. Furthermore, influenza also has an economic impact resulting from work time loss, reduced productivity, and increased use of medical resources.
Empirical estimates of the full global influenza disease burden have not yet been published, primarily due to challenges of associating non-respiratory endpoints with influenza, especially when laboratory confirmation is rare. However, at the country level, excess mortality studies using regression techniques to estimate the influenza-attributable disease burden have been able to do this. For example, an excess mortality study performed between 1997 and 2007 in the US showed that most influenza-associated mortality was due to respiratory and circulatory causes, and that influenza also substantially contributed to mortality from other diseases [32]. In this study, average annual influenza-associated mortality rates per 100,000 individuals were estimated to be 11.92 (95% CI: 10.17, 13.67) for all underlying causes, 1.73 (1.53, 1.93) for pneumonia and influenza, 3.58 (3.04, 4.14) for all respiratory causes, 3.82 (3.21, 4.4) for heart disease, 4.6 (3.79, 5.39) for all circulatory causes, 0.87 (0.68, 1.05) for cancer, 0.33 (0.26, 0.39) for diabetes, and 0.41 (0.3, 0.52) for Alzheimer’s disease.
Further studies will be required to assess the mechanistic basis for these non-respiratory outcomes, as it is not entirely known how infection leads to many of these complications. They may be a product of the genetic, physiological, or immunological background of the host, may be associated with specific strains of influenza viruses, or confounded by other factors and environmental conditions that are difficult to take into account.
The data discussed in this review should be interpreted in light of several limitations. First, our review is a non-systematic review and the studies described were subjectively selected. Second, although we recognize that the robustness of the different methodologies used in these studies is variable, we did not systematically assess the quality of the studies described. For instance, some of the studies mentioned had a small sample size or risks of bias. Third, we found only limited data on associations between influenza burden and several pre-existing medical conditions such as COPD.
In conclusion, better understanding of the broader impact of influenza should help provide greater support to health authorities regarding the needs for vaccination programs. Indeed, in most countries, influenza vaccination coverage rates among at-risk populations are far below the 75% recommended by the WHO [6]. Increasing vaccination coverage to reach this target will require an integrated strategy that reinforces awareness among healthcare policymakers and the public and encourages healthcare workers to become active vaccination advocates. Generating high-quality real-world evidence in big data studies may be crucial to achieve this goal. Additionally, structured communication campaigns targeting at-risk populations and strategies to reduce out-of-pocket expenses associated with vaccination will also be necessary. These measures to reduce the burden of seasonal influenza are supported in the WHO’s recent Global Influenza Strategy 2019–2030, especially for low- and middle-income countries [108].
CRediT authorship contribution statement
Alejandro E. Macias: Investigation, Writing - original draft, Writing - review & editing. Janet E. McElhaney: Investigation, Writing - original draft, Writing - review & editing. Sandra S. Chaves: Investigation, Writing - review & editing. Joshua Nealon: Conceptualization, Investigation, Writing - original draft, Writing - review & editing, Project administration. Marta C. Nunes: Investigation, Writing - original draft, Writing - review & editing. Sandrine I. Samson: Conceptualization, Investigation, Writing - original draft, Writing - review & editing. Bruce T. Seet: Conceptualization, Investigation, Writing - original draft, Writing - review & editing. Thomas Weinke: Investigation, Writing - original draft, Writing - review & editing. Hongjie Yu: Investigation, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: JN, SSC, SIS, and BTS are employees of Sanofi Pasteur. AEM has received honoraria for lectures or advisory boards from Sanofi Pasteur, Stendhal Pharma, Roche, and Pfizer. JEM has received honoraria for participation in advisory boards or lectures and related travel support from Sanofi, and participation in data monitoring boards or advisory boards for GSK, Pfizer, Merck, and Medicago. MCN has received honoraria for lectures or advisory boards from Sanofi Pasteur and Pfizer, and research grants from MedImmune. TW has received honoraria for lectures or advisory boards from MSD, Sanofi Pasteur, and Seqirus. HY has received investigator-initiated research funding from Sanofi Pasteur, GlaxoSmithKline, Yichang HEC Changjiang Pharmaceutical Company, and Roche (Shanghai).
