Key Points
Question
Does severe acute respiratory syndrome coronavirus disease 2 (SARS-CoV-2) exist in the ocular tissues of a patient with coronavirus disease 2019 (COVID-19)?
Findings
In this case study, nucleocapsid protein antigens were detected on the cells of the conjunctiva, iris, and trabecular meshwork of a patient with a COVID-19 infection, and these antigens were absent on the specimens from the control patient. In addition, angiotensin-converting enzyme 2 receptor proteins were detected in the conjunctiva cells of this patient and a control participant.
Meaning
Nucleocapsid protein antigens of SARS-CoV-2 existed in the inner ocular tissues of a patient previously infected with COVID-19, which implied that SARS-CoV-2 can infect ocular tissues as well as the respiratory system.
This case study compares a patient previously infected with coronavirus disease 2019 with a control participant in assessment of nucleocapsid protein antigens on the cells of the conjunctiva, iris, and trabecular meshwork.
Abstract
Importance
Coronavirus disease 2019 (COVID-19) has been recognized as a pandemic by the World Health Organization. Whether severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can also infect tissues besides the respiratory system, such as the ocular tissues, remains unclear.
Objective
To determine whether SARS-CoV-2 exists intracellularly in the ocular tissues of a patient previously infected with COVID-19.
Design, Setting, and Participants
This case study analyzed a patient previously infected with COVID-19 who had an acute glaucoma attack during her rehabilitation. Plasma samples and tissue specimens, including ones from the conjunctiva, anterior lens capsular, trabecular meshwork, and iris, were collected during phacoemulsification and trabeculectomy surgery. Specimens from another patient who had glaucoma but not COVID-19 were used as a negative control.
Main Outcomes and Measures
Specimens were analyzed using hematoxylin-eosin staining. The nucleocapsid protein antigen of SARS-CoV-2 was measured in the conjunctiva, trabecular meshwork, and iris using immunofluorescence and immunohistochemistry. The expression of angiotensin-converting enzyme 2 receptor on the conjunctiva was measured using immunohistochemistry.
Results
The patient with a previous COVID-19 infection was female and 64 years old, and the control patient without a COVID-19 infection history was male and 61 years old. The nucleocapsid protein antigen of SARS-CoV-2 was detected on the cells of the conjunctiva, trabecular, and iris of the patient infected with COVID-19 but not in the control participant, while angiotensin-converting enzyme 2 receptor proteins were detected on the conjunctiva of both patients.
Conclusions and Relevance
The nucleocapsid protein antigen of SARS-CoV-2 existed intracellularly in the ocular tissues of a patient previously infected with COVID-19. Thus, SARS-CoV-2 can also infect ocular tissues in addition to the respiratory system.
Introduction
Coronavirus disease 2019 (COVID-19), the pathogen for which is officially named the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been recognized as a pandemic by the World Health Organization.1 However, whether SARS-CoV-2 can infect tissues other than those in the respiratory system, such as the ocular tissues, remains unclear.
Some previously published reports2,3,4 have shown that SARS-CoV-2 can be detected in the conjunctival sac of patients with confirmed or suspected COVID-19 using reverse transcriptase–polymerase chain reaction (RT-PCR). However, they found relatively low conjunctival sac infection rates in 3 of 67 individuals,2 1 of 72 individuals,3 and 2 of 12 individuals.4 Furthermore, Colavita et al5 reported that SARS-CoV-2 was successfully isolated from ocular secretions of a patient with COVID-19. However, no direct evidence showed that SARS-CoV-2 existed in the inner ocular tissues.5 Since patients with COVID-19 have viremia during the acute phase,6 the positive result of a SARS-CoV-2 RNA test likely resulted from the virus in the exudation of conjunctivitis.7
This study provides a case report of a patient who had an acute glaucoma attack after recovering from COVID-19 infection. Since she needed to receive ophthalmic surgery, surgical ocular specimen removal and analysis were performed. Samples were also taken from a control participant.
