Abstract
Schistosomiasis, caused by helminth flatworms of the genus Schistosoma, is a neglected tropical disease that afflicts over 230 million people worldwide. Currently, treatment is achieved with only one drug, praziquantel (PZQ). In this regard, the roots of Solidago microglossa (Asteraceae) and Aristolochia cymbifera (Aristolochiaceae) are popularly used as anthelmintic. Despite their medicinal use against helminthiasis, such as schistosomiasis, A. cymbifera, and S. microglossa have not been evaluated against S. mansoni. Then, in this work, the in vitro antischistosomal activity of the crude extracts of A. cymbifera (Ac) and S. microglossa (Sm) and their isolated compounds were investigated against S. mansoni adult worms. Sm (200 μg/mL) and Ac (100–200 μg/mL) were lethal to all male and female worms at the 24 h incubation. In addition, Sm (10–50 μg/mL) and Ac (10 μg/mL) caused significant reduction in the parasite's movements, showing no significant cytotoxicity to Vero cells at the same range of schistosomicidal concentrations. Confocal laser scanning microscopy revealed that Sm and Ac caused tegumental damages and reduced the numbers of tubercles of male schistosomes. Chromatographic fractionation of Sm leads to isolation of bauerenol, α-amirin, and spinasterol, while populifolic acid, cubebin, 2-oxopopulifolic acid methyl ester, and 2-oxopopulifolic acid were isolated from Ac. At concentrations of 25–100 μM, bauerenol, α-amirin, spinasterol, populifolic acid, and cubebin showed significant impact on motor activity of S. mansoni. 2-oxopopulifolic acid methyl ester and 2-oxopopulifolic acid caused 100% mortality and decreased the motor activity of adult schistosomes at 100 μM. This study has reported, for the first time, the in vitro antischistosomal effects of S. microglossa and A. cymbifera extracts, also showing promising compounds against adult schistosomes.
1. Introduction
Schistosomiasis, a chronic parasitic disease caused by helminth flatworms of the genus Schistosoma, afflicts over 250 million people worldwide [1–3], being the second-most important human parasitic disease in terms of public health [1, 4]. There is currently no vaccine for schistosomiasis, and chemotherapy relies on one drug only, praziquantel (PZQ) [2, 5]. Although PZQ is safe, it exhibits lack of activity against juvenile worms and limited effects on liver and spleen lesions, and its use over the last decades may contribute to emerging PZQ-resistance development [3, 6]. Therefore, the lack of any other effective and safe schistosomicidal compounds has raised the urgent need for new antischistosomal drugs that could either complement or replace PZQ chemotherapy [7]. As a result, the search for antischistosomal compounds, especially from natural sources, has been increased [2].
In this regard, several Solidago (Asteraceae) species are used in folk medicines of all over the world for many medicinal purposes, including as antiparasitic and antiseptic [8, 9]. Solidago microglossa De Candolle (Asteraceae), synonymy S. chilensis Meyen, is a medicinal plant known as “arnica-do-campo” and “erva-lanceta” that possess a very widely popular therapeutic applications in South America, including as anti-inflammatory [10, 11] and anthelmintic [12, 13]. Due to its high medicinal importance in Brazil, since 2009, S. microglossa is included in the National List of Medicinal Plants of Interest to the Brazilian Unified Health System (RENISUS) [10, 11, 13].
Also, in Brazil, mainly in the South States, the roots decoctions of S. microglossa are popularly used as anthelmintic for the complementary and alternative treatment of some parasitic diseases [13–16]. Although S. microglossa roots are employed for the treatment of helminthiasis, this popular indication against helminths, such as Schistosoma, which arise from the traditional knowledge, has not been supported by any scientific evidence so far.
In addition, studies have shown that the roots decoctions of some Aristolochia species known as “cipó-mil-homens,” such as A. triangularis and A. cymbifera, with suggested oral doses varying of 0.1 to 2 mL up to three times daily, have been used in the traditional medicine of South America as anthelmintic and for the treatment of malaria and general infectious [8, 9, 14–19]. A. cymbifera Mart. & Zucc (Aristolochiaceae), synonymy A. esperanzae Kuntze, is a medicinal plant used in Brazilian folk medicine for the treatment of infectious diseases, malaria, wounds, fever, diarrhea, snakebite, and as anthelmintic [8, 9, 14, 16–19]. Previous studies showed antibacterial, antifungal, trypanocidal, and antileishmanial activities for A. cymbifera extracts [20–22]. Due to its significance in the traditional medicine, A. cymbifera is included in the first edition of the Brazilian Official Pharmacopoeia [23]. Nowadays, the roots of A. cymbifera are sold in several Brazilian markets, either alone or in combination with other plants, as herbal remedies for the treatment of helminthiasis, such as schistosomiasis and general infections [8, 9, 17, 19]. However, despite its medicinal use against helminths, A. cymbifera have not been evaluated against Schistosoma.
Then, based on their traditional use as anthelmintic, we wondered whether the roots of S. microglossa and A. cymbifera and their isolated compounds possess effects against S. mansoni. Thus, in this work, we evaluated the in vitro antischistosomal effects of the crude extracts and isolated compounds from the roots of S. microglossa and A. cymbifera.
2. Materials and Methods
2.1. Plant Material and Extraction
This study was developed in line with Brazilian Federal Law number 13.123/2015 on Access to Genetic Heritage, registered under number AE32DB3. Roots of S. microglossa (650 g) and A. cymbifera (1300 g) were collected at Faculty of Pharmacy's garden from the Federal University of Juiz de Fora. Exsiccates of the plant species were deposited in the Herbarium Leopoldo Krieger of the Federal University of Juiz de Fora, MG, Brazil, under the numbers #64488 (S. microglossa) and #50054 (A. cymbifera). After collected, roots were dried at 40°C, pulverized, and extracted, by maceration, using ethanol : water (8 : 2 v/v) as solvent. Next, the solvent was removed under reduced pressure to yield 15 g of the crude hydroalcoholic extract of the roots of S. microglossa (Sm) and 40 g of the hydroalcoholic extract from the roots of A. cymbifera (Ac).
2.2. Isolation and Identification of Compounds
The crude extract Sm (10 g) was chromatographed over silica gel (70–230 mesh, Merck) under the vacuum liquid chromatography system (VLC, glass columns with 5–10 cm id), using hexane: EtOAc mixtures in increasing proportions as eluent, furnishing nine fractions. The resulting fraction IV (hexane: EtOAc 85 : 15; 1.2 g) was submitted to classic column chromatography over silica gel, using hexane: EtOAc in increasing proportions as eluent, furnishing the compounds 1 (0.23 g), 2 (0.20 g), and 3 (0.05 g).