Acknowledgments
Acknowledgments
Medical writing was provided by Drs. Jonathan Pitt and Julie Harriague (4Clinics, Paris, France) and paid for by Sanofi Pasteur. The authors would like to thank Jason Lee (Sanofi Pasteur, Toronto, Canada) for developing Fig. 1. HY acknowledges financial support from the National Science Fund for Distinguished Young Scholars (No. 81525023) and National Science and Technology Major Project of China (No. 2017ZX10103009-005, 2018ZX10713001-007).
Funding
This work was funded by Sanofi Pasteur. Employees of Sanofi Pasteur participated in drafting and editing the article, deciding to submit the article for publication, and approving the final, submitted version.
Footnotes
This publication was produced with support from Sanofi Pasteur. Manuscripts were accepted after rigorous peer review process that was managed by an expert Guest Editor independently appointed by the Editor-in-Chief.
Abbreviations: CI, confidence interval; ICU, intensive care unit; ILI, influenza-like illness; MI, myocardial infarction; LRTI, lower respiratory tract infection; WHO, World Health Organization.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2020.09.048.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Paules C., Subbarao K. Influenza. Lancet. 2017;390(10095):697–708. doi: 10.1016/s0140-6736(17)30129-0. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Influenza (Seasonal). 2018; Available from: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal). Accessed: 17 July 2019.
- 3.Hayward A.C., Fragaszy E.B., Bermingham A., Wang L., Copas A., Edmunds W.J., et al. Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med. 2014;2(6):445–454. doi: 10.1016/S2213-2600(14)70034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2017 Influenza Collaborators. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 2019;7(1):69-89. doi: 10.1016/s2213-2600(18)30496-x. [DOI] [PMC free article] [PubMed]
- 5.Nickol M.E., Kindrachuk J. A year of terror and a century of reflection: perspectives on the great influenza pandemic of 1918–1919. BMC Infect Dis. 2019;19(1):117. doi: 10.1186/s12879-019-3750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Prevention and control of influenza pandemics and annual epidemics. Geneva; 2003. http://www.who.int/immunization/sage/1_WHA56_19_Prevention_and_control_of_influenza_pandemics.pdf; 2003, [accessed 18 July 2018].
- 7.World Health Organization. Pandemic Influenza Risk Management: a WHO guide to inform & harmonize national & international pandemic preparedness and response. 2017; Available from: https://www.who.int/influenza/resources/publications/manual_burden_of_disease/en/. Accessed: 22 June 2020.
- 8.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iuliano A.D., Roguski K.M., Chang H.H., Muscatello D.J., Palekar R., Tempia S., et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–1300. doi: 10.1016/s0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glezen W.P., Couch R.B. Interpandemic influenza in the Houston area, 1974–76. N Engl J Med. 1978;298(11):587–592. doi: 10.1056/nejm197803162981103. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan K.M., Monto A.S., Longini I.M., Jr. Estimates of the US health impact of influenza. Am J Public Health. 1993;83(12):1712–1716. doi: 10.2105/ajph.83.12.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokars J.I., Olsen S.J., Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis. 2018;66(10):1511–1518. doi: 10.1093/cid/cix1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Somes M.P., Turner R.M., Dwyer L.J., Newall A.T. Estimating the annual attack rate of seasonal influenza among unvaccinated individuals: A systematic review and meta-analysis. Vaccine. 2018;36(23):3199–3207. doi: 10.1016/j.vaccine.2018.04.063. [DOI] [PubMed] [Google Scholar]
- 14.Nair H., Brooks W.A., Katz M., Roca A., Berkley J.A., Madhi S.A., et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011;378(9807):1917–1930. doi: 10.1016/s0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- 15.Center for disease control and prevention. People at high risk for flu complications. 2018; Available from: https://www.cdc.gov/flu/highrisk/index.htm. Accessed: 11 October 2019.
