Abstract
BACKGROUND
Many natural products confer health benefits against diverse diseases through their antioxidant activities. Carbon tetrachloride (CCl4) is often used in animal experiments to study the effects of substances on liver injury and the related mechanisms of action, among which oxidative stress is a major pathogenic factor.
AIM
To compare antioxidant and hepatoprotective activities of ten herbs and identify and quantify phytochemicals for the one with strongest hepatoprotection.
METHODS
The antioxidant activity of ten medicinal herbs was determined by both ferric-reducing antioxidant power and Trolox equivalent antioxidant capacity assays. The total phenolic and flavonoid contents were determined by Folin–Ciocalteu method and aluminum chloride colorimetry, respectively. Their effects on CCl4-induced oxidative liver injury were evaluated and compared in a mouse model by administrating each water extract (0.15 g/mL, 10 mL/kg) once per day for seven consecutive days and a dose of CCl4 solution in olive oil (8%, v/v, 10 mL/kg). The herb with the strongest hepatoprotective performance was analyzed for the detailed bioactive components by using high-performance liquid chromatography-electrospray ionization source-ion trap tandem mass spectrometry.
RESULTS
The results revealed that all tested herbs attenuated CCl4-induced oxidative liver injury; each resulted in significant decreases in levels of serum alanine transaminase, aspartate transaminase, alkaline phosphatase, and triacylglycerols. In addition, most herbs restored hepatic superoxide dismutase and catalase activities, glutathione levels, and reduced malondialdehyde levels. Sanguisorba officinalis (S. officinalis) L., Coptis chinensis Franch., and Pueraria lobata (Willd.) Ohwi root were the three most effective herbs, and S. officinalis L. exhibited the strongest hepatoprotective effect. Nine active components were identified in S. officinalis L. Gallic acid and (+)-catechin were quantified (7.86 ± 0.45 mg/g and 8.19 ± 0.57 mg/g dried weight, respectively). Furthermore, the tested herbs displayed a range of in vitro antioxidant activities proportional to their phenolic content; the strongest activities were also found for S. officinalis L.
CONCLUSION
This study is of value to assist the selection of more effective natural products for direct consumption and the development of nutraceuticals or therapeutics to manage oxidative stress-related diseases.
Keywords: Antioxidant activity, CCl4-induced liver injury, Medicinal herbs, Hepatoprotection, Sanguisorba officinalis L., Coptis chinensis Franch
Core Tip: Many natural products confer health benefits against diverse diseases through their antioxidant activities. In this study, ten medicinal herbs were selected for an evaluation and comparison of their effects on carbon tetrachloride (CCl4)-induced oxidative liver injury. Sanguisorba officinalis (S. officinalis) L. exhibited the strongest hepatoprotective effect, and the strongest in vitro activities were also found for S. officinalis L. Our results provided valuable information for the selection of more efficient herbs to protect against CCl4-induced liver injury and to support the direct application of herbs or the development of novel therapies for the management of oxidative stress-related diseases.
INTRODUCTION
Redox reactions are involved in numerous physiological and pathological processes; moreover, cellular homeostasis depends on the interaction between oxidants and the defense system, which includes reductants and antioxidant enzymes[1,2]. The prevalence of free radicals, such as reactive oxygen species and reactive nitrogen species, at a desirable level can contribute to cell growth and differentiation[2]. However, the overproduction of free radicals is destructive, resulting in oxidative stress and contributing to various diseases, such as cardiovascular diseases, cancer, diabetes, obesity, neurodegenerative disorders, and liver diseases[3-6].
Many factors can cause liver damage. In addition to physical factors (e.g., radiation) and biological factors (e.g., viruses), some chemicals are hepatotoxic. Carbon tetrachloride (CCl4) is often used in animal experiments to study the effects of substances on liver injury and the related mechanisms of action[7-9]. The causal link between CCl4 and liver diseases has been well established[9,10]. During the metabolism of CCl4 in the liver, hepatotoxic metabolites and excessive free radicals such as trichloromethyl radical (∙CCl3) and trichloromethylperoxy radical (∙OOCCl3) are generated, accompanied by other free radicals (e.g., O2– and H2O2). Consequently, reductants (e.g., glutathione, GSH) are depleted, and antioxidant enzymes [e.g., superoxide dismutase (SOD) and catalase (CAT)] are inhibited, inducing oxidative stress[9]. Meanwhile, the toxic metabolites and free radicals bind to phospholipid molecules embedded in the membranes of mitochondria, the endoplasmic reticulum, and hepatocytes, which leads to lipid peroxidation and membrane dysfunction or damage[9]. In addition, they can also bind with other macromolecules, such as proteins and DNA, and result in cell damage or death. Such a condition may aggravate hypoxia, induce the accumulation of more lipids, facilitate gut leakage and bacterial translocation, promote cytokine release, and increase hepatic iron accumulation, which exacerbates the production of highly reactive radicals[4,11]. Therefore, oxidative stress is a major pathogenic factor for CCl4-induced liver injury.
Through their antioxidant activities, many natural products have been shown to exert protective effects against various oxidative stress-related diseases, such as cardiovascular disease, cancer, and liver diseases[4,12,13]. Notably, based on observations from many cultures over many years, numerous foods and herbs, including staple foods, vegetables, seasonings, and herbal teas[14,15], have been reported to protect the liver. Subsequently, scientific evidence has shown that many related products and specific bioactive components have protective effects in the liver against oxidative injuries[8,16]. In this study, ten medicinal foods and herbs were selected for an evaluation and comparison of their effects on CCl4-induced oxidative liver injury. In addition, the herb with the strongest hepatoprotective performance was analyzed for the detailed bioactive components by using high-performance liquid chromatography-electrospray ionization source-ion trap tandem mass spectrometry (HPLC-ESI-ITMS/MS). The in vitro antioxidant capacities, total phenolic content (TPC), and total flavonoid content (TFC) of the ten herbs were also determined. This study aimed to provide valuable information for the selection of more efficient herbs to protect against CCl4-induced liver injury and to support the direct application of herbs or the development of novel therapies for the management of oxidative stress-related diseases.
MATERIALS AND METHODS
Chemicals
CCl4, sodium chloride, and acetic acid were obtained from Damao Chemical Reagent Factory (Tianjin, China). Olive oil was purchased from Aladdin Industrial Corporation (Shanghai, China). Methanol (99.9%, HPLC/ACS grade) and formic acid (≥ 90%, guaranteed grade) were purchased from Amethyst Chemicals (Beijing, China) and Kermel Chemical Factory (Tianjin, China), respectively. The phenolic standards, 2,4,6-tripyridyl-s-triazine (TPTZ), 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), Folin and Ciocalteu’s phenol, and 2,2’-azino-bis(3-ethylbenothiazoline-6-sulphonic acid) diammonium salt (ABTS) were purchased from Sigma-Aldrich (St. Louis, MO, United States). Gallic acid (> 98%) and (+)-catechin (> 98%) were obtained from Chengdu Derick Biotechnology Co. Ltd. (Sichuan, China).
All chemicals were of analytical or chromatographical grade. Double-distilled water was used in all experiments. Bifendate was obtained from the Beijing Union Pharmaceutical Factory. Detection kits for total protein, malondialdehyde (MDA), triglyceride (TG), GSH, SOD, and CAT were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Preparation of water extract
Ten medicinal foods and herbs, Acanthopanax senticosus (Rupr. et Maxim.) harms (root and rhizome), Amomum villosum Lour. (fruit), Amomum kravanh Pierre ex Gagnep. (fruit), Artemisia capillaris Thunb. (herb), Cimicifuga heracleifolia Kom. (rhizome), Coptis chinensis Franch. (rhizome), Glycyrrhiza uralensis Fisch. (root and rhizome), Pueraria lobata (Willd.) Ohwi (flower), P. lobata (Willd.) Ohwi (root), and Sanguisorba officinalis L. (root) were purchased from Tong Ren Tang Chinese Medicine Co., Ltd. (Guangzhou, Guangdong, China). The samples were processed in accordance with a published method[17] but with minor modifications. The finely ground powder of each sample was filtered through a 100-mesh sieve. Then, 10.00 g of filtered powder was mixed with 100 mL of water (room temperature, 30 min) before the mixture was decocted in a water bath (98 °C, 30 min). The cooled mixtures were then centrifuged (4200 g, 10 min), and the supernatant was collected. The extraction was conducted twice, and the supernatants were combined for further use.