In addition, the crude extract Ac (35 g) was firstly chromatographed over silica gel (VLC system, 70–230 mesh, Merck glass columns with 5–10 cm i.d) using hexane: EtOAc mixtures to furnish 8 fractions. Next, fraction II (Ac. II, hexane: EtOAc 9 : 1; 10.2 g) was additionally chromatographed over VLC system with silica gel with hexane: EtOAc mixtures as eluent, giving 6 subfractions, affording 4 (1.7 g, from fraction Ac. II.d). Also, fraction V was submitted to flash chromatography using DCM: EtOAc (97 : 3, v/v) as eluent, furnishing the compound 5 (0.07 g). Fraction VI was submitted to semipreparative reverse-phase HPLC purification (HPLC-DAD Waters 2998), binary HPLC pump, column ODS 250 × 10 mm, 5 μm, UV-DAD detector at 220 nm) using MeOH-H2O 75 : 25 v/v as mobile phase, affording 6 (0.02 g) and 7 (0.01 g).
Chemical structures of all compounds were established by 1H- and 13C-NMR analysis in comparison with the literature. 1H- and 13C- NMR spectra were recorded in CDCl3 solutions on a Bruker 500 Advance spectrometer (500 MHz for 1H NMR and 125 MHz for 13C NMR) with chemical shifts (δ) reported in parts per million (ppm) relative to trimethylsilane (TMS) as internal standard and coupling constants (J) in Hertz (Hz). The purity of all isolated compounds was predicted to be higher than 95% by 13C- and 1H-NMR data analysis.
Compound 1 (baurenol): the NMR spectroscopic data are according to the literature [24]. 1H NMR (500 MHz, CDCl3) δ (ppm): 3.24 (1H, s, OH); 5.40 (2H, m, H-7 and H8). 13C NMR (125 MHz, CDCl3) δ (ppm): 37.0 (C-1); 27.8 (C-2); 79.4 (C-3); 39.0 (C-4); 50.5 (C-5); 24.3 (C-6); 116.5 (C-7); 145.4 (C-8); 48.3 (C-9); 35.3 (C-10); 16.9 (C-11); 32.5 (C-12); 37.8 (C-13); 41.3 (C-14); 28.9 (C-15); 37.8 (C-16); 32.1 (C-17); 55.0 (C-18); 35.4 (C-19); 32.1 (C-20); 29.3 (C-21); 31.6 (C-22); 27.6 (C-23); 14.8 (C-24); 13.1 (C-25); 23.5 (C-26); 22.8 (C-27); 39.7 (C-28); 25.7 (C-29); 22.7 (C-30).
Compound 2 (α-amirin): the NMR spectroscopic data are according to the literature [25]. 1H NMR (500 MHz, CDCl3) δ (ppm): 4.5 (1H, s, OH); 5.12 (1H, dt, H-12). 13C NMR (125 MHz, CDCl3) δ (ppm): 38.1 (C-1); 27.8 (C-2); 79.4 (C-3); 39.0 (C-4); 55.0 (C-5); 18.5 (C-6); 32.5 (C-7); 41.3 (C-8); 48.3 (C-9); 37.0 (C-10); 23.8 (C-11); 124.5 (C-12); 139.7 (C-13); 41.6 (C-14); 28.2 (C-15); 26.3 (C-16); 33.8 (C-17); 59.2 (C-18); 38.9 (C-19); 39.8 (C-20); 31.4 (C-21); 41.6 (C-22); 29.0 (C-23); 15.7 (C-24); 15.8 (C-25); 16.9 (C-26); 23.5 (C-27); 28.8 (C-28); 16.9 (C-29); 21.5 (C-30).
Compound 3 (spinasterol): the NMR spectroscopic data are according to the literature [26]. 1H NMR (500 MHz, CDCl3) δ (ppm): 3.60 (1H, s, OH); 5.02 (1H, dd, J1 = 8.0 Hz and J2 = 15.0 Hz, H-23); 5.15 (2H, m, H-7 and H-22). 13C NMR (125 MHz, CDCl3) δ (ppm): 37.2 (C-1); 31.6 (C-2); 71.2 (C-3); 38.1 (C-4); 40.4 (C-5); 29.7 (C-6); 117.6 (C-7); 139.7 (C-8); 49.5 (C-9); 34.3 (C-10); 21.5 (C-11); 39.6 (C1-2); 43.4 (C-13); 55.2 (C-14); 23.1 (C-15); 28.6 (C-16); 56.0 (C-17); 12.2 (C-18); 13.1 (C-19); 40.9 (C-20); 21.5 (C-21); 138.3 (C-22); 129.5 (C-23); 51.4 (C-24); 32.0 (C-25); 21.2 (C-26); 21.2 (C-27); 25.5 (C-28); 12.4 (C-29).
Compound 4 (populifolic acid): the NMR spectroscopic data are according to the literature [27]. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.71 (3H, s, H-20); 0.79 (3H, d, J = 6.5 Hz, H-17); 0.98 (3H, d, J = 7.2 Hz, H-16); 1.00 (3H, s, H-18); 1.57 (3H, d, J = 1.7 Hz, H-19); 5.18 (1H, s, H-3). 13C NMR (125 MHz, CDCl3) δ (ppm): 18.2 (C-1); 26.8 (C-2); 120.3 (C-3); 144.4 (C-4); 38.1 (C-5); 36.8 (C-6); 27.5 (C-7); 36.2 (C-8); 38.5 (C-9); 46,4 (C-10); 35.4 (C-11); 29.4 (C-12); 30.8 (C-13); 41.4 (C-14); 179.2 (C-15, C=O); 19.9 (C-16); 15.9 (C-17); 19.8 (C-18); 17.9 (C-19); 18.4 (C-20).
Compound 5 (cubebin): the NMR spectroscopic data are according to the literature [28]. 1H NMR (500 MHz, CDCl3) δ (ppm): 2.01 (1H, m, H-8); 2.43 (2H, m, H-7 and H-8′); 2.69 (1H, m, H-7′); 4.11 (1H, t, J = 7.8 Hz, H-9′a); 3.59 (1H, t, J = 7.8 Hz, H-9′b); 5.24 (1H, s, H-9); 5.94 (4H, s, 2x OCH2O); 6.63 (6H, m, H-2, H-5, H-6 and H-2′, H-5′, H-6′). 13C NMR (125 MHz, CDCl3) δ (ppm): 133.2 (C-1); 108.9 (C-2); 147.6 (C-3); 145.7 (C-4); 108.2 (C-5); 121.4 (C-6); 38.4 (C-7); 53.0 (C-8); 103.3 (C-9); 134.1 (C-1′); 109.1 (C-2′); 147.5 (C-3′); 145.9 (C-4′); 108.0 (C-5′); 121.7 (C-6′); 39.2 (C-7′); 45.8 (C-8′); 72.1 (C-9′); 100.8 (2 x OCH2O).