- 16.Committee on infectious diseases. Recommendations for prevention and control of influenza in children, 2018-2019. Pediatrics 2018;142(4). doi: 10.1542/peds.2018-2367. [DOI] [PubMed]
- 17.Dao C.N., Kamimoto L., Nowell M., Reingold A., Gershman K., Meek J., et al. Adult hospitalizations for laboratory-positive influenza during the 2005–2006 through 2007–2008 seasons in the United States. J Infect Dis. 2010;202(6):881–888. doi: 10.1086/655904. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec. 2012;87(47):461–476. [PubMed] [Google Scholar]
- 19.Fell D.B., Azziz-Baumgartner E., Baker M.G., Batra M., Beaute J., Beutels P., et al. Influenza epidemiology and immunization during pregnancy: Final report of a World Health Organization working group. Vaccine. 2017;35(43):5738–5750. doi: 10.1016/j.vaccine.2017.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madhi S.A., Cutland C.L., Kuwanda L., Weinberg A., Hugo A., Jones S., et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med. 2014;371(10):918–931. doi: 10.1056/NEJMoa1401480. [DOI] [PubMed] [Google Scholar]
- 21.Zaman K., Roy E., Arifeen S.E., Rahman M., Raqib R., Wilson E., et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359(15):1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 22.Tapia M.D., Sow S.O., Tamboura B., Teguete I., Pasetti M.F., Kodio M., et al. Maternal immunisation with trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: a prospective, active-controlled, observer-blind, randomised phase 4 trial. Lancet Infect Dis. 2016;16(9):1026–1035. doi: 10.1016/S1473-3099(16)30054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinhoff M.C., Katz J., Englund J.A., Khatry S.K., Shrestha L., Kuypers J., et al. Year-round influenza immunisation during pregnancy in Nepal: a phase 4, randomised, placebo-controlled trial. Lancet Infect Dis. 2017;17(9):981–989. doi: 10.1016/S1473-3099(17)30252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roth G.A., Johnson C., Abajobir A., Abd-Allah F., Abera S.F., Abyu G., et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins S.D. Excess mortality from causes other than influenza and pneumonia during influenza epidemics. Public Health Rep. 1932;47:2159–2179. [Google Scholar]
- 26.Blackburn R., Zhao H., Pebody R., Hayward A., Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis. 2018;67(1):8–17. doi: 10.1093/cid/cix1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reichert T.A., Simonsen L., Sharma A., Pardo S.A., Fedson D.S., Miller M.A. Influenza and the winter increase in mortality in the United States, 1959–1999. Am J Epidemiol. 2004;160(5):492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- 28.Fischer W.A., 2nd, Gong M., Bhagwanjee S., Sevransky J. Global burden of influenza as a cause of cardiopulmonary morbidity and mortality. Glob Heart. 2014;9(3):325–336. doi: 10.1016/j.gheart.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sellers S.A., Hagan R.S., Hayden F.G., Fischer W.A., 2nd The hidden burden of influenza: A review of the extra-pulmonary complications of influenza infection. Influenza Other Respir Viruses. 2017;11(5):372–393. doi: 10.1111/irv.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen J.L., Yang W., Ito K., Matte T.D., Shaman J., Kinney P.L. Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol. 2016;1(3):274–281. doi: 10.1001/jamacardio.2016.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ang L.W., Yap J., Lee V., Chng W.Q., Jaufeerally F.R., Lam C.S.P., et al. Influenza-associated hospitalizations for cardiovascular diseases in the tropics. Am J Epidemiol. 2017;186(2):202–209. doi: 10.1093/aje/kwx001. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein E., Viboud C., Charu V., Lipsitch M. Improving the estimation of influenza-related mortality over a seasonal baseline. Epidemiology. 2012;23(6):829–838. doi: 10.1097/EDE.0b013e31826c2dda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smeeth L., Thomas S.L., Hall A.J., Hubbard R., Farrington P., Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 34.Kwong J.C., Schwartz K.L., Campitelli M.A., Chung H., Crowcroft N.S., Karnauchow T., et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378(4):345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 35.Warren-Gash C., Smeeth L., Hayward A.C. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis. 2009;9(10):601–610. doi: 10.1016/S1473-3099(09)70233-6. [DOI] [PubMed] [Google Scholar]