Animals and experimental design
Each of the water extracts was freeze dried in a FreeZone Freeze Dryer (Labconco FreeZone®, United States), and the dried crude extract was dissolved in water to give a concentration of 0.15 g/mL. Therefore, when gavaged with 10 mL/kg water extract, each mouse received 1.5 g dried weight (DW)/kg, which is equivalent to the desired human dose recommended by National Administration of Traditional Chinese Medicine[18-20].
SPF Kunming mice (male, weight 18–22 g) were provided by the Laboratory Animal Center of Sun Yat-Sen University, Guangzhou, China. With free access to water and rodent chow, the animals were kept in a controlled environment (22 °C ± 0.5 °C, 40%–60% relative humidity, and a 12 h light-dark cycle). All animal procedures were approved by the Animal Ethics Committee of the School of Public Health at Sun Yat-Sen University (No. 2017-011). The mice were randomly assigned into 13 groups each containing eight mice: control group, model group, positive control, and ten treatment groups. Each herb extract (15 mg/mL, 10 mL/kg) was administered to the mice by gavage once per day for seven consecutive days; bifendate (150 mg/kg) was administered in the positive control group[21], and an equivalent volume of water was administered in the control and model groups. One hour after the final administration, CCl4 solution in olive oil (8%, v/v, 10 mL/kg)[22,23] was administered by intraperitoneal injection to the mice in the model group, positive control group, and ten treatment groups; an equivalent concentration of olive oil (10 mL/kg) was administered to the control group. After 16 h, the mice were anesthetized, blood samples were taken, and the livers were harvested.
The blood samples were centrifuged twice at 3600 g for 15 min, and the serum was separated and analyzed by using a Beckman Coulter Chemistry Analyzer (AU5821, Tokyo, Japan), and the following serum biomarkers were analyzed: alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), total bilirubin (TBIL), and TG. Two slides of liver were sampled from the middle of the left lobe of the liver, one for histopathologic examination (hematoxylin and eosin staining) and the other for biomarker assessment. Liver homogenate was prepared from liver tissue (0.2 g) and physiological saline (ice-cold, 1.8 mL), centrifuged at 2500 g for 10 min and then examined for the activities of SOD and CAT and the levels of GSH, MDA, and TG in accordance with the manufacturer’s instructions and as described in our previous publications[24].
HPLC-ESI-ITMS/MS
The decoction of the herb with the strongest hepatoprotective effect (S. officinalis L.) was analyzed by reverse-phase HPLC using a Shim-pack GIS C18 column (5 µm, 4.6 mm × 250 mm; Shimadzu) maintained at 30 °C and a gradient elution (solvent A: H2O containing 0.1% (v/v) formic acid; solvent B, methanol containing 0.1% (v/v) formic acid). The phytochemical compounds were separated using a 70-min linear gradient from 20% to 70% solvent B at a flow rate of 1.0 mL/min and detected at 280 nm. The injection volumes were 20 µL (sample without dilution) for HPLC analysis and 50 µL (sample with 10-fold dilution) for HPLC-MS/MS analysis.
The MS analysis was performed by using an Ion Trap Mass Spectrometer (ITMS; Thermo Scientific, United States) equipped with an ESI under the following conditions: ESI source temperature, 320 °C; source voltages, 4.0 kV for positive mode and 3.5 kV for negative mode; capillary voltages, 24.0 V for positive polarity, and -12.0 V for negative polarity; desolvation gas, nitrogen; full scan mass range, m/z 50–1500. Tandem mass spectrometry analyses were performed using nitrogen as the collision gas with a collision energy of 35 eV. The MS/MS spectrum was obtained to the tenth most intense ion from ITMS.
Evaluation of in vitro antioxidant activities
The ferric-reducing antioxidant power (FRAP) assay was performed to assess the antioxidant activity (Fe3+-reducing capability) of the tested herbs using a slightly modified version of a published method[25]. In short, FRAP reagent was freshly prepared and kept in a water bath at 37 °C until use with the following three solutions (10:1:1, v/v/v): (1) Sodium acetate buffer (300 mmol/L, pH 3.6); (2) TPTZ solution (10 mmol/L, solvent: 40 mmol/L HCl); and (3) Ferric chloride solution (20 mmol/L). Then, the mixture of the water extract of each sample (100 μL) and FRAP reagents (3 mL) were incubated at room temperature for 4 min. The absorbance of each mixture at 593 nm was recorded. The results were expressed in the form of μmol Fe2+/g DW, with reference to ferrous sulfate as the standard.
The Trolox equivalent antioxidant capacity (TEAC) assay was also conducted to measure the antioxidant activities (free radical, i.e., ABTS•+-scavenging capability) of the tested herbs and was performed using a slightly modified version of a published method[26]. Briefly, the ABTS free radical (ABTS•+) solution was a 1:1 (v/v) solution of ABTS stock solution (7 mmol/L) and potassium persulfate (2.45 mmol/L). The mixture was kept in the dark (room temperature, more than 16 h) for no more than 2 d. The ABTS•+ solution was diluted with ethanol until the solution had an absorbance of 0.710 ± 0.050 at 734 nm. Each water extract (100 μL) was mixed with diluted ABTS•+ solution (3.8 mL) and allowed to react for 6 min; subsequently, the absorbance was recorded, and the results were presented as μmol Trolox equivalent per gram DW (μmol TE/g DW).
Determination of TPC and TFC
The TPC was determined in accordance with the Folin–Ciocalteu method[27]. Briefly, a properly diluted sample (0.5 mL) was mixed with Folin–Ciocalteu reagent (2.5 mL, 0.2 M). After 4 min, a saturated sodium carbonate solution (2 mL, 75 g/L) was added, and the mixture was kept at room temperature for 2 h. The absorbance at 760 nm was recorded. The results were presented as mg gallic acid equivalent per gram DW (mg GAE/g DW).
The TFC was determined by using a slightly modified version of a published aluminum chloride colorimetric method[28]. Specifically, ethanol (1.5 mL, 95%, v/v), aluminum chloride (0.1 mL, 10%, w/v), potassium acetate (0.1 mL, 1 mol/L), and water (2.8 mL) were added to the diluted sample (0.5 mL), and the mixture was kept at room temperature for 30 min. Absorbance was recorded at 415 nm. The results were expressed in terms of mg quercetin equivalent per gram DW (mg QE/g DW).
Statistical analysis
All data were expressed as the mean ± standard deviation. IBM SPSS Statistics 20.0 (SPSS Inc., Chicago, IL, United States) was used for statistical analysis. Analysis of variance and the least significant difference test were applied to examine the differences in means. Systematic cluster analysis with online analytical processing was also performed. For all tests, P < 0.05 and P < 0.01 were defined as two levels of statistical significance. The statistical methods of this study were reviewed by Chan-Juan Zhao from Department of Bio-statistics, School of Public Health, Hainan Medical University, Hainan Province, China.