Compound 6 (2-oxopopulifolic acid methyl ester): the NMR spectroscopic data are according to the literature [29–31]. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.65 (6 H, m, H-17 and H-20); 0.96 (3 H, d, J = 6.0 Hz, H-16); 1.06 (3H, s, H-19); 1.80 (3H, s, H-18); 3.67 (3H, s, OCH3); 5.65 (1H, s, H-3). 13C NMR (125 MHz, CDCl3) δ (ppm): 35.4 (C-1); 188.9 (C-2, C=O); 137.2 (C-3); 172.2 (C-4); 38.1 (C-5); 35.2 (C-6); 26.3 (C-7); 37.5 (C-8); 38.8 (C-9); 50.9 (C-10); 35.1 (C-11); 32.2 (C-12); 30.9 (C-13); 38.1 (C-14); 178.6 (C-15, CO2H); 19.9 (C-16); 15.2 (C-17); 18.4 (C-18); 19.6 (C-19); 17.3 (C-20); 26.3 (OCH3).
Compound 7 (2-oxopopulifolic acid): the NMR spectroscopic data are according to the literature [29–31]. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.80 (3H, s, H-20); 0.96 (3H, d, J = 6.0 Hz, H-16); 1.10 (6H, s, H-17 and H-19); 1.87 (3H, s, H-18); 5.70 (1H, s, H-3); 7.60 (1H, s, COOH). 13C NMR (125 MHz, CDCl3) δ (ppm): 34.2 (C-1); 189.0 (C-2, C=O); 137.3 (C-3); 172.2 (C-4); 41.5 (C-5); 37.3 (C-6); 26.2 (C-7); 37.2 (C-8); 37.9 (C-9); 50.0 (C-10); 34.2 (C-11); 35.2 (C-12); 31.0 (C-13); 41.5 (C-14); 172.2 (C-15, CO2H); 20.0 (C-16); 15.4 (C-17); 17.8 (C-18); 18.3 (C-19); 17.3 (C-20).
2.3. Antischistosomal Assays
2.3.1. Maintenance of the S. mansoni Life Cycle
Schistosoma mansoni (BH strain) was maintained by passage through Biomphalaria glabrata, as the intermediate host and Swiss female mice (Anilab, São Paulo, Brazil) as definitive host as previously described [32]. Both mice and snails were kept under environmentally controlled conditions (temperature, 25°C; humidity, 50%), with unrestricted access to rodent food and water.
2.3.2. In Vitro Antischistosomal Assay
Seven weeks after infection, S. mansoni were removed from the hepatic portal system and cultured in RPMI 1640 culture medium (supplemented with 5% inactivated fetal calf serum and 100 U/mL penicillin and 100 μg/mL streptomycin (Vitrocell, Campinas, SP, Brazil) at 37°C in an atmosphere of 5% CO2 until usage. For the determination of activity against adult schistosomes, all samples were initially tested at the concentration of 100 μM (compounds) or 100 μg/mL (extracts), using DMSO stock solutions (10 mM) diluted in supplemented RPMI 1640 medium within 24 flat bottom well plates (Tissue Culture Plastics, TPP, St. Louis, MO) with a final volume of 2 mL per well [33]. Samples were tested in triplicate with two worms of both sexes placed into each well. Wells with the highest concentration of DMSO in medium (0.5%) served as controls. Praziquantel (2 μM) served as positive control. Parasites were kept for 48 h (37°C, 5% CO2), and their viability was assessed via microscopic readout (Leica Microsystems, Wetzlar, Germany) [34]. Next, samples presenting antischistosomal activity were tested at lower concentrations, as described above, and each experiment was performed at least three times [35].
2.3.3. Microscopy Studies
Adult schistosomes were monitored by light microscopy using a Leica Microsystems EZ4E (Wetzlar, Germany) [34, 36]. Also, tegumental alteration and quantification of the number of tubercles were performed in adult male schistosomes for Sm and Ac (10, 25, 50, and 100 μm) using a confocal laser scanning microscope (Laser Scanning Microscope, LSM 510 META, Carl Zeiss, Göttingen, Vertrieb, Germany). For confocal studies, after the occurrence of death, helminths were fixed in a formalin-acetic acid-alcohol solution and analyzed under a confocal microscope with autofluorescence excited at 488 nm and emitted light at 505 nm [36].
2.3.4. Assessment of the Schistosome Egg Output
Sexual fitness of adult worms exposed to nonlethal concentrations of samples and parasites were monitored in order to determine the schistosome egg output by counting the number of eggs for five days using an inverted microscope, as previously described [37]. After 48 h of drug exposure and to analyze reversible effect on egg output, the medium containing samples was removed and worms were carefully rinsed with RPMI to prevent separation of the pairs. Then, worms were incubated continuously in the medium without drug and monitored for five days [37].
2.3.5. Ethics Statement
All experiments were conducted in conformity with the Brazilian law for Guidelines for Care and Use of Laboratory Animals (Law 11790/2008). The protocol for experimental design was approved by the Comissão de Ética no Uso de Animais (CEUA), Brazil (Protocols ≠ CEUA, 11.794/08). Animal studies are reported in compliance with the ARRIVE guidelines.
2.4. In Vitro Cytotoxicity Studies
Mammalian Vero cells (African green monkey kidney fibroblast) used in this study were obtained from the American Type Culture Collection (ATCC CCL-81; Manassas, VA) and provided by Instituto Butantan (São Paulo, Brazil). Cytotoxicity of the samples was determined using the MTT assay [35]. The values of cytotoxic concentration reducing 50% of viable cells (CC50) were obtained using GraFit Version 5 software.
2.5. Statistical Analysis
The statistical tests were performed with using Graph Pad Prism software 5.0 (Graphpad software Inc., La Jolla, CA, USA). Significant differences were determined by one-way analysis of variance (ANOVA) and applying Tukey's test for multiple comparisons with a level of significance set at P < 0.05.
3. Results and Discussion
Schistosomiasis is a neglected disease with a huge impact in public health. Also, there is only one available drug to treat schistosomiasis, and due to the urgent need to identify new drugs, several natural compounds have been recently investigated against S. mansoni [2, 38]. In this regard, WHO encouraged the study and development of new pharmaceutical products on medicinal plants, especially in underdeveloped countries, as a relevant approach for the experimental treatment of schistosomiasis [39, 40].
In this context, we have highlighted the in vitro antischistosomal activity of S. microglossa and A. cymbifera extracts and their isolated compounds against S. mansoni, which have not been reported in the literature.
According to the literature [37, 39, 40], in vitro assays are essential tools to the initial selection of a potential anthelmintic drug. Then, after preparation, the crude extracts Sm and Ac were assayed against S. mansoni. Effects on mortality rate and motor activity of parasites after incubation with Sm and Ac, at concentrations of 10–200 μg/mL, are shown in Table 1. Sm (200 μg/mL) and Ac (100–200 μg/mL) were lethal to all male and female worms at the 24 h incubation, while Sm (100 μg/mL) and Ac (25–50 μg/mL) caused death in half of adult worms and significant reduction in motor activity together with tegumental alterations. In addition, concentrations of 10–50 μg/mL of Sm and 10 μg/mL of Ac were not lethal to schistosomes but caused significant reduction in the parasite's movements (Table 1). Furthermore, Sm and Ac showed no significant cytotoxicity to Vero cells at the same range of schistosomicidal concentrations, as shown in Table 1.