- 36.Boehme A.K., Luna J., Kulick E.R., Kamel H., Elkind M.S.V. Influenza-like illness as a trigger for ischemic stroke. Ann Clin Transl Neurol. 2018;5(4):456–463. doi: 10.1002/acn3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vejpongsa P., Kitkungvan D., Madjid M., Charitakis K., Anderson H.V., Arain S., et al. Outcomes of acute myocardial infarction in patients with influenza and other viral respiratory infections. Am J Med. 2019 doi: 10.1016/j.amjmed.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Modin D., Jorgensen M.E., Gislason G., Jensen J.S., Kober L., Claggett B., et al. Influenza vaccine in heart failure. Circulation. 2019;139(5):575–586. doi: 10.1161/circulationaha.118.036788. [DOI] [PubMed] [Google Scholar]
- 39.Mohseni H., Kiran A., Khorshidi R., Rahimi K. Influenza vaccination and risk of hospitalization in patients with heart failure: a self-controlled case series study. Eur Heart J. 2017;38(5):326–333. doi: 10.1093/eurheartj/ehw411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacIntyre C.R., Mahimbo A., Moa A.M., Barnes M. Influenza vaccine as a coronary intervention for prevention of myocardial infarction. Heart. 2016;102(24):1953–1956. doi: 10.1136/heartjnl-2016-309983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feikin D.R., Scott J.A., Gessner B.D. Use of vaccines as probes to define disease burden. Lancet. 2014;383(9930):1762–1770. doi: 10.1016/s0140-6736(13)61682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Udell J.A., Zawi R., Bhatt D.L., Keshtkar-Jahromi M., Gaughran F., Phrommintikul A., et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA. 2013;310(16):1711–1720. doi: 10.1001/jama.2013.279206. [DOI] [PubMed] [Google Scholar]
- 43.Samson S.I., Leventhal P.S., Salamand C., Meng Y., Seet B.T., Landolfi V., et al. Immunogenicity of high-dose trivalent inactivated influenza vaccine: a systematic review and meta-analysis. Expert Rev Vaccines. 2019;18(3):295–308. doi: 10.1080/14760584.2019.1575734. [DOI] [PubMed] [Google Scholar]
- 44.DiazGranados C.A., Dunning A.J., Kimmel M., Kirby D., Treanor J., Collins A., et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 45.DiazGranados C.A., Robertson C.A., Talbot H.K., Landolfi V., Dunning A.J., Greenberg D.P. Prevention of serious events in adults 65 years of age or older: A comparison between high-dose and standard-dose inactivated influenza vaccines. Vaccine. 2015;33(38):4988–4993. doi: 10.1016/j.vaccine.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Lee J.K.H., Lam G.K.L., Shin T., Kim J., Krishnan A., Greenberg D.P., et al. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: a systematic review and meta-analysis. Expert Rev Vaccines. 2018;17(5):435–443. doi: 10.1080/14760584.2018.1471989. [DOI] [PubMed] [Google Scholar]
- 47.Frobert O., Gotberg M., Angeras O., Jonasson L., Erlinge D., Engstrom T., et al. Design and rationale for the Influenza vaccination After Myocardial Infarction (IAMI) trial. A registry-based randomized clinical trial. Am Heart J. 2017;189:94–102. doi: 10.1016/j.ahj.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Loeb M., Dokainish H., Dans A., Palileo-Villanueva L.M., Roy A., Karaye K., et al. Randomized controlled trial of influenza vaccine in patients with heart failure to reduce adverse vascular events (IVVE): Rationale and design. Am Heart J. 2019;212:36–44. doi: 10.1016/j.ahj.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mereckiene J, Cotter S, Nicoll A, Levy-Bruhl D, Ferro A, Tridente G, et al. National seasonal influenza vaccination survey in Europe, 2008. Euro Surveill 2008;13(43). doi: 10.2807/ese.13.43.19017-en. [DOI] [PubMed]
- 50.Centers for Disease Control and Prevention. Flu and people with asthma. 2019; Available from: https://www.cdc.gov/flu/highrisk/asthma.htm. Accessed: 22 July 2019.