RESULTS
Effects of tested foods and herbs on CCl4-induced injury
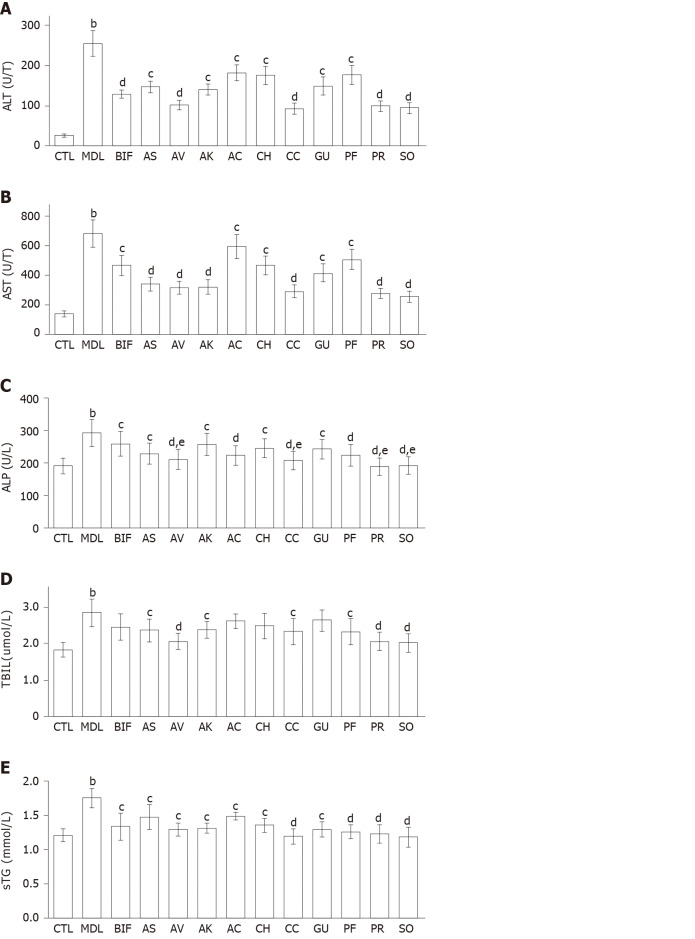
Effects on serum biomarkers: Compared with the control group, significantly increased serum levels of ALT, AST, ALP, TBIL, and TG were observed in the model group (all P < 0.01), indicating that liver injury was successfully induced by CCl4 (Figure 1). Bifendate is often used to lower the levels of serum transaminases in the treatment of hepatitis[21,29]. Compared with the model group, bifendate significantly decreased ALT, AST, ALP, and TG, and moderately reduced TBIL. As Figure 1A and 1B showed, each herb significantly decreased ALT and AST levels (P < 0.05 or P < 0.01). Notably, four herbs (A. villosum, C. chinensis, P. lobata root, and S. officinalis) were more effective in reducing both ALT and AST (each P < 0.01). Compared with the model group, each herb significantly reduced ALP, with the lowest values found after treatment with P. lobata root and S. officinalis (Figure 1C). Moreover, four herbs (A. villosum, C. chinensis, P. lobata root, and S. officinalis) exhibited better ALP-lowering effects than bifendate (each P < 0.05). Furthermore, A. senticosus, A. villosum, A. kravanh, C. chinensis, P. lobata flower, P. lobata root, and S. officinalis significantly decreased TBIL compared with the model group (Figure 1D). Specifically, better performance was found for S. officinalis, P. lobata root, and A. villosum (each P < 0.01). In addition, A. capillaris, C. heracleifolia, and G. uralensis, only slightly decreased TBIL. All herbs remarkably reduced TG compared with the model group (P < 0.05 or P < 0.01) (Figure 1E).
Figure 1.
Effects of ten herbs on serum biomarkers (n = 8). A: Alanine transaminase ; B: Aspartate transaminase; C: Alkaline phosphatase; D: Total bilirubin; E: Triglyceride. The values are presented as the mean ± standard deviation. aP < 0.05, bP < 0.01 vs control; cP < 0.05, dP < 0.01 vs model; eP < 0.05, fP < 0.01 vs bifendate. ALT: Alanine transaminase; AST: Aspartate transaminase; ALP: Alkaline phosphatase; TBIL: Total bilirubin; TG: Triglyceride; CTL: Control; MDL: Model; BIF: Bifendate; AS: Acanthopanax senticosus; AV: Amomum villosum; AK: Amomum kravanh; AC: Artemisia capillaris; CH: Cimicifuga heracleifolia; CC: Coptis chinensis; GU: Glycyrrhiza uralensis; PF: Pueraria lobata flower; PR: Pueraria lobata root; SO: Sanguisorba officinalis.
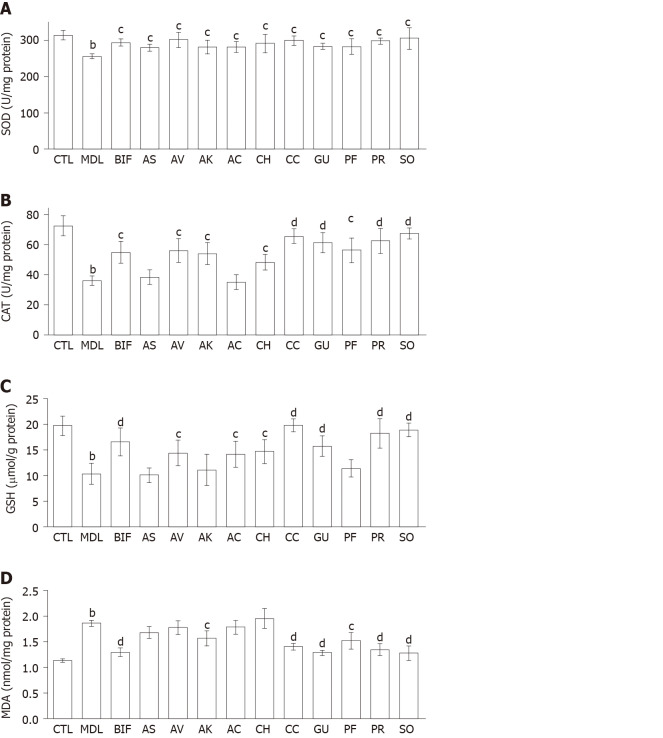
Effects of herbs on hepatic antioxidant enzymes, GSH, and lipid peroxidation in the liver: As shown in Figure 2, a significant decrease was observed in SOD and CAT activities and GSH level in the CCl4 model group (each P < 0.01) as well as a significant increase in the MDA level (P < 0.01), indicating that oxidative liver injury had been successfully induced. Bifendate and all herbs restored SOD activity compared with the model group (each P < 0.05) (Figure 2A). In addition, each herb significantly increased CAT activity (P < 0.05 or P < 0.01), except for A. senticosus and A. capillaris (Figure 2B). Moreover, compared with the model group, significant increases in GSH were found in the S. officinalis, P. lobata root, C. chinensis, G. uralensis, A. capillaris, C. heracleifolia, and A. villosum groups and moderate elevations of GSH in the A. kravanh, P. lobata flower, and A. senticosus group (Figure 2C). Overall, S. officinalis, P. lobata root, and C. chinensis were more effective than the other herbs in restoring the hepatic antioxidant activity in CCl4-induced liver injury. As shown in Figure 2D, only S. officinalis, G. uralensis, P. lobata root, C. chinensis, P. lobata flower, and A. kravanh ameliorated the increase in MDA. S. officinalis, P. lobata root, C. chinensis and G. uralensis showed better lipid peroxidation-reducing effects than the other herbs (each P < 0.01).
Figure 2.
Effects of ten herbs on hepatic antioxidant enzymes, glutathione, and malondialdehyde (n = 8). A: Superoxide dismutase; B: Catalase; C: Glutathione; D: Malondialdehyde. The values are presented as the mean ± standard deviation. aP < 0.05, bP < 0.01 vs control; cP < 0.05, dP < 0.01 vs model; eP < 0.05, fP < 0.01 vs bifendate. SOD: Superoxide dismutase; CAT: Catalase; GSH: Glutathione; MDA: Malondialdehyde; CTL: Control; MDL: Model; BIF: Bifendate; AS: Acanthopanax senticosus; AV: Amomum villosum; AK: Amomum kravanh; AC: Artemisia capillaris; CH: Cimicifuga heracleifolia; CC: Coptis chinensis; GU: Glycyrrhiza uralensis; PF: Pueraria lobata flower; PR: Pueraria lobata root; SO: Sanguisorba officinalis.
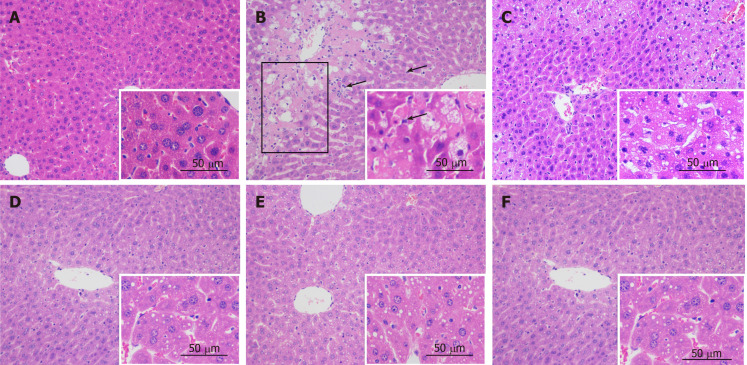
Histopathological analysis: No visible histological abnormalities were found in the control group (Figure 3A). The liver pathology of the CCl4 model showed necrosis and ballooning degeneration in the perivenular zone as a result of severe cell injury, obvious massive inflammatory cells, and the accumulation of lipid droplets in hepatocytes (Figure 3B). These findings indicated the occurrence of CCl4-induced liver injury. Meanwhile, bifendate and the ten tested materials ameliorated the morphological changes by mitigating necrosis, attenuating inflammatory cell infiltration, and resulting in lower lipid droplet accumulation, as shown below, for C. chinensis, P. lobata root, and S. officinalis, respectively (Figure 3D-F).
Figure 3.