Table 1.
In vitro schistosomicidal and cytotoxic activities of Sm and Ac.
| Groups | Dead worms (%)a,c | Motor activity reduction (%)a | Worms with tegumental alterations (%)a | Cytotoxicity CC50 (μg/mL)d | ||
|---|---|---|---|---|---|---|
| Slight | Significant | Partial | Extensive | |||
| Controlb | — | — | — | — | — | — |
| PZQ (2 μM) | 100 | — | 100 | — | 100 | — |
| DMSO 0.5% | — | — | — | — | — | — |
| Smc | >200 | |||||
| 200 | 100 | — | 100 | — | 100 | |
| 100 | 50 | — | 100 | — | 100 | |
| 50 | — | 50 | 50 | 50 | 50 | |
| 25 | — | 50 | 50 | 50 | 50 | |
| 10 | — | — | — | — | — | |
| Acc | >200 | |||||
| 200 | 100 | — | 100 | — | 100 | |
| 100 | 100 | — | 100 | — | 100 | |
| 50 | 50 | 50 | 50 | 50 | 50 | |
| 25 | 25 | 50 | 50 | 50 | 50 | |
| 10 | — | 25 | — | |||
aPercentages relative to the 20 worms investigated. bRPMI 1640. cIncubation period: 24 h with concentrations in μg/m. dCC50 values (50% cytotoxicity concentration) on Vero cells.
In addition, given the importance of the worm's tegument in the action of new drugs, confocal laser microscopy studies were performed to evaluate morphological damages, tegument structures, and their alterations at the male surface of worms exposed to the plant extracts Sm and Ac (Figure 1). Along with dead, treatment with Sm (100–200 μg/mL) and Ac (100–200 μg/mL) (Figure 1) also caused evident damage at tegument of male schistosomes, in which destruction of tubercles was observed in a dose-dependent manner (Figure 1). Additionally, male adult schistosomes treated with Sm (200 μg/mL) and Ac (200 μg/mL) showed apparent rupture and/or disintegration of tubercles, which appeared eroded and deformed along the surface of worms, while nontreated adult worms showed intact surface (Figure 1).
Figure 1.
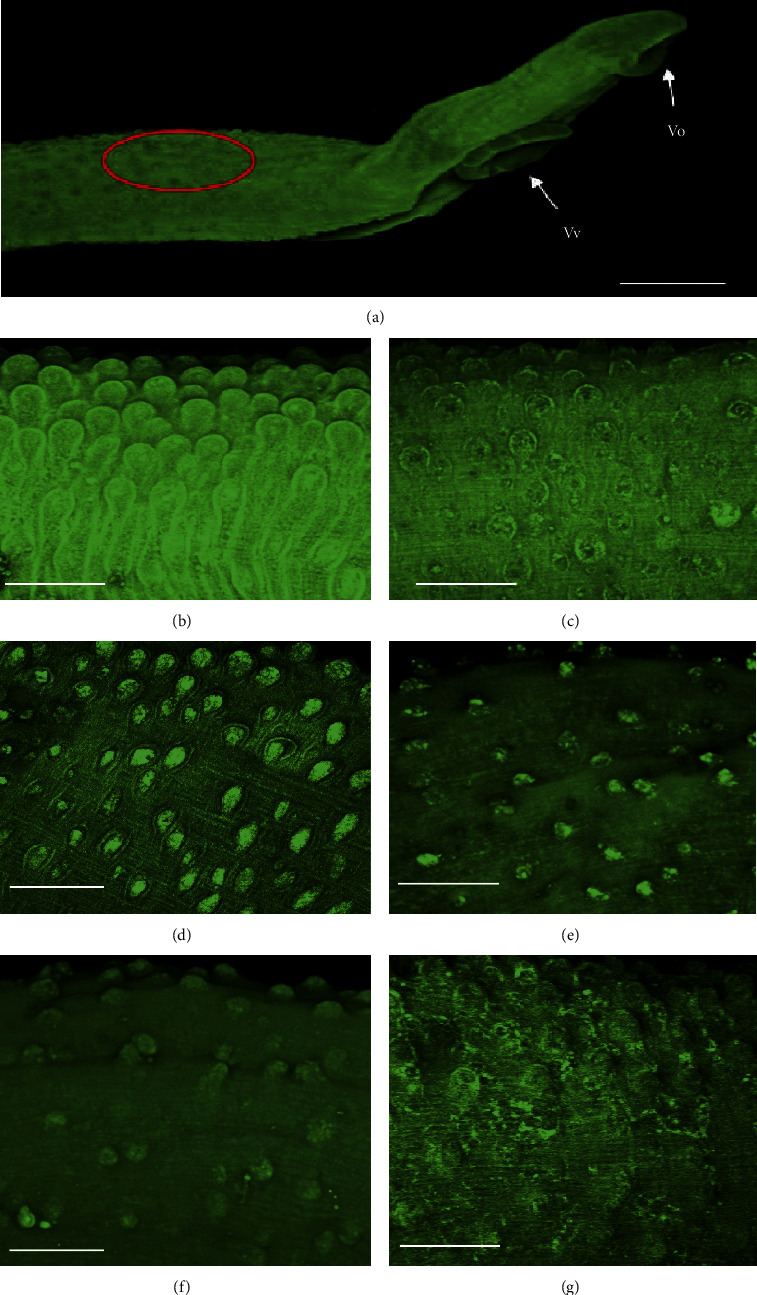
Confocal laser scanning microscopy observations of S. mansoni male worms after in vitro incubation with Sm and Ac. (a) General view of the anterior worm region showing, in red, the location where tegument was analyzed. (b) Control containing RPMI 1640 with DMSO 0.5%. (c) 2 μM PZQ. (d) Sm 100 μg/mL. (e) Sm 200 μg/mL. (f) Ac 100 μg/mL. (g) Ac 200 μg/mL. Scale bars, 200 μm.
Schistosoma' tegument of is well-recognized as an important drug target and model of study in schistosomiasis [41]. In this regard, tegument is pivotal for the survival of Schistosoma not only because it is one of the major route for nutrient absorption, but also because it is important for the protection of schistosomes, since tegumental changes might result in exposure of parasite antigens to the host immune system [41, 42].