- 51.Schwarze J., Openshaw P., Jha A., Del Giacco S.R., Firinu D., Tsilochristou O., et al. Influenza burden, prevention, and treatment in asthma-A scoping review by the EAACI Influenza in asthma task force. Allergy. 2018;73(6):1151–1181. doi: 10.1111/all.13333. [DOI] [PubMed] [Google Scholar]
- 52.Trinh P., Jung T.H., Keene D., Demmer R.T., Perzanowski M., Lovasi G. Temporal and spatial associations between influenza and asthma hospitalisations in New York City from 2002 to 2012: a longitudinal ecological study. BMJ Open. 2018;8(9) doi: 10.1136/bmjopen-2017-020362. e020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bramley A.M., Dasgupta S., Skarbinski J., Kamimoto L., Fry A.M., Finelli L., et al. Intensive care unit patients with 2009 pandemic influenza A (H1N1pdm09) virus infection - United States, 2009. Influenza Other Respir Viruses. 2012;6(6):e134–e142. doi: 10.1111/j.1750-2659.2012.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Myles P.R., Semple M.G., Lim W.S., Openshaw P.J., Gadd E.M., Read R.C., et al. Predictors of clinical outcome in a national hospitalised cohort across both waves of the influenza A/H1N1 pandemic 2009–2010 in the UK. Thorax. 2012;67(8):709–717. doi: 10.1136/thoraxjnl-2011-200266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Myles P., Nguyen-Van-Tam J.S., Semple M.G., Brett S.J., Bannister B., Read R.C., et al. Differences between asthmatics and nonasthmatics hospitalised with influenza A infection. Eur Respir J. 2013;41(4):824–831. doi: 10.1183/09031936.00015512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Veerapandian R., Snyder J.D., Samarasinghe A.E. Influenza in asthmatics: for better or for worse? Front Immunol. 2018;9:1843. doi: 10.3389/fimmu.2018.01843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mallia P., Johnston S.L. Influenza infection and COPD. Int J Chron Obstruct Pulmon Dis. 2007;2(1):55–64. doi: 10.2147/copd.2007.2.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wedzicha J.A., Seemungal T.A. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi: 10.1016/s0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rohde G., Wiethege A., Borg I., Kauth M., Bauer T.T., Gillissen A., et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yap F.H., Ho P.L., Lam K.F., Chan P.K., Cheng Y.H., Peiris J.S. Excess hospital admissions for pneumonia, chronic obstructive pulmonary disease, and heart failure during influenza seasons in Hong Kong. J Med Virol. 2004;73(4):617–623. doi: 10.1002/jmv.20135. [DOI] [PubMed] [Google Scholar]
- 61.Schanzer D.L., Langley J.M., Tam T.W. Role of influenza and other respiratory viruses in admissions of adults to Canadian hospitals. Influenza Other Respir Viruses. 2008;2(1):1–8. doi: 10.1111/j.1750-2659.2008.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kopsaftis Z., Wood-Baker R., Poole P. Influenza vaccine for chronic obstructive pulmonary disease (COPD) Cochrane Database Syst Rev. 2018 doi: 10.1002/14651858.CD002733.pub3. 6:CD002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.International Diabetes Federation. IDF Diabetes Atlas. Eighth edition 2017. 2017; Available from: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/134-idf-diabetes-atlas-8th-edition.html. Accessed: 06 Mar 2020.