Histopathological findings showing the effects of ten tested materials on carbon tetrachloride-induced liver injury (200 × and 400 × magnification) (n = 8). A: Control; B: carbon tetrachloride (CCl4) model; C: Bifendate + CCl4; D: Coptis chinensis + CCl4; E: Pueraria lobata root + CCl4; F: Sanguisorba officinalis + CCl4. Scale bar, 50 μm in 400×; Box, necrotic area; arrow, inflammatory cell. CCl4: Carbon tetrachloride.
Antioxidant activities, TPC, and TFC of tested herbs
Antioxidant activities of herbs: The FRAP values of the ten medicinal foods and herbs ranged from 29.80 ± 0.29 to 1141.88 ± 81.16 μmol Fe2+/g DW, and the TEAC values varied from 37.34 ± 1.02 to 1554.48 ± 68.58 μmol TE/g DW (Table 1). The three highest FRAP values (in decreasing order) were found in S. officinalis (1141.88 ± 81.16 μmol Fe2+/g DW), C. chinensis (557.04 ± 4.73 μmol Fe2+/g DW), and P. lobata root (554.38 ± 3.92 μmol Fe2+/g DW). The lowest two were found in G. uralensis and A. kravanh (29.85 ± 2.10 and 29.80 ± 0.29 μmol Fe2+/g DW, respectively). Meanwhile, the top three TEAC values were found in S. officinalis (1554.48 ± 68.58 μmol TE/g DW), P. lobata root (454.06 ± 8.14 μmol TE/g DW), C. chinensis (450.36 ± 27.23 μmol TE/g DW), and the lowest two were also found in G. uralensis and A. kravanh (53.09 ± 2.59 and 37.34 ± 1.02 μmol TE/g DW, respectively).
Table 1.
Ferric-reducing antioxidant power, Trolox equivalent antioxidant capacity, total phenolic content, and total flavonoid content values of ten medicinal herbs (n = 8)
| Scientific name of original medicinal plant | Parts with medicinal properties | FRAP value | TEAC value | TPC value | TFC value |
| (μmol Fe2+/g DW) | (μmol TE/g DW) | (mg GAE/g DW) | (mg QE/g DW) | ||
| Acanthopanax senticosus (Rupr. et Maxim.) Harms | Root and rhizome | 53.07 ± 0.23e | 54.70 ± 3.73d | 2.05 ± 0.16d | 0.44 ± 0.01e |
| Amomum villosum Lour. | Fruit | 219.85 ± 14.44c | 210.18 ± 23.88c | 18.42 ± 0.25c | 1.98 ± 0.07c,d |
| Amomum kravanh Pierre ex Gagnep. | Fruit | 29.80 ± 0.29e | 37.34 ± 1.02d | 18.49 ± 0.70c | 0.32 ± 0.01e |
| Artemisia capillaris Thunb. | Herb | 146.71 ± 2.91d | 208.16 ± 1.42c | 19.06 ± 0.21c | 1.55 ± 0.11d |
| Cimicifuga heracleifolia Kom. | Rhizome | 191.25 ± 6.38c,d | 214.60 ± 25.17c | 17.07 ± 0.28c | 0.40 ± 0.06e |
| Coptis chinensis Franch. | Rhizome | 557.04 ± 4.73b | 450.36 ± 27.23b | 50.15 ± 1.14b | 7.39 ± 0.39b |
| Glycyrrhiza uralensis Fisch. | Root and rhizome | 29.85 ± 2.10e | 53.09 ± 2.59d | 5.70 ± 0.04d | 3.71 ± 0.19c |
| Pueraria lobata (Willd.) Ohwi | Flower | 216.53 ± 11.34c | 213.25 ± 9.65c | 14.18 ± 0.11c | 2.86 ± 0.35c |
| Pueraria lobata (Willd.) Ohwi | Root | 554.38 ± 3.92b | 454.06 ± 8.14b | 45.06 ± 0.07b | 6.84 ± 0.08b |
| Sanguisorba officinalis L. | Root | 1141.88 ± 81.16a | 1554.48 ± 68.58a | 91.59 ± 0.00a | 14.93 ± 0.24a |
a,b,c,d,eP < 0.05, different superscript letters indicate statistical significance. DW: dried weight; FRAP: ferric-reducing antioxidant power; GAE: gallic acid equivalent; QE: quercetin equivalent; TE: Trolox equivalent; TEAC: Trolox equivalent antioxidant capacity; TFC: total flavonoid content; TPC: total phenolic content.
TPC and TFC of herbs: The TPC and TFC of the ten materials were determined (Table 1). The TPC values ranged from 2.05 ± 0.16 to 91.59 ± 0.00 mg GAE/g DW, which was a difference of more than 40-fold. The three highest TPC values were found for S. officinalis (91.59 ± 0.00 mg GAE/g DW), C. chinensis (50.15 ± 1.14 mg GAE/g DW), and P. lobata root (45.06 ± 0.07 mg GAE/g DW). The lowest TPC values were found for G. uralensis and A. senticosus; both were below 10 mg GAE/g DW. The highest three TFC values were found for S. officinalis (14.93 ± 0.24 mg QE/g DW), C. chinensis (7.39 ± 0.39 mg QE/g DW), and P. lobata root (6.84 ± 0.08 mg QE/g DW); in contrast, the lowest three TFC values were found in A. senticosus, C. heracleifolia, and A. kravanh, all of which were below 1 mg QE/g DW. Our results revealed that S. officinalis showed the strongest in vitro antioxidant activities, possibly because it contained the highest contents of phenols and flavonoids.
Correlations between FRAP, TEAC, TPC, and TFC values: Correlation analyses were performed to identify the strength of relationships between FRAP, TEAC, TPC, and TFC values (Table 2). A strong correlation between FRAP and TEAC (R2 = 0.9340) indicated that the herbs possessed the ability to reduce Fe3+ to Fe2+ and to scavenge ABTS•+. In addition, the FRAP and TEAC values were both found to correlate with the TPC values (R2 = 0.9600 for FRAP and TPC; R2 = 0.9013 for TEAC and TPC), implying that the phenolic components contributed to the Fe3+-reducing and ABTS•+-scavenging activities[30]. Moreover, a correlation between the TPC and TFC values (R2 = 0.8829) suggested that flavonoids may be the major phenolic compounds but were not the only ones. Correlations were also found between FRAP/TEAC and TFC (R2 = 0.9139 for FRAP and TFC; R2 = 0.8729 for TEAC and TFC). In summary, the antioxidants in the tested medicinal herbs were able to both reduce oxidants (e.g., Fe3+) and scavenge free radicals (e.g., ABTS•+), which was largely dependent on their TPC.
Table 2.
Correlation analysis between ferric-reducing antioxidant power, Trolox equivalent antioxidant capacity, total phenolic content, and total flavonoid content values
| Correlation Coefficient (R2) | FRAP value (μmol Fe2+/g DW) | TEAC value (μmol TE/g DW) | TPC value (mg GAE/g DW) | TFC value (mg QE/g DW) |
| FRAP value | 1 | 0.9340 | 0.9600 | 0.9139 |
| TEAC value | - | 1 | 0.9013 | 0.8729 |
| TPC value | - | - | 1 | 0.8829 |
| TFC value | - | - | - | 1 |
DW: Dried weight; FRAP: Ferric-reducing antioxidant power; GAE: Gallic acid equivalent; TE: Trolox equivalent; TEAC: Trolox equivalent antioxidant capacity; TFC: Total flavonoid content; TPC: Total phenolic content; QE: quercetin equivalent.