Furthermore, a quantitative analysis of the number of intact tubercles on male parasites was performed (Figure 2). Results showed dose-response effects by Sm (Figure 2(a)) and Ac (Figure 2(b)) on the tubercles of the male worm teguments. Remarkable, after exposure to 200 μg/mL of Sm (Figure 2(a)) a reduction was observed in the intact tubercles of 82.2% (P < 0.001), while PZQ (5 μM) and Ac (50 μg/mL) caused 71.1% (P < 0.001) and 64.4% (P < 0.001) of reduction.
Figure 2.
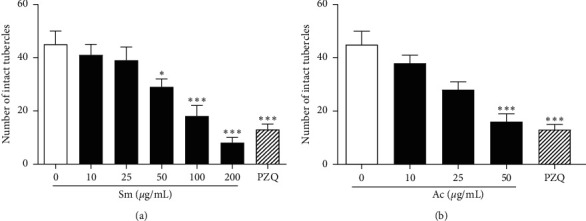
Effect of Sm and Ac on tubercles of male schistosomes. Quantification was performed using confocal microscopy. Intact tubercles was measured in a 20,000 μm2 of area calculated with the Zeiss LSM Image Browser software. Praziquantel (PZQ, 2 μM) was used as reference compound. A minimum of three tegument areas of each parasite were assessed. Values are mean ± SD (bars) of ten male adult worms. ∗P < 0.05 and ∗∗∗P < 0.001 compared with untreated groups.
In addition, the schistosomicidal activity can also be assessed by the ability of samples in suppressing female oviposition [43]. Regarding egg production, groups of parasites were incubated with Sm and Ac, and the number of eggs by adult worms of S. mansoni was monitored for 120 hours (Figure 3). The egg production in S. mansoni adult females was inhibited significantly after 48 hours exposure of Sm (25–50 μg/mL) and after 120 hours with Ac (10 μg/mL) (Figure 3). Following 120 hours exposure with Sm (50 μg/mL) and Ac (10 μg/mL), egg laying was decreased significantly in 65.9% and 27.5% in comparison to the negative control group. Results showed a suppression of egg laying in all sublethal concentrations of Sm and Ac. These are important observed antischistosomal effects, since the pathology of human schistosomiasis is directly associated to the large number of eggs, which become trapped in the hosts tissues, resulting in immunopathological lesions that are characterized by inflammation and fibrosis in the target organs [44]. Other plant extracts active on the sexual reproductive fitness of schistosomes are from the leaves of Clerodendrum umbellatum (Verbenaceae) [45] and from the roots of Zingiber officinale (Zingiberaceae) [46].
Figure 3.
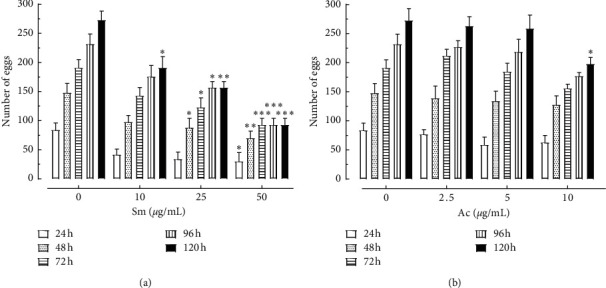
Effect of Sm and Ac on oviposition of S. mansoni. Adult worm couples were incubated with nonlethal concentrations of Sm and Ac and, at the indicated time periods, and the cumulative number of eggs was assessed using an inverted microscope. Values are mean ± SD (bars) of ten worm couples. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 compared with untreated groups.
Additionally, Sm was submitted to chromatographic fractionation, yielding three isolated compounds (Figure 4), which were chemically identified by 13C- and 1H- NMR data analysis in comparison to those in the literature as follows: baurenol (1) [24], α-amirin (2) [25], and spinasterol (3) [26]. Similarly, populifolic acid (4) [27], cubebin (5) [28], 2-oxopopulifolic acid methyl ester (6) [29–31], and 2-oxopopulifolic acid (7) [29–31] were isolated after chromatographic fractionation of Ac (Figure 4). Purity of all isolated compounds was predictable to be higher than 95% by 13C- and 1H- NMR data analysis.
Figure 4.
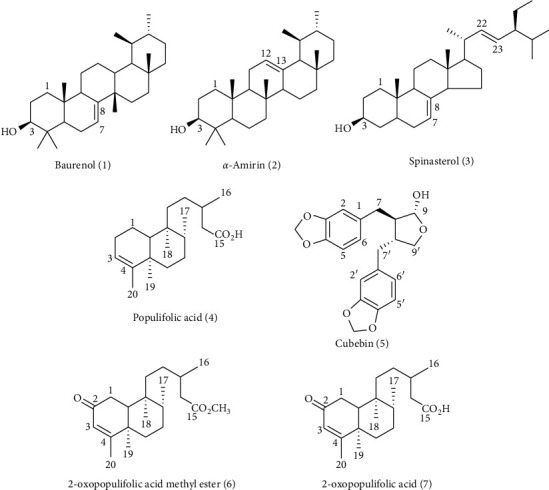
Chemical structures of compounds isolated from the crude extracts of S. microglossa (1–3) and A. cymbifera (4–7).
All isolated compounds (25–100 μM) were also evaluated against S. mansoni adult worms (Table 2). Regarding compounds from Sm, bauerenol (1), α-amirin (2), and spinasterol (3) were able to decrease the motor activity of adult schistosomes in a dose-dependent manner (Table 1), without causing lethal effects on schistosomes, even when tested at 100 μM. Although bauerenol (1), α-amirin (2), and spinasterol (3) were not lethal to schistosomes, they showed significant impact on worm motor activity of S. mansoni, like the crude Sm extract.
Table 2.
In vitro schistosomicidal and cytotoxic activities of isolated compounds from Sm and Ac.
| Groups | Dead worms (%)a, | Motor activity reduction (%)a | Worms with tegumental alterations (%)a | Cytotoxicity CC50 (μM)d | ||
|---|---|---|---|---|---|---|
| Slight | Significant | Partial | Extensive | |||
| Controlb | — | — | — | — | — | — |
| PZQc 2 μM | 100 | — | 100 | 25 | 75 | — |
| DMSO 0.5% | — | — | — | — | — | — |
| Compoundsc | ||||||
| α-Amirin (1) | >100 | |||||
| 100 | — | — | 100 | — | — | |
| 50 | — | 50 | 50 | — | — | |
| 25 | — | — | — | — | — | |
| Baurenol (2) | >100 | |||||
| 100 | — | — | 100 | — | — | |
| 50 | — | 50 | 50 | — | — | |
| 25 | — | — | — | — | — | |
| Spinasterol (3) | >100 | |||||
| 100 | — | 100 | — | — | — | |
| 50 | — | — | — | — | — | |
| 25 | — | — | — | — | — | |
| Populifolic acid (4) | >100 | |||||
| 100 | — | — | 100 | — | — | |
| 50 | — | — | — | — | — | |
| 25 | — | — | — | — | — | |
| Cubebin (5) | >100 | |||||
| 100 | — | — | 100 | — | — | |
| 50 | — | 50 | 50 | — | — | |
| 25 | — | 50 | 50 | — | — | |
| 2-oxopopulifolic acid methyl ester (6) | >100 | |||||
| 100 | 100 | — | 100 | 100 | — | |
| 50 | 50 | — | 100 | — | — | |
| 25 | — | 50 | — | — | — | |
| 2-oxopopulifolic acid (7) | >100 | |||||
| 100 | 100 | — | 100 | 100 | — | |
| 50 | 50 | — | 100 | — | — | |
| 25 | — | 50 | — | — | — | |
aPercentages relative to the 20 worms investigated. bRPMI 1640. cIncubation period: 24 h, tested at μM. dCC50 values CC50 values (50% cytotoxic concentration) on Vero cells.