- 64.Goeijenbier M., van Sloten T.T., Slobbe L., Mathieu C., van Genderen P., Beyer W.E.P., et al. Benefits of flu vaccination for persons with diabetes mellitus: A review. Vaccine. 2017;35(38):5095–5101. doi: 10.1016/j.vaccine.2017.07.095. [DOI] [PubMed] [Google Scholar]
- 65.Diepersloot R.J., Bouter K.P., Hoekstra J.B. Influenza infection and diabetes mellitus. Case for annual vaccination. Diabetes Care. 1990;13(8):876–882. doi: 10.2337/diacare.13.8.876. [DOI] [PubMed] [Google Scholar]
- 66.Hulme K.D., Gallo L.A., Short K.R. Influenza Virus and glycemic variability in diabetes: a killer combination? Front Microbiol. 2017;8:861. doi: 10.3389/fmicb.2017.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mertz D., Kim T.H., Johnstone J., Lam P.P., Science M., Kuster S.P., et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347 doi: 10.1136/bmj.f5061. f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allard R., Leclerc P., Tremblay C., Tannenbaum T.N. Diabetes and the severity of pandemic influenza A (H1N1) infection. Diabetes Care. 2010;33(7):1491–1493. doi: 10.2337/dc09-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moghadami M., Honarvar B., Sabaeian B., Zamiri N., Pourshahid O., Rismanchi M., et al. H1N1 influenza infection complicated with diabetic ketoacidosis. Arch Iran Med. 2012;15(1):55–58. doi: 012151/AIM.0015. [PubMed] [Google Scholar]
- 70.Cano M., Iglesias P., Perez G., Diez J.J. Influenza A virus (H1N1) infection as a cause of severe diabetic ketoacidosis in type 1 diabetes. Endocrinol Nutr. 2010;57(1):37–38. doi: 10.1016/S1575-0922(10)70008-5. [DOI] [PubMed] [Google Scholar]
- 71.Samson S.I., Konty K., Lee W.N., Quisel T., Foschini L., Kerr D., et al. Quantifying the impact of influenza among persons with type 2 diabetes mellitus: a new approach to determine medical and physical activity impact. J Diabetes Sci Technol. 2019;1932296819883340 doi: 10.1177/1932296819883340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klekotka R.B., Mizgala E., Krol W. The etiology of lower respiratory tract infections in people with diabetes. Pneumonol Alergol Pol. 2015;83(5):401–408. doi: 10.5603/PiAP.2015.0065. [DOI] [PubMed] [Google Scholar]
- 73.Pearson-Stuttard J., Blundell S., Harris T., Cook D.G., Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016;4(2):148–158. doi: 10.1016/S2213-8587(15)00379-4. [DOI] [PubMed] [Google Scholar]
- 74.Ekstrand J.J. Neurologic complications of influenza. Semin Pediatr Neurol. 2012;19(3):96–100. doi: 10.1016/j.spen.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 75.Vellozzi C., Iqbal S., Broder K. Guillain-Barre syndrome, influenza, and influenza vaccination: the epidemiologic evidence. Clin Infect Dis. 2014;58(8):1149–1155. doi: 10.1093/cid/ciu005. [DOI] [PubMed] [Google Scholar]
- 76.Sanz Fadrique R., Martin Arias L., Molina-Guarneros J.A., Jimeno Bulnes N., Garcia O.P. Guillain-Barre syndrome and influenza vaccines: current evidence. Rev Esp Quimioter. 2019;32(4):288–295. [PMC free article] [PubMed] [Google Scholar]
- 77.Klein E.Y., Monteforte B., Gupta A., Jiang W., May L., Hsieh Y.H., et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2016;10(5):394–403. doi: 10.1111/irv.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morris D.E., Cleary D.W., Clarke S.C. Secondary bacterial infections associated with influenza pandemics. Front Microbiol. 2017;8:1041. doi: 10.3389/fmicb.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salomon A., Berry I., Tuite A.R., Drews S., Hatchette T., Jamieson F., et al. Influenza increases invasive meningococcal disease risk in temperate countries. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 80.Polgreen P.M., Yang M., Bohnett L.C., Cavanaugh J.E. A time-series analysis of clostridium difficile and its seasonal association with influenza. Infect Control Hosp Epidemiol. 2010;31(4):382–387. doi: 10.1086/651095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Allard R., Couillard M., Pilon P., Kafka M., Bedard L. Invasive bacterial infections following influenza: a time-series analysis in Montreal, Canada, 1996–2008. Influenza Other Respir Viruses. 2012;6(4):268–275. doi: 10.1111/j.1750-2659.2011.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hendriks W., Boshuizen H., Dekkers A., Knol M., Donker G.A., van der Ende A., et al. Temporal cross-correlation between influenza-like illnesses and invasive pneumococcal disease in The Netherlands. Influenza Other Respir Viruses. 2017;11(2):130–137. doi: 10.1111/irv.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Christopoulou I., Roose K., Ibanez L.I., Saelens X. Influenza vaccines to control influenza-associated bacterial infection: where do we stand? Expert Rev Vaccines. 2015;14(1):55–67. doi: 10.1586/14760584.2015.957191. [DOI] [PubMed] [Google Scholar]