Methods
Ethical approval was obtained from the Ethics Committee of the General Hospital of the Central Theatre. Written informed consent was given by both patients.
A 64-year-old woman presented with dry cough for 5 days and diarrhea for 9 days before she went to the hospital on January 31, 2020. On the day of admission, her temperature was 37.8 °C and her oxygen saturation was 98%. Laboratory tests showed that her C-reactive protein concentration was elevated to 1.25 mg/dL (normal range, 0-1.0 mg/dL [to convert to milligrams per liter, multiply by 10]). A complete blood cell count showed a leukocyte count of 7270 cells/μL (normal range, 400-10 000 cells/μL [to convert to cells × 109 per liter, multiply by 0.001]). Computed tomography scans of her chest showed ground-glass opacity in her lower bilateral lobes. Based on RT-PCR analysis of throat swab samples (eMethods 1 in the Supplement), she was diagnosed with COVID-19. She was then admitted to the hospital to receive antiviral and antibacterial treatment, as well as supplemental oxygen, as empirical therapy. During her hospitalization, the patient had no serious respiratory or ocular symptoms. By day 18, her symptoms were fully resolved, and the throat swab RT-PCR tests, which were performed on February 18 and 20, indicated negative results.
On February 28, she began experiencing persistent left eye pain and visual acuity loss. Three days later, the same symptoms developed in her right eye. She was admitted to the ophthalmology clinic on March 8. At presentation, her visual acuity was light perception bilaterally. The intraocular pressure was 50 mm Hg in both eyes. A slitlamp examination showed bilaterally conjunctival hyperemia, corneal edema, dilated (5-mm diameter) and fixed pupils, flat anterior chambers, and lens opacity (grade 3, according to the Lens Opacities Classification System III grades criteria8). Her fundus was unclear because of the corneal edema. She was diagnosed with acute angle-closure glaucoma and cataract.
Because her intraocular pressure could not be lowered by medication, phacoemulsification surgery was performed on her left and right eyes on March 14 and 15, respectively, and tissue samples of the anterior lens capsular were collected during this surgery. Because of the uncontrollable intraocular pressure in her right eye after phacoemulsification surgery, she underwent trabeculectomy surgery on April 10. Samples of the conjunctiva, trabecular, and iris were collected during this surgery. Further details of the trabeculectomy surgery are available in eMethods 3 in the Supplement.
During a trabeculectomy surgery also performed on April 10, control specimens were collected from another patient (male; 61 years old) with glaucoma. This patient had not had COVID-19 infection (based on 2 negative RT-PCR throat swabs and negative chest computed tomography and blood test results).
Fasting blood samples of approximately 5 mL were drawn from both participants to detect SARS-CoV-2 IgG and IgM antibodies (Innovita Biological Technology Co Ltd) and nucleocapsid protein (NP) antigen (Bioeasy Biotechnology Co Ltd). All specimens were placed in a freezing box at 4 °C and transported to the laboratory within 30 minutes. The specimens were embedded in paraffin, cut into 4-μm slices, and mounted onto polylysine-coated glass slides stained with hematoxylin-eosin. The NP antigen of SARS-CoV-2 was detected on the conjunctiva, trabecular, and iris specimens using immunochemistry or immunofluorescence, while angiotensin-converting enzyme 2 (ACE2) receptor protein on the conjunctiva was detected using immunochemistry. Additional details are available in eMethods 2 in the Supplement.