Furthermore, along with the isolated compounds from Ac, populifolic acid (4) and cubebin (5) were not lethal at concentrations of 25–100 μM but caused significant reduction in motor activity and movements of parasites (Table 2). Populifolic acid (4) and cubebin (5) have been previously isolated from A. cymbifera [18, 29], while populifolic acid (4) is a diterpene widely found in Aristolochia species [18]. In addition, cubebin (5) is a lignan previously evaluated against S. mansoni. Recently, Parreira et al., showed that cubebin (5) (at 100 μM) can separate adult worm pairs and reduce egg laying, without causing death in adult schistosomes [47]. PZQ (2 μM) was lethal to 100% of the worms after 24 h of incubation, while all worms incubated in RPMI-1640 stayed alive until the end of the experiment.
On the other hand, 2-oxopopulifolic acid methyl ester (6) and 2-oxopopulifolic acid (7) showed the best in vitro antischistosomal activity, causing death and decrease of motor activity in all adult schistosomes at 100 μM after 24 h of incubation (Table 2). Results suggested dose-response effects by 2-oxopopulifolic acid methyl ester (6) and 2-oxopopulifolic acid (7) on the mortality rate of schistosomes (Table 2). 2-Oxopopulifolic acid methyl ester (6) and 2-oxopopulifolic acid (7) have been previously isolated from Aristolochia species [18]. Previous report showed that 2-oxopopulifolic acid (7) isolated from A. cymbifera showed antimicrobial activity against Staphylococcus sp and Pseudomonas aeruginosa [48, 49].
Diterpenes are a class of plant-derived compounds that display a broad spectrum of biological activities, including antiparasitic effects against parasites of neglected tropical diseases [50]. Previous reports show that some diterpenes possess schistosomicidal activity against S. mansoni [33], such as pimaradienoic acid [51], isolated from Viguiera Arenaria (Asteraceae) and 7-ceto-sempervirol, obtained from Lycium chinense [52]. Also, although the knowledge about the schistosomicidal properties of diterpenes is limited, the scientific literature has pointed out that some diterpenes may be potentially employed as prototypes for further in vitro and in vivo investigations against S. mansoni, such as the diterpene phytol [32] and other acid diterpenes from Copaiba species [53].
The schistosomicidal results of methyl-2-oxopopulifoloate (6) and 2-oxopopulifolic acid (7) suggest that these diterpenes may be important candidates for further antischistosomal investigations.
4. Conclusion
In this study, we have reported, for the first time, the in vitro antischistosomal effects of S. microglossa and A. cymbifera extracts, with no cytotoxicity on mammalian cells. Also, we have isolated and identified compounds from these active extracts that demonstrate in vitro properties against adult schistosomes. Finally, our findings identified some diterpenes as promising lead antischistosomal compounds to further antiparasitic investigations.
Acknowledgments
The authors are grateful to FAPEMIG (Grants PPM-00296-16 and APQ 02015/14), CNPq (Grant 487221/2012-5), and FAPESP (Grant 2016/22488-3) for financial support as well as to PIBIC/CNPq/UFJF, CAPES, and CNPq for fellowships. They are also grateful to Mr. Jefferson S. Rodrigues for excellent technical assistance with S. mansoni life cycle maintenance at the Adolfo Lutz Institute (São Paulo, SP, Brazil). They also thank Dr. Henrique K. Roffato and Dr. Ronaldo Z. Mendonça (Butantan Institute, São Paulo, SP, Brazil) for expert help with confocal microscope studies (Grant number 00/11624-5). The authors are also grateful to Lorena R. Riani, Lívia M. Silva, and Carolina M. Gasparetto (CENTRALBIO/Faculdade de Farmácia/UFJF) for technical assistance.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.de Moraes J., Geary T. G. FDA-approved antiparasitic drugs in the 21st century: a success for helminthiasis? Trends in Parasitology. 2020;36(7):573–575. doi: 10.1016/j.pt.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Lago E. M., Xavier R. P., Teixeira T. R., Silva L. M., Da Silva Filho A. A., de Moraes J. Antischistosomal agents: state of art and perspectives. Future Medicinal Chemistry. 2018;10(1):89–120. doi: 10.4155/fmc-2017-0112. [DOI] [PubMed] [Google Scholar]
- 3.Vale N., Gouveia M. J., Rinaldi G., Brindley P. J., Gärtner F., da Costa J. M. C. Praziquantel for schistosomiasis: single-drug metabolism revisited, mode of action, and resistance. Antimicrobial Agents and Chemotherapy. 2017;61(5):1–16. doi: 10.1128/aac.02582-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tucker M. S., Karunaratne L. B., Lewis F. A., Freitas T. C., Liang Y. Schistosomiasis. Current Protocols in Immunology. 2013;103(1):19.1.1–19.1.58. doi: 10.1002/0471142735.im1901s103. [DOI] [PubMed] [Google Scholar]
- 5.Doenhoff M. J., Sabah A. A. A., Fletcher C., Webbe G., Bain J. Evidence for an immune-dependent action of praziquantel on Schistosoma mansoni in mice. Transactions of The Royal Society of Tropical Medicine and Hygiene. 1987;81(6):947–951. doi: 10.1016/0035-9203(87)90360-9. [DOI] [PubMed] [Google Scholar]
- 6.Bergquist R., Utzinger J., Keiser J. Controlling schistosomiasis with praziquantel: how much longer without a viable alternative? Infectious Diseases of Poverty. 2017;6(74):1–10. doi: 10.1186/s40249-017-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santiago E. D. F., de Oliveira S. A., de Oliveira Filho G. B., et al. Evaluation of the anti-Schistosoma mansoni activity of thiosemicarbazones and thiazoles. Antimicrobial Agents and Chemotherapy. 2014;58(1):352–363. doi: 10.1128/aac.01900-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bieski I. G. C., Leonti M., Arnason J. T., et al. Ethnobotanical study of medicinal plants by population of valley of Juruena region, Legal Amazon, Mato Grosso, Brazil. Journal of Ethnopharmacology. 2015;173:383–423. doi: 10.1016/j.jep.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro R. V., Bieski I. G. C., Balogun S. O., Martins D. T. D. O. Ethnobotanical study of medicinal plants used by Ribeirinhos in the North Araguaia microregion, Mato Grosso, Brazil. Journal of Ethnopharmacology. 2017;205:69–102. doi: 10.1016/j.jep.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Malpezzi-Marinho E. L. A., Molska G. R., Freire L. I. G. P., et al. Effects of hydroalcoholic extract of Solidago chilensis Meyen on nociception and hypernociception in rodents. BMC Complementary Medicine and Therapies. 2019;19(72):1–9. doi: 10.1186/s12906-019-2478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gastaldi B., Catalán C. A. N., Silva-Sofrás F. M., González S. B. Solidago chilensis Meyen (Asteraceae), a medicinal plant from South America. A comprehensive review: ethnomedicinal uses, phytochemistry and bioactivity. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromaticas. 2018;17(1):17–29. [Google Scholar]