- 84.Morgan D.J., Casulli J., Chew C., Connolly E., Lui S., Brand O.J., et al. Innate immune cell suppression and the link with secondary lung bacterial pneumonia. Front Immunol. 2018;9:2943. doi: 10.3389/fimmu.2018.02943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siegel S.J., Roche A.M., Weiser J.N. Influenza promotes pneumococcal growth during coinfection by providing host sialylated substrates as a nutrient source. Cell Host Microbe. 2014;16(1):55–67. doi: 10.1016/j.chom.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee L.N., Dias P., Han D., Yoon S., Shea A., Zakharov V., et al. A mouse model of lethal synergism between influenza virus and Haemophilus influenzae. Am J Pathol. 2010;176(2):800–811. doi: 10.2353/ajpath.2010.090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Christenson B., Lundbergh P., Hedlund J., Ortqvist A. Effects of a large-scale intervention with influenza and 23-valent pneumococcal vaccines in adults aged 65 years or older: a prospective study. Lancet. 2001;357(9261):1008–1011. doi: 10.1016/S0140-6736(00)04237-9. [DOI] [PubMed] [Google Scholar]
- 88.Lekan D.A. Frailty and other emerging concepts in care of the aged. South Online J Nurs Res. 2009;9(3):1–29. [Google Scholar]
- 89.Creditor M.C. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219–223. doi: 10.7326/0003-4819-118-3-199302010-00011. [DOI] [PubMed] [Google Scholar]
- 90.Andrew M.K., Gilca V., Waite N., Pereira J.A. EXamining the knowledge, Attitudes and experiences of Canadian seniors Towards influenza (the EXACT survey) BMC Geriatr. 2019;19(1):178. doi: 10.1186/s12877-019-1180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barker W.H., Borisute H., Cox C. A study of the impact of influenza on the functional status of frail older people. Arch Intern Med. 1998;158(6):645–650. doi: 10.1001/archinte.158.6.645. [DOI] [PubMed] [Google Scholar]
- 92.Gozalo P.L., Pop-Vicas A., Feng Z., Gravenstein S., Mor V. Effect of influenza on functional decline. J Am Geriatr Soc. 2012;60(7):1260–1267. doi: 10.1111/j.1532-5415.2012.04048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Andrew M., MacDonald S., Ye L., Ambrose A., Boivin G., Diaz-Mitoma F., et al. Impact of frailty on influenza vaccine effectiveness and clinical outcomes: experience from the Canadian immunization research network (CIRN) serious outcomes surveillance (SOS) network 2011/12 season. Open Forum Infectious Diseases. 2016 doi: 10.1093/ofid/ofw172.573. 3. [DOI] [Google Scholar]
- 94.McConeghy K.W., Lee Y., Zullo A.R., Banerjee G., Daiello L., Dosa D., et al. Influenza illness and hip fracture hospitalizations in nursing home residents: are they related? J Gerontol A Biol Sci Med Sci. 2018;73(12):1638–1642. doi: 10.1093/gerona/glx200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fraenkel M., Yitshak-Sade M., Beacher L., Carmeli M., Mandelboim M., Siris E., et al. Is the association between hip fractures and seasonality modified by influenza vaccination? An ecological study. Osteoporos Int. 2017;28(9):2611–2617. doi: 10.1007/s00198-017-4077-1. [DOI] [PubMed] [Google Scholar]