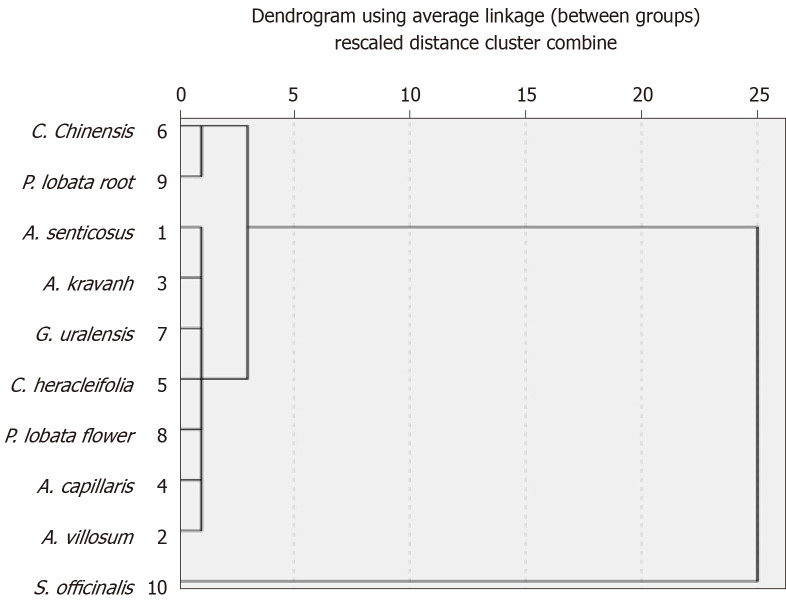
Relationship between in vitro antioxidant activity, effects on liver injury and in vivo antioxidant activity: To investigate the relationship between in vivo antioxidant activity of the tested herbs, their effects on liver injury, and in vitro antioxidant activity, systematic cluster analyses were performed (range of solutions, 2–6 cluster numbers) for clinical indicators of CCl4-induced liver injury and the values of FRAP, TEAC, and TPC (Table 3 and Figure 4). Then, a cluster number of 3 was used for online analytical processing and analysis of variance. With the exception of seven herbs in Cluster 1, Cluster 2 included P. lobata root and C. chinensis, and Cluster 3 contained S. officinalis. Herbs in Clusters 2 and 3 (P. lobata root, C. chinensis, and S. officinalis) showed stronger hepatoprotective effects and in vivo antioxidant activity, and they exhibited stronger in vitro antioxidant activities, together with higher TPC and TFC.
Table 3.
Online analytical processing cubes based on systematic cluster analysis for indicators of CCl4-induced liver injury, antioxidant activity, total phenolic content, and total flavonoid content (cluster number = 3)
| ALT | AST | ALP | TBIL | sTG | SOD | CAT | GSH | MDA | FRAP | TEAC | TPC | TFC | ||
| Clt 1 | Sum | 1080.59 | 3017.02 | 1640.70 | 16.89 | 9.48 | 1997.54 | 349.62 | 91.24 | 11.58 | 887.06 | 991.32 | 94.97 | 11.26 |
| AV | n | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| AS | mean | 154.37 | 431.00 | 234.39 | 2.41 | 1.35 | 285.36 | 49.95 | 13.03 | 1.65 | 126.72 | 141.62 | 13.57 | 1.61 |
| AK | SD | 27.92 | 82.89 | 15.86 | 0.20 | 0.09 | 7.67 | 9.92 | 2.17 | 0.22 | 87.09 | 87.42 | 6.89 | 1.33 |
| GU | % of TS | 78.9% | 78.6% | 73.5% | 72.5% | 72.0% | 68.9% | 64.1% | 61.6% | 74.2% | 28.2% | 28.7% | 33.7% | 27.9% |
| AC | % of TN | 70.0% | 70.0% | 70.0% | 70.0% | 70.0% | 70.0% | 70.0% | 70.0% | 70.0% | 70.0% | 70.0% | 70.0% | 70.0% |
| CH | ||||||||||||||
| PF | ||||||||||||||
| Clt 2 | Sum | 193.77 | 567.29 | 398.53 | 4.39 | 2.46 | 595.96 | 128.15 | 37.99 | 2.74 | 1111.42 | 904.42 | 95.21 | 14.23 |
| CC | n | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| PR | mean | 96.89 | 283.65 | 199.27 | 2.20 | 1.23 | 297.98 | 64.08 | 19.00 | 1.37 | 555.71 | 452.21 | 47.61 | 7.12 |
| SD | 4.79 | 9.30 | 13.09 | 0.19 | 0.06 | 1.27 | 2.10 | 1.10 | 0.04 | 1.88 | 2.62 | 3.60 | 0.39 | |
| % of TS | 14.1% | 14.8% | 17.9% | 18.8% | 18.7% | 20.6% | 23.5% | 25.7% | 17.6% | 35.4% | 26.2% | 33.8% | 35.2% | |
| % of TN | 20.0% | 20.0% | 20.0% | 20.0% | 20.0% | 20.0% | 20.0% | 20.0% | 20.0% | 20.0% | 20.0% | 20.0% | 20.0% | |
| Clt 3 | Sum | 95.34 | 256.05 | 192.78 | 2.03 | 1.23 | 304.69 | 67.44 | 18.85 | 1.28 | 1141.88 | 1554.48 | 91.59 | 14.93 |
| SO | n | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| mean | 95.34 | 256.05 | 192.78 | 2.03 | 1.23 | 304.69 | 67.44 | 18.85 | 1.28 | 1141.88 | 1554.48 | 91.59 | 14.93 | |
| SD | / | / | / | / | / | / | / | / | / | / | / | / | / | |
| % of TS | 7.0% | 6.7% | 8.6% | 8.7% | 9.3% | 10.5% | 12.4% | 12.7% | 8.2% | 36.4% | 45.1% | 32.5% | 36.9% | |
| % of TN | 10.0% | 10.0% | 10.0% | 10.0% | 10.0% | 10.0% | 10.0% | 10.0% | 10.0% | 10.0% | 10.0% | 10.0% | 10.0% | |
| Total | Sum | 1369.70 | 3840.36 | 2232.01 | 23.31 | 13.17 | 2898.19 | 545.21 | 148.08 | 15.60 | 3140.36 | 3450.22 | 281.77 | 40.42 |
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| mean | 136.97 | 384.04 | 223.20 | 2.33 | 1.32 | 289.82 | 54.52 | 14.81 | 1.56 | 314.04 | 345.02 | 28.18 | 4.04 | |
| SD | 36.16 | 101.81 | 22.67 | 0.22 | 0.10 | 9.71 | 11.01 | 3.38 | 0.23 | 348.53 | 449.84 | 27.02 | 4.59 | |
| % of TS | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | |
| % of TN | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | |
ALT: Alanine transaminase; AST: Aspartate transaminase; ALP: Alkaline phosphatase; TBIL: Total bilirubin; sTG: Serum triglycerides; SOD: Superoxide dismutase; CAT: Catalase; GSH: Glutathione; MDA: Malondialdehyde; FRAP: Ferric-reducing antioxidant power; TEAC: Trolox equivalent antioxidant capacity; TPC: Total phenolic content; TFC: Total flavonoid content; Clt: Cluster; SD: Standard deviation; TN: Total number; TS: Total sum; AS: Acanthopanax senticosus; AV: Amomum villosum; AK: Amomum kravanh; AC: Artemisia capillaris; CH: Cimicifuga heracleifolia; CC: Coptis chinensis; GU: Glycyrrhiza uralensis; PF: Pueraria lobata flower; PR: Pueraria lobata root; SO: Sanguisorba officinalis.
Figure 4.
Dendrogram using average linkage (between groups) from systematic cluster analysis of ten medicinal herbs.
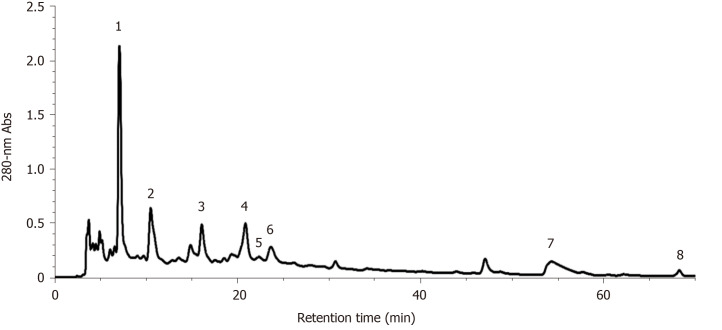
Identification and quantification of phytochemical compounds in S. officinalis
As S. officinalis showed the strongest hepatoprotective effect against CCl4-induced liver injury compared with the other tested medicinal foods and herbs, its phytochemical compounds in the decoction were identified by HPLC-ESI-ITMS/MS using external standards and/or according to MS and MS/MS information found in references[31-33]. As shown in Figure 5, eight main peaks were observed on the HPLC chromatogram; these corresponded to nine main compounds in the S. officinalis decoction (Table 4). The four major peaks (Peak 1-4) were identified as gallic acid (Peak 1), galloyl-methylglucoside, procyanidin C2 (Peak 2), (+)-catechin (Peak 3), and procyanidin B3 3-O-gallate (Peak 4)[33]. In addition, the quantification of unambiguously identified compounds was performed by HPLC using corresponding standards; i.e., gallic acid (Peak 1) and (+)-catechin (Peak 3) were quantified as 7.86 ± 0.45 and 8.19 ± 0.57 mg/g DW, respectively.
Figure 5.
Reverse-phase high-performance liquid chromatography analysis of Sanguisorba officinalis decoction.
Table 4.