- 12.Vila R., Mundina M., Tomi F., et al. Composition and antifungal activity of the essential oil of Solidago chilensis. Planta Medica. 2002;68(2):164–167. doi: 10.1055/s-2002-20253. [DOI] [PubMed] [Google Scholar]
- 13.Mercandeli A. A., Bessa G. P., Ronchi S. N., Segato T. P. S., Silva A. G. Evidence for the safe use of the extract from the Brazilian arnica, Solidago chilensis Meyen, in primary health care. Chinese Medicine. 2012;68(3):4–8. doi: 10.4236/cm.2012.31002. [DOI] [Google Scholar]
- 14.Stasi L. C. D., Hiruma-Lima C. A. Plantas Medicinais na Amazônia e na Mata Atlântica. São Paulo, Brazil: UNESP; 2002. [Google Scholar]
- 15.Duke J. A. Duke’s Handbook of Medicinal Plants of Latin America. Boca Rato, FL, USA: CRC Press; 2009. [Google Scholar]
- 16.Garlet T. M. B. Plantas Medicinais Nativas de Uso Popular no Rio Grande do Sul. Santa Maria, Brazil: Universidade Federal de Santa Maria; 2019. [Google Scholar]
- 17.Heinrich M., Chan J., Wanke S., Neinhuis C., Simmonds M. S. J. Local uses of aristolochia species and content of nephrotoxic aristolochic acid 1 and 2-a global assessment based on bibliographic sources. Journal of Ethnopharmacology. 2009;125(1):108–144. doi: 10.1016/j.jep.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Wu T.-S., Damu A. G., Su C.-R., Kuo P.-C. Terpenoids of Aristolochia and their biological activities. Natural Product Reports. 2004;21(5):594–624. doi: 10.1039/b401950d. [DOI] [PubMed] [Google Scholar]
- 19.Bitu V. C. N., Bitu V. C. N., Matias E. F. F., et al. Ethnopharmacological study of plants sold for therapeutic purposes in public markets in Northeast Brazil. Journal of Ethnopharmacology. 2015;172:265–272. doi: 10.1016/j.jep.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Tempone A. G., Sartorelli P., Teixeira D., et al. Brazilian flora extracts as source of novel antileishmanial and antifungal compounds. Memórias do Instituto Oswaldo Cruz. 2008;103(5):443–449. doi: 10.1590/s0074-02762008000500006. [DOI] [PubMed] [Google Scholar]
- 21.Alviano W. S., Alviano D. S., Diniz C. G., et al. In vitro antioxidant potential of medicinal plant extracts and their activities against oral bacteria based on Brazilian folk medicine. Archives of Oral Biology. 2008;53(6):545–552. doi: 10.1016/j.archoralbio.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Sartorelli P., Salomone Carvalho C., Quero Reimão J., Lorenzi H., Tempone A. Antitrypanosomal activity of a diterpene and lignans isolated from Aristolochia cymbifera. Planta Medica. 2010;76(13):1454–1456. doi: 10.1055/s-0029-1240952. [DOI] [PubMed] [Google Scholar]
- 23.Brandão M. G. L., Cosenza G. P., Grael C. F. F., Netto Junior N. L., Monte-Mór R. L. M. Traditional uses of American plant species from the 1st edition of Brazilian official pharmacopoeia. Revista Brasileira de Farmacognosia. 2009;19(2):478–487. doi: 10.1590/s0102-695x2009000300023. [DOI] [Google Scholar]
- 24.Raga D. D., Herrera A. A., Shen C. C., Ragasa C. Y. Triterpenes from Ardisia cf. Elliptica (subgenus tinus) limit vascular density and promote von Willebrand factor expression on duck chorioallantoic membrane. Pharmaceutical Chemistry Journal. 2015;49(1):24–29. doi: 10.1007/s11094-015-1216-x. [DOI] [Google Scholar]
- 25.Lima M. P., Braga P. A. C., Macedo M. L., et al. Phytochemistry of Trattinnickia burserifolia, T. rhoifolia, and Dracryodes hopkinsii: chemosystematic implications. Journal of the Brazilian Chemical Society. 2004;15(3):387–394. doi: 10.1590/s0103-50532004000300008. [DOI] [Google Scholar]
- 26.Kokima H., Sato N., Hatano A., Ogura H. Sterol glucides from Prunela vulgaris. Phytochemistry. 1990;70:2351–2355. [Google Scholar]
- 27.Leitào G. G., Kaplan M. A. C., Galeffi C. Epi-populifolic acid from Aristolochia cymbifera. Phytochemistry. 1992;31(9):3277–3279. doi: 10.1016/0031-9422(92)83495-k. [DOI] [Google Scholar]
- 28.Depascoli I., Nascimento I., Lopes L. Configurational analysis of cubebins and bicubebin from Aristolochia lagesiana and Aristolochia pubescens. Phytochemistry. 2006;67(7):735–742. doi: 10.1016/j.phytochem.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 29.Pacheco A. G., Machado de Oliveira P., Piló-Veloso D., Flávio de Carvalho Alcântara A. 13C-NMR data of diterpenes isolated from aristolochia species. Molecules. 2009;14(3):1245–1262. doi: 10.3390/molecules14031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pacheco A. G., Silva T. M., Manfrini R. M., et al. Chemical study and antibacterial activity of stem of Aristolochia esperanzae Kuntze (aristolochiaceae) Química Nova. 2010;33(8):1649–1652. doi: 10.1590/s0100-40422010000800006. [DOI] [Google Scholar]
- 31.Urones J. G., Basabe P., Marcos I. S., et al. Ring a functionalized neo-clerodane diterpenoids from Cistus populifolius. Tetrahedron. 1994;50(36):10791–10802. doi: 10.1016/s0040-4020(01)89271-1. [DOI] [Google Scholar]