Results
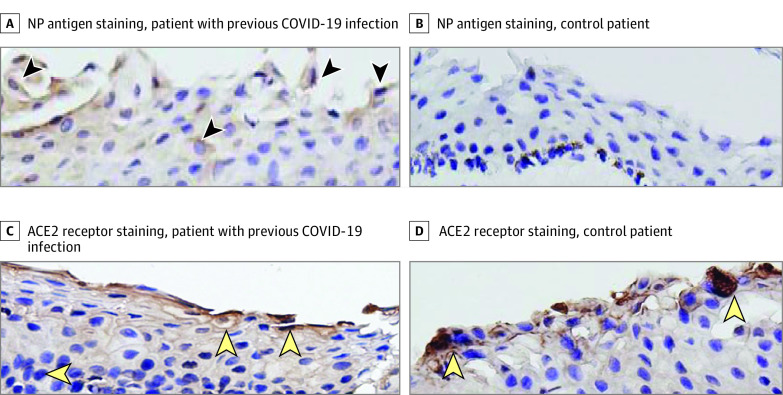
For both patients, the NP antigen of SARS-CoV-2 was not detected in the plasma. Additionally, IgG antibody tests had a positive result and IgM antibody tests had a negative result in the patient with COVID-19, while IgG and IgM antibody tests in the control participant both had negative results. Since no cell structure was found in the anterior capsule of the lens under hematoxylin-eosin staining, neither immunohistochemical nor immunofluorescence measurements were performed. Immunohistochemistry was performed for conjunctiva specimens from both patients; the results are shown in Figure 1. The NP antigen was present intracellularly in the conjunctiva collected from the patient previously infected with COVID-19 but absent in the conjunctiva tissue collected from the control patient. The ACE2 receptor proteins could be detected in the conjunctiva cells from both patients.
Figure 1. Immunohistochemistry Assays of Conjunctiva Samples.
A, Conjunctiva from a patient with coronavirus disease 2019 (COVID-19) infection, stained for nucleocapsid protein (NP) antigen. Brown staining indicates the NP antigen positive cells (arrowheads). B, Conjunctiva from the control patient. No NP antigen staining is present. C and D, Conjunctiva from a patient infected with COVID-19 (C) and a control participant (D), stained for angiotensin-converting enzyme 2 (ACE2) receptor. Brown staining indicates the ACE2 receptor–positive cells (arrowheads). The original magnification was ×40.
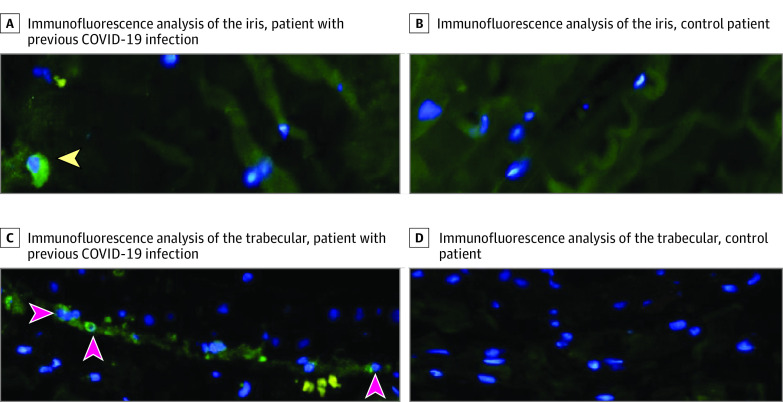
Immunofluorescence analysis was performed for the iris and trabecular from both patients; the results are shown in Figure 2. Iris and trabecular meshwork samples from the patient previously infected with COVID-19 showed positive staining for the NP antigen, while the iris and trabecular meshwork samples from the control participant did not show NP antigen staining.
Figure 2. Immunofluorescence Analysis of Iris and Trabecular Meshwork.
A, Iris from patient infected with coronavirus disease 2019 (COVID-19), stained for nucleocapsid protein (NP) antigen. Green staining indicates the NP antigen–positive cells (arrowheads). B, Iris sample from the control patient, with no NP antigen staining indicated. C, Trabecular meshwork from patient infected with COVID-19, stained for NP antigen. Green staining indicates the NP antigen–positive cells (arrowheads). D, Trabecular meshwork sample from the control patient with no NP antigen staining indicated. The original magnification was ×40.