Identification of the main phytochemical components in Sanguisorba officinalis decoction using high-performance liquid chromatography-electrospray ionization source-ion trap tandem mass spectrometry (positive or negative mode)
| Peak | RT (min) | λmax (nm) | [M+H]+ | [M-H]- | MS/MS fragments | MW | Putative compound | Ref. |
| 1 | 7.10 | 270 | / | 169 | 125 | 170 | Gallic acid1 | [33] |
| 2 | 10.52 | 275 | / | 345; 865 | 313, 169, 151, 125; 739, 695, 577, 543, 407, 287 | 346; 866 | Galloyl-methylglucoside; Procyanidin C2 | [33] |
| 3 | 16.10 | 278 | 291 | / | 273, 165, 151, 139, 123 | 290 | (+)-Catechin1 | [33] |
| 4 | 20.87 | 275 | / | 729 | 577, 559, 407 | 730 | Procyanidin B3 3-O-gallate | [33] |
| 5 | 22.35 | 274 | 563 | / | 545, 423, 411, 435, 393, 271 | 562 | Fisetinidol-(4α/β→8)-(+)-catechin | [33] |
| 6 | 23.65 | 272 | / | 1103 | 1059, 935, 633, 469 | 1104 | Sanguiin H-2 | [31] |
| 7 | 54.30 | 264/362 | / | 277 | 197, 182, 111 | 278 | Methoxygallic acid methyl ester 5-O-sulfate | [32] |
| 8 | 68.30 | 243/372 | 345 | / | 330, 313 | 344 | 3,3’,4’-O-trimethylellagic acid | [33] |
DISCUSSION
AST and ALT are distributed predominantly in the liver cells. When the liver is damaged, they enter the bloodstream. Clinically, elevations in serum ALT and AST can be regarded as indicators of liver diseases, although ALT is more specific than AST[21]. In our study, all the herbs significantly decreased ALT and AST levels showing their hepatoprotection, with more effectiveness found for A. villosum, C. chinensis, P. lobata root, and S. officinalis. Clinically, increased ALP often indicates cholestasis caused by liver injury[34,35]. The ALP-lowering effects were also found for each herb. TBIL is also a sensitive indicator of bilirubin metabolic disorders, which are often increased in acute liver injury[36-38]. A. senticosus, A. villosum, A. kravanh, C. chinensis, P. lobata flower, P. lobata root, and S. officinalis significantly decreased TBIL compared with the model group, particularly S. officinalis, P. lobata root, and A. villosum. Serum TG is another biomarker of liver dysfunction and is found to be elevated if the liver is damaged. In this study, all herbs reduced TG significantly compared with the model group. Histopathological examinations confirmed the hepatoprotective effects of the ten tested medicinal foods and herbs by improving the degenerative morphological changes induced by CCl4 (Figure 3).
Many chemicals can cause liver injury, and the mechanisms of action involved in CCl4-induced liver disease have been investigated widely[4]. Briefly, CCl4 is metabolized in the liver by cytochrome P450 enzymes, biotransformed into ∙CCl3, and then oxygenated to ∙OOCCl3; both of these radicals are highly reactive and can induce the depletion of reductants, inhibit antioxidant enzymes, induce lipid peroxidation, hypomethylate proteins, and mutate nucleic acids, resulting in oxidative stress, inflammation, apoptosis, and necrosis[23].
Whether oxidative injury occurs is dependent on the outcome of the interaction between oxidants and the protective system. In the body, there is a complex defense system consisting of antioxidant enzymes that can protect against oxidative damage, such as SOD, CAT, and glutathione peroxidase and some nonenzymatic antioxidants, including GSH, vitamins, and ubiquinone, that can also help to maintain the redox balance[39]. The metabolism of CCl4 results in the accumulation of reactive oxygen species, mainly O2– and H2O2, which can be scavenged by SOD and CAT, respectively[40]. The depletion of reduced endogenous antioxidants (such as GSH) can therefore increase the sensitivity of hepatocytes to oxidative stress[41]. Therefore, the activities of SOD and CAT as well as levels of GSH, are often used to evaluate in vivo antioxidant activity[30,42]. Free radicals are often highly reactive, and they can quickly bind to other molecules or atoms to form reactive metabolites, leading to lipid peroxidation, which results in elevated MDA[11]. Thus, MDA is often used to evaluate lipid peroxidation status[30,42].
Compared with the model group, all herbs restored SOD activity, and each herb significantly increased CAT activity, except for A. senticosus and A. capillarys. Significant increases in GSH were also found in the S. officinalis, P. lobata root, C. chinensis, G. uralensis, A. capillaris, C. heracleifolia, and A. villosum groups. Additionally, S. officinalis, G. uralensis, P. lobata root, C. chinensis, P. lobata flower, and A. kravanh ameliorated the increase in MDA. Overall, S. officinalis, P. lobata root, and C. chinensis were more effective than the other herbs in restoring the hepatic antioxidant activity and reducing lipid peroxidation in CCl4-induced liver injury.
The chemical composition of most natural products is complex. Moreover, the antioxidant activities of medicinal herbs are often multifunctional and may be influenced by various factors; as such, more than one method is preferred to evaluate the antioxidant activity of natural products[43,44]. In this study, the antioxidant activities of selected medicinal herbs were determined by using the FRAP assay and the TEAC assay. The former indicates the antioxidant activity that is dependent on the capacity to reduce [Fe (TPTZ)2]3+ to [Fe (TPTZ)2]2+[25], whereas the latter is based on the ability to scavenge ABTS•+[24,44].
The FRAP and TEAC values of the ten medicinal foods and herbs ranged with a nearly 40-fold difference, both of which S. officinalis ranked first, and C. chinensis and P. lobata root were the second or the third. Similar results were found for TPC and TFC. Because bioactive components, especially phenols and flavonoids, are the main contributors to the antioxidant activities of natural products[17,45], our results revealed that S. officinalis showed the strongest in vitro antioxidant activities, which was possibly because it contained the highest contents of phenols and flavonoids. The correlations between FRAP, TEAC, TPC, and TFC values indicated that the antioxidants in the tested medicinal herbs possessed both oxidant-reducing and free radical-scavenging capability, mainly according to their TPC. Moreover, relationship between in vitro antioxidant activity, effects on liver injury, and in vivo antioxidant activity revealed that P. lobata root, C. chinensis, and S. officinalis performed better hepatoprotection with in vivo antioxidant activity; meanwhile, they also showed stronger in vitro antioxidant activities containing higher TPC and TFC.
As S. officinalis showed the best performance in protecting the liver from CCl4-induced injury among the tested herbs, the identification and quantification of phytochemical compounds in its decoction was conducted. Nine main compounds were identified, and (+)-catechin and gallic acid were also quantified. According to the Chinese Pharmacopeia, the gallic acid content was a crucial criterion to evaluate the quality of S. officinalis, and it should be no less than 0.60%[46]. The gallic acid content quantified in our study was in this desirable range. The phytochemical compounds identified in our study, such as gallic acid and catechin, have been regarded as strong antioxidants that can confer health benefits and may contribute to the hepatoprotective activity[47-49].
S. officinalis has a cucumber-like flavor, and many parts are edible. For example, the young leaves and flower buds are often used in salads, the fresh or dried leaves are used to make tea, and the root can be cooked as a constituent in porridge or soups. It is an alpine plant that grows at high elevation and is adapted to harsh conditions, such as low temperatures, strong ultraviolet radiation, and dryness, which result in the altitude-dependent accumulation of antioxidants[50]. The phytochemical compounds identified in our study, such as gallic acid and catechin, have been regarded as strong antioxidants that can provide health benefits and may contribute to the hepatoprotective actions and in vitro antioxidant activities[47-49].
CONCLUSION
In conclusion, the results of our study indicated that all ten medicinal foods and herbs could individually protect against CCl4-induced oxidative liver injury, and S. officinalis L., C. chinensis Franch., and P. lobata (Willd.) Ohwi root were more effective than the others. In addition, S. officinalis L. exhibited the strongest hepatoprotective effect, and nine components of its decoction were identified, among which gallic acid and (+)-catechin were quantified. It was also revealed that the tested materials exhibited various in vitro antioxidant activities, proportionate to their phenolic content and that the highest values were found in S. officinalis L. The results of this study are valuable for the selection of more effective natural products to be consumed directly or developed into nutraceuticals or therapeutics for the prevention and treatment of oxidative stress-related diseases.