- 96.Bartley J.M., Pan S.J., Keilich S.R., Hopkins J.W., Al-Naggar I.M., Kuchel G.A., et al. Aging augments the impact of influenza respiratory tract infection on mobility impairments, muscle-localized inflammation, and muscle atrophy. Aging (Albany NY) 2016;8(4):620–635. doi: 10.18632/aging.100882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fragaszy E.B., Warren-Gash C., White P.J., Zambon M., Edmunds W.J., Nguyen-Van-Tam J.S., et al. Effects of seasonal and pandemic influenza on health-related quality of life, work and school absence in England: Results from the Flu Watch cohort study. Influenza Other Respir Viruses. 2018;12(1):171–182. doi: 10.1111/irv.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McLaughlin J.M., McGinnis J.J., Tan L., Mercatante A., Fortuna J. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev. 2015;36(4):259–273. doi: 10.1007/s10935-015-0394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Putri W., Muscatello D.J., Stockwell M.S., Newall A.T. Economic burden of seasonal influenza in the United States. Vaccine. 2018;36(27):3960–3966. doi: 10.1016/j.vaccine.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 100.Preaud E., Durand L., Macabeo B., Farkas N., Sloesen B., Palache A., et al. Annual public health and economic benefits of seasonal influenza vaccination: a European estimate. BMC Public Health. 2014;14:813. doi: 10.1186/1471-2458-14-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schanzer D.L., Schwartz B. Impact of seasonal and pandemic influenza on emergency department visits, 2003–2010, Ontario, Canada. Acad Emerg Med. 2013;20(4):388–397. doi: 10.1111/acem.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barnighausen T., Bloom D.E., Cafiero-Fonseca E.T., O'Brien J.C. Valuing vaccination. Proc Natl Acad Sci U S A. 2014;111(34):12313–12319. doi: 10.1073/pnas.1400475111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jit M., Hutubessy R., Png M.E., Sundaram N., Audimulam J., Salim S., et al. The broader economic impact of vaccination: reviewing and appraising the strength of evidence. BMC Med. 2015;13:209. doi: 10.1186/s12916-015-0446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ng C., Ye L., Noorduyn S.G., Hux M., Thommes E., Goeree R., et al. Resource utilization and cost of influenza requiring hospitalization in Canadian adults: A study from the serious outcomes surveillance network of the Canadian Immunization Research Network. Influenza Other Respir Viruses. 2018;12(2):232–240. doi: 10.1111/irv.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Doyon-Plourde P., Fakih I., Tadount F., Fortin E., Quach C. Impact of influenza vaccination on healthcare utilization - A systematic review. Vaccine. 2019;37(24):3179–3189. doi: 10.1016/j.vaccine.2019.04.051. [DOI] [PubMed] [Google Scholar]
- 106.Meier C.R., Napalkov P.N., Wegmuller Y., Jefferson T., Jick H. Population-based study on incidence, risk factors, clinical complications and drug utilisation associated with influenza in the United Kingdom. Eur J Clin Microbiol Infect Dis. 2000;19(11):834–842. doi: 10.1007/s100960000376. [DOI] [PubMed] [Google Scholar]
- 107.Klugman K.P., Black S. Impact of existing vaccines in reducing antibiotic resistance: Primary and secondary effects. Proc Natl Acad Sci U S A. 2018;115(51):12896–12901. doi: 10.1073/pnas.1721095115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.World Health Organization. Global Influenza Strategy 2019-2030. 2019; Available from: https://apps.who.int/iris/bitstream/handle/10665/311184/9789241515320-eng.pdf?ua=1. Accessed: 18 July 2019.
- 109.Barnes M., Heywood A.E., Mahimbo A., Rahman B., Newall A.T., Macintyre C.R. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart. 2015;101(21):1738–1747. doi: 10.1136/heartjnl-2015-307691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhu T., Carcaillon L., Martinez I., Cambou J.P., Kyndt X., Guillot K., et al. Association of influenza vaccination with reduced risk of venous thromboembolism. Thromb Haemost. 2009;102(6):1259–1264. doi: 10.1160/TH09-04-0222. [DOI] [PubMed] [Google Scholar]
- 111.Fonarow G.C., Abraham W.T., Albert N.M., Stough W.G., Gheorghiade M., Greenberg B.H., et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008;168(8):847–854. doi: 10.1001/archinte.168.8.847. [DOI] [PubMed] [Google Scholar]
- 112.Rezkalla S.H., Kloner R.A. Influenza-related viral myocarditis. WMJ. 2010;109(4):209–213. [PubMed] [Google Scholar]
- 113.Watanabe T. Renal complications of seasonal and pandemic influenza A virus infections. Eur J Pediatr. 2013;172(1):15–22. doi: 10.1007/s00431-012-1854-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.