Discussion
The patient under study was in the recovery period of infection, and thus the 3-time nucleic acid testing of her swabs had negative results. The method used to detect the SARS-CoV-2 NP antigen in this study was similar to the method used in a previous investigation of lung and kidney autopsy specimens.9 The specimens from the patient without an infection in this study showed no SARS-CoV-2 NP antigen expression; the evidence provided was relatively reliable.
In the patient previously infected with COVID-19, the SARS-CoV-2 NP antigen was found in the conjunctival, trabecular, and iris tissues. This indicated that SARS-CoV-2 may exist intracellularly in the inner ocular tissues as well as on the ocular surface. Based on these results, the eye is also one of the target organs for the viral infection in addition to the lungs. Although the method by which the virus enters the eye is still unclear, it could theoretically enter the inner eye tissues in 2 ways. First, SARS-CoV-2 could enter the inner eye tissue via the ACE2 receptor on the surface of the conjunctiva.10 This study’s results showed that ACE2 receptor proteins are expressed on the conjunctival cell, seemingly supporting this hypothesis. However, this hypothesis can only be confirmed through animal experiments—for example, ones that detect the virus in the intraocular tissue after conjunctiva inoculation. Second, the virus may spread systemically to end organs as a result of the primary respiratory infection. However, when the patient presented at the ophthalmology clinic, the possible viremia was already in the recovery stage, and the SARS-CoV-2 NP antigen test and IgM antibody in the plasma showed negative results. These findings do not seem to support this second transmission-route hypothesis.
Limitations
The patient under study was not in the acute period of infection. The transmission route was short of definite evidence.
Conclusions
The viral antigen detected in the eye of the patient 2 months after infection should prompt future investigations. These investigations should aim to determine whether the viral NP antigens that remain in the eye over time cause damage to the ocular structure or function, represent the presence of active viral residues in organs (not only the eyes), and are still infectious.
eMethods 1. The details of the RT-PCR analysis
eMethods 2. The protocol of immunohistochemistry
eMethods 3. The procedure of the trabeculectomy surgery
References
- 1.Zhu N, Zhang D, Wang W, et al. ; China Novel Coronavirus Investigating and Research Team . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou Y, Zeng Y, Tong Y, et al. Ophthalmologic evidence against the interpersonal transmission of 2019. novel coronavirus through conjunctiva. Published online February 12, 2020. https://www.medrxiv.org/content/10.1101/2020.02.11.20021956v1
- 3.Zhang X, Chen X, Chen L, et al. The evidence of SARS-CoV-2 infection on ocular surface. Ocul Surf. 2020;18(3):360-362. doi: 10.1016/j.jtos.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei province, China. JAMA Ophthalmol. 2020;138(5):575-578. doi: 10.1001/jamaophthalmol.2020.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colavita F, Lapa D, Carletti F, et al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann Intern Med. 2020;173(3):242-243. doi: 10.7326/M20-1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng Y, Zhou YH. Is novel coronavirus disease (COVID-19) transmitted through conjunctiva? J Med Virol. Published March 16, 2020. doi: 10.1002/jmv.25753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta M, Ram J, Jain A, Sukhija J, Chaudhary M. Correlation of nuclear density using the Lens Opacity Classification System III versus Scheimpflug imaging with phacoemulsification parameters. J Cataract Refract Surg. 2013;39(12):1818-1823. doi: 10.1016/j.jcrs.2013.05.052 [DOI] [PubMed] [Google Scholar]
- 9.Diao B, Wang CH, Wang RS, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Published April 10, 2020. https://www.medrxiv.org/content/10.1101/2020.03.04.20031120v4 [DOI] [PMC free article] [PubMed]
- 10.Sun Y, Liu L, Pan X, Jing M. Mechanism of the action between the SARS-CoV S240 protein and the ACE2 receptor in eyes. Int J Ophthalmol-Chi. 2006;6(4):783-786. doi: 10.3969/j.issn.1672-5123.2006.04.014 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. The details of the RT-PCR analysis
eMethods 2. The protocol of immunohistochemistry
eMethods 3. The procedure of the trabeculectomy surgery