ARTICLE HIGHLIGHTS
Research background
Many natural products confer health benefits against diverse diseases through their antioxidant activities. Carbon tetrachloride (CCl4) is often used in animal experiments to study the effects of substances on liver injury and the related mechanisms of action, among which oxidative stress is a major pathogenic factor.
Research motivation
The antioxidant activities of ten herbs were evaluated both in vitro and in vivo. Their hepatoprotective effects were also evaluated in order to elect more effective natural products for direct consumption and the development of nutraceuticals or therapeutics to manage oxidative stress-related diseases.
Research objectives
To compare antioxidant and hepatoprotective activities of ten herbs and identify and quantify phytochemicals for the one with strongest hepatoprotection, which could be helpful in the prevention and treatment of oxidative liver injury.
Research methods
The antioxidant activity of ten medicinal herbs was determined by both ferric-reducing antioxidant power and Trolox equivalent antioxidant capacity assays. The total phenolic and flavonoid contents were determined by Folin–Ciocalteu method and aluminum chloride colorimetry, respectively. Their effects on CCl4-induced oxidative liver injury were evaluated and compared in a mouse model by administrating each water extract [(0.15 g/mL, 10 mL/kg) once per day] for seven consecutive days and a dose of CCl4 solution in olive oil (8%, v/v, 10 mL/kg). The herb with the strongest hepatoprotective performance was analyzed for the detailed bioactive components by using high-performance liquid chromatography-electrospray ionization source-ion trap tandem mass spectrometry.
Research results
The results revealed that all tested herbs attenuated CCl4-induced oxidative liver injury; each resulted in significant decreases in levels of serum alanine transaminase, aspartate transaminase, alkaline phosphatase, and triacylglycerols. In addition, most herbs restored hepatic superoxide dismutase and catalase activities, glutathione levels, and reduced malondialdehyde levels. Sanguisorba officinalis (S. officinalis) L., Coptis chinensis Franch., and Pueraria lobata (Willd.) Ohwi root were the three most effective herbs, and S. officinalis L. exhibited the strongest hepatoprotective effect. Nine active components were identified in S. officinalis, and gallic acid and (+)-catechin were quantified (7.86 ± 0.45 mg/g and 8.19 ± 0.57 mg/g dried weight, respectively). Furthermore, the tested herbs displayed a range of in vitro antioxidant activities proportional to their phenolic content; the strongest activities were also found for S. officinalis L.
Research conclusions
The results of this study indicated that all ten medicinal foods and herbs could individually protect against CCl4-induced oxidative liver injury, and S. officinalis L., C. chinensis Franch., and P. lobata (Willd.) Ohwi root were more effective than the others. In addition, S. officinalis L. exhibited the strongest hepatoprotective effect, and nine components of its decoction were identified, among which gallic acid and (+)-catechin were quantified. It was also revealed that the tested materials exhibited various in vitro antioxidant activities proportionate to their phenolic content, and that the highest values were found in S. officinalis L. The results are valuable for the selection of more effective natural products to be consumed directly or developed into nutraceuticals or therapeutics for the prevention and treatment of oxidative stress-related diseases.
Research perspectives
This study is of value to assist the selection of more effective natural products for direct consumption and the development of nutraceuticals or therapeutics to manage oxidative stress-related diseases. In the future, methods to identify and quantify the phytochemical compounds in medicinal herbs must be investigated more comprehensively. The bioavailability, desirable dose range, and side effects should also be clarified.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Ms Ping-Ping Wu for her assistance in raising animals.
Footnotes
Institutional animal care and use committee statement: All experimental procedures were performed based on the approval of Animal Ethics Committee of School of Public Health, Sun Yat-Sen University (No. 2017-011).
Conflict-of-interest statement: The authors declare no conflict of interest.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Manuscript source: Unsolicited manuscript
Corresponding Author’s Membership in Professional Societies: UK Nutrition Society, 54434; Chinese Nutrition Society, M102918830M.
Peer-review started: June 12, 2020
First decision: July 25, 2020
Article in press: September 9, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Boeckxstaens G, Ryan E S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
Contributor Information
Xiao Meng, Department of Nutrition, School of Public Health, Sun Yat-Sen University, Guangzhou 510080, Guangdong Province, China. mengx7@mail2.sysu.edu.cn.
Guo-Yi Tang, Department of Nutrition, School of Public Health, Sun Yat-Sen University, Guangzhou 510080, Guangdong Province, China.
Pin-He Liu, Department of Nutrition, School of Public Health, Sun Yat-Sen University, Guangzhou 510080, Guangdong Province, China.
Chan-Juan Zhao, Department of Bio-statistics, School of Public Health, Hainan Medical University, Haikou 571199, Hainan Province, China.
Qing Liu, Department of Nutrition, School of Public Health, Sun Yat-Sen University, Guangzhou 510080, Guangdong Province, China.
Hua-Bin Li, Department of Nutrition, School of Public Health, Sun Yat-Sen University, Guangzhou 510080, Guangdong Province, China.
Data sharing statement
No additional data are available.
References
- 1.Bardaweel SK, Gul M, Alzweiri M, Ishaqat A, ALSalamat HA, Bashatwah RM. Reactive Oxygen Species: the Dual Role in Physiological and Pathological Conditions of the Human Body. Eurasian J Med. 2018;50:193–201. doi: 10.5152/eurasianjmed.2018.17397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenberg D, Pinchuk I. Oxidative stress, the term and the concept. Biochem Biophys Res Commun. 2015;461:441–444. doi: 10.1016/j.bbrc.2015.04.062. [DOI] [PubMed] [Google Scholar]
- 4.Meng X, Li Y, Li S, Gan RY, Li HB. Natural products for prevention and treatment of chemical-induced liver injuries. Compr Rev Food Sci Food Saf. 2018;17:472–495. doi: 10.1111/1541-4337.12335. [DOI] [PubMed] [Google Scholar]
- 5.Ke Y, Xu C, Lin J, Li Y. Role of Hepatokines in Non-alcoholic Fatty Liver Disease. J Transl Int Med. 2019;7:143–148. doi: 10.2478/jtim-2019-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li C, Qu L, Farragher C, Vella A, Zhou B. MicroRNA Regulated Macrophage Activation in Obesity. J Transl Int Med. 2019;7:46–52. doi: 10.2478/jtim-2019-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahn M, Park JS, Chae S, Kim S, Moon C, Hyun JW, Shin T. Hepatoprotective effects of Lycium chinense Miller fruit and its constituent betaine in CCl4-induced hepatic damage in rats. Acta Histochem. 2014;116:1104–1112. doi: 10.1016/j.acthis.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Peng WH, Chen YW, Lee MS, Chang WT, Tsai JC, Lin YC, Lin MK. Hepatoprotective Effect of Cuscuta campestris Yunck. Whole Plant on Carbon Tetrachloride Induced Chronic Liver Injury in Mice. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17122056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slater TF, Cheeseman KH, Ingold KU. Carbon tetrachloride toxicity as a model for studying free-radical mediated liver injury. Philos Trans R Soc Lond B Biol Sci. 1985;311:633–645. doi: 10.1098/rstb.1985.0169. [DOI] [PubMed] [Google Scholar]
- 10.Clawson GA. Mechanisms of carbon tetrachloride hepatotoxicity. Pathol Immunopathol Res. 1989;8:104–112. doi: 10.1159/000157141. [DOI] [PubMed] [Google Scholar]
- 11.Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015;12:231–242. doi: 10.1038/nrgastro.2015.35. [DOI] [PubMed] [Google Scholar]
- 12.Cao SY, Li Y, Meng X, Zhao CN, Li S, Gan RY, Li HB. Dietary natural products and lung cancer: Effects and mechanisms of action. J Funct Foods. 2019;52:316–331. [Google Scholar]
- 13.Tang GY, Meng X, Li Y, Zhao CN, Liu Q, Li HB. Effects of Vegetables on Cardiovascular Diseases and Related Mechanisms. Nutrients. 2017;9 doi: 10.3390/nu9080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding RB, Tian K, Huang LL, He CW, Jiang Y, Wang YT, Wan JB. Herbal medicines for the prevention of alcoholic liver disease: a review. J Ethnopharmacol. 2012;144:457–465. doi: 10.1016/j.jep.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 15.Song G, Zhang C. Statistical analysis of the drugs used in the ancient anti-alcohol prescriptions. Shizhen Guoyi Guoyao. 2009;20:216–217. [Google Scholar]
- 16.Huang GJ, Deng JS, Huang SS, Shao YY, Chen CC, Kuo YH. Protective effect of antrosterol from Antrodia camphorata submerged whole broth against carbon tetrachloride-induced acute liver injury in mice. Food Chem. 2012;132:709–716. [Google Scholar]
- 17.Fu L, Xu BT, Xu XR, Gan RY, Zhang Y, Xia EQ, Li HB. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011;129:345–350. doi: 10.1016/j.foodchem.2011.04.079. [DOI] [PubMed] [Google Scholar]
- 18.USFDA. Guidance for Industry: Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. 2005. [Accessed on 2 Jan 2020] . Available from: URL: https://www.fda.gov/media/72309/download. [Google Scholar]
- 19.China National Administration of Traditional Chinese Medicine. Hovenia dulcis seeds. 2015. [Accessed on 2 April 2020] Available from: https://baike.baidu.com/item/%E6%9E%B3%E6%A4%87%E5%AD%90/758262?fr=aladdin.