- 32.de Moraes J., de Oliveira R. N., Costa J. P., et al. Phytol, a diterpene alcohol from chlorophyll, as drug against neglected tropical disease Schistosomiasis mansoni. PLoS Neglected Tropical Diseases. 2014;8(1) doi: 10.1371/journal.pntd.0002617.e2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mafud A. C., Silva M. P. N., Monteiro D. C., et al. Structural parameters, molecular properties, and biological evaluation of some terpenes targeting Schistosoma mansoni parasite. Chemico-Biological Interactions. 2016;244:129–139. doi: 10.1016/j.cbi.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 34.de Castro C. C. B., Costa P. S., Laktin G. T., et al. Cardamonin, a schistosomicidal chalcone from Piper aduncum L. (Piperaceae) that inhibits Schistosoma mansoni ATP diphosphohydrolase. Phytomedicine. 2015;22(10):921–928. doi: 10.1016/j.phymed.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Carvalho L. S. A., Queiroz L. S., Alves Junior I. J., et al. In vitro schistosomicidal activity of the alkaloid-rich fraction from Ruta graveolens L. (Rutaceae) and its characterization by UPLC-QTOF-MS. Evidence-Based Complementary and Alternative Medicine. 2019;2019:8. doi: 10.1155/2019/7909137.7909137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mafud A. C., Silva M. P. N., Nunes G. B. L., et al. Antiparasitic, structural, pharmacokinetic, and toxicological properties of riparin derivatives. Toxicology in Vitro. 2018;50:1–10. doi: 10.1016/j.tiv.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 37.de Moraes J., Nascimento C., Yamaguchi L. F., Kato M. J., Nakano E. Schistosoma mansoni:in vitro schistosomicidal activity and tegumental alterations induced by piplartine on schistosomula. Experimental Parasitology. 2012;132(2):222–227. doi: 10.1016/j.exppara.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 38.de Brito M. R. M., Peláez W. J., Faillace M. S., et al. Cyclohexene-fused 1,3-oxazines with selective antibacterial and antiparasitic action and low cytotoxic effects. Toxicology in Vitro. 2017;44:273–279. doi: 10.1016/j.tiv.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 39.de Oliveira R. N., Rehder V. L. G., Oliveira A. S. S., Jeraldo V. D. L. S., Linhares A. X., Allegretti S. M. Anthelmintic activity in vitro and in vivo of Baccharis trimera (less) DC against immature and adult worms of Schistosoma mansoni. Experimental Parasitology. 2014;139:63–72. doi: 10.1016/j.exppara.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Tekwu E. M., Bosompem K. M., Anyan W. K., et al. In vitro assessment of anthelmintic activities of Rauwolfia vomitoria (Apocynaceae) stem bark and roots against parasitic stages of Schistosoma mansoni and cytotoxic study. Journal of Parasitology Research. 2017;2017:11. doi: 10.1155/2017/2583969.2583969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guimarães M. A., de Oliveira R. N., de Almeida R. L., et al. Epiisopilosine alkaloid has activity against Schistosoma mansoni in mice without acute toxicity. PLoS One. 2018;13(5):1–19. doi: 10.1371/journal.pone.0196667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao S., Binggui S., Chollet J., Tanner M. Tegumental changes in 21-day-old Schistosoma mansoni harboured in mice treated with artemether. Acta Tropica. 2000;75(3):341–348. doi: 10.1016/s0001-706x(00)00067-x. [DOI] [PubMed] [Google Scholar]
- 43.de Oliveira R. N., Rehder V. L. G., Santos Oliveira A. S., et al. Schistosoma mansoni: in vitro schistosomicidal activity of essential oil of Baccharis trimera (less) DC. Experimental Parasitology. 2012;132(2):135–143. doi: 10.1016/j.exppara.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Costain A. H., MacDonald A. S., Smits H. H. Schistosome egg migration: mechanisms, pathogenesis and host immune responses. Frontiers in Immunology. 2018;9:1–16. doi: 10.3389/fimmu.2018.03042.3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jatsa H. B., Kenfack C. M., Simo D. N., et al. Schistosomicidal, hepatoprotective and antioxidant activities of the methanolic fraction from Clerodendrum umbellatum poir leaves aqueous extract in Schistosoma mansoni infection in mice. BMC Complementary and Alternative Medicine. 2015;15(248):1–9. doi: 10.1186/s12906-015-0788-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanderson L., Bartlett A., Whitfield P. J. In vitro and in vivo studies on the bioactivity of a ginger (Zingiber officinale) extract towards adult schistosomes and their egg production. Journal of Helminthology. 2002;76(3):241–247. doi: 10.1079/joh2002116. [DOI] [PubMed] [Google Scholar]
- 47.Parreira R. L. T., Costa E. S., Heleno V. C. G., et al. Evaluation of lignans from Piper cubeba against Schistosoma mansoni adult worms: a combined experimental and theoretical study. Chemistry and Biodiversity. 2018;16(1):1–13. doi: 10.1002/cbdv.201800305. [DOI] [PubMed] [Google Scholar]
- 48.de Barros Machado T., Leal I. C. R., Kuster R. M., et al. Brazilian phytopharmaceuticals—evaluation against hospital bacteria. Phytotherapy Research. 2005;19(6):519–525. doi: 10.1002/ptr.1696. [DOI] [PubMed] [Google Scholar]
- 49.Kuo P.-C., Li Y.-C., Wu T.-S. Chemical constituents and pharmacology of the Aristolochia (馬兜鈴 mădōu ling) species. Journal of Traditional and Complementary Medicine. 2012;2(4):249–266. doi: 10.1016/s2225-4110(16)30111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alencar M. V. O. B., Sousa J. M. C., Rolim H. M. L., et al. Diterpenes as lead molecules against neglected tropical diseases. Phytotherapy Research. 2017;31(2):175–201. doi: 10.1002/ptr.5749. [DOI] [PubMed] [Google Scholar]
- 51.Porto T. S., da Silva Filho A. A., Magalhães L. G., et al. Fungal transformation and schistosomicidal effects of pimaradienoic acid. Chemistry & Biodiversity. 2012;9(8):1465–1474. doi: 10.1002/cbdv.201100336. [DOI] [PubMed] [Google Scholar]
- 52.Edwards J., Brown M., Peak E., Bartholomew B., Nash R. J., Hoffmann K. F. The diterpenoid 7-keto-sempervirol, derived from Lycium chinense, displays anthelmintic activity against both Schistosoma mansoni and Fasciola hepatica. PLoS Neglected Tropical Diseases. 2015;9(3):1–19. doi: 10.1371/journal.pntd.0003604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borges C. H. G., Cruz M. G., Carneiro L. J., et al. Copaifera duckei oleoresin and its main nonvolatile terpenes: in vitro schistosomicidal properties. Chemistry & Biodiversity. 2016;13(10):1348–1356. doi: 10.1002/cbdv.201600065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