- 20.Walpole SC, Prieto-Merino D, Edwards P, Cleland J, Stevens G, Roberts I. The weight of nations: an estimation of adult human biomass. BMC Public Health. 2012;12:439. doi: 10.1186/1471-2458-12-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu SK, Zhang N, Shen XR, Mei WW, He Y, Ge WH. Preparation of total flavonoids from loquat flower and its protective effect on acute alcohol-induced liver injury in mice. J Food Drug Anal. 2015;23:136–143. doi: 10.1016/j.jfda.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borkham-Kamphorst E, van de Leur E, Zimmermann HW, Karlmark KR, Tihaa L, Haas U, Tacke F, Berger T, Mak TW, Weiskirchen R. Protective effects of lipocalin-2 (LCN2) in acute liver injury suggest a novel function in liver homeostasis. Biochim Biophys Acta. 2013;1832:660–673. doi: 10.1016/j.bbadis.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Scholten D, Trebicka J, Liedtke C, Weiskirchen R. The carbon tetrachloride model in mice. Lab Anim. 2015;49:4–11. doi: 10.1177/0023677215571192. [DOI] [PubMed] [Google Scholar]
- 24.Xu XY, Zheng J, Meng JM, Gan RY, Mao QQ, Shang A, Li BY, Wei XL, Li HB. Effects of Food Processing on In Vivo Antioxidant and Hepatoprotective Properties of Green Tea Extracts. Antioxidants (Basel) 2019;8 doi: 10.3390/antiox8120572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 26.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Froilan R, Hernandez-Ledesma B, Camara M, Perez-Rodriguez ML. Evaluation of the antioxidant potential of mixed fruit-based beverages: A new insight on the Folin-Ciocalteu method. Food Anal Meth. 2018;11:2897–2906. [Google Scholar]
- 28.Kalia K, Sharma K, Singh HP, Singh B. Effects of extraction methods on phenolic contents and antioxidant activity in aerial parts of Potentilla atrosanguinea Lodd. and quantification of its phenolic constituents by RP-HPLC. J Agric Food Chem. 2008;56:10129–10134. doi: 10.1021/jf802188b. [DOI] [PubMed] [Google Scholar]
- 29.Zeng Y, He YJ, He FY, Fan L, Zhou HH. Effect of bifendate on the pharmacokinetics of cyclosporine in relation to the CYP3A4*18B genotype in healthy subjects. Acta Pharmacol Sin. 2009;30:478–484. doi: 10.1038/aps.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q, Tang GY, Zhao CN, Gan RY, Li HB. Antioxidant Activities, Phenolic Profiles, and Organic Acid Contents of Fruit Vinegars. Antioxidants (Basel) 2019;8 doi: 10.3390/antiox8040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kool MM, Comeskey DJ, Cooney JM, McGhie TK. Structural identification of the main ellagitannins of a boysenberry (Rubus loganbaccus×baileyanus Britt.) extract by LC–ESI-MS/MS, MALDI-TOF-MS and NMR spectroscopy. Food Chem. 2010;119:1535–1543. [Google Scholar]
- 32.Li KP, Gao CK, Li WM. Analysis of vitexin and isorhamnetin-3-O-β-D-rutinoside by UPLC-ESI-Q-TOF-MS-MS. Zhongguo Zhongyao Zazhi. 2011;36:180–184. [PubMed] [Google Scholar]
- 33.Xu WT, Huo ZP, Lei L, Shi JW, Wang Y, He Y. Chemical constituent cluster of decoction of Sanguisorbae Radix by HPLC-IT-TOF/MS. Zhongcaoyao. 2018;49:1277–1288. [Google Scholar]
- 34.Mauro P, Renze B, Wouter W. Enzymes. In: Burtis CA, Ashwood ER, Bruns DE, editors. Tietz text book of clinical chemistry and molecular diagnostics. 6th ed. St Louis, Missouri, USA: Elsevier, 2006: 604-616. [Google Scholar]
- 35.Djiambou-Nganjeu H. Relationship Between Portal HTN and Cirrhosis as a Cause for Diabetes. J Transl Int Med. 2019;7:79–83. doi: 10.2478/jtim-2019-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koca TT. Clinical Significance of Serum Bilirubin in Behçet’s Disease. J Transl Int Med. 2018;6:185–188. doi: 10.2478/jtim-2018-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu X, Bai Z, Zhao Q, Li H, Shi Q, Deng J, Zhang J, Guo X, Qi X. Successful Pharmacotherapy for Multiple Acute Decompensation Events in a Cirrhotic Patient with Acute-on-chronic Liver Failure: A Case Report. J Transl Int Med. 2018;6:189–193. doi: 10.2478/jtim-2018-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pratt D. Sleisenger and Fordtran´s Gastrointestinal and Liver Disease: Pathophysiology/Diagnosis/Management. 10th ed. Philadelphia, USA: Elsevier Saunders, 2016. [Google Scholar]
- 39.Grasselli E, Compalati AD, Voci A, Vecchione G, Ragazzoni M, Gallo G, Borro P, Sumberaz A, Testino G, Vergani L. Altered oxidative stress/antioxidant status in blood of alcoholic subjects is associated with alcoholic liver disease. Drug Alcohol Depend. 2014;143:112–119. doi: 10.1016/j.drugalcdep.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 40.Cederbaum AI. Cytochrome P450 2E1-dependent oxidant stress and upregulation of anti-oxidant defense in liver cells. J Gastroenterol Hepatol. 2006;21 Suppl 3:S22–S25. doi: 10.1111/j.1440-1746.2006.04595.x. [DOI] [PubMed] [Google Scholar]
- 41.Hirano T, Kaplowitz N, Tsukamoto H, Kamimura S, Fernandez-Checa JC. Hepatic mitochondrial glutathione depletion and progression of experimental alcoholic liver disease in rats. Hepatology. 1992;16:1423–1427. doi: 10.1002/hep.1840160619. [DOI] [PubMed] [Google Scholar]
- 42.Zhao CN, Tang GY, Liu Q, Xu XY, Cao SY, Gan RY, Zhang KY, Meng SL, Li HB. Five-Golden-Flowers Tea: Green Extraction and Hepatoprotective Effect against Oxidative Damage. Molecules. 2018;23 doi: 10.3390/molecules23092216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 44.Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J. 2013;21:143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Liu YM, Qi ZM, Wang SY, Liu SX, Li X, Wang HJ, Xia XC. An overview on natural polysaccharides with antioxidant properties. Curr Med Chem. 2013;20:2899–2913. doi: 10.2174/0929867311320230006. [DOI] [PubMed] [Google Scholar]
- 46.Chinese Pharmacopoecia Commission. Sanguisorbae Radix. Zhongguo Yaodian. 2015:126–127. [Google Scholar]
- 47.Wang J, Tang L, White J, Fang J. Inhibitory effect of gallic acid on CCl4-mediated liver fibrosis in mice. Cell Biochem Biophys. 2014;69:21–26. doi: 10.1007/s12013-013-9761-y. [DOI] [PubMed] [Google Scholar]
- 48.Quan M, Li Q, Zhao P, Tian C. Chemical composition and hepatoprotective effect of free phenolic extract from barley during malting process. Sci Rep. 2018;8:4460. doi: 10.1038/s41598-018-22808-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Lu JF, Wen XY, Kan J, Jin CH. Antioxidant and protective effect of inulin and catechin grafted inulin against CCl4-induced liver injury. Int J Biol Macromol. 2015;72:1479–1484. doi: 10.1016/j.ijbiomac.2014.09.066. [DOI] [PubMed] [Google Scholar]
- 50.Wildi B, Lutz C. Antioxidant composition of selected high alpine plant species from different altitudes. Plant Cell Environ. 1996;19:138–146. